Abstract
We have studied the effects of granulocyte colony-stimulating factor (G-CSF) administration to normal individuals on a variety of functional and biochemical neutrophil characteristics that relate to host defense. G-CSF adversely affected neutrophil (polymorphonuclear leukocyte [PMN]) chemotaxis. While this could be partially explained by reduced assembly of neutrophil F-actin, we also recognized an elevated cytosolic calcium mobilization and a normal upregulation of neutrophil CD11b. G-CSF resulted in reduced PMN killing of Staphylococcus aureus with a 10:1 (bacteria:neutrophil) ratio and normal killing with a 1:1 ratio. In association with this, we demonstrated divergent effects on the respiratory burst of intact cells and divergent effects on the content of marker proteins for neutrophil granules. While G-CSF may have resulted in increased content of cytochrome b558 in the cell membrane, it did not alter the amounts of cytosolic oxidase components. After therapy, there was normal content of the azurophilic granule marker, myeloperoxidase, decreased content of the specific granule marker, lactoferrin, and normal content of lysozyme (found in both granules classes). Finally, G-CSF therapy markedly reduced the apoptotic rate of the isolated neutrophil. Therefore, considering disparate functional and biochemical activities, the real benefit of G-CSF therapy may lie in enhanced number and survival of neutrophils.
GRANULOCYTE colony-stimulating factor (G-CSF) is a lineage-specific hematopoietic cytokine widely used to combat the risk of infection resulting from both congenital and acquired neutropenias.1-4 G-CSF stimulates the proliferation and differentiation of hematopoietic precursors5 and modifies functional, biochemical, and survival characteristics of mature phagocytic cells.6 It has been assumed, therefore, that the therapeutic benefits of G-CSF relate both to a greater number of circulating neutrophils and to an enhanced phagocyte function providing protection against microbial invasion.
Neutrophil (polymorphonuclear leukocyte [PMN]) function is a complicated synthesis of biochemical processes serving as a primary mechanism in defense of the host. PMN cellular activity includes motility, allowing the phagocyte to reach and ingest invading microbes, and killing, which involves both oxygen-dependent and independent mechanisms. Inherent in this process, the mature neutrophil has a short life span during which time it either becomes involved in an inflammatory process or undergoes spontaneous cell death by apoptosis.7
G-CSF modulates many of these neutrophil activities. In vitro, G-CSF acts as a chemotactic stimulus for isolated neutrophils,8primes the neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase for subsequent activation,9and induces an increased content of neutrophil alkaline phosphatase.10,11 Incubation of isolated neutrophils, with G-CSF, prolongs survival as measured by a decrease in neutrophil apoptosis.12,13 Therapy with G-CSF decreases the bone marrow transit time for neutrophils,14 but may not prolong neutrophil survival in vivo.14 G-CSF treatment modifies the neutrophil expression of β2integrins6,15 and receptors for IgG,6,16reduces neutrophil chemotaxis,6 and enhances microbicidal killing3,17,18 and antibody-dependent cellular cytotoxicity.19
Despite these data, the in vivo capacity of the neutrophil produced during exogenous administration of G-CSF, to provide host defense, is not well understood. A more complete description of the circulating PMN is required to understand the mechanisms by which G-CSF therapy influences the end products of myelopoiesis. We have undertaken a systematic study of the effects of G-CSF administration to normal individuals on a variety of neutrophil functional and biochemical characteristics that relate to host defense. These results describe novel changes in the circulating neutrophil produced during G-CSF administration.
MATERIALS AND METHODS
Materials.
Human recombinant G-CSF was obtained from Amgen Inc, Thousand Oaks, CA. Superoxide dismutase (SOD), cytochrome c, Ficoll Hypaque, diisopropyl fluorophosphate (DFP), phenylmethylsulfonyl fluoride (PMSF), acetyl-leu-leu-arginine-AL (leupeptin), formyl-methionyl-leucyl-phenylalanine (fMLP), phorbol myristate acetate (PMA), platelet activating factor (PAF), bovine intestinal alkaline phosphatase, -S-GTP, dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS), and NADPH were purchased from the Sigma Chemical Co (St Louis, MO). Rabbit antihuman lactoferrin (IgG) was purchased from US Biochemicals (Cleveland, OH). Protein assay reagents and standards were obtained from Pierce (Rockford, IL). Polyclonal antibodies for the neutrophil NADPH oxidase components p67-phox, p47-phox, and p40-phox were obtained from Dr Michael Kleinberg (Greenebaum Cancer Center and University of Maryland School of Medicine, Baltimore, MD), Dr Harry Malech (National Institutes of Health [NIH], Bethesda, MD) and Dr Tony Segal (University of London Hospital, London, UK), respectively.
Study population.
Healthy adult volunteers receiving no medications and with no infections within the prior 2 weeks were enrolled into the study. All subjects gave informed consent for this study through a protocol, which was approved by the Colorado Multiple Institutional Review Board. As experimental controls, we studied healthy adult volunteers not receiving cytokine administration who also satisfied enrollment criteria. Each treatment volunteer [referred to as treated volunteer(s)] was paired to a sex-matched control volunteer [referred to as control(s)]. G-CSF was administered once daily by subcutaneous injection (10 μg/kg/d) for 7 consecutive days. Fourteen treated volunteers and controls were studied, nine men and five women, in each study group. The administration of G-CSF was well-tolerated. Prominent side effects included bone pain and headache in most treated volunteers (grade I and II, mild and moderate severity). These symptoms were responsive to nonnarcotic analgesics (ibuprofen and acetaminophen). In one treated volunteer, G-CSF administration was discontinued on the fifth day because of severe bone pain. All of the symptoms resolved within 24 to 48 hours of discontinuing G-CSF. All treated volunteers demonstrated a significant increase in total leukocyte count from a mean of 4.87 ± 0.28 on day 0 to a mean of 31.61 ± 1.54 on day 4 (106 cells/mL; mean ± standard error of mean [SEM]), a mean absolute neutrophil count of 2.64 ± 0.17 on day 0 to 24.92 ± 1.43 on day 4 (106 cells/mL; mean ± SEM) and a mean percent band count of 1.1 ± 0.7 on day 0 (range, 0% to 7%) to 18.6 ± 2.4 on day 4 (range, 5% to 39%). By day 1, the mean percent band count was 15.3 ± 2; by day 2, 19.9 ± 3, and by day 3, 13.1 ± 2. Absolute neutrophil and band counts were based on manual differential counts.
Cell isolation.
Heparinized (1 U/mL) peripheral blood samples were obtained before cytokine administration on the first day of the protocol (day 0, D0) and on the fifth day of administration (day 4, D4) from both study groups (controls and treated volunteers). Blood samples were drawn approximately 1 hour after the administration of G-CSF on day 4. Neutrophils were isolated by dextran sedimentation, Ficoll-Hypaque centrifugation, and hypotonic cell lysis, as previously described.20 These cells were greater than 98% viable as determined by the trypan blue exclusion test. Intact cells were used immediately for functional and biochemical assays or stored at 2.5 × 107 cells/mL in 0.1% Triton X-100, at −70°C for granule marker enzyme measurement.
Neutrophil chemotaxis, F-actin determination, and expression of CD11b.
Neutrophil chemotaxis was determined by the leading front method using a modified Boyden chamber technique, as previously described.21,22 Zymosan activated serum (2% by volume) was used as the chemotactic stimulus. To determine F-actin content, isolated neutrophils (1 × 106 cells), loaded with NBD-phallacidin (Molecular Probes, Eugene, OR), were incubated with fMLP (10−7 mol/L) in DMSO for 10 minutes at 37°C. F-actin content was then measured by NBD-phallacidin staining, as previously published.23 Flow cytometric analysis was performed using a BD FACScan (Becton Dickinson, Mountain View, CA) calibrated with an excitation wavelength of 488 nm. A total of 2,500 events was analyzed for each experiment. Results were displayed on a log scale for FL1 mean channel fluorescence versus cell count and were expressed as geometric mean channel fluorescence of gated cells in FL1. The mean channel fluorescence was arbitrarily set to 10 for unstimulated control. This produced a more useful fluorescence curve allowing better interpretation of the shape of the curve. Other samples were normalized accordingly, which allowed us to compare changes in F-actin assembly on stimulation with greater confidence; however, it did not allow a true interpretation of baseline F-actin content in treated volunteers. The resting and stimulated expression of neutrophil CD11b in response to three stimuli was also determined. Briefly, isolated neutrophils (1 × 106/mL) were incubated with fMLP (10−6mol/L), PMA (200 ng/mL) or PAF (2 × 10−6 mol/L) for 5 minutes at 37°C. After stopping the reaction by adding ice-cold Krebs-Ringer-Phosphate with Dextrose: 12.5 mmol/L Na2HPO4, 3 mmol/L NaH2PO4, 4.8 mmol/L KCL, 120 mmol/L NaCl, 1.3 mmol/L CaCl2, 1.2 mmol/L MgSO4·7H2O + 0.2% dextrose (KRPD), a phycoerythrin-labeled mouse antihuman CD11b antibody (Becton Dickinson) was added and allowed to incubate for 30 minutes at 4°C. The cells were then fixed with paraformaldehyde (1.6%). Cell surface CD11b was detected with direct immunofluorescence using flow cytometry (Becton Dickinson) by standard techniques. Results were displayed on log scale for FL2 mean channel fluorescence versus cell count and expressed as geometric mean channel fluorescence of gated cells in FL2. The mean channel fluorescence was arbitrarily set to 10 for unstimulated control CD11b and other samples were normalized accordingly.
Mobilization of cytosolic-free calcium.
The concentration of cytosolic calcium [Ca2+]c was determined using the calcium binding fluorometric dye indo-1,AM.24 Cells (2.5 × 107) were diluted to 5 × 106 cells/mL in KRPD and incubated with 25 μg indo-1,AM (5 μmol/L final concentration; Molecular Probes, Eugene, OR) for 10 minutes at 37°C. Cells were centrifuged for 8 minutes at 1,000g at room temperature and resuspended at 1 × 106 cells/mL in fresh KRPD at 37°C. Changes in the fluorescence ratio were measured at the excitation wavelength of 355 nm and emission wavelength of 485 nm and 405 nm in a Perkin-Elmer LS50B spectrofluorometer (Perkin-Elmer Corp, Norwalk, CT). For these measurements, 2 × 106 cells were diluted with 1 mL KRPD (final volume 3 mL) in a standard cuvette with stirring at 37°C. Stimuli of 1 μmol/L fMLP or 40 nmol/L PAF were added to the cells in the cuvette in the spectrofluorometer. The Ca2+-saturated signal (r max) was determined by lysing the cells with 0.1 mmol/L digitonin, and the Ca2+-free signal (r min) was measured by subsequently adding 5 mol/L EGTA, pH 7.35. [Ca2+]c was calculated using Perkin Elmer Fluorescence Data Manager software and the Grynkiewicz equation.25
Neutrophil bactericidal assay.
Bactericidal activity was measured as previously described.22,26 27 Briefly, 1 × 106neutrophils were incubated, at 37°C, with Staphylococcus aureus (American Type Culture Collection [ATCC], Rockville, MD; #2059) at 1:1 and, in some experiments, at 10:1 ratios (bacteria:neutrophils) in the presence of 10% (by volume) pooled normal serum. At 0, 30, 60, 90, and 120 minutes, 50-μL aliquots were removed from the reaction tube and added to sterile H2O to lyse the neutrophils. After serial dilutions appropriate to the initial bacterial colony count, 50 μL of the resultant suspension was mixed with trypticase soy agar in a sterile Petri dish. After incubation at 37°C overnight, bacterial colonies were counted and the surviving bacteria at each sample time were expressed as a percent of initial values.
Analysis of the neutrophil NADPH oxidase.
O2− production by intact neutrophils was measured as the maximum initial rate of the SOD-inhibitable reduction of ferricytochrome c.28 Neutrophils were stimulated by the addition of 200 ng/mL PMA and 1 μmol/L fMLP. An additional stimulation sequence included preincubation with 2 μmol/L PAF for 3 minutes at 37°C and then stimulation with 1 μmol/L fMLP. O2− was also measured after PMN exposure to opsonized zymosan (OZ), 1 mg/mL.20 Neutrophil subcellular fractions were prepared in the presence of protease inhibitors DFP, PMSF, and leupeptin by the previously published technique of nitrogen cavitation and discontinuous sucrose centrifugation.28,29 The maximal rate of O2− production over 5 minutes was assayed in the SDS cell-free system in the presence of NADPH (200 μmol/L) and γS-GTP (10 μmol/L) by the SOD-inhibitable reduction of cytochrome c,28 and was normalized for the protein content of both the cytosol and membrane fractions. Subcellular fractions (membrane and cytosol) were added together (patient + patient) or were mixed with control fractions (patient + control) to correct for any abnormalities documented.28 Three cytosolic components of the neutrophil oxidase (p40-phox, p47-phox, and p67-phox) were analyzed by Western blot using an enhanced chemiluminescence (ECL) detection system (Amersham Corp, Buckinghamshire, UK). The total content of cytochrome b558 in specific granules was measured as the sodium dithionite reduced-minus oxidized spectrum using the absorbance coefficient of 160 mmol/L−1 cm−1 at 428 nm by spectrophotometric analysis.28 30
Granule proteins.
Myeloperoxidase (MPO), lysozyme, and alkaline phosphatase were measured by previously described spectrophotometric techniques.29,31Lactoferrin content was determined with a competitive enzyme-linked immunosorbent assay (ELISA).29 32 Whole cell and subcellular protein concentration was measured with the BCA protein assay (Pierce, Rockford, IL) and granule marker protein content and alkaline phosphatase were normalized for whole cell protein content.
Neutrophil apoptosis.
Polymorphonuclear leukocytes were isolated from heparinized whole blood by centrifugation over polymorphprep gradients (GIBCO-BRL Life Technologies, Grand Island, NY). On day 4, the PMN isolated from treated volunteers tended to be less dense. After two washes in RPMI-1640 (Sigma), PMN were cultured at 3 × 106cells/mL in tissue culture medium containing RPMI-1640, 10% fetal bovine serum (Gemini Bioproducts, Calabasas, CA), 2 mmol/L l-glutamine (Sigma), and 50 μg/mL gentamicin (Sigma). PMN were cultured in the presence or absence of G-CSF (25 ng/mL). Apoptosis was assessed at regular intervals between 0 hours and 48 hours or until there was greater than 90% apoptosis. Apoptosis was determined morphologically, as described previously.33 The percent of apoptotic cells was determined by counting a minimum of 100 cells.
Statistical methods.
Results were analyzed by analysis of variance appropriate for repeated measures data34 using the computer statistical software package SAS (SAS Institute Inc, Cary, NC). Neutrophil apoptosis was interpreted by logistic regression to predict the expected time to 50% apoptosis and these results were used in an analysis of variance model suitable for repeated measures data.34 The statistical significance reported here represents the comparison (day 0 vday 4 for treated volunteers) unless otherwise expressed.
RESULTS
Neutrophil motility.
Neutrophil directed migration measured in a modified boyden chamber was significantly reduced in treated volunteers after G-CSF administration compared with pretreatment values and controls values (*P = .0001) (Fig 1). Although nondirected migration was also decreased, this was not statistically significant. The ratio of directed to nondirected migration in treated volunteers was significantly reduced from 2.21 ± 0.14 on day 0 to 1.66 ± 0.16 on day 4 (P = .02).
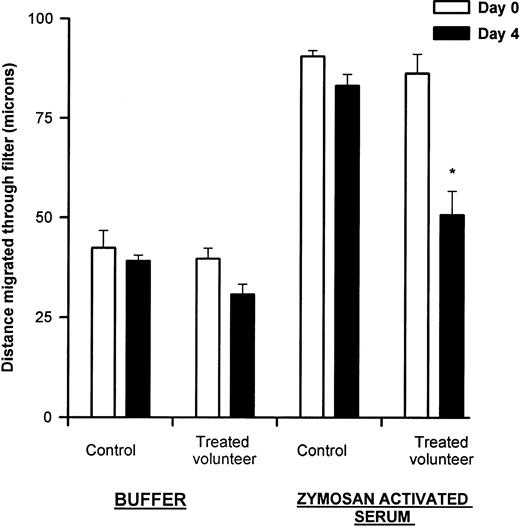
Neutrophil chemotaxis. Neutrophil chemotaxis was measured to zymosan-activated serum as described in Materials and Methods. There was significantly reduced chemotaxis after 5 days of G-CSF therapy (*P = .0001). There was also a trend toward less nondirected migration (buffer) after G-CSF therapy, but this difference was not significant (P = .18). Results are expressed as distance migrated through filter (microns) and represent mean ± SEM, n = 8.
Neutrophil chemotaxis. Neutrophil chemotaxis was measured to zymosan-activated serum as described in Materials and Methods. There was significantly reduced chemotaxis after 5 days of G-CSF therapy (*P = .0001). There was also a trend toward less nondirected migration (buffer) after G-CSF therapy, but this difference was not significant (P = .18). Results are expressed as distance migrated through filter (microns) and represent mean ± SEM, n = 8.
To further evaluate the basis for the chemotactic defect, stimulus-induced increase of neutrophil F-actin and expression of CD 11b were measured. F-actin assembly in response to fMLP was reduced after G-CSF administration in vivo. The content of F-actin, in response to fMLP, was 16.43 ± 0.99 on day 4 (mean channel fluorescence ± SEM, n = 6) compared with 18.81 ± 0.60 on day 0 (P = .04). Results for PMNs from controls on day 0 and day 4 were 19.84 ± 0.29 and 18.33 ± 0.98, respectively. In two treated volunteers, the baseline F-actin content was determined on day 4. In both, the baseline content of F-actin was increased (14.64 and 13.53; mean channel fluorescence).
Resting and stimulated expression of neutrophil CD11b were also examined. The agonists evaluated were PMA, fMLP, and PAF. No significant effect of G-CSF administration on the upregulation of this receptor was detected; the ratio of unstimulated:stimulated expression was the same after administration as before (Table 1). Thus, the impaired directed migration of neutrophils demonstrated during treatment with G-CSF was associated with diminished actin assembly, but normal β2integrin expression.
Expression of CD11b During Stimulation of Neutrophils
| Condition . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| PMA (200 ng/mL) | 2.72 ± 0.34 | 2.77 ± 0.35 | 2.5 ± 0.43 | 2.36 ± 0.27 |
| fMLP (10−6mol/L) | 3.53 ± 0.61 | 2.61 ± 0.31 | 2.71 ± 0.33 | 2.09 ± 0.23 |
| PAF (2 × 10−6 mol/L) | 5.39 ± 0.77 | 4.15 ± 0.52 | 3.83 ± 0.36 | 3.58 ± 0.07 |
| Condition . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| PMA (200 ng/mL) | 2.72 ± 0.34 | 2.77 ± 0.35 | 2.5 ± 0.43 | 2.36 ± 0.27 |
| fMLP (10−6mol/L) | 3.53 ± 0.61 | 2.61 ± 0.31 | 2.71 ± 0.33 | 2.09 ± 0.23 |
| PAF (2 × 10−6 mol/L) | 5.39 ± 0.77 | 4.15 ± 0.52 | 3.83 ± 0.36 | 3.58 ± 0.07 |
Neutrophil upregulation of CD11b was measured in response to PMA (200 ng/mL), fMLP (1 × 10−6 mol/L) and PAF (2 × 10−6 mol/L) as described in Materials and Methods. There appeared to be a reduced upregulation of CD11b on treated volunteer day 4, but this was not statistically significant for any stimulus. There also appeared a difference between control day 0 and control day 4 for the PAF-stimulated expression of CD11b, but this was not statistically significant. Data represent the ratio of stimulated CD11b expression to baseline CD11b expression (mean channel fluorescence); n = 6 (matched pairs); mean ± SEM.
Abbreviations: C, control; P, treated volunteer.
Mobilization of cytosolic-free calcium.
We studied changes in [Ca2+]c after incubation of neutrophils with fMLP and PAF to ascertain if Ca2+ mobilization might explain the observed chemotactic defect. Figure 2 presents a representative study of changes in cytosolic calcium Ca2+ in response to fMLP for one treated volunteer, before and after G-CSF administration. There is a clear increase in the maximum [Ca2+]c reached after G-CSF administration, while the rate of increase is the same compared with results obtained before G-CSF administration began. Controls reached maximum Ca2+ concentrations almost identical to results obtained for the treated volunteer on day 0 (data not shown). Summary data for the study groups (Table 2) show the significant enhancing effect that treatment with G-CSF in vivo had on the mean [Ca2+]c mobilization of isolated neutrophils compared with day 0 values (P ≤ .0005) and compared with control values (P ≤ .003) for both PAF and fMLP. There was a slight change in baseline values of [Ca2+]c for the PAF experiments (not statistically significant) (Table 2). No changes were observed in baseline values for fMLP, or in the rates of change for fMLP or PAF for any group at any time of study.
Neutrophil cytosolic calcium response. Cytosolic calcium concentration (nmol/L) was measured over time in response to fMLP (1 μmol/L) as described in Materials and Methods. These results demonstrate a representative study of one treated volunteer, of the six pairs studied. There is a clear increase in peak [Ca2+]c after G-CSF therapy in response to fMLP compared with the response to fMLP before G-CSF.
Neutrophil cytosolic calcium response. Cytosolic calcium concentration (nmol/L) was measured over time in response to fMLP (1 μmol/L) as described in Materials and Methods. These results demonstrate a representative study of one treated volunteer, of the six pairs studied. There is a clear increase in peak [Ca2+]c after G-CSF therapy in response to fMLP compared with the response to fMLP before G-CSF.
Cytoplasmic Free Calcium [Ca2+]c Levels in Resting and Stimulated Neutrophils
| . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| fMLP (baseline) | 0.03 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| fMLP (peak) | 0.75 ± 0.06 | 0.99 ± 0.07 | 0.99 ± 0.17 | 2.35 ± 0.24*,† |
| PAF (baseline) | 0.07 ± 0.03 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.11 ± 0.03 |
| PAF (peak) | 1.43 ± 0.32 | 1.55 ± 0.20 | 1.80 ± 0.36 | 3.03 ± 0.65*,† |
| . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| fMLP (baseline) | 0.03 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| fMLP (peak) | 0.75 ± 0.06 | 0.99 ± 0.07 | 0.99 ± 0.17 | 2.35 ± 0.24*,† |
| PAF (baseline) | 0.07 ± 0.03 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.11 ± 0.03 |
| PAF (peak) | 1.43 ± 0.32 | 1.55 ± 0.20 | 1.80 ± 0.36 | 3.03 ± 0.65*,† |
Neutrophil [Ca2+] mobilization was assayed as described in Materials and Methods. There was a slight increase in baseline calcium in the PAF stimulus experiments. On day 4 of G-CSF administration, there was significant enhancement of both PAF and fMLP-stimulated peak mobilization compared with treated day 0 levels (P ≤ .0005) and compared with control day 4 levels (P ≤ .003). Results represent μmol/L concentration of cytosolic calcium and are expressed as mean ± SEM, n = 6 (matched pairs).
P ≤ .0005.
P ≤ .003.
Neutrophil bactericidal activity.
The capacity to ingest and kill S aureus was studied to address whether administration with G-CSF affected bacterial microbicidal function. Bactericidal activity was assayed at a bacteria:neutrophil ratio of 1:1 in five treated volunteers and was unaffected by G-CSF administration (Fig 3A). However, on day 4 of G-CSF treatment, PMN bactericidal activity with ratios of 10:1 (bacteria:neutrophil) was reduced at all time points reaching statistical significance at 30 minutes (*P = .0023) and 90 minutes (**P = .028) (Fig 3B).
Neutrophil microbicidal activity. Neutrophil bactericidal activity was measured as described in Materials and Methods. At a 1:1 ratio (bacteria:neutrophil) (A), the PMN bactericidal capacity was normal after G-CSF therapy in vivo (normal, n = 15; treated volunteers, n = 5). At 10:1 ratio (bacteria:neutrophil), the PMN bactericidal capacity was reduced at all time points after G-CSF treatment in vivo (B). The difference reached statistical significance at 30 minutes (*P = .002) and at 90 minutes (**P = .028). Results are expressed as a percentage of the initial bacterial count and represent mean ± SEM (normal, n = 13; treated volunteers, n = 3).
Neutrophil microbicidal activity. Neutrophil bactericidal activity was measured as described in Materials and Methods. At a 1:1 ratio (bacteria:neutrophil) (A), the PMN bactericidal capacity was normal after G-CSF therapy in vivo (normal, n = 15; treated volunteers, n = 5). At 10:1 ratio (bacteria:neutrophil), the PMN bactericidal capacity was reduced at all time points after G-CSF treatment in vivo (B). The difference reached statistical significance at 30 minutes (*P = .002) and at 90 minutes (**P = .028). Results are expressed as a percentage of the initial bacterial count and represent mean ± SEM (normal, n = 13; treated volunteers, n = 3).
Neutrophil NADPH oxidase and granule marker proteins.
G-CSF has been shown to enhance the activity of the neutrophil NADPH oxidase.2 35-37 We analyzed O2− production in intact cells, as well as in the cell free system to further define any changes induced by G-CSF. NADPH oxidase activity of intact neutrophils in response to several different stimuli is summarized in Table 3. PMA-induced superoxide anion (O2−) production was significantly reduced on day 4 of G-CSF treatment (P ≤ .0001), as was the PAF-primed/fMLP stimulated respiratory burst (P ≤ .0001). However, the production of O2−after fMLP stimulation was significantly higher on day 4 of G-CSF administration (P = .004). No differences were observed during G-CSF administration on the respiratory burst stimulated with opsonized zymosan (Table 3).
Production of Superoxide Anion by Intact Neutrophils
| Condition . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| PMA (200 ng/mL) | 15.2 ± 1.3 | 13.8 ± 0.8 | 13.4 ± 0.7 | 6.8 ± .53-150 |
| fMLP (10−6 mol/L) | 3.6 ± 1.3 | 2.7 ± 0.6 | 4.8 ± 1.4 | 7.3 ± 1.13-151 |
| PAF/fMLP (2 × 10−6 mol/L PAF) | 33.2 ± 7.4 | 31.3 ± 3.6 | 28.2 ± 3.9 | 16.5 ± 0.83-150 |
| Opsonized zymosan (1 mg/mL) | 5.9 ± 0.7 | 5.5 ± 0.4 | 6.2 ± 0.5 | 5.5 ± 0.4 |
| Condition . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| PMA (200 ng/mL) | 15.2 ± 1.3 | 13.8 ± 0.8 | 13.4 ± 0.7 | 6.8 ± .53-150 |
| fMLP (10−6 mol/L) | 3.6 ± 1.3 | 2.7 ± 0.6 | 4.8 ± 1.4 | 7.3 ± 1.13-151 |
| PAF/fMLP (2 × 10−6 mol/L PAF) | 33.2 ± 7.4 | 31.3 ± 3.6 | 28.2 ± 3.9 | 16.5 ± 0.83-150 |
| Opsonized zymosan (1 mg/mL) | 5.9 ± 0.7 | 5.5 ± 0.4 | 6.2 ± 0.5 | 5.5 ± 0.4 |
Intact cell superoxide anion production was measured as described in Materials and Methods. PMN on day 4 showed reduced NADPH oxidase activity in response to PMA compared with day 0 P ≤ .0001. PMN respiratory burst was increased on day 4 compared with day 0 in response to fMLP (P = .004), while PAF priming of the fMLP response was reduced on day 4 (P ≤ .0001). Superoxide anion production with opsonized zymosan was unaffected by G-CSF therapy. Results represent nmol superoxide anion/min and are expressed as mean ± SEM, n = 8 (matched pairs).
P ≤ .0001.
P ≤ .004.
On cellular activation, the membrane and cytosolic components of the oxidase assemble in the plasma membrane or membrane of the phagolysosome. We examined the activity of the neutrophil oxidase in plasma membrane isolates in the cell-free system, as described in Materials and Methods, to determine if any of the changes in oxidase activity of intact cells reflect alterations in oxidase components. Neutrophil membrane isolated from treated volunteers, when mixed with autologous cytosol, produced 7.4 ± 1.9 nmol O2-/min/mg protein on day 0 and 11.1 ± 1.5 nmol O2-/min/mg protein on day 4 (mean ± SEM, n = 8) (not statistically different). However, neutrophil membrane isolated from treated volunteers, when mixed with cytosol isolated from control neutrophils, produced 5.0 ± 0.7 nmol O2−/min/mg protein on day 0 and 10.7 ± 1.1 nmol O2−/min/mg protein on day 4 (P = .01). These data suggest that the capacity of membrane to support oxidase activity had increased after G-CSF therapy. Additional studies evaluated cytosolic oxidase components. Figure 4 is a representative study of two treated volunteers and an untreated control and demonstrates similar amounts of p67-phox, p47-phox, and p40-phox in cytosol before and after the administration of G-CSF. Despite an apparent decrease in p40-phox in #7 D0, there was no statistically significant effect on the quantity of any oxidase protein studied, as measured by densitometry (data not shown). This experiment was completed in eight treated volunteers (data not shown).
Neutrophil cytosolic NADPH oxidase components. Neutrophil cytosolic oxidase components were examined as described in Materials and Methods. (A) Results with antibodies to p47-phox and p67-phox. (B) Results with an antibody to p40-phox. Despite an apparent decrease in p40-phox in #7 D0, there was no statistically significant effect on the quantity of the oxidase proteins studied, as measured by densitometry (data not shown). These results demonstrate a representative study of two treated volunteers and a control. C, control; #7 and #8 denote treated volunteers, on day 0 and day 4.
Neutrophil cytosolic NADPH oxidase components. Neutrophil cytosolic oxidase components were examined as described in Materials and Methods. (A) Results with antibodies to p47-phox and p67-phox. (B) Results with an antibody to p40-phox. Despite an apparent decrease in p40-phox in #7 D0, there was no statistically significant effect on the quantity of the oxidase proteins studied, as measured by densitometry (data not shown). These results demonstrate a representative study of two treated volunteers and a control. C, control; #7 and #8 denote treated volunteers, on day 0 and day 4.
Neutrophil lactoferrin, lysozyme, and myeloperoxidase were measured in whole cell lysates as markers for two major granule classes. G-CSF administration resulted in a significantly reduced whole cell lactoferrin content (P = .0004) (Table 4), while no effect of G-CSF administration was detected on the quantity of lysozyme or myeloperoxidase. Neutrophil alkaline phosphatase, which is located in secretory vesicles and neutrophil plasma membrane, was significantly increased after five doses of G-CSF (P = .0001) (Table 4). Content of cytochrome b558, the membrane associated component of the NADPH oxidase, was measured in the specific granule subcellular fraction (as described in Materials and Methods). Cytochrome b558 was significantly reduced on day 4 at 0.43 ± 0.06 compared with 0.68 ± 0.10 on day 0 (nmol/mg specific granule protein; mean ± SEM, n = 7; P = .026) and 0.70 ± 0.07 in controls (n = 7).
Content of Granule Marker Proteins
| Assay . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| Lactoferrin (μg/mg protein) | 46.4 ± 6.8 | 65.4 ± 0.7 | 60.4 ± 8.7 | 23.3 ± 3.34-150 |
| Alkaline phosphatase (OD/min/mg protein) | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.1 | 4.5 ± 1.04-151 |
| Myeloperoxidase (OD/min/mg protein) | 4.0 ± 0.6 | 5.6 ± 0.3 | 5.8 ± 0.6 | 5.3 ± 0.6 |
| Lysozyme (OD/min/mg protein) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Assay . | C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . |
|---|---|---|---|---|
| Lactoferrin (μg/mg protein) | 46.4 ± 6.8 | 65.4 ± 0.7 | 60.4 ± 8.7 | 23.3 ± 3.34-150 |
| Alkaline phosphatase (OD/min/mg protein) | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.1 | 4.5 ± 1.04-151 |
| Myeloperoxidase (OD/min/mg protein) | 4.0 ± 0.6 | 5.6 ± 0.3 | 5.8 ± 0.6 | 5.3 ± 0.6 |
| Lysozyme (OD/min/mg protein) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
Total protein and granule marker proteins were measured as described in Materials and Methods.
Lactoferrin content was reduced after 5 days of G-CSF therapy compared with pretreatment levels (P = .0002). There was an increased amount of alkaline phosphatase after 5 days of G-CSF therapy (P = .0009). No alteration in the content of myeloperoxidase or lysozyme was recognized with G-CSF therapy. Neutrophil whole cell protein, in treated volunteers, was 1.8 ± 0.1 on day 0 and 1.7 ± 0.1 on day 4 (mg/mL). Results are expressed as mean ± SEM, n = 13 (matched pairs).
P = .0002.
Neutrophil apoptosis.
G-CSF added to neutrophils in vitro prolongs neutrophil survival.12 13 We addressed the survival of neutrophils after the in vivo administration of G-CSF. Neutrophils isolated from untreated controls and G-CSF–treated volunteers were placed in culture and assayed for apoptotic characteristics at multiple time points. Neutrophil survival was significantly longer after G-CSF administration. The impact of G-CSF administration on the survival of PMN was apparent by 9 hours of incubation (Fig 5). Using logistic regression, as described in Materials and Methods, we predicted the time to 50% apoptosis for all study groups (Table 5). Neutrophils isolated from treated volunteers on day 4 had a time to 50% apoptosis of 30.8 ± 2.9 hours, which was nearly twice that measured before administration (P = .007; Table 5). When G-CSF was added to cultures in vitro, there was a further enhancement of survival of PMN obtained after 5 days of cytokine administration when compared with day 0 survival (P = .02) (Table 5).
Neutrophil apoptosis. Neutrophil apoptosis was determined as described in Materials and Methods. Apoptosis of isolated neutrophils, after 5 days of G-CSF therapy, was significantly reduced by 9 hours of culture and remained lengthened throughout the assay time (30 hours). Results are expressed as percent apoptosis and represent mean ± SEM, n = 8 (matched pairs); C, control; P, treated volunteer.
Neutrophil apoptosis. Neutrophil apoptosis was determined as described in Materials and Methods. Apoptosis of isolated neutrophils, after 5 days of G-CSF therapy, was significantly reduced by 9 hours of culture and remained lengthened throughout the assay time (30 hours). Results are expressed as percent apoptosis and represent mean ± SEM, n = 8 (matched pairs); C, control; P, treated volunteer.
Neutrophil Apoptosis Before and During G-CSF Therapy
| Ex Vivo Addition of G-CSF . | Time to 50% Apoptosis (h) . | |||
|---|---|---|---|---|
| C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . | |
| (−) G-CSF | 17.3 ± 1.0 | 18.3 ± 0.8 | 17.1 ± 0.8 | 30.8 ± 2.95-150 |
| (+) G-CSF | 37.6 ± 1.6 | 41.4 ± 2.2 | 42.0 ± 4.7 | 55.1 ± 4.45-151 |
| Ex Vivo Addition of G-CSF . | Time to 50% Apoptosis (h) . | |||
|---|---|---|---|---|
| C Day 0 . | P Day 0 . | C Day 4 . | P Day 4 . | |
| (−) G-CSF | 17.3 ± 1.0 | 18.3 ± 0.8 | 17.1 ± 0.8 | 30.8 ± 2.95-150 |
| (+) G-CSF | 37.6 ± 1.6 | 41.4 ± 2.2 | 42.0 ± 4.7 | 55.1 ± 4.45-151 |
Neutrophils were isolated from controls and treated volunteers. Apoptosis was assayed as described in Materials and Methods. The predicted time to 50% apoptosis was calculated using a logistic regression model and the results represent the time (hours) to 50% apoptosis. There was a significantly prolonged time to 50% apoptosis (P = .007) after 5 days of G-CSF therapy in vivo without G-CSF added to the culture system. This effect was enhanced further when G-CSF (25 ng/mL) was added to the culture suspension in vitro at time 0 (P = .02). Results are expressed, in hours, as mean ± SEM, n = 8 (matched pairs).
P = .007.
P = .02.
DISCUSSION
Many clinical benefits of G-CSF have been documented. G-CSF therapy reduces the length of neutropenia after chemotherapy38 and bone marrow transplantation.39 It reduces the incidence of hospital admissions for chemotherapy-related fever and neutropenia.38 Its impact on the quality of life in patients with congenital neutropenias is also well described.3,40,41 G-CSF has specificity for polymorphonuclear neutrophils with demonstrated effects on their morphology,42 activities,35,43 and survival.44 Interpretation and correlation of currently available data regarding the effect of G-CSF on neutrophils are difficult because information gained from in vitro studies may not correlate with results obtained during administration of the drug. Interpretation of available data into routine therapeutic strategies needs to recognize the baseline function of the neutrophil population studied6 and the dose, timing, and route of G-CSF administration. Furthermore, many studies have focused on a limited number of evaluation parameters. This report presents a comprehensive evaluation of neutrophils from normal individuals treated with G-CSF. We describe functional and biochemical properties of the circulating neutrophil produced during G-CSF administration in vivo and suggest a rationale for some of the unique characteristics, which differentiate the neutrophil produced during exogenous G-CSF administration from the neutrophil produced under standard in vivo conditions.
In this report, one of the most significant findings was the reduction in directed migration of isolated neutrophils. Associated with this defect, we recognized a reduced ability to upregulate F-actin and an increased peak [Ca2+]c in response to the same chemotactic stimulus. Baseline CD11b expression has been reported as decreased after five doses of G-CSF administration.45However, the data presented here demonstrate a normal ability to upregulate neutrophil CD11b after the administration of five doses of G-CSF. Overall evidence6,14 is in agreement with our motility data after G-CSF exposure in vivo, but the reason for this abnormality remains unexplained. Assembly of actin to filamentous form is reflective of neutrophil chemotactic potential.23 A neutrophil with increased F-actin may be less mobile and have less reserve for motion in response to chemotactic stimuli, as in newborn neutrophils46 and neutrophils from patients with severe congenital neutropenia.1 Thus, our demonstration of reduced assembly and increased baseline content (in only two volunteers) of F-actin, may partially account for some reduced neutrophil chemotaxis. The impact of G-CSF administration on mobilization of cytoplasmic calcium was studied because [Ca2+]c is involved in receptor-linked activation of a variety of functions, including chemotaxis.1 Our observation of an enhanced stimulus-induced calcium mobilization after G-CSF is in agreement with previous data in neutrophils from patients with glycogenosis Ib as described by McCawley.47 However, the enhanced calcium mobilization in these PMN has not been associated with any improvement in neutrophil chemotaxis.47 48 The association between an enhanced stimulus-induced calcium mobilization and reduced neutrophil chemotaxis and altered F-actin content remains unclear.
Using S aureus, we examined neutrophil microbicidal activity27 in standard and stress conditions by varying bacteria:neutrophil ratios. Microbicidal activity remained intact at 1:1 (bacteria:neutrophil) ratio, but was mildly diminished in three treated volunteers under the stress conditions of 10:1 ratio. Contrary to reports by Liles18 and Fossat,17 our results suggest a normal to mildly deficient killing activity after G-CSF administration, albeit we used different organisms and different organism:neutrophil ratios. Killing of an ingested microbe results from the simultaneous and synergistic action of toxic oxygen species (O2−, H2O2, .OH, HClO−),49 with multiple oxygen independent microbicidal mechanisms supplied by the contents of the specific and azurophilic granules.50,51 Oxygen-dependent killing reflects the ability of the neutrophil NADPH oxidase to produce O2-. G-CSF has been reported to enhance oxidase activity.6,36,43 In our study, however, we found divergent effects depending on the stimulus used. The response to fMLP was enhanced in cells, which developed under the influence of G-CSF. The physiological stimulus OZ produced a normal response after G-CSF therapy. We recognized diminished PAF priming for the fMLP response, while the PMA-induced respiratory burst was also reduced after G-CSF therapy, in agreement with Macey.52 These last two findings may relate to a reduction in overall neutrophil responsiveness after partial activation by in vivo cytokine administration, as described by Khwaja.53 Furthermore, immature neutrophils have higher levels of protein kinase C (PKC) type III, an isoenzyme that is not responsible for cellular activity.54 If the neutrophils we described are less mature than normal, PMA, an activator of PKC, may induce a less than maximal respiratory burst.
These divergent effects on oxidase activity in intact cells could, of course, be the result of alterations of NADPH oxidase constituents; and to explore this, we examined the oxidase in more detail. We demonstrated overall normal generation of O2− in the cell-free assay; however, in cross-mixing experiments, an increased amount of O2− was generated when neutrophil membrane from treated volunteers (day 4) was mixed with control cytosol (day 4). Increased cytochrome b558 in PMN membrane after G-CSF administration might be inferred from this finding. However, membrane content of this heterodimer was not measured. NADPH oxidase cytosolic component proteins were unaffected by G-CSF treatment. Thus, the oxidase components remain intact with G-CSF therapy, although there appears to be indirect evidence for increased membrane cytochrome b558. Accordingly, the differing oxidase activities in response to various agonists may be related to accessory pathways required to transform stimulus coupled signals to assembly and activation of the oxidase.
Microbicidal activity is also dependent on the integrity of neutrophil granules. We examined the status of azurophilic and specific granules indirectly by measuring several marker proteins. G-CSF is known to increase the transcription and amount of neutrophil alkaline phosphatase,11,55 an enzyme stored in secretory vesicles and membrane.51 This characteristic feature of G-CSF–exposed neutrophils was reconfirmed here. A reduced specific granule marker, lactoferrin, and a normal myeloperoxidase are consistent with reduced specific granules possibly due to degranulation. However, degranulation alone is unlikely to fully explain the apparent changes in lactoferrin for several reasons. We recognized equal amounts of neutrophil lysozyme before and after cytokine treatment, which would be expected to be lower in states of specific granule degranulation.26 Transcobalamin 2 binding protein, another indicator of secondary granule content, is reported to be normal during G-CSF therapy.56 Also, PMN isolated after G-CSF administration continue to recruit normal amounts of CD11b/CD18 (also stored in specific granules) to the cell surface in response to cell stimuli, as we and others6 have demonstrated. Lastly, if reduced cytochrome b558, as we described here, were the exclusive result of degranulation, then the amount of cytochrome b558 per mg of specific granule protein should remain the same. This is not the case in these experiments. Hence, a specific effect of G-CSF on production of neutrophil lactoferrin and possibly other proteins remains possible and merits further exploration.
Neutrophils die by apoptosis. In vitro exposure of neutrophils to inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor (TNF), IL-6, interferon-γ, and GM-CSF, as well as lipopolysaccharide (LPS) all decrease neutrophil apoptosis.13 G-CSF also has significant effects on neutrophil apoptotic rates in vitro7,12 and has some documented effects on neutrophil apoptosis after in vivo administration.57 However, G-CSF therapy in vivo is reported not to alter the circulation half-life of neutrophils.14 Neutrophils, which expressbcl-2,58 also demonstrate normal neutrophil survival kinetics, despite the inhibiting effect this proto-oncogene has on neutrophil apoptosis. We have shown here that in vivo administration of G-CSF markedly decreased the apoptotic rate of the isolated peripheral blood neutrophil, in agreement with results by Adachi et al.57 Also, there was an additive effect of exogenous G-CSF, reducing apoptotic rate in PMN collected after G-CSF therapy. In additional studies not reported here, we have demonstrated a protective effect of G-CSF administration on neutrophil apoptosis when neutrophils are exposed to cycloheximide.59 The in vivo and in vitro effects delaying apoptosis, exhibited by G-CSF, have implications for the prolonged survival of neutrophils at an inflammatory site.
Early in vitro studies describing the effects of G-CSF on the neutrophil led to the concept that G-CSF administration resulted in cells with markedly enhanced function. Our studies demonstrate that G-CSF administration imparts only modest effects on neutrophil function and biochemistry and results in a diverse pattern of activities not always consistent with increased function. Inherent in enhancing the activity of neutrophils is a risk for tissue injury and this risk is presumably avoided by G-CSF, given the modest effects we describe. Given the diverse functional and biochemical effects on neutrophils, the clinical benefit of G-CSF most likely lies in an increased absolute number of neutrophils with enhanced survival characteristics providing improved host defense.
ACKNOWLEDGMENT
We are grateful to Dr Richard B. Johnston, Jr, and Dr Arthur Verhoeven for their critical comments on this project and to Flo Usechek for her secretarial assistance in preparation of this manuscript.
Supported by the Margery Wilson Transfusion Medicine Fellowship, Bonfils Blood Center, The Stacy Marie True Memorial Trust, a Transfusion Medicine Academic Award, National Heart, Lung and Blood Institute, National Institutes of Health (K07-HL02036), Public Health Services research Grant No. 5 01 RR00051 from the Division of Research Resources, a grant from Amgen, Inc, Thousand Oaks, CA, and a Clinical Associate Physician Award (M01-RR00069) from the General Clinical Research Centers Program, National Centers for Research Resources, National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Daniel R. Ambruso, MD, Bonfils Blood Center, 4200 E Ninth Ave, B-128, Denver, CO 80262.

![Fig. 2. Neutrophil cytosolic calcium response. Cytosolic calcium concentration (nmol/L) was measured over time in response to fMLP (1 μmol/L) as described in Materials and Methods. These results demonstrate a representative study of one treated volunteer, of the six pairs studied. There is a clear increase in peak [Ca2+]c after G-CSF therapy in response to fMLP compared with the response to fMLP before G-CSF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/11/10.1182_blood.v92.11.4366/5/m_blod42323002x.jpeg?Expires=1763496712&Signature=ZBUOCfuZ7M9SQQ4gB8dfiC6hyWOqmEivHjnfzkL5tDFeROWC-gzb-Iss0QX3qVtBamPtb0bf4APJr8eeKNVrUKH-oe2cqtLfB-NNWfimJ-iryVDq40OMBlZKzx12BkU4hQ9ldaluy4~Ce8hSuArPmezfD066Yz0bK2-zbK-VN1RYyLus~1ED7gG1xP2yrbr5NaT8hpBY5aAD3Ys4qbtvz7BPACdllkeCJOJ7ExXy3XOxj497oKXa5yuqc1SxVmrOZyE~XeFplG2dms~EM05WI~uIqVE--TL4YlZJo0kXIvgyNaeIOj9i7DZl8Pr626NYI5l62KatdzGSgGRrZ3kDLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal