Abstract
Recently it has been shown that dendritic cells (DC) can develop from peripheral blood monocytes when grown in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). However, it is unclear whether DC can also develop from monocytes in absence of these cytokines. We therefore analyzed the effect of Flt-3 ligand (Flt3L) and of CD40 ligand on the development of human DC from blood monocytes in the absence of GM-CSF. Adherent peripheral blood mononuclear cells (PBMNC) were cultured in the presence of different cytokine combinations and analyzed for the expression of surface molecules and antigen presenting capacity. For functional analyses, cells were tested for their ability to stimulate allogeneic T lymphocytes in a mixed lymphocyte reaction (MLR), to present soluble antigens, and to induce primary HIV-peptide–specific cytotoxic T-cell (CTL) responses in vitro. Furthermore, expression of DC-CK1, a recently identified chemokine with specific expression in DC, and of IL-18 (IGIF), a growth and differentiation factor for Th 1 lymphocytes, was analyzed by reverse-transcription polymerase chain reaction (RT-PCR). In our study, Flt3L alone was not sufficient to generate DC and required addition of IL-4. DC generated with Flt3L and IL-4 underwent maturation after stimulation with tumor necrosis factor- (TNF-) or CD40L, characterized by CD83 expression, upregulation of MHC, adhesion, and costimulatory molecules as well as increased allogeneic proliferative response. In contrast, CD40 ligation alone promoted differentiation of adherent blood monocytes into functional DC in the absence of GM-CSF and IL-4. These cells displayed all phenotypic and functional characteristics of mature DC and were potent stimulatory cells in priming of major histocompatibility complex (MHC) class I–restricted CTL responses against an HIV-peptide, whereas their ability to present soluble protein antigens was reduced. Using a semiquantitative RT-PCR, DC-CK1 and IL-18 transcripts were detected in all generated DC populations, independent of growth factors used. Our findings provide further evidence for the importance of CD40-CD40L interaction for initiation and maintenance of T-cell responses and confirm the emerging concept that blood monocytes provide an additional source of DC depending on external stimuli.
DENDRITIC CELLS (DC) are key regulators in immune responses, capable of priming naive resting T cells and initiating primary T-cell responses when pulsed with antigenic peptides or proteins.1-12 In vitro, DC can be generated from human CD34+ bone marrow, cord blood, and peripheral blood progenitor cells after culture with different cytokine combinations including granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and either interleukin-4 (IL-4) or tumor necrosis factor-α (TNF-α).4 13-18
Recently it was shown that DC can also develop from CD14+blood monocytes when grown in the presence of GM-CSF and IL-4. These cells have the characteristics of immature DC and can be further induced to mature by inflammatory stimuli like TNF-α, IL-1, lipopolysaccharide (LPS), or by monocyte-conditioned medium.19-23
There is experimental evidence from murine studies showing that GM-CSF does not seem to be the major growth factor for DC development because GM-CSF transgenic mice and mice carrying a null allele of the GM-CSF gene do not have aberrant numbers of DC, and other stimuli are sufficient to generate DC from hematopoetic progenitor cells.24 25 However, the conditions for the development of DC are different depending on the species analyzed, and caution is warranted comparing mouse and human DC development.
As shown in a previous report, treatment of mice with Flt3 ligand (Flt3L) resulted in a dramatic numerical increase of functionally mature DC in vivo.26 In vitro, addition of Flt3L to culture medium can increase the yield of DC generated from bone marrow precursors and CD34+ peripheral blood progenitor cells.17,27,28 CD40 ligation of CD34+hematopoetic cells induces their proliferation and differentiation into cells with dendritic phenotype and function.18
The induction of an efficient immune response requires a coordinated collective cell to cell interaction of a variety of cell types, and CD40-CD40L interaction seems to play a central role in antigen presentation, development of T-cell–dependent effector functions, and activation of macrophages and dendritic cells.29-32
We analyzed the effect of CD40L and Flt3L on the development of DC from human peripheral blood monocytes in the absence of GM-CSF. We show that Flt3L alone was not sufficient to induce differentiation of monocytes and required addition of IL-4. In contrast, CD40 ligation alone promoted differentiation of peripheral blood monocytes into functional DC in the absence of GM-CSF and IL-4, thus confirming the importance of CD40 ligation for the induction of primary immune reponses.
MATERIALS AND METHODS
Cell isolation and cultures.
Peripheral blood mononuclear cells (PBMNC) were isolated by Ficoll/Paque (GIBCO-BRL, Grand Island, NY) density gradient centrifugation of heparinized blood obtained from buffy coat of healthy volunteers from the blood bank of the University of Tübingen. These PBMNC were plated (1 × 107 cells/3 mL per well) into 6-well plates (Costar, Cambridge, MA) in RP10 medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum [FCS], 2 mmol/L L-glutamine, 50 μmol/L 2-mercaptoethanol, and antibiotics). For some experiments we used serum-free X-VIVO 20 medium (Bio Whittaker, Walkersville, MD), as indicated in Results. After 2 hours of incubation at 37°C, nonadherent cells were removed and the adherent cells (yield, 19 ± 3% of incubated cells; n = 6) were cultured. The population of the adherent cells remaining in the wells comprised of 94 ± 3.6% CD14+ cells, 4 ± 2.6% CD3+ cells, and 1 ± 1.5% CD19+ cells. The percentage of CD1a+ or CD83+ cells was less than 1%. These cells were used as a starting population and cultured in RP10 medium supplemented with various combinations of cytokines or CD40 ligand transfectants for 7 days.
The following cytokines obtained from Genzyme (Cambridge, MA) were used: IL-4 (1,000 IU/mL), IL-6 (50 ng/mL), and TNF-α (10 ng/mL). Flt3 ligand (100 ng/mL) was purchased from PreproTech (Rocky Hill, NJ) and human recombinant GM-CSF (Leukomax, 100 ng/mL) from Novartis (Basel, Switzerland).
For stimulation of adherent cells with CD40L we used mouse fibroblastic Ltk cells (L cells) transfected with either CD40L (LCD40L) or with CD32 (LCD32) as a control (kindly provided by Schering-Plough, Dardilly, France18 32). Cultured fibroblastic cells were detached, washed, irradiated with 150 Gy, and added to the cultures (5 × 105 cells per well).
The DC cultures were fed with fresh medium and cytokines every other day, and cell differentiation was monitored by light microscopy. The antigen-presenting capacity and expression of cell surface molecules were analyzed after 7 days of culture.
DC, CD34+, and CD14+ cells were purified using MACS isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the cells used in the experiments was 96% to 99% as analyzed by flow cytometry.
To analyze that CD40 ligation results in DC maturation in the absence of GM-CSF, we performed experiments to neutralize any induced GM-CSF using a neutralizing polyclonal antibody obtained from Genzyme (50 μg/mL). The antibody was added every other day for 7 days.
Immunostaining.
Cell staining was performed using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse monoclonal antibodies (MoAbs) against CD86, CD40, CD44, CD33, CD154, and CLA (all purchased from Pharmingen, Hamburg, Germany); CD80, HLA-DR, CD54, CD14, CD3, CD19, CD56 (Becton Dickinson, Heidelberg, Germany); APO-1/FAS (APO 1-3; Alexis, San Diego, CA), HLA-A, -B, -C (W6/32; Dako, Glostrup, Denmark), CD83 (Coulter-Immunotech Diagnostics, Hamburg, Germany), CD1a (OKT6; Ortho Diagnostic Systems Neckargemund, Germany, and T6-RD1; Coulter Immunology, Hialeah, FL), and mouse IgG isotype controls (Becton Dickinson). Samples were analyzed on a FACScan Calibur (Becton Dickinson).
Mixed lymphocyte reaction (MLR) assay.
A total of 105 responding cells either from allogeneic PBMNC was cultured in 96 flat-bottom microplates (Nunc, Wiesbaden, Germany) with 103 irradiated stimulator cells (DC or PBMNC). To use the identical number of DC in the assays, the DC were quantitated based on fluorescence-activated cell sorter (FACS) data (cells expressing CD1a and/or CD83 on the cell surface) and confirmed by counting of the cells after staining with trypan blue under a light microscope. Thymidine incorporation was measured on day 5 by a 16-hour pulse with 3H-thymidine (1 μCi/well; Amersham Life Science, Buckingham, UK).
Soluble protein presentation.
To analyze the ability of generated DC to uptake and to present soluble antigens, 1.5 × 105 PBMNC were incubated with 104 irradiated DC or PBMNC with tetanus toxoid (Behringwerke, Marburg, Germany) in 96-well plates. Each well was obtained after 7 days following a 16-hour pulse with 1 μCi tritiated thymidine. Results are expressed as mean cpm of triplicate wells ± SD.
Induction of antigen-specific cytotoxic T-cell (CTL) response using HLA-A2 restricted synthetic peptides.
The influenza matrix peptide (IMP) 58-66: GILGFVFTL and pol HIV-1 reverse transcriptase peptide (HIV) 476-484, ILKEPVHGV, were synthesized using standard Fmoc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reverse-phase HPLC and mass spectrometry. For CTL induction 5 × 105 DC were pulsed with 25 μg/mL of the synthetic HIV peptide for 2 hours, washed, and incubated with 2.5 × 106 autologous PBMNC in RP10 medium. Cells were restimulated after 7 days of culture and 1 ng/mL human recombinant IL-2 (Genzyme) was added every other day. The cytolytic activity of induced CTL was analyzed on day 5 after the last restimulation in a standard51Cr-release assay.
CTL assay.
The standard 51Cr-release assay was performed with some modifications as described.32 Target cells (T2 cells, 174 × CEM.T2 hybridoma, TAP1 and TAP2 deficient) were pulsed with 25 μg/mL peptide for 2 hours and labeled with [51Cr]-sodium chromate in RP10 for 1 hour at 37°C. 104 cells were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTL were added to give a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay supernatants (50 μL/well) were obtained and counted in a microbeta counter (Wallac, Turku, Finland). The percent specific lysis was calculated as: 100 × (Experimental Release − Spontaneous Release/Maximal Release − Spontaneous Release). Spontaneous and maximal release were determined in the presence of either medium or 1% Triton X-100, respectively.
Reverse-transcription polymerase chain reaction (RT-PCR).
Semiquantitative RT-PCR was performed with some modifications as recently described.33,34 Total RNA was isolated from cell lysates using Qiagen RNeasy anion-exchange spin columns (Qiagen GmbH, Hilden, Germany) according to the instructions of the manufacturer. Five hundred nanograms of total RNA was subjected to first-strand cDNA using an optimized protocol described by Life Technologies (SuperScript Preamplification System, GIBCO-BRL, Eggenstein, Germany), using Oligo(dT) as primer. Two microliters of cDNA obtained from the reverse transcriptase reaction was subjected to the DC-CK1 and IL-18 PCR amplification. To control the integrity of the isolated RNA, 1 μL of cDNA was amplified by an intron-spanning primer pair for the 2-microglobulin gene. There was no sample from which genomic 2-microglobulin DNA sequences were amplified. To further exclude genomic DNA contamination, we analyzed in parallel samples obtained from first-strand cDNA synthesis without addition of reverse-transcriptase enzyme. Primer sequences were deduced from published cDNA sequences: DC-CK ACAAAGAGCTCTGCTGCCTC (sense) and CCCACTTCTTATTGGGGTCA (anti-sense); IL-1837GCTTGAATCTAAATTATCAGTC and GAAGATTCA-AATTGCATCTTAT; IL-12 GAGAAATGGTGGTCCTCACCTGTG and GAG-TGTAGCAGCTCCGCACGTC; β2-microglobulin GGGTTTCATCCATCCGA-CAT and GATGCTGCTTACATGTCTCGA. Each 50-μL PCR reaction contained 1.25 U AmpliTaq DNA polymerase (Perkin Elmer, Weiterstadt, Germany), 200 μmol/L of each dNTP, and 60 pmol of each oligonucleotide primer for the DC-CK1 and IL-18 transcripts and 25 pmol for the 2-microglobulin transcript in PCR buffer (10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.001% gelatin). Reactions were amplified in a DNA thermal cycler (GeneAmp PCR System 2400; Perkin Elmer) for 28 cycles. The temperature profiles were as follows: 5 minutes at 94°C pretreatment, 60°C for 30 seconds annealing for the DC-CK1, and IL-12 primers and 55°C for the IL-18 and β2-microglobulin primers, 72°C for 30 seconds synthesis, and 94°C for 30 seconds denaturation. Finally, a single posttreatment was performed at 72°C for 5 minutes. Ten microliters of the RT-PCR products was electrophoresed through a 3% agarose gel and stained with ethidium bromide for visualization under ultraviolet light. The specificity of the amplified PCR products was confirmed by restriction enzyme digest of the PCR products.
Statistical analysis.
Each experiment was performed at least three times. Representative experiments are shown. Unpaired t-tests were performed to evaluate the significance of the results.
RESULTS
Phenotype of cells differentiating from adherent PBMNC using cytokine cominations including Flt3 ligand (Flt3L).
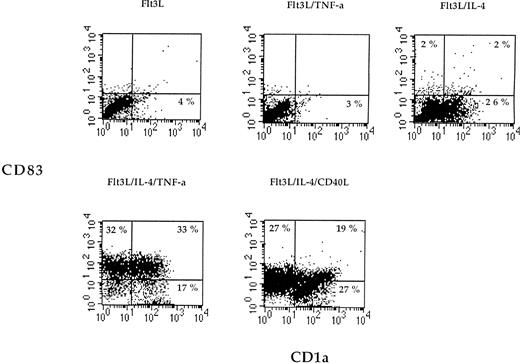
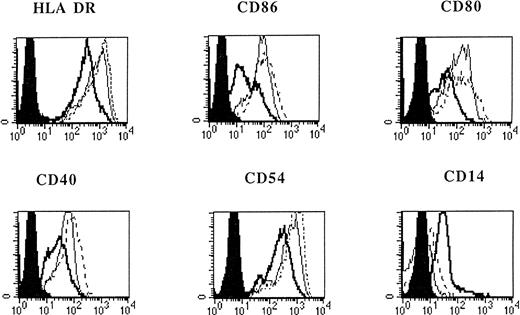
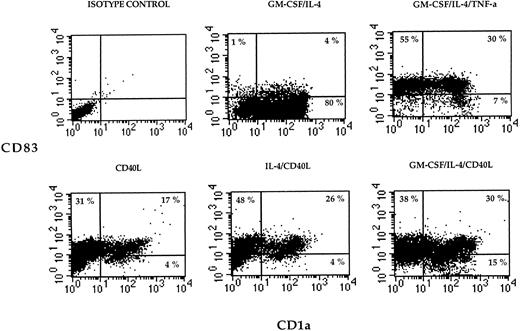
Flt3L alone or in combination with TNF-α was not sufficient to induce differentiation of monocytes (Fig 1). However, when IL-4 was added to the medium containing Flt3L, a marked increase of CD1a expression on cultured cells was detected (Fig 1). When adherent PBMNC were grown in FLT3L and IL-4 and further stimulated by TNF-α or CD40L, the cultured cells showed an increased expression of MHC, costimulatory, and adhesion molecules (Fig 2). The cells became CD83 positive and CD14 low or negative, corresponding to an activated phenotype of DC (Figs 1 and 2). All DC generated from adherent PBMNC expressed FAS/APO-1 (CD95) on cell surface independent of the stimuli used for induction (data not shown).
Two-color dot plot of cultured cells labeled with CD83-PE (y-axis) and CD1a-FITC (x-axis). Adherent PBMNC were cultured with the indicated combinations of cytokines or CD40L for 7 days, and cell surface phenotype was examined by flow cytometry. The numbers indicate the percentage of cells in each quadrant. Data are presented from one representative experiment out of three.
Two-color dot plot of cultured cells labeled with CD83-PE (y-axis) and CD1a-FITC (x-axis). Adherent PBMNC were cultured with the indicated combinations of cytokines or CD40L for 7 days, and cell surface phenotype was examined by flow cytometry. The numbers indicate the percentage of cells in each quadrant. Data are presented from one representative experiment out of three.
Phenotypic analysis of in vitro–generated DC. PBMNC were cultured in presence of Flt3L/IL-4 (bold solid line), Flt3L/IL-4/ TNF- (thin solid line), or Flt3L/IL-4/CD40L (dotted line). Overlay diagrams show expression of indicated molecules after 7 days of culture. Solid histograms: labeling with isotype matched irrelevant MoAb.
Phenotypic analysis of in vitro–generated DC. PBMNC were cultured in presence of Flt3L/IL-4 (bold solid line), Flt3L/IL-4/ TNF- (thin solid line), or Flt3L/IL-4/CD40L (dotted line). Overlay diagrams show expression of indicated molecules after 7 days of culture. Solid histograms: labeling with isotype matched irrelevant MoAb.
CD40 ligation also resulted in a significant increase of absolute numbers of generated DC compared with cultures without CD40L, as shown in Table 1. The presence of CD32 transfectants used as a control in the experiments had no effect on number, phenotype, or function of generated cells (data not shown).
Numbers of DC Generated From Adherent PBMNC
| Cytokines* . | %DC . | (Absolute Numbers, ×103)† . | %CD3+ . | %CD19+ . |
|---|---|---|---|---|
| Flt3L/IL-4 | 31 ± 5.8 | (249 ± 73) | 4 ± 1.2 | 1 ± 1.6 |
| Flt3L/IL-4/TNF-α | 73 ± 9.1 | (884 ± 65) | 3 ± 1.7 | 1 ± 1 |
| Flt3L/IL-4/CD40L | 75 ± 7.0 | (1202 ± 211)‡ | 6 ± 3.1 | 2 ± 1.5 |
| CD40L | 55 ± 10 | (747 ± 102) | 4 ± 1.5 | 1 ± 0.8 |
| CD40L/IL-4 | 71 ± 9.0 | (1087 ± 153)1-153 | 5 ± 1.7 | 4 ± 1 |
| CD40L/IL-4/GM-CSF | 81 ± 11 | (1345 ± 241)1-155 | 2 ± 1.6 | 1 ± 0.6 |
| GM-CSF/IL-4/TNF-α | 85 ± 9.8 | (1276 ± 96) | 6 ± 1.4 | 1 ± 1.6 |
| GM-CSF/IL-4 | 76 ± 8.2 | (964 ± 120) | 4 ± 1.8 | 2 ± 1.2 |
| Cytokines* . | %DC . | (Absolute Numbers, ×103)† . | %CD3+ . | %CD19+ . |
|---|---|---|---|---|
| Flt3L/IL-4 | 31 ± 5.8 | (249 ± 73) | 4 ± 1.2 | 1 ± 1.6 |
| Flt3L/IL-4/TNF-α | 73 ± 9.1 | (884 ± 65) | 3 ± 1.7 | 1 ± 1 |
| Flt3L/IL-4/CD40L | 75 ± 7.0 | (1202 ± 211)‡ | 6 ± 3.1 | 2 ± 1.5 |
| CD40L | 55 ± 10 | (747 ± 102) | 4 ± 1.5 | 1 ± 0.8 |
| CD40L/IL-4 | 71 ± 9.0 | (1087 ± 153)1-153 | 5 ± 1.7 | 4 ± 1 |
| CD40L/IL-4/GM-CSF | 81 ± 11 | (1345 ± 241)1-155 | 2 ± 1.6 | 1 ± 0.6 |
| GM-CSF/IL-4/TNF-α | 85 ± 9.8 | (1276 ± 96) | 6 ± 1.4 | 1 ± 1.6 |
| GM-CSF/IL-4 | 76 ± 8.2 | (964 ± 120) | 4 ± 1.8 | 2 ± 1.2 |
107 PBMNC were stimulated with the indicated combinations of cytokines or by CD40 ligation for 7 days.
The percentage of DC (cells expressing CD1a and/or CD83), CD3+, and CD19+ cells was analyzed by flow cytometry. The absolute number of DC was calculated based on FACS data. Results show the means and standard deviations of six different experiments.
P < .0001 compared with Flt3L/IL-4.
P < .0001 compared with Flt3L/IL-4.
P = .0061 compared with GM-CSF/IL-4.
CD40 ligation alone promotes differentiation of monocytes into DC.
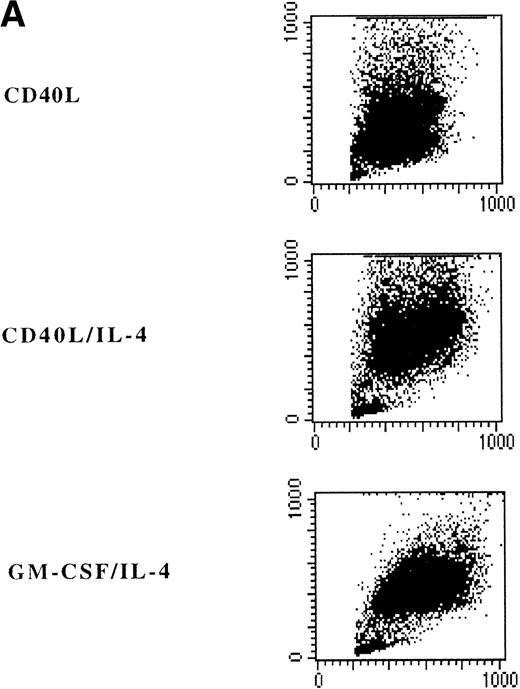
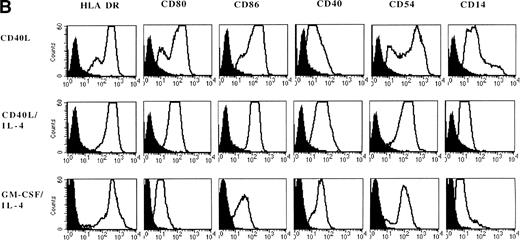
Adherent PBMNC cultured with CD40L-transfected L cells alone revealed small clusters of cells that started to detach after 5 to 7 days. In a typical experiment, after 7 days of culture with CD40L-transfected L cells alone, 50% of the cultured cells appeared as clumps of cells with dendritic morphology and phenotype. Analysis of surface markers showed that CD40 ligation resulted in expression of high levels of MHC class II molecules, CD80, CD86, CD40, and CD54 (Fig 3B). Moreover, DC were positive for CLA, CD11b, CD33, CD44, and CD45RO expression (data not shown). However, about half of the cells still expressed high levels of CD14 representing the monocytes/macrophages in the cell cultures (Fig 3A and B). The percentage of DC in these cultures could be further increased to 60% to 90% by addition of IL-4 with or without GM-CSF, which was mainly due to a selective loss of the CD14brightmonocyte/macrophage population (Fig 3, Fig4, Table 1).
Phenotypic analysis of in vitro–generated DC. PBMNC were cultured in presence of CD40L, CD40L/IL-4, or GM-CSF/IL-4. Dot plots (A) show the forward- and side-scatter profiles of the cells. Histograms (B) show expression of indicated cell surface molecules after 7 days of culture. Solid histograms: labeling with isotype matched irrelevant MoAb.
Phenotypic analysis of in vitro–generated DC. PBMNC were cultured in presence of CD40L, CD40L/IL-4, or GM-CSF/IL-4. Dot plots (A) show the forward- and side-scatter profiles of the cells. Histograms (B) show expression of indicated cell surface molecules after 7 days of culture. Solid histograms: labeling with isotype matched irrelevant MoAb.
Two-color cytograms of cultured cells labeled with CD83-PE (y-axais) and CD1a-FITC (x-axis). Adherent PBMNC were cultured with the indicated combinations of cytokines or CD40L for 7 days, and cell surface phenotype was examined by flow cytometry. The numbers indicate the percentage of cells in each quadrant.
Two-color cytograms of cultured cells labeled with CD83-PE (y-axais) and CD1a-FITC (x-axis). Adherent PBMNC were cultured with the indicated combinations of cytokines or CD40L for 7 days, and cell surface phenotype was examined by flow cytometry. The numbers indicate the percentage of cells in each quadrant.
To analyze the effects of serum to our findings, the same experiments were repeated under serum-free conditions using X-VIVO 20 medium. The expression of CD83, CD1a, and costimulatory molecules did not differ when monocyte-derived DC were generated in FCS or in X-VIVO medium, in contrast to results reported when DC were induced in medium complemented with human serum (data not shown).
To show that CD40 ligation results in DC maturation in the absence of GM-CSF, we used a neutralizing anti–GM-CSF antibody to block any induced endogenous GM-CSF. The addition of this antibody to the culture medium had no effect on the generation of DC by CD40 ligation, whereas the same antibody reduced the numbers of DC up to 70% in cultures grown in the presence of IL-4/GM-CSF (data not shown).
DC generated from CD14+ monocytes strongly stimulate allogeneic T cells.
Cultured DC or freshly isolated PBMNC were analyzed for their capacity to stimulate alloreactive T cells. A total of 103 DC or PBMNC from the same donor was cultured with 105 allogeneic T cells. As shown in Table 2, DC generated in presence of TNF-α or CD40L induced an increased thymidine incorporation of allogeneic T-cells compared with unstimulated DC (P < .05) or to PBMNC (P < .001).
Stimulatory Capacity of Mononuclear Cells Cultured With Various Cytokine Combinations
| Cytokines* . | Allogeneic MLR† . |
|---|---|
| Flt3L/IL-4 | 20,341 ± 1,815 |
| Flt3L/IL-4/TNF-α | 32,387 ± 2,610 |
| Flt3L/IL-4/CD40L | 37,532 ± 2,815 |
| CD40L | 28,419 ± 3,301 |
| CD40L/IL-4 | 33,080 ± 5,528 |
| CD40L/IL-4/GM-CSF | 44,130 ± 3,421 |
| GM-CSF/IL-4/TNF-α | 38,761 ± 957 |
| GM-CSF/IL-4 | 21,213 ± 2,030 |
| PBMNC | 6,349 ± 259 |
| Medium | 484 ± 64 |
| Cytokines* . | Allogeneic MLR† . |
|---|---|
| Flt3L/IL-4 | 20,341 ± 1,815 |
| Flt3L/IL-4/TNF-α | 32,387 ± 2,610 |
| Flt3L/IL-4/CD40L | 37,532 ± 2,815 |
| CD40L | 28,419 ± 3,301 |
| CD40L/IL-4 | 33,080 ± 5,528 |
| CD40L/IL-4/GM-CSF | 44,130 ± 3,421 |
| GM-CSF/IL-4/TNF-α | 38,761 ± 957 |
| GM-CSF/IL-4 | 21,213 ± 2,030 |
| PBMNC | 6,349 ± 259 |
| Medium | 484 ± 64 |
Adherent PBMNC were cultured with the indicated combinations of cytokines for 7 days.
105 responding cells from allogeneic PBMNC were cultured with 103 irradiated stimulator cells (DC or PBMNC). Thymidine incorporation was measured on day 5 by a 16-hour pulse with3H-thymidine. The assay was conducted in triplicate and results show the means ± SD in cpm from one representative experiment out of four.
Stimulation of DC with TNF-α or by CD40 ligation results in a reduced capacity to present soluble antigens.
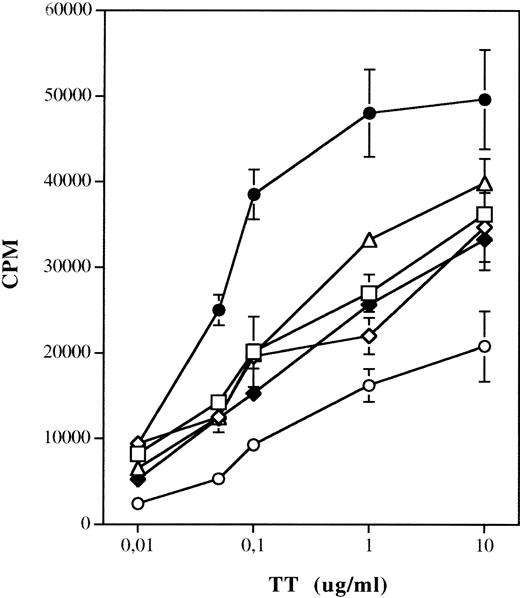
To analyze the ability of different antigen-presenting cell (APC) populations to uptake and present soluble antigens, DC cells generated from monocytes using different stimuli were incubated with or without tetanus toxoid (TT) and autologous PBMNC and harvested after 7 days. As shown in Fig 5, activation of DC with TNF-α or CD40L results in reduced stimulation of TT-specific T-lymphocyte proliferation as compared with DC grown in the presence of GM-CSF and IL-4 (P < .005). The same results were obtained when DC generated in presence of Flt3L and IL-4, and stimulated by TNF-α or CD40L, were used as APC in the assay (data not shown). Interestingly, we observed some increased background proliferation of autologous T lymphocytes, particularly in cultures stimulated with TNF-α or by CD40 ligation, which may be due to the activity against the FCS proteins.
Presentation of TT by DC generated using different culture conditions. 1.5 × 105 PBMNC were incubated with 104 irradiated APC with TT in 96-well plates. The absolute number of DC was determined by FACS staining for each culture condition. Each well was obtained after 7 days following a 12-hour pulse with tritiated thymidine. Results are expressed as mean cpm of triplicate wells ± SD. Thimidine incorporation of autologous T lymphocytes stimulated by DC and PBMNC not pulsed with TT: PBMNC, 724 ± 96 cpm; GM-CSF/IL-4, 1,244 ± 147 cpm; GM-CSF/IL-4/TNF-, 1,920 ± 141 cpm; GM-CSF/IL-4/CD40L, 1,770 ± 240 cpm; CD40L, 1,870 ± 96 cpm; CD40L/IL-4, 1,917 ± 366 cpm. (□) GM-CSF/IL-4/TNF-; (◊) GM-CSF/IL-4/CD40L; (•) GM-CSF/IL-4; (▵) CD40L; (⧫) CD40L/IL-4; (○) PBMNC.
Presentation of TT by DC generated using different culture conditions. 1.5 × 105 PBMNC were incubated with 104 irradiated APC with TT in 96-well plates. The absolute number of DC was determined by FACS staining for each culture condition. Each well was obtained after 7 days following a 12-hour pulse with tritiated thymidine. Results are expressed as mean cpm of triplicate wells ± SD. Thimidine incorporation of autologous T lymphocytes stimulated by DC and PBMNC not pulsed with TT: PBMNC, 724 ± 96 cpm; GM-CSF/IL-4, 1,244 ± 147 cpm; GM-CSF/IL-4/TNF-, 1,920 ± 141 cpm; GM-CSF/IL-4/CD40L, 1,770 ± 240 cpm; CD40L, 1,870 ± 96 cpm; CD40L/IL-4, 1,917 ± 366 cpm. (□) GM-CSF/IL-4/TNF-; (◊) GM-CSF/IL-4/CD40L; (•) GM-CSF/IL-4; (▵) CD40L; (⧫) CD40L/IL-4; (○) PBMNC.
DC activated with CD40L or TNF-α are strong stimulators of primary MHC class I–restricted T-cell responses.
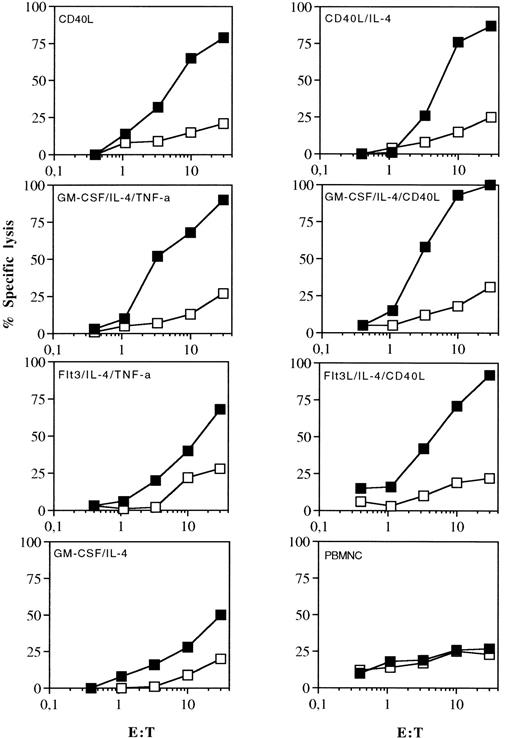
DC generated from adherent HLA-A2 positive PBMNC were pulsed with a synthetic peptide derived from pol HIV-1 reverse transcriptase 476-484, ILKEPVHGV, and used as APC to induce a primary CTL response in vitro. As shown in Fig 6, CTL lines obtained after 3 weekly restimulations showed high peptide-specific killing. It was observed that T cells exhibited a cytotoxic response only against targets coated with the cognate HIV-peptide, but they did not recognize targets coated with an irrelevant HLA-A2 binding peptide derived from IMP. CTL induced with DC generated in presence of TNF-α or CD40L elicited a higher cytotoxic activity when stimulated with the cognate peptide compared with CTL induced with DC grown in GM-CSF and IL-4 alone. Peptide-pulsed PBMNC did not elicit any measurable antigen specific cytotoxic activity.
Induction of CTL responses by peptide pulsed DC or freshly isolated PBMNC. PBMNC from a HLA A2 positive donor were cultured with the indicated combinations of cytokines for 7 days. 5 × 105 DC pulsed with the synthetic peptides derived from pol HIV-1 reverse transcriptase were used as APC to induce an MHC class I–restricted CTL response in vitro. Cytotoxic activity of induced CTL was determined in a standard 51Cr-release assay using T2 cells as targets pulsed for 2 hours with 25 μg of the cognate HIV (closed symbols) or irrelevant IMP peptide (open symbols). Data are from one representative experiment out of three.
Induction of CTL responses by peptide pulsed DC or freshly isolated PBMNC. PBMNC from a HLA A2 positive donor were cultured with the indicated combinations of cytokines for 7 days. 5 × 105 DC pulsed with the synthetic peptides derived from pol HIV-1 reverse transcriptase were used as APC to induce an MHC class I–restricted CTL response in vitro. Cytotoxic activity of induced CTL was determined in a standard 51Cr-release assay using T2 cells as targets pulsed for 2 hours with 25 μg of the cognate HIV (closed symbols) or irrelevant IMP peptide (open symbols). Data are from one representative experiment out of three.
Expression of DC-CK1.
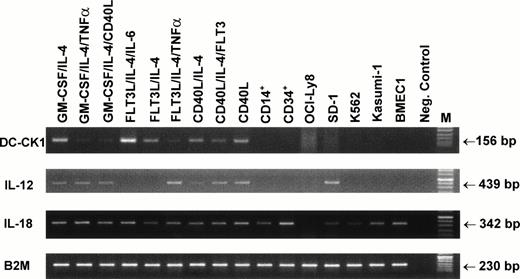
Recently, a dendritic cell–derived chemokine DC-CK1 was identified.35 This chemokine has a specific expression in DC with a preferential chemotactic activity for naive T cells. We analyzed the expression of DC-CK1 in generated DC populations using a semiquantitative RT-PCR and primers specific for DC-CK1. DC-CK1 expression was readily detected in all generated DC populations, independent of the cytokines used in the experiments (Fig 7). DC-CK1 transcripts were not detectable in samples obtained from purified CD14+peripheral blood monocytes, isolated CD34+ peripheral blood progenitor cells, and human tumor cell lines OCI-Ly8 (B-cell lymphoma), SD-1 (acute lymphoblastic leukemia), K562 (erythroleukemia), Kasumi-1 (AML, M2), and BMEC 1 (human bone marrow–derived endothelial cell line) after 28 rounds of amplification. Interestingly, activation of DC grown in GM-CSF and IL-4 containing medium with TNF-α or CD40L resulted in reduced DC-CK1 mRNA expression.
Expression of DC-CK1, IL-12, and IL-18 by in vitro–generated DC. PBMNC were cultured with the indicated combinations of cytokines for 7 days. Total RNA was isolated from cultured DC, purified CD14+ and CD34+ cells, or human tumor cell lines. DC-CK1, IL-12, and IL-18 expression was examined by semiquantitative RT-PCR. Twenty-eight rounds of amplification using primers specific for DC-CK1 and IL-18, 32 cycles for IL-12 amplification, and 23 cycles for β2-microglobulin were performed. PCR products were run on a 3% agarose gel and visualized by ethidium bromide staining. Samples containing no cDNA were used as negative control.
Expression of DC-CK1, IL-12, and IL-18 by in vitro–generated DC. PBMNC were cultured with the indicated combinations of cytokines for 7 days. Total RNA was isolated from cultured DC, purified CD14+ and CD34+ cells, or human tumor cell lines. DC-CK1, IL-12, and IL-18 expression was examined by semiquantitative RT-PCR. Twenty-eight rounds of amplification using primers specific for DC-CK1 and IL-18, 32 cycles for IL-12 amplification, and 23 cycles for β2-microglobulin were performed. PCR products were run on a 3% agarose gel and visualized by ethidium bromide staining. Samples containing no cDNA were used as negative control.
Expression of IL-18 (interferon-γ inducing factor [IGIF]) by in vitro–generated DC.
DC were shown to be potent inducers of Th 1–directed immune responses, probably as a result of IL-12 production.4 Like IL-12, IL-18 has been described as a growth and differentiation factor for Th 1 lymphocytes. To this end, only activated macrophages and keratinocytes were identified as a source of IL-18.36-40 In conrast to IL-12 expression, IL-18 transcripts were detected in all DC populations generated from peripheral blood monocytes (Fig 7). Interestingly, IL-18 mRNA was also found in CD34+peripheral blood progenitor cells, CD14+ purified blood monocytes, and in all tested tumor cell lines with the exception of the OCI-Ly8 B lymphoma cell line.
DISCUSSION
DC are recognized as the most efficient professional APC for the induction of primary immune responses. Several previous studies showed that DC can develop from CD14+ blood monocytes cultured with GM-CSF and IL-4. Here we show that DC can develop from adherent CD14+ monocytes in the absence of GM-CSF by using Flt3L and IL-4, or by CD40-CD40 ligand interactions.
Flt3L shows structural homology with SCF and induces in vitro proliferation of purified immature and committed myeloid colony-forming cells41 and seems to play an important role in DC development.26-28 In our study, Flt3L alone was not sufficient to induce differentiation of adherent peripheral blood monocytes and required addition of IL-4. The absolute number of DC generated using this cytokine combination was lower as compared with cells grown in GM-CSF and IL-4. However, when these cells were grown in the presence of CD40L, a significant increase of DC numbers was observed, even when compared with DC generated with GM-CSF/IL-4.
The CD40 receptor ligand (CD40L) belongs to the emerging tumor necrosis factor receptor family.29-32 CD40L is mainly expressed on activated CD4+ T cells but can also be found on natural killer (NK) cells, monocytes, mast cells, basophils, and some CD8+ T cells. Recent work has shown that CD40-CD40L interactions play a central role in antigen presentation, autoimmunity, development of T-cell–dependent effector functions, and activation of macrophages and dendritic cells. The in vivo importance of CD40 and CD40L interactions in the development of humoral and T-cell–mediated immune responses has been clearly shown in patients suffering from hyper IgM syndrome, characterized by mutations in CD40L, and in studies using CD40L and CD40 knock out mice.29-32
Here we show for the first time that DC can develop from adherent blood monocytes solely upon CD40-CD40L interactions. These cells showed all the phenotypic and functional characteristics of mature DC and were potent inducers of antigen specific CTL responses (see Fig 3 through6).
To make the statement that CD40 ligation results in DC maturation in absence of GM-CSF, we blocked any induced GM-CSF in these cultures using a neutralizing antibody. The presence of this antibody had no effect on phenotype and yield of generated DC in cultures stimulated with CD40L.
The finding that DC can be generated from blood monocytes upon CD40 ligation is in contrast to a previous report using the same CD40L transfectants, but elutriated monocytes. These cells did not display the typical DC morphology after 2 to 6 days. In our study, we used adherent instead of elutriated blood monocytes, and functional and phenotypical analyses of the cultured cells were performed after 7 days of culture. This was the time point when the adherent cells started to detach in sufficient numbers. It seems unlikely that prior adherence explains this difference, but we cannot completely exclude that the low number of contaminating B and T cells directly or indirectly might contribute to our observations.
To further confirm the presence of DC in these cultures, we examined the expression of DC-CK1,35 a recently identified chemokine, which was shown to be exclusively expressed in DC. In our study, DC-CK1 transcripts were detected by RT-PCR in all generated DC populations independent of growth factors used, whereas no expression was found in purified CD14+ monocytes, isolated CD34+ cells, and human tumor cell lines derived from B and T lymphocytes, myelomonocytes, and bone marrow endothelial cells. These data suggest that all cytokines used were able to induce DC development from adherent blood monocytes in vitro. Interestingly, stimulation of immature DC resulted in reduced expression of DC-CK1 mRNA.
DC were shown to be potent inducers of Th 1–directed immune responses, probably due to production of IL-12.4 IL-18 (IGIF), another potent growth and differentiation factor for Th 1 lymphocytes, is a recently described cytokine that shares functional properities of IL-12. Like IL-12, IGIF is a potent inducer of IFN-γ from NK cells, T lymphocytes, and activated B cells. To this end, only activated macrophages, Kupffer cells, or keratinocytes were identified as a source of IL-18.37-40 Using RT-PCR, IL-18 transcripts were detected in all DC populations generated from peripheral blood monocytes, indicating that besides IL-12, DC may also produce IL-18. Interestingly, IL-18 mRNA was also found in CD34+ cells, CD14+ blood monocytes, and in all tested tumor cell lines with the exception of the OCI-Ly8 B lymphoma cell line, suggesting that this cytokine is broadly expressed (at least at the mRNA level). However, further studies analyzing protein production are needed to confirm this assumption.
Recently, several reports have shown that peptides generated from exogenous proteins can gain access to the cytosol and therefore be presented on class I MHC molecules.42,43 In vivo studies revealed that bone marrow–derived APC can present class I–restricted peptides after endocytosis of particulate or soluble exogenous protein antigens and induce antigen specific CTL responses.44 In vitro results suggest that macrophages and DC may be involved in the cross-priming phenomenon seen in vivo.43,45-48 For the induction of CD8+ cytotoxic T lymphocytes by cross-priming cognate CD4+ T cell help was shown to be required.49 Because of the observed cognitive nature of this process it was further suggested that CD4+ T cells actively modify the APC, converting it into an effective stimulator for successful priming of CTL precursor.
One possible sequence of events derived from our data and suggested by Grewal and Flavell30 would be that monocytes/macrophages activate T cells at the site of inflammation by presentation of captured and processed protein antigens and cytokine secretion, which results in upregulation of CD40L on T cells. The coupling and interaction of CD40L on T cells and CD40 on monocytes/macrophages induces a functional and phenotypic change of these cells. They upregulate their costimulatory capacity and differentiate into CD83+ mature DC capable of migrating to the peripheral lymph nodes and of efficiently initiating CD8+ and CD4+ T-cell responses.
In conclusion, our findings provide further evidence for the importance of CD40-CD40L interaction for initiation and maintenance of T-cell responses and confirm the emerging concept that monocytes provide an additional source of DC, depending on external stimuli.
ACKNOWLEDGMENT
We thank Dr Stefan Stevanovic for providing the synthetic HIV-peptides and Prof Dr H.-G. Rammensee for reading the manuscript and for helpful discussion. We are grateful to Prof Rammensee and Alexandra Muhm for the help provided in performing the Cr-release assays. We are thankful to Stefanie Kurtz for excellent technical assistance.
Supported by grants from Deutsche Forschungsgemeinschaft (SFB 510), Deutsche Krebshilfe, and fortune Program of the University of Tübingen.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Wolfram Brugger, MD, Department of Hematology, Oncology and Immunology, University of Tübingen, Otfried-Müller-Strasse-10, D-72076 Tübingen, Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal