CLINICAL INDICATIONS for use of recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF) have expanded considerably since the drug first became available in the early 1990s for acceleration of myeloid engraftment in neutropenic patients. Initial clinical trials of rHuGM-CSF were based on prevailing knowledge of the biologic effects of endogenous GM-CSF at the time and therefore concentrated on the drug’s myeloproliferative effects in myelosuppressed patients. As additional information accumulated from in vitro research and from results of clinical trials, it became apparent that rHuGM-CSF had diverse biologic effects and played a vital role in various functions of the immune system, including responses to inflammation and infection, as well as in hematopoiesis. Consequently, a variety of potential clinical uses for rHuGM-CSF are under investigation, such as prophylaxis or adjunctive treatment of infection in high-risk settings or immunosuppressed patient populations, use as a vaccine adjuvant, and use as immunotherapy for malignancies.
The molecular sequence of endogenous human GM-CSF was first identified in 1985; within a few years, three different synthetic human GM-CSFs were produced using recombinant DNA technology and bacterial,1 mammalian,2 and yeast expression systems.3 Sargramostim is yeast-derived rHuGM-CSF produced using Saccharomyces cerevisiae; bacterially derived rHuGM-CSF is produced using Escherichia coli and is termed molgramostim; and mammalian-derived rHuGM-CSF is produced using Chinese hamster ovary cells (CHO) and is termed regramostim. These preparations are not identical and are differentiated by their specific amino acid sequences and degree of glycosylation.1-3 Sargramostim has an amino acid sequence identical to that of endogenous human GM-CSF, except that it contains leucine instead of proline at position 23 and may have a different carbohydrate moiety. Sargramostim is glycosylated to a lesser extent than regramostim, and molgramostim is not glycosylated. The degree of glycosylation of rHuGM-CSF may be an important characteristic, because it can affect pharmacokinetics, biologic activity, antigenicity, and toxicity.4-7
This review discusses current knowledge concerning the biologic effects, pharmacokinetics, and emerging clinical uses of rHuGM-CSF, with a focus on the yeast-derived rHuGM-CSF, sargramostim, the only form of synthetic rHuGM-CSF commercially available in the United States. Information on molgramostim, the form of rHuGM-CSF available in Europe, is also provided. Because literature reports do not always indicate the form of rHuGM-CSF used, the term “rHuGM-CSF” is used throughout this review to describe the drug when the expression system was not identified or when multiple studies using different forms of rHuGM-CSF reported similar findings.
PHARMACOLOGY OF SARGRAMOSTIM
Biologic effects.
GM-CSF was first identified based on its ability to stimulate the clonal proliferation of myeloid precursors in vitro.8Endogenous GM-CSF, a heavily glycosylated polypeptide, was the first human myeloid hematopoietic growth factor to be molecularly cloned, which allowed the expression of large quantities of the protein. More than a decade of in vitro and in vivo research using murine GM-CSF and synthetic rHuGM-CSFs has shown that the name of this CSF is restrictive, because it describes only one aspect of the numerous biologic effects that have now been attributed to GM-CSF. Although GM-CSF plays a vital role in hematopoiesis by inducing the growth of several different cell lineages, it also enhances numerous functional activities of mature effector cells involved in antigen presentation and cell-mediated immunity, including neutrophils, monocytes, macrophages, and dendritic cells.9-20
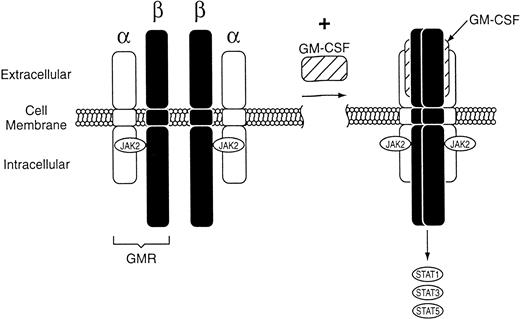
The biologic effects of GM-CSF are mediated via binding to receptors expressed on the surface of target cells. The GM-CSF receptor is expressed on granulocyte, erythrocyte, megakaryocyte, and macrophage progenitor cells as well as mature neutrophils, monocytes, macrophages, dendritic cells, plasma cells, certain T lymphocytes, vascular endothelial cells, uterine cells, and myeloid leukemia cells.21-27 Molecular cloning studies have shown that the GM-CSF receptor is composed of two distinct subunits, α and common β (βc; Fig1).28 The α-subunit binds GM-CSF with low affinity. The βc has no detectable binding affinity for GM-CSF on its own, but forms a heterodimer with the α-subunit that has high affinity for GM-CSF. Whereas the α-subunit is unique to the GM-CSF receptor, βc is shared with the receptors for interleukin-3 (IL-3) and IL-5.29
Schematic representation of the GM-CSF receptor (GMR), which is composed of two distinct subunits, and β. Binding of rHuGM-CSF to GMR leads to formation of the signaling complex and activation of a Janus kinase (JAK2). Regulation of gene expression by JAK2 activates transcription proteins STAT1, STAT3, and STAT5.
Schematic representation of the GM-CSF receptor (GMR), which is composed of two distinct subunits, and β. Binding of rHuGM-CSF to GMR leads to formation of the signaling complex and activation of a Janus kinase (JAK2). Regulation of gene expression by JAK2 activates transcription proteins STAT1, STAT3, and STAT5.
The signal transduction pathways that occur after rHuGM-CSF binds to the GM-CSF receptor are under evaluation. There appear to be at least two distinct signaling pathways, each involving a distinct region of βc.30 The first, which leads to induction of c-myc and activation of DNA replication, involves activation of a Janus kinase (JAK2) that is physically associated with βc.31 Regulation of gene expression by JAK2 appears to be mediated by production of a DNA-binding complex containing the signal transducer and activator of transcription (STAT) proteins STAT1, STAT3, and STAT5.32,33 The second pathway involves activation of ras34 and mitogen-activated protein kinases,35 with consequent induction of c-fos and c-jun, which are genes involved in regulation of hematopoietic differentiation.31
Pharmacokinetics.
Information regarding the pharmacokinetics of rHuGM-CSF after intravenous or subcutaneous administration is available from studies in healthy adults,36 adults with malignancy or myelodysplastic syndrome,6,37-40 and children with recurrent or refractory solid tumors.41,42 Because evidence exists from animal and clinical studies that the degree of glycosylation of synthetic rHuGM-CSFs influences pharmacokinetic parameters,4-6 data regarding the pharmacokinetics of sargramostim and molgramostim are presented separately.
Studies have determined that the pharmacokinetics of sargramostim are similar among healthy individuals and patients.39 The pharmacokinetics of sargramostim are dependent on the route of administration. Table 1 compares pharmacokinetic parameters after intravenous and subcutaneous administration of sargramostim in healthy adult males.36Peak serum concentrations are higher after intravenous administration; however, bioavailability (as determined by the area under the concentration-versus-time curve) of sargramostim is similar between administration routes. The elimination of sargramostim occurs principally by nonrenal mechanisms.39 Serum concentrations are more prolonged after subcutaneous administration than after intravenous administration.36,42 The magnitude of the percentage of increase in absolute neutrophil count with a specific dose of sargramostim is greater after subcutaneous injection than after 2-hour intravenous infusion.39
Pharmacokinetic Parameters After Intravenous and Subcutaneous Administration of Sargramostim in Healthy Adult Males
| Pharmacokinetic Parameter . | Sargramostim at 250 μg/m2 . | |
|---|---|---|
| IV . | SC . | |
| Cmax (ng/mL) | 5.0-5.4 | 1.5 |
| AUC (ng/mL · min) | 640-677 | 501-549 |
| Clearance (mL/min/m2) | 420-431 | 529-549 |
| t1/2β (min) | 60 | 162 |
| Pharmacokinetic Parameter . | Sargramostim at 250 μg/m2 . | |
|---|---|---|
| IV . | SC . | |
| Cmax (ng/mL) | 5.0-5.4 | 1.5 |
| AUC (ng/mL · min) | 640-677 | 501-549 |
| Clearance (mL/min/m2) | 420-431 | 529-549 |
| t1/2β (min) | 60 | 162 |
Abbreviations: Cmax, peak plasma concentration; AUC, area under the concentration-versus-time curve; t1/2β, terminal elimination half-life; IV, intravenous; SC, subcutaneous.
The pharmacokinetics of molgramostim (0.3 to 30 μg/kg) also were studied after subcutaneous and intravenous administration.37 Maximum serum concentrations and area under the concentration-versus-time curve increased with dose for both routes of adminstration, but appeared larger after intravenous administration in comparison to the same dose administered subcutaneously. However, rHuGM-CSF concentrations greater than 1 ng/mL were maintained longer after subcutaneous administration. Immunoreactive molgramostim was detected in the urine of patients, ranging from 0.001% to 0.2% of the injected dose, supporting nonrenal elimation. The half-life after intravenous adminstration ranged from 0.24 to 1.18 hours; the mean half life was 3.16 hours after subcutaneous adminstration.
USE IN ENHANCING HEMATOPOIETIC RECOVERY AFTER CANCER CHEMOTHERAPY AND BONE MARROW TRANSPLANTATION (BMT)
rHuGM-CSF is classified as a multilineage CSF because it stimulates the proliferation and differentiation of hematopoietic progenitor cells of neutrophil, eosinophil, and monocyte colonies.43 Parenteral administration of rHuGM-CSF induces a dose-dependent increase in peripheral blood neutrophil counts.19,44 Sargramostim alters the kinetics of myeloid progenitor cells within the bone marrow, causing rapid entry of cells into the cell cycle and decreasing the cell-cycle time by as much as 33%.45 The leukocyte response to rHuGM-CSF is reflected in peripheral blood principally as an increase in segmented neutrophils, but also involves an increase in monocytes and eosinophils.19,46-48Leukocyte differentials generally demonstrate a shift to the left; myelocytes, promyelocytes, and myeloblasts may be present. When rHuGM-CSF is discontinued, leukocyte counts gradually decrease to pretreatment levels.19 43
The myeloproliferative effects of rHuGM-CSF are also the result of its interaction with other cytokines. rHuGM-CSF functions in conjunction with erythropoietin and IL-3 to promote the proliferation and differentiation of erythroid and megakaryocytic progenitors, respectively.8,49 The addition of thrombopoietin to early acting cytokines, such as rHuGM-CSF, increases the overall in vitro megakaryocyte expansion compared with thrombopoietin alone and also generates different subpopulations of CD41+ megakaryocyte progenitors, with much less coexpression of CD42b and CD34 and slightly more coexpression of c-kit.50 In addition, the overall number of CD34+ cells increases approximately fivefold with the combination of thrombopoietin and early acting cytokines. A trial in sublethally irradiated nonhuman primates showed that coadministration of sargramostim and thrombopoietin augmented megakaryocyte, erythrocyte, and neutrophil recovery compared with either cytokine alone.51
Enhancing neutrophil proliferation is an important aspect of rHuGM-CSF function; however, effects of this multilineage growth factor on other cells of the immune system, including monocytes and macrophages, have been identified. Administration of rHuGM-CSF not only increases the number of circulating monocytes, but also increases the function of monocytes and macrophages, including oxidative metabolism, cytotoxicity, and Fc-dependent phagocytosis.19,52,53rHuGM-CSF enhances dendritic cell maturation, proliferation, and migration.20,54,55 In addition, class II major histocompatibility complex (MHC) expression on macrophages and dendritic cells is increased by rHuGM-CSF, enhancing the function of antigen-presenting cells.56
Combined, these effects of rHuGM-CSF not only increase hematopoietic cell counts, but also enhance immune function. The ability of rHuGM-CSF to accelerate myeloid recovery and to prevent infection has resulted in multiple approved indications for sargramostim and molgramostim in their respective countries. The drugs are used in patients after autologous BMT (AuBMT), peripheral blood progenitor cell (PBPC) transplantation, induction therapy for acute myelogenous leukemia (AML), engraftment delay or failure after BMT, and chemotherapy-induced neutropenia. These uses are well established and have been recently reviewed.57-61 Research has expanded in some of these settings to investigate new uses of rHuGM-CSF, including use in combination with granulocyte colony-stimulating factor (G-CSF) for PBPC mobilization, to prime leukemic cells before or during chemotherapy for AML, and as an adjunct to increase chemotherapy dose intensity.
PBPC mobilization in combination with G-CSF.
There has been increasing interest in combining rHuGM-CSF with other cytokines, especially G-CSF, as a means of improving mobilization without having to administer chemotherapy. This is especially true in the allogeneic transplant setting, where a nontoxic mobilization regimen that allows for collection of a sufficient number of cells to promote engraftment in a minimum number of leukaphereses is most critical. Lane et al62 evaluated the PBPC mobilization efficacy of G-CSF at 10 μg/kg/d (n = 8), sargramostim at 10 μg/kg/d (n = 5), or sargramostim plus G-CSF each at 5 μg/kg/d (n = 5) in normal donors. The median CD34+ cell yield with the combination regimen and with G-CSF was significantly higher than for rHuGM-CSF alone (101 × 106, 119 × 106, and 12.6 × 106, respectively;P < .01 for both comparisons).
An analysis of CD34+ cell subsets showed some interesting differences between the different mobilization regimens. A higher proportion of cells in the combination regimen were CD34+CD38− and CD34+CD38− HLA-DR+(Table 2). Pluripotent progenitor cells are characterized as CD34+CD38− and are likely responsible for long-term hematopoietic reconstitution after transplantation. These cells can be further subdivided according to the presence or absence of HLA-DR, with HLA-DR+ cells giving rise to lymphoid and myeloid precursors.63 The greater percentage of this subpopulation of cells mobilized by the combination regimen translated into a higher overall number of CD34+CD38−HLA-DR+ cells in leukapheresis products than products from subjects mobilized with either G-CSF or rHuGM-CSF alone (1.41 × 106, 0.36 × 106, and 0.12 × 106, respectively; P < .05 for all comparisons). Moreover, the plating efficiency of colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit-erythroid (BFU-E) was higher in cells stimulated by rHuGM-CSF than in those stimulated by G-CSF. Whether this would correlate with more rapid engraftment is not known, although the investigators have reported that PBPCs mobilized by the combination regimen successfully engrafted after allogeneic PBPC transplantation.64 Ali et al65 also compared mobilization of PBPCs in normal donors using rHuGM-CSF at 5 μg/kg/d plus G-CSF at 10 μg/kg/d (n = 15) versus G-CSF at 10 μg/kg/d alone (n = 35). They found a statistically insignificant increase in CD34+ cells in the leukapheresis products from donors mobilized with the combination of cytokines; however, the number of CD3+ cells in the leukapheresis product was significantly lower with the combination regimen than with G-CSF alone, ie, 160 versus 328 × 106/kg.
CD34+ Cell Subsets in Leukapheresis Harvests From Normal Donors Treated With Sargramostim and G-CSF62
| Subsets . | Sargramostim (n = 3) . | G-CSF (n = 4) . | Sargramostim + G-CSF (n = 3) . |
|---|---|---|---|
| CD34+ | 0.24 ± 0.22* | 1.19 ± 0.33 | 0.34 ± 0.18 |
| CD34+/CD38− | 4.42 ± 3.40 | 0.81 ± 0.22 | 4.73 ± 2.72* |
| CD34+/HLA-DR− | 20.3 ± 2.9 | 20.7 ± 6.9 | 24.0 ± 9.3 |
| CD34+/HLA-DR−/CD38− | 1.10 ± 0.22* | 0.37 ± 0.19 | 1.86 ± 0.34* |
| Subsets . | Sargramostim (n = 3) . | G-CSF (n = 4) . | Sargramostim + G-CSF (n = 3) . |
|---|---|---|---|
| CD34+ | 0.24 ± 0.22* | 1.19 ± 0.33 | 0.34 ± 0.18 |
| CD34+/CD38− | 4.42 ± 3.40 | 0.81 ± 0.22 | 4.73 ± 2.72* |
| CD34+/HLA-DR− | 20.3 ± 2.9 | 20.7 ± 6.9 | 24.0 ± 9.3 |
| CD34+/HLA-DR−/CD38− | 1.10 ± 0.22* | 0.37 ± 0.19 | 1.86 ± 0.34* |
Values are percentages.
P < .05 v G-CSF.
Investigations are ongoing to determine optimal doses and sequence of administration of the cytokines in combination.66,67 In a follow-up study to that reported by Lane et al,62 healthy volunteers received either sargramostim at 10 μg/kg/d for 3 or 4 days followed by G-CSF at 10 μg/kg/d for 2 days.66 In comparison to the results of single-agent G-CSF for 4 days and combination rHuGM-CSF and G-CSF for 5 days, sequential administration failed to demonstrate any differences in the extent of mobilization as measured by CD34+ cells. In addition, the proportion of the CD38− subset, which contains the more primitive hemotopoietic cells, was higher with the combination of sargramostim and G-CSF for 5 days.
Molgramostim also has been studied in combination or in sequence with G-CSF for mobilization of PBPCs.67 The combination of the two cytokines resulted in dramatic and sustained increases in the number of CFU-GM per kilogram collected per harvest, with administration of G-CSF to patients already receiving molgramostim increasing the hematopoietic progenitor cell content nearly 80-fold. A randomized trial comparing combination therapy versus G-CSF or molgramostim (10 μg/kg) alone is ongoing. Additional trials are required to determine the optimal scheduling of cytokine adminstration as well as apheresis scheduling.
Priming effect before or during chemotherapy for AML.
Myeloid leukemic cells and their precursors have GM-CSF receptors, and there is in vitro evidence that the proliferation and differentiation of these cells is supported by exposure to rHuGM-CSF.68-71Thus, recruitment of chemoresistant resting leukemic cells into sensitive phases of the cell cycle by rHuGM-CSF may enhance the antileukemic effect of chemotherapy. The rHuGM-CSF–induced increases in leukemic cells in S phase and intracellular phosphorylation of cytarabine have been shown to promote drug-induced cell kill.72 In contrast to enhancing cytarabine cytotoxicity, Lotem and Sachs73 found that the typical features of apoptosis were prevented by rHuGM-CSF and G-CSF in a murine leukemic cell line. The growth factors also inhibited apoptosis induced by cytarabine, but the overall clonogenic cell reduction was not reduced. Because contradictory laboratory data exist, it has not been possible to predict the clinical benefit of cytokine priming before results of studies in patients with AML.
In a multicenter, randomized trial of 114 patients (17 to 75 years of age) with newly diagnosed AML, Büchner et al74compared use of chemotherapy alone with use of chemotherapy administered in conjunction with sargramostim priming. Sargramostim at 250 μg/m2 was administered once daily by subcutaneous injection starting 24 hours before chemotherapy and continuing until neutrophil recovery occurred after the induction courses, consolidation course, and first two maintenance courses. Overall, 79% of sargramostim-treated patients and 84% of controls achieved disease remission; persistent leukemia was observed in 4% and 18% of patients, respectively. In patients younger than 60 years of age, complete remissions were achieved in 82% of sargramostim-treated patients and 73% of controls, with fewer relapses in the sargramostim-treated patients during the first 6 months (3% and 22%, respectively).74
Similar studies in patients with newly diagnosed AML receiving induction chemotherapy have been conducted with molgramostim.47 75-77 Patients received molgramostim at 250 μg/m2 or 5 μg/kg/d starting either on days 1 to 3 before chemotherapy or with induction chemotherapy. Although a trend toward benefit in disease-free survival was observed, the use of rHuGM-CSF during induction therapy of AML does not appear to have a significant impact on treatment outcome. The use of rHuGM-CSF for priming remains an intriuging therapeutic approach for AML. Because negative effects on the course of AML were rarely observed, additional studies are being conducted to determine the benefits and risks of growth factors administered before or concurrently with chemotherapy regimens in the treatment of AML. However, the definitive results of an ongoing ECOG trial are awaited before use of rHuGM-CSF for priming effects can be recommended outside of a clinical trial.
Adjunctive use to increase chemotherapy dose intensity.
Adjunctive use of rHuGM-CSF may allow an increase in the dose intensity of combination chemotherapy regimens including drugs with a primary toxicity of myelosuppression; however, the benefits associated with rHuGM-CSF in patients receiving dose-intensive chemotherapy may be limited to early courses of therapy, because late-cycle thrombocytopenia is not prevented.78-83 The ability of sargramostim to support a multiple-cycle high-dose chemotherapy regimen was evaluated in a phase III, double-blind, randomized trial of 56 patients with lymphoma or breast cancer. Patients received submyeloablative doses of cyclophosphamide, etoposide, and cisplatin (DICEP) and were randomized to receive sargramostim (250 μg/m2) or placebo subcutaneously every 12 hours.83 Sargramostim-treated patients had a significantly decreased duration of neutropenia after the first course of chemotherapy in comparison to patients who received placebo (10v 12 days; P = .01), but the difference did not achieve statistical significance after the second or third courses. Sargramostim-treated patients experienced a statistically significant 1.5-day delay in platelet recovery during the second course. There was no difference between the groups in numbers of hospitalizations for febrile neutropenia or the incidence of bacteremia, although any potential difference might have been obscured, because prophylactic oral ciprofloxacin was administered to all patients during neutropenia. However, the duration of hospitalization for neutropenic fever was shorter for sargramostim-treated patients in the first course. Importantly, the primary endpoint of this study was duration of neutropenia during the first course of therapy. The study was stopped because of a significant difference in duration of neutropenia; therefore, the small number of patients potentially obscures other clinical benefits of sargramostim therapy.83
The feasibility of EAP (etoposide, doxorubicin, cisplatin) dose escalation using molgramostim at 10 μg/kg/d starting on day 4 and continuing unitl recovery of granulocyte count was studied by Ford et al.78 Intolerable myelosuppression, including grade 4 neutropenia or thrombocytopenia lasting at least 7 days, occurred in 4 of 5 patients receiving escalated doses of the EAP regimen. At the lowest doses of each agent, 3 of 6 patients had intolerable myelosuppression. The investigators concluded that molgramostim did not permit dose escalation of EAP.78 In contrast, molgramostim at 5 μg/kg/d allowed dose escalation of 5-fluorouracil (5-FU) with leucovorin to 425 mg/m2/d, with further 5-FU dose escalation according to individual tolerance.79
USE IN INFECTIOUS DISEASE
Modulation of host defense against bacterial and fungal infections.
There are several mechanisms by which rHuGM-CSF may enhance host defense mechanisms against bacterial and fungal infection. Exposure of neutrophils to rHuGM-CSF in vitro and in vivo has been shown to enhance expression of cell surface adhesion molecules, such as β-integrins, as well as receptors for the Fc portion of IgG, and receptors for activated complement components.84,85 Other effects of rHuGM-CSF on neutrophils include enhanced chemotaxis,17phagocytosis,10 leukotriene B4 synthesis, release of arachidonic acid,86,87 and superoxide anion generation.44,88 The upregulation of neutrophil surface antigens combined with the induction of phagocyte migration and increased phagocytic activity contribute to a role for rHuGM-CSF in host defense. Sargramostim also prolongs neutrophil survival from 96 hours to at least 216 hours by preventing apoptosis.89Finally, sargramostim induces the expression of class II MHC molecules on neutrophils, which could potentially allow neutrophils to act as antigen-presenting cells much like B cells, macrophages, and dendritic cells.90 91
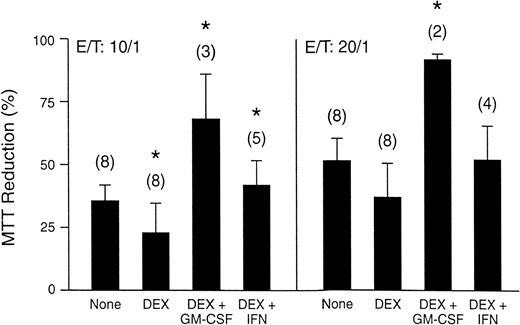
As a result of its multilineage activity, similar functional effects of rHuGM-CSF have been observed in monocytes and macrophages. Administration of rHuGM-CSF increases the level of expression of a number of receptors found on macrophages, such as CD11a, CD11b, and CD11c, that augment adhesion-dependent phenomena and FcγRII (CDw32) receptors that bind Ig during phagocytosis.92-94Upregulation of these receptors would be expected to aid the phagocytic ability of macrophages. Additonally, rHuGM-CSF enhances antibody-dependent cell cytotoxic activity, respiratory burst, and superoxide anion generation by macrophages and monocytes.13,17,19,52 Moreover, sargramostim significantly counteracted dexamethasone-induced inhibition of superoxide anion release by monocytes, and the fungicidal activity of dexamethasone-treated monocytes against Aspergillus fumigatus was enhanced (Fig2).95
(A) Fumigatus hyphal damage induced by elutriated human monocytes incubated with 500 nmol/L dexamethasone (DEX) alone and with either 5 ng/mL sargramostim (rHuGM-CSF) or 1.2 ng/mL interferon-γ (IFN). Vertical bars denote standard errors of means, and the number of experiments performed are shown in parentheses. *P < .05. (Reprinted with permission.95)
(A) Fumigatus hyphal damage induced by elutriated human monocytes incubated with 500 nmol/L dexamethasone (DEX) alone and with either 5 ng/mL sargramostim (rHuGM-CSF) or 1.2 ng/mL interferon-γ (IFN). Vertical bars denote standard errors of means, and the number of experiments performed are shown in parentheses. *P < .05. (Reprinted with permission.95)
Substantial evidence exists from in vitro and in vivo studies that rHuGM-CSF activates and enhances the ability of neutrophils and macrophages to phagocytize and destroy bacteria and fungi. Enhancement of the microbicidal activity of neutrophils by rHuGM-CSF was shown in vitro against Staphylococcus aureus,96,97Torulopsis glabrata,98 and Candida albicans.16,88,99 Neutrophils treated with rHuGM-CSF killed 90% of intracellular C albicans in comparison to 50% of intracellular yeast cells killed by untreated neutrophils.99 Similarly, enhancement of the microbicidal activity of monocytes by rHuGM-CSF was shown in vitro against C albicans,16A fumigatus,95,100Histoplasma capsulatum,101Cryptococcus neoformans,102 and Trypanosoma cruzi.103 Functional studies of neutrophils and monocytes isolated from patients treated with rHuGM-CSF at 250 μg/m2/d indicate that phagocytic and cytotoxic activity against S aureus is increased.97,104 The percentage of S aureus phagocytosed or killed after 20 minutes significantly increased from 62% before rHuGM-CSF treatment to 72% during treatment (P = .0028).97
Sargramostim also promotes killing of Mycobacterium aviumcomplex.105-108 Significant growth inhibition ofMycobacterium avium complex was observed in human macrophages treated with sargramostim or tumor necrosis factor α (TNFα; Table 3).108 A similar effect was observed using a mouse model of disseminated Mycobacterium avium complex infection. Significantly (P = .04) lower concentrations of Mycobacterium avium complex were present in the liver and spleen of mice treated with sargramostim for 14 days compared with control mice.105 These data also suggest that sargramostim enhances the antimycobacterial effect of clarithromycin, azithromycin, amikacin, and ofloxacin.105 107
Growth Inhibition of Mycobacterium AviumComplex in Human Macrophages Treated With Sargramostim or TNF108
| Cytokine (dose) . | Percentage of Inhibition3-150 . |
|---|---|
| rHuGM-CSF (1 U/mL) | 36 ± 6 |
| rHuGM-CSF (10 U/mL) | 58 ± 4 |
| rHuGM-CSF (100 U/mL) | 50 ± 4 |
| TNFα (50 U/mL) | 37 ± 6 |
| TNFα (500 U/mL) | 51 ± 6 |
| TNFα (5000 U/mL) | 44 ± 4 |
| rHuGM-CSF (10 U/mL) + TNFα (500 U/mL) | 41 ± 8 |
| Cytokine (dose) . | Percentage of Inhibition3-150 . |
|---|---|
| rHuGM-CSF (1 U/mL) | 36 ± 6 |
| rHuGM-CSF (10 U/mL) | 58 ± 4 |
| rHuGM-CSF (100 U/mL) | 50 ± 4 |
| TNFα (50 U/mL) | 37 ± 6 |
| TNFα (500 U/mL) | 51 ± 6 |
| TNFα (5000 U/mL) | 44 ± 4 |
| rHuGM-CSF (10 U/mL) + TNFα (500 U/mL) | 41 ± 8 |
Percentage of inhibition = (CFU in control well − CFU in well with each cytokine)/CFU in control well × 100. Values are means ± SEM.
In other animal studies, enhancement of microbicidal activity by rHuGM-CSF has been confirmed. Survival was significantly (P < .05) improved in neonatal rats when sargramostim was administered 6 hours before inoculation of a lethal dose of S aureus.109 Similarly, in neutropenic mice, administration of molgramostim 1 to 5 μg/d protected against lethal infections of S aureus and Pseudomonas aeruginosa; survival was significantly increased in molgramostim-treated mice infected with either S aureus (70% v 20%,P < .05) or P aeruginosa (50% v 0%, P< .01).110
Recombinant murine GM-CSF protected 62% of neutropenic rats from a lethal inoculum of C albicans and reduced lung injury. Importantly, there was no effect of murine GM-CSF on the neutrophil count, suggesting that the protective mechanism involved led to enhanced host defense mechanisms.111
Adjunctive treatment of fungal infections.
The effect of sargramostim on the incidence and severity of fungal infections was observed in randomized, double-blind studies of the drug in patients undergoing AuBMT and in patients with AML.112,113 Fungal infections developed in 4 AuBMT patients who received placebo in comparison to 2 AuBMT patients who received sargramostim.112 Two of the infections in placebo-treated patients were disseminated aspergillosis. In the phase III ECOG trial of 99 elderly patients undergoing chemotherapy for AML, sargramostim significantly (P = .02) reduced mortality due to fungal infection.113 One of 8 patients who received sargramostim died as a result of fungal infection, whereas 9 of 12 placebo-treated patients developed fatal fungal infections.
A pilot study of molgramostim as adjuvant therapy for fungal infections was conducted in cancer patients with proven major-organ or disseminated fungal infection.114 Of 8 evaluable patients, 6 had a neutrophil response to molgramostim; 4 of these patients were completed cured of the fungal infection and the other 2 had a partial response. Several case series have reported a response to adjunctive treatment with sargramostim for fungal infections. Three human immunodeficiency virus (HIV)-infected patients with oropharyngeal candidiasis refractory to fluconazole at doses of 200 mg daily or greater for at least 14 days were treated with sargramostim at 125 μg/m2/d.115 Fluconazole treatment was maintained at the same dose. All patients experienced improvement in signs and symptoms of oropharyngeal candidiasis by week 2. No significant adverse events occurred, including no upregulation of HIV-1 replication, and therapy was well tolerated. When sargramostim was discontinued, 2 of 3 patients relapsed.115
Sargramostim also has been administered to patients with rhinocerebral and disseminated mucormycosis, a rare opportunistic infection associated with a mortality rate exceeding 50%.116 Three of four patients with mucormycosis have been successfully treated with sargramostim (doses ranging from 250 to 500 μg/d for 14 days to 6 months) in combination with traditional surgical and medical treatment. The three patients were disease-free at periods of 6 months, 18 months, and 3 years after surgery.
HIV infection.
Initial use of sargramostim in patients with HIV infection focused on its ability to ameliorate drug-induced myelosuppression.117-119 In a phase I/II study in patients with Kaposi’s sarcoma who became neutropenic while receiving zidovudine and interferon α, administration of sargramostim resulted in a prompt increase in absolute neutrophil count in all patients and an absolute neutrophil count greater than 1,000 cells/μL within 7 days; there was no increase in p24 antigen levels.118Sargramostim (250 or 500 μg/m2/d administered by subcutaneous injection) also has been used to ameliorate chemotherapy-induced neutropenia in patients with acquired immunodeficiency syndrome (AIDS)-related Kaposi’s sarcoma receiving a regimen of doxorubicin, bleomycin, and vincristine (ABV).117 Although both sargramostim doses allowed the chemotherapy regimen to be continued without a dose reduction, the lower sargramostim dose was better tolerated. In a recent study in 12 patients with advanced HIV infection (CD4+ cell count ≤200/μL) who were receiving zidovudine (300 to 1,200 mg/d), administration of sargramostim in a dosage of 50, 125, or 250 μg/m2/d by subcutaneous injection resulted in significant increases in absolute neutrophil count and monocyte counts at all three dosage levels.119
Potential uses for the drug in HIV patients were expanded when it became evident that rHuGM-CSF activates and enhances the ability of neutrophils and macrophages to phagocytize bacteria, fungi, and intracellular parasites, which has important implications for the prophylaxis and treatment of opportunistic infections in this patient population.
Although there were initial concerns that use of rHuGM-CSF in HIV-infected patients might stimulate HIV replication and increase viral load, these concerns have not been substantiated. In vitro studies are somewhat conflicting regarding the effect of rHuGM-CSF on HIV replication. Numerous studies have demonstrated enhancement of viral replication when HIV-infected monocytes or macrophages are exposed to rHuGM-CSF120-124; however, three studies have reported suppression of HIV expression by rHuGM-CSF.125-127 An additional finding from in vitro studies is that rHuGM-CSF enhances the antiretroviral activity of some dideoxynucleoside antiretroviral agents, such as zidovudine and stavudine, possibly by increasing intracellular phosphorylation of these agents to their active metabolite.124,128 129
Early clinical trials evaluating the effect of rHuGM-CSF on viral replication in HIV patients failed to show an increase in viral load as determined by serum p24 antigen levels as long as patients received concurrent zidovudine117,118,130-132; however, in studies in which molgramostim was administered without an antiretroviral, some patients did experience an increase in serum p24 antigen levels.133,134 Subsequent trials using a more sensitive polymerase chain reaction assay for viral load determination confirmed that rHuGM-CSF does not result in an increase in viral load during or after CSF therapy in HIV patients receiving concurrent zidovudine.119,135 More recently, administration of sargramostim to patients on stable, highly active antiretroviral therapy including a protease inhibitor has been shown to result in no upregulation of viral load by polymerase chain reaction.136 137 These HIV-positive patients receiving sargramostim have experienced a significant (P = .0372) increase in CD4 count and decrease in viral load ≥0.5 log.
Interestingly, the studies by Massari et al126 and Matsuda et al127 that reported suppression of HIV expression by rHuGM-CSF were conducted before the role of coreceptors for HIV infection was appreciated. Indeed, the investigators examined the effects of rHuGM-CSF on CD4 expression as a potential mechanism for the reduction in HIV expression, but found CD4 expression to be unchanged. A recent study provides a possible mechanism for their findings. Exposure to sargramostim has recently been shown to downregulate expression of the C-C chemokine receptor CCR5, a β-chemokine receptor on macrophages, and to reduce the susceptibility of macrophages to infection by a macrophage-tropic strain of HIV.125 138 CCR5 has been shown to be the major coreceptor required for infection of macrophages by macrophage-tropic strains of HIV. Sargramostim-stimulated monocytes produced high levels of β-chemokines, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β in the medium. This medium was able to protect bystander cells from entry by JRFL, a macrophage-tropic strain of HIV.
Another possible role for sargramostim in HIV-infected patients is in the prevention or treatment of opportunistic infections. Based on results of in vitro studies demonstrating that rHuGM-CSF promotes killing of Mycobacterium avium-intracellulare105,107 and in vitro and murine studies indicating that rHuGM-CSF can enhance the antimycobacterial effects of some antimicrobial agents, including azithromycin, ofloxacin, and clarithromycin,105,107 investigations of sargramostim for the adjunctive treatment of Mycobacterium avium-intracellulare were initiated. In a small study, AIDS patients with disseminated Mycobacterium avium-intracellularewere randomized to receive azithromycin with or without sargramostim for 6 weeks.139 Mycobacteremia and monocyte function were assessed biweekly. Mean superoxide anion production was significantly increased in monocytes obtained from all 4 patients receiving sargramostim (53% to 199% relative to controls) and these patients had a 60% reduction in the number of viable intracellularMycobacterium avium-intracellulare per milliliter at the end of treatment. Patients receiving azithromycin alone had no increase in superoxide anion production and only a 28% reduction in viableMycobacterium avium-intracellulare per milliliter. These data indicate that sargramostim activates monocytes in AIDS patients withMycobacterium avium-intracellulare bacteremia and deserves further study as adjunctive therapy in these patients.
A multicenter phase III randomized, double-blind, placebo-controlled trial of sargramostim in patients with advanced HIV disease is ongoing to compare the incidence and time to first opportunistic infection or death. Patients with CD4 count ≤50 cells/μL and receiving a stable antiretroviral regimen before and during the study are eligible. Patients are randomized to receive either sargramostim 250 μg/d 3 days per week for a minimum of 24 weeks or placebo. Secondary objectives include incidence of AIDS-related opportunistic malignancies and esophageal candidiasis; survival; pharmacoeconomic and quality-of-life parameters; changes in HIV viral load or CD4+ lymphocyte counts; incidence, degree, and duration of neutropenia; and concurrent use of open-label cytokines.
USE AS A VACCINE ADJUVANT
rHuGM-CSF is the principal mediator of proliferation, maturation, and migration of dendritic cells, important antigen-presenting cells that play a major role in the induction of primary and secondary T-cell immune responses.14,20,140 Dendritic cells display antigens on their surface in conjunction with class II major histocompatibility complex (MHC). rHuGM-CSF also increases class II MHC expression.12 Once presented, the antigen can be recognized by helper CD4+ T cells,141 which provide support for the development of B cells and cytotoxic CD8+ T cells. By augmenting antigen presentation to lymphocytes by dendritic cells, rHuGM-CSF stimulates T-cell immune responses.12,14rHuGM-CSF has been demonstrated to augment the primary in vitro immune response to sheep red blood cells by murine spleen cells.142 rHuGM-CSF also is important to the immune response to vaccination, because it enhances expression of costimulatory molecules such as B7 and adhesion molecules (eg, intercellular adhesion molecule [ICAM]) that are necessary for the interaction of antigen-presenting cells with T cells; it also enhances production of other cytokines such as IL-1, TNF, and IL-6, which promote expansion and differentiation of B and T lymphocytes. In addition, rHuGM-CSF primes T cells for IL-2–induced proliferation143 and augments lymphokine-activated killer (LAK) cell generation in conjunction with IL-2.15 144 The important role of rHuGM-CSF in the maturation and function of antigen-presenting cells, such as dendritic cells and macrophages, as well as its ability to affect T-cell immunity, provides the basis for its potential evaluation as a vaccine adjuvant in new immunotherapy strategies for infectious diseases and cancer.
Local injection of rHuGM-CSF would be expected to enhance vaccine immunogenicity and would likely be well tolerated based on clinical experience in other uses. Disis et al145 evaluated the use of sargramostim as an adjuvant for protein- and peptide-based vaccines in rats. Tetanus toxoid was used as the foreign antigen system, and peptides derived from a self antigen, rat neu protein, were used as the tumor antigen system. A series of initial experiments demonstrated that intradermal injections of sargramostim every 24 hours for a total of five inoculations increased the number of class II MHC+ cells in regional lymph nodes that peaked at the fourth inoculation, whereas subcutaneous injections of sargramostim on the same schedule increased these cells with a peak after the second inoculation. This conditioning schema was then used, with tetanus toxoid administered at the beginning or end of the immunization cycle. Intradermal immunization was more effective than subcutaneous immunization in eliciting specific immunity to the tetanus toxoid antigen. In addition, intradermal injection of sargramostim as a single dose with antigen was similarly effective in eliciting specific antibody and cellular immunity as the use of Freund’s adjuvant or alum (Fig 3). Inoculation with rat neupeptides and sargramostim elicited a strong delayed-type hypersensitivity response, whereas the peptides alone were nonimmunogenic. Sargramostim was as effective as Freund’s adjuvant in generating rat neu-specific delayed-type hypersensitivity responses after immunization with the peptide-based vaccine. These studies demonstrated that sargramostim was an effective adjuvant for elicitation of immunity to both antigen systems, comparing favorably with other standard adjuvants.
rHuGM-CSF, as an adjuvant, elicits delayed-type hypersensitivity (DTH) responses to tetanus toxoid (tt) similar to those seen in animals immunized with a standard adjuvant. Rats were injected with Freund’s adjuvant (CFA) subcutaneously (sq), alum sq, rHuGM-CSF intradermally (id) or sq (5 μg), and phosphate-buffered saline (PBS) sq with tt at a concentration of 3 limit flocculation (Lf ) units. Immunizations were administered on 1 day only with no repeated administration of rHuGM-CSF. Six rats were included in each experimental group. Figure represents data collected from two separate experiments. Twenty days after immunization, a DTH response was measured in the immunized animals. Antigen was applied to rat ear and responses measured at 48 hours. Ear swelling of experimental compared with control ear was measured. Results are shown as the mean and standard deviation of measurements taken from each experimental group. (Reprinted with permission.145)
rHuGM-CSF, as an adjuvant, elicits delayed-type hypersensitivity (DTH) responses to tetanus toxoid (tt) similar to those seen in animals immunized with a standard adjuvant. Rats were injected with Freund’s adjuvant (CFA) subcutaneously (sq), alum sq, rHuGM-CSF intradermally (id) or sq (5 μg), and phosphate-buffered saline (PBS) sq with tt at a concentration of 3 limit flocculation (Lf ) units. Immunizations were administered on 1 day only with no repeated administration of rHuGM-CSF. Six rats were included in each experimental group. Figure represents data collected from two separate experiments. Twenty days after immunization, a DTH response was measured in the immunized animals. Antigen was applied to rat ear and responses measured at 48 hours. Ear swelling of experimental compared with control ear was measured. Results are shown as the mean and standard deviation of measurements taken from each experimental group. (Reprinted with permission.145)
Results of several preliminary studies using molgramostim in conjunction with hepatitis B141,146 and tetravalent influenzae virus vaccine147 suggest that rHuGM-CSF may have a potential role as an antiviral vaccine adjuvant; however, further evaluation is needed in this setting. Its evaluation as an adjuvant to vaccines and other immunotherapies for tumors is promising and is discussed in the subsequent section.
USE IN ANTITUMOR THERAPY
Antitumor effects.
The functional effects of granulocytes, lymphocytes, and macrophages are important in patients with malignancies because of the ability of these cells to exhibit antitumor activity. In vitro, rHuGM-CSF has been shown to slightly enhance the cytotoxic activity of peripheral blood monocytes and lymphocytes and markedly increase antibody-dependent cellular cytotoxicity148 and to enhance monocyte cytotoxicity against a malignant melanoma cell line.149rHuGM-CSF has also been shown to augment the cytotoxic activity of peripheral blood monocytes in antibody-dependent cellular cytotoxicity against numerous human tumor cells in the presence of various monoclonal antibodies150 and to enhance IL-2–mediated LAK cell function.151,152 In tumor-infiltrating macrophages, it also increases secretion of matrix metalloelastase with subsequent production of angiostatin, which inhibits angiogenesis and suppresses the growth of lung metastases.153 rHuGM-CSF may also enhance the immunogenicity of tumor cells through facilitation of tumor antigen presentation.56 In a comparative study in mice, the most potent stimulator of specific antitumor immunity was tumor cells engineered to secrete GM-CSF.154 Also, as previously noted, sargramostim has been shown in rats to be an excellent adjuvant for generation of immune responses to tumor antigen-derived peptides.145 Thus, rHuGM-CSF might enhance functions of cells critical for immune activation against tumor cells, alone or with other cytokines or monoclonal antibodies, making it potentially useful in the biotherapy of malignant diseases.
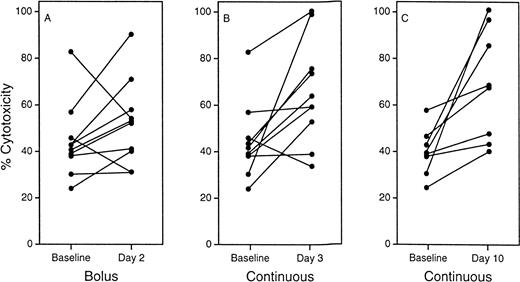
In a phase I study in patients with cancer, administration of sargramostim enhanced monocyte antibody-dependent cellular cytotoxicity (Fig 4) and increased secretion of both TNFα and interferon.19 In another study in patients with metastatic solid tumors, sargramostim was administered once daily for 14 days every 28 days; monocyte cytotoxicity against HT29 tumor cells was enhanced by the cytokine treatment.46 No clinical effects on tumor regression were apparent in either study. Sargramostim is under evaluation in an open-label, phase II trial as surgical adjuvant therapy in patients with advanced melanoma at very high risk of recurrence.155 Sargramostim at 125 μg/m2/d was administered subcutaneously for 14 days every 28 days beginning within 60 days of the last evidence of tumor. Treatment is continued until recurrence or a tumor-free interval of 1 year. An interim analysis of 25 patients demonstrated a significant prolongation of disease-free survival (P = .04) and survival (P= .02) compared with 50 matched historical control patients.155 These initial results are encouraging; long-term follow-up is needed.
Antibody-dependent cellular cytotoxicity (ADCC) of monocytes after treatment with sargramostim. Monocytes were collected from patients 2 days after a bolus infection (A) and 3 (B) and 10 (C) days after the start of a continuous infusion of sargramostim. Antibody-dependent cellular cytotoxicity activity was measured against antibody-coated chicken erythrocytes by a Cr51-release assay. Experimental results were compared statistically with the average of two baseline assays. (Reprinted with permission.19)
Antibody-dependent cellular cytotoxicity (ADCC) of monocytes after treatment with sargramostim. Monocytes were collected from patients 2 days after a bolus infection (A) and 3 (B) and 10 (C) days after the start of a continuous infusion of sargramostim. Antibody-dependent cellular cytotoxicity activity was measured against antibody-coated chicken erythrocytes by a Cr51-release assay. Experimental results were compared statistically with the average of two baseline assays. (Reprinted with permission.19)
A phase Ib trial was conducted in 20 patients with metastatic melanoma to evaluate the use of sargramostim as an adjuvant to R24, a murine monoclonal antibody that mediates complement-dependent and antibody-dependent cellular cytotoxicity of melanoma tumor targets.156 The rationale for this combination was the hypothesis that upregulation of monocyte and granulocyte antibody-dependent cellular cytotoxicity induced by sargramostim might enhance antitumor activity. Sargramostim (150 μg/m2/d administered by subcutaneous injection for 21 days) was administered alone or in conjunction with R24 (10 or 50 mg/m2administered by continuous intravenous infusion on days 8 through 15). Measurement of direct cytotoxicity and antibody-dependent cellular cytotoxicity indicated that sargramostim enhanced monocyte and granulocyte cytotoxicity by week 3 in all evaluable patients.156 Of the 6 patients who received sargramostim alone, 3 had no response (2 had stable disease) and 3 had disease progression; in the 14 patients who received sargramostim plus R24, 2 had a partial response, 6 patients had no response (3 had stable disease), and 6 developed progressive disease.156
The Pediatric Oncology Group performed a phase II study to evaluate the use of sargramostim to enhance antibody-dependent cellular cytotoxicity of a chimeric anti-GD2 monoclonal antibody (ch14.18) in the treatment of recurrent or refractory neuroblastoma.157 Sargramostim was administered in a dosage of 10 μg/kg daily for 14 days with 5-hour infusions of ch14.18 at 50 mg/m2 daily for 4 days. Thirty-two patients who had failed to respond to 1 to 4 therapeutic regimens, including BMT in 18 patients, received 70 courses of treatment. In 27 patients evaluable for response, there were 1 complete response, 3 partial responses, 1 mixed response, and 2 stable disease. When analyzed by site of disease, in 18 patients with marrow disease, there were 4 complete responses and 1 partial response; in 21 patients with bone involvement, there were 1 complete response and 2 partial responses. Two patients with large tumor masses had greater than 60% reduction in tumor size. Among the responding patients, 4 were alive at follow-up ranging from 9 to 20 months, whereas those with progressive disease had a median survival of 3 months. All responding patients had an increase in neutrophil-mediated antibody-dependent cellular cytotoxicity to greater than 20 lytic units, whereas 9 of 12 patients with progressive disease had peak antibody-dependent cellular cytotoxicity activity less than 20 lytic units. These findings were the basis for a recommendation that a phase III trial in the setting of minimal residual disease is warranted.157
Adjuvant to tumor vaccines.
Based on enhancement of functional effects on monocytes, macrophages, and antigen-presenting cells (dendritic cells, macrophages),15 142 sargramostim has been studied for its potential to enhance the immune response to antitumor immunotherapies, including autologous tumor cell vaccines, recombinant peptide tumor vaccines, and autologous Id-KLH tumor vaccines.
Leong et al158 administered sargramostim at 125 to 250 μg as an adjuvant to a melanoma vaccine that consisted of irradiated autologous melanoma cells with Bacillus Calmette-Guérin vaccine (BCG vaccine) in 20 stage IV melanoma patients. Patients received multiple cycles that consisted of vaccine plus sargramostim on day 1, with local injection of sargramostim alone in the vaccine site on days 2 to 5; 48 hours before cycles 1, 3, and 4, cyclophosphamide at 300 mg/m2 was administered. Four patients showed partial to complete responses (20%), 4 had stable disease (20%), and the remaining 12 patients had disease progression (60%). In the responding patients, regression of visceral metastases was observed. The results demonstrated the ability of patients bearing a significant tumor burden to respond specifically to their autologous melanoma.
Based on the fact that autologous tumor-derived Ig idiotype proteins (Id) have been shown to induce effective antitumor activity in experimental models and B-cell lymphoma, a vaccine containing autologous Id-KLH (keyhole limpet hemocyanin, a foreign protein used as a vaccine adjuvant) conjugates was administered to patients with multiple myeloma with either rHuGM-CSF or IL-2 as an adjuvant.159 Results of skin testing with autologous and unrelated Id were used to assess the specificity of the immune response; rHuGM-CSF appeared to be a better adjuvant than IL-2 in these patients.
Immunotherapy for AML.
Future directions in the treatment of AML may include immunotherapy based on the effect of rHuGM-CSF on T-lymphocyte cytotoxic functions and surface adhesion proteins. Several preliminary investigations have been conducted using molgramostim in patients with AML. The effect of molgramostim at 5 μg/kg/d on activated killer cell activity was studied in 20 patients with AML undergoing AuBMT.160Activated killer cell function was investigated before AuBMT, during rHuGM-CSF therapy, and after withdrawal. The actuarial risk of relapse was also analyzed and compared with a historical control group of 20 patients transplanted before initiation of this study. Activated killer cell function was significantly enhanced with rHuGM-CSF (P < .001); during rHuGM-CSF treatment, median activated killer cell function increased from 1.8% before AutoBMT to 35% and remained increased after withdrawal of rHuGM-CSF (median, 20%). After a median follow-up of 24 months, the actuarial risk of relapse was 37.4% in rHuGM-CSF–treated patients compared with 49.5% in controls (P= .05). Additionally, none of the 7 patients with activated killer cell activity ≥20% in the first 2 to 5 weeks after AutoBMT have relapsed, compared with 6 of 9 patients with activated killer cell activity less than 20% (P < .02).
Exposure of AML cells to rHuGM-CSF upregulates expression of ICAM-1 (CD54) and lymphocyte function associated molecule-3 (LFA-3; CD58), but does not increase their sensitivity to lysis by IL-2–activated natural killer cells.161 rHuGM-CSF induces a significantly greater upregulation of ICAM-1 on leukemic CD34+ cells than their CD34− counterparts. When AML cells are exposed to rHuGM-CSF before incubation with killer cells, their subsequent clonogenic activity is significantly reduced. These data suggest that administration of effector cell activators, such as IL-2, and target cell modulators, such as rHuGM-CSF, may have therapeutic benefit in patients with minimal residual myeloid leukemia.
OTHER POTENTIAL USES
Mucositis, stomatitis, and diarrhea.
Mucositis, stomatitis, and diarrhea are frequent complications of high-dose chemoradiotherapy. Mucosal epithelial cells in the gastrointestinal tract are susceptible to direct damage from these therapies, resulting in dysphagia and decreased oral intake and potentially leading to airway compromise. Mucosal damage may be further aggravated by infections or hemorrhage related to myelosuppression. rHuGM-CSF has been shown to stimulate the migration and proliferation of endothelial cells and promote keratinocyte growth, suggesting that the growth factor has a direct effect on mucosal cells.162 163 In addition, by decreasing the severity and duration of neutropenia, rHuGM-CSF may reduce the severity and duration of mucositis.
Several clinical trials evaluating sargramostim for hematopoietic support have shown a coincidental benefit of the drug on mucositis. In addition to enhancing myeloprotection and permitting dose-intensification of chemotherapy, the incidence of mucositis was reduced in sarcoma patients who received sargramostim after chemotherapy.164 In a phase III placebo-controlled trial of sargramostim in patients undergoing allogeneic BMT, 8% of sargramostim-treated patients compared with 29% of placebo-treated patients developed grade 3 or 4 mucositis (P = .005).165
Based on these results, several prospective trials have been conducted to evaluate the effect of rHuGM-CSF on mucositis. In a nonrandomized trial, the effect of sargramostim on oral mucositis was assessed in pediatric patients undergoing stem cell transplant.166Children who received thiotepa, etoposide, and total body irradiation followed by sargramostim experienced a significantly shorter duration of mucositis than those children who did not receive sargramostim (12.2v 20.3 days, P = .02). However, the severity of mucositis was similar between the groups. In this same study, patients treated with thiotepa, etoposide, and cyclophosphamide as the preparative regimen experienced a similar duration and severity of mucositis regardless of whether they received sargramostim. Although recovery of neutrophils was faster in sargramostim-treated patients, there was no correlation between recovery of neutrophils and resolution of mucositis.
A phase I trial of sargramostim as a mucoprotectant was conducted in 10 patients with advanced head and neck cancer who received adjuvant radiation after surgery or chemotherapy.167 Four patients developed grade 3 or worse mucositis, and the remaining 6 patients had grade 1 or 2 mucositis. In comparison to 13 historical control patients, grade 3 or worse mucositis was reduced by half from 85% to 40% with sargramostim administration. In a phase I trial of colorectal cancer patients receiving escalating doses of 5-FU, sargramostim used in conjunction with leucovorin resulted in decreased rates of diarrhea relative to historical patients.168
Topical application of molgramostim in the treatment or prevention of mucositis has also been investigated.169,170 Of 10 BMT patients who received molgramostim (400 μg) in 100 mL of water administered as a mouthwash and then swallowed, only grade 1 or 2 mucositis was observed; in comparison, 8 of 10 patients who did not receive the mouthwash developed grade 4 mucositis.170 When the molgramostim mouthwash was used as a 2-minute rinse and not swallowed, there was no benefit with regard to mucositis; however, a positive correlation between molgramostim dose and leukocyte recovery was observed, providing evidence for systemic absorption and a hematopoietic effect.169
Wound healing.
As mentioned previously, rHuGM-CSF has been shown to enhance the migration and proliferation of endothelial cells and to promote keratinocyte growth.162,163 Animal studies have also shown that local application of rHuGM-CSF to wounds results in increased formation of granulation tissue, increased breaking strength of incisional wounds, and reversal of wound contraction in infected wounds, resulting in a faster time to wound healing.171-173Intradermal injections of rHuGM-CSF (molgramostim and regramostim) in humans with lepromatous leprosy resulted in enlarged keratinocytes, keratinocyte proliferation, thickening of the epidermis, accumulation of Langerhans cells, and enhanced healing.174,175 A number of case reports and small series reports have been published on the use of rHuGM-CSF as a treatment for nonhealing wounds and ulcers.176-178 Using various routes of rHuGM-CSF administration (subcutaneously around the wound, incubated with skin grafts, and as a topical application in sterile water), signs of wound healing occurred rapidly and total wound closure was achieved between 10 days and 5 weeks after treatment.
The safety and feasibility of using molgramostim to treat patients with vascular leg ulcers was evaluated by Arnold et al.179 Ten patients were treated with four intradermal injections of molgramostim at 50 μg around the perimeter of their ulcers every 2 weeks for a total of 12 weeks. No hematological abnormalities were observed and the injections were reported to be relatively painless. Although this study was not designed to determine efficacy, some patients demonstrated complete or partial healing of their ulcers.
In a double-blind, placebo-controlled study, 40 patients with chronic leg ulcers were randomized to receive either 400 μg of rHuGM-CSF or a similar volume of saline; equal-dose injections were administered into four quadrants around the wound.180 The study was unblinded prematurely and data on 25 treated patients were reported. By day 8 after treatment, a significant (P < .005) difference in mean ulcer surface area reduction was observed between the two arms in favor of rHuGM-CSF. Complete healing by week 8 was observed in 8 of 16 patients treated with rHuGM-CSF and 1 of 9 placebo-treated patients. No significant side effects were reported during the trial. These findings from case reports and small studies indicate that some patients with nonhealing ulcers may benefit from rHuGM-CSF therapy. More research is needed to determine the appropriate dose, optimal dosing frequency, and efficacy of rHuGM-CSF in different types of wounds and ulcers.
Hypercholesterolemia.
Elevated cholesterol concentrations result from disordered lipid metabolism. The liver is the major site of cholesterol biosynthesis and excretion; however, macrophages produce factors that activate cholesterol biosynthesis or excretion, and mononuclear phagocytes play an important role in the processing and transport of cholesterol.181 Activated T-cell products also can affect the synthesis and accumulation of cholesterol by mononuclear phagocytes. Therefore, rHuGM-CSF could indirectly affect cholesterol levels by stimulating the activity of macrophages in the liver or phagocytic cells in the circulation or present at the site of an atherosclerotic plaque.181
The ability of regramostim to lower serum cholesterol concentrations was reported almost a decade ago.181 Since then, efforts have focused on determining the mechanism(s) of this effect. In rabbits, the reduction in serum cholesterol is accompanied by an increase in the levels of mRNA for very low density lipoprotein (VLDL) receptors in muscle; the levels of LDL receptor mRNA in liver are unchanged. These findings suggest that the cholesterol-lowering effect of rHuGM-CSF (molgramostim) may be mediated by enhancement of macrophage functions in lipid metabolism and the increase in mRNA for VLDL receptor.182
Treatment of pulmonary alveolar proteinosis.
Pulmonary alveolar proteinosis includes a heterogenous group of diseases, both congenital and acquired, that are characterized by accumulation of large quantities of lipid- and protein-rich eosinophilic material (ie, surfactant) within the alveoli and airways.183 Murine studies indicate that mice carrying a null allele of the GM-CSF gene develop a pulmonary abnormality that resembles alveolar proteinosis, suggesting that GM-CSF regulates the clearance or catabolism of surfactant proteins and lipids.184-186 Additionally, dysregulation of inflammatory cell activity due to the lack of GM-CSF may have detrimental effects on host defense and contribute to further lung injury.183 An anecdotal report of rHuGM-CSF use in an adult with this disease has been published and indicates some improvement in symptoms with cytokine therapy.187
SUMMARY AND CONCLUSIONS
rHuGM-CSF stimulates the proliferation and differentiation of multiple hematopoietic progenitor cells in the myeloid lineage and activates or augments many of the functional activities of mature neutrophils, monocytes/macrophages, and dendritic cells, enhancing host defenses against a broad spectrum of invading microorganisms. These properties have greatly expanded the possible therapeutic benefits of the cytokine in a wide variety of settings (Table 4), particularly those in which prevention of infection is desirable. The drug may be useful as prophylaxis or adjunctive treatment of bacterial or fungal infections in immunocompromised individuals, including cancer patients receiving myelosuppressive chemotherapy and patients with advanced HIV infection. In addition, exposure to rHuGM-CSF has recently been shown to reduce the susceptibility of macrophages to infection by HIV. Sargramostim is being evaluated as a vaccine adjuvant against infectious diseases and malignancies and as immunotherapy in the treatment of various malignancies, including melanoma and neuroblastoma.
Summary of Emerging Applications of rHuGM-CSF
| Therapeutic Use . | Preclinical Actions of rHuGM-CSF . | References . | Clinical Results With rHuGM-CSF . | References . |
|---|---|---|---|---|
| Fungal infections | Increases receptor expression on macrophages. Enhances fungicidal activity againstAspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum, Torulopsis glabrata. Counteracts dexamethasone-induced inhibition of superoxide anion release by monocytes. | 16, 88, 92-95, 98-102 | Decreases incidence of fungal infections versus placebo in AuBMT patients. Reduces mortality due to fungal infections in elderly patients with AML. As an adjunct to amphotericin B, improves recovery from Candida andAspergillus infections. Improves oropharyngeal candidiasis refractory to fluconazole in HIV-infected patients. | 112-116 |
| HIV infection and its complications | Suppresses HIV expression. Enhances antiretroviral activity of zidovudine and stavudine. Downregulates expression of CCR5, reducing the susceptibility of macrophages to HIV infection. Promotes killing of Mycobacterium avium-intracellular(MAC). | 105, 107, 124-129 | Increases CD4 count. Decreases viral load. As adjunctive treatment of MAC, reduces the number of viable intracellular MAC/mL. | 136, 137, 139 |
| Vaccine adjuvant | Increases class II MHC expression and stimulates T-cell immune responses. Augments the primary in vitro immune response to sheep red blood cells by murine spleen cells. Enhances expression of costimulatory molecules and adhesion molecules and enhances production of other cytokines. Primes T cells for IL-2–induced proliferation. Augments LAK cell generation in conjunction with IL-2. | 12, 14, 15, 142-145 | Enhances antibody response to hepatitis B vaccine. Increases the percent of patients who seroconverted to all three strains of flu vaccine. | 141, 146, 147 |
| Antitumor therapy | Enhances monocyte cytotoxicity against human tumor cells. Enhances IL-2–mediated LAK cell function. Increases secretion of matrix metalloelastase with subsequent production of angiostatin. Facilitates tumor antigen presentation. | 56, 149-153 | Prolongs disease-free survival and overall survival compared with historical controls in patients with advanced melanoma. Of 20 stage IV melanoma patients who received sargramostim as an adjuvant to a melanoma vaccine, 4 had partial to complete responses. | 155-157 |
| Immunotherapy for AML | Enhances activated killer cell function. Upregulates expression of intercellular adhesion molecule-1 and lymphocyte function associated molecules. | 160, 161 | Decreases risk of relapse compared with controls (37.4% v 49.5%). | 160 |
| Mucositis, stomatitis, diarrhea | Stimulates the migration and proliferation of endothelial cells and promotes keratinocyte growth. | 162, 163 | Reduces incidence and severity of mucositis in patients with sarcoma, advanced head and neck cancer, and those undergoing allogeneic BMT. Shortens the duration of mucositis in children undergoing stem cell transplant. Decreases rates of diarrhea in colorectal cancer patients receiving 5-FU. | 164-168, 170 |
| Wound healing | Increases formation of granulation tissue. Increases breaking strength of incisional wounds. Decreases time to wound healing. | 171-173 | Intradermal injections of rHuGM-CSF results in enlarged keratinocytes, keratinocyte proliferation, thickening of the epidermis, accumulation of Langerhans cells, and enhances healing. Reduces mean ulcer surface area versus saline in patients with chronic leg ulcers. | 174-180 |
| Therapeutic Use . | Preclinical Actions of rHuGM-CSF . | References . | Clinical Results With rHuGM-CSF . | References . |
|---|---|---|---|---|
| Fungal infections | Increases receptor expression on macrophages. Enhances fungicidal activity againstAspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Histoplasma capsulatum, Torulopsis glabrata. Counteracts dexamethasone-induced inhibition of superoxide anion release by monocytes. | 16, 88, 92-95, 98-102 | Decreases incidence of fungal infections versus placebo in AuBMT patients. Reduces mortality due to fungal infections in elderly patients with AML. As an adjunct to amphotericin B, improves recovery from Candida andAspergillus infections. Improves oropharyngeal candidiasis refractory to fluconazole in HIV-infected patients. | 112-116 |
| HIV infection and its complications | Suppresses HIV expression. Enhances antiretroviral activity of zidovudine and stavudine. Downregulates expression of CCR5, reducing the susceptibility of macrophages to HIV infection. Promotes killing of Mycobacterium avium-intracellular(MAC). | 105, 107, 124-129 | Increases CD4 count. Decreases viral load. As adjunctive treatment of MAC, reduces the number of viable intracellular MAC/mL. | 136, 137, 139 |
| Vaccine adjuvant | Increases class II MHC expression and stimulates T-cell immune responses. Augments the primary in vitro immune response to sheep red blood cells by murine spleen cells. Enhances expression of costimulatory molecules and adhesion molecules and enhances production of other cytokines. Primes T cells for IL-2–induced proliferation. Augments LAK cell generation in conjunction with IL-2. | 12, 14, 15, 142-145 | Enhances antibody response to hepatitis B vaccine. Increases the percent of patients who seroconverted to all three strains of flu vaccine. | 141, 146, 147 |
| Antitumor therapy | Enhances monocyte cytotoxicity against human tumor cells. Enhances IL-2–mediated LAK cell function. Increases secretion of matrix metalloelastase with subsequent production of angiostatin. Facilitates tumor antigen presentation. | 56, 149-153 | Prolongs disease-free survival and overall survival compared with historical controls in patients with advanced melanoma. Of 20 stage IV melanoma patients who received sargramostim as an adjuvant to a melanoma vaccine, 4 had partial to complete responses. | 155-157 |
| Immunotherapy for AML | Enhances activated killer cell function. Upregulates expression of intercellular adhesion molecule-1 and lymphocyte function associated molecules. | 160, 161 | Decreases risk of relapse compared with controls (37.4% v 49.5%). | 160 |
| Mucositis, stomatitis, diarrhea | Stimulates the migration and proliferation of endothelial cells and promotes keratinocyte growth. | 162, 163 | Reduces incidence and severity of mucositis in patients with sarcoma, advanced head and neck cancer, and those undergoing allogeneic BMT. Shortens the duration of mucositis in children undergoing stem cell transplant. Decreases rates of diarrhea in colorectal cancer patients receiving 5-FU. | 164-168, 170 |
| Wound healing | Increases formation of granulation tissue. Increases breaking strength of incisional wounds. Decreases time to wound healing. | 171-173 | Intradermal injections of rHuGM-CSF results in enlarged keratinocytes, keratinocyte proliferation, thickening of the epidermis, accumulation of Langerhans cells, and enhances healing. Reduces mean ulcer surface area versus saline in patients with chronic leg ulcers. | 174-180 |
Abbreviations: CCR5, β-chemokine receptor on macrophages; MHC, major histocompatibility complex; LAK, lymphokine-activated killer; 5-FU, fluorouracil.
Based on the increasing variety of biologic effects being attributed to endogenous GM-CSF, additional clinical uses for sargramostim and molgramostim are under investigation. Because rHuGM-CSF has been shown to stimulate the migration and proliferation of endothelial cells and local application of rHuGM-CSF in animal studies has shown faster wound healing times, clinical trials have evaluated rHuGM-CSF in patients susceptible to mucosal damage, such as mucositis, stomatitis, and diarrhea, and those with nonhealing wounds and ulcers. It is likely that the future will see applicaton of rHuGM-CSF in a variety of settings beyond those classically associated with myelosuppression.
REFERENCES
Author notes
Address reprint requests to James O. Armitage, MD, University of Nebraska Medical Center, 600 S 42nd St, Omaha, NE 68198-3332.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal