Abstract
We describe a long-term, in vitro culture system initiated with CD34+ or CD34+CD38− umbilical cord blood hematopoietic progenitors that supports normal human B-lineage development, including the production of mature Ig-secreting B cells. In the first stage (human B-progenitor long-term culture [HB-LTC]), CD34+ hematopoietic progenitors are cultured on the murine stromal cell line, S17, leading to the sustained production of large numbers of CD10+, CD19+early B progenitors. Reverse transcriptase-polymerase chain reaction (RT-PCR) and three-parameter flow cytometry for VpreB (surrogate light chain), cytoplasmic μ chain, and surface IgM expression were used to characterize the CD19+ B progenitors present within these cultures. This analysis showed distinct B-lineage subpopulations, including pro-B cells, cycling pre-B cells, and IgM+, IgD−/+ immature B cells. The limited expansion of IgM+ B cells and the immature surface phenotype of this population (IgM+, IgD+, CD10+, CD38+) suggested that HB-LTC conditions were unable to provide appropriate signals for further differentiation. A second culture stage was used to determine if these immature B cells were functionally competent. Purified CD19+ cells were transferred onto fibroblasts expressing human CD40-ligand in the presence of IL-10 and IL-4. This lead to cell proliferation, modulation of the IgM+ cell surface phenotype to one consistent with an activated mature B cell, secretion of Ig, and isotype switching. Notably, IgM and IgG producing B cells were also generated using two-stage cultures established with highly purified multipotent CD34+CD38−hematopoietic stem cell progenitors. This culture model should permit detailed in vitro analysis and genetic manipulation of the major transition points in human B ontogeny, beginning with commitment to the B lineage and leading to development and activation of mature B cells.
B LYMPHOPOIESIS is a developmentally regulated process characterized by expression of specific regulatory genes, cell surface molecules, and somatic gene rearrangements leading ultimately to production of Ig-secreting plasma B cells. These events are controlled through cell-cell interactions, by hematopoietic growth factors, and by signaling through the pre-B– and B-cell antigen receptors. These receptors regulate two key steps during B-lineage development: (1) the pre-B to immature B cell and (2) the naive to activated B-cell transitions.1-5 Pre-B– and B-cell antigen receptor-dependent signals are essential for Ig chain allelic exclusion and proliferation of bone marrow pre-B cells and for activation and proliferation of naive B cells after encounter with antigen in the peripheral lymphoid tissues. The timing, dosage, and cellular microenvironmental context of antigen receptor engagement further modulates these events. Dysregulation of these signals can lead, alternatively, to humoral immunodeficiencies or to autoimmunity or malignancy.6-8
The complexity of mammalian bone marrow and lymphoid tissues has impeded detailed in vivo molecular and phenotypic analysis of events regulating both primary (bone marrow-dependent) and secondary (peripheral lymphoid tissue-dependent) B-lineage development. Analysis of normal and altered human B lymphopoiesis must rely primarily on in vitro or SCID/Hu in vivo animal models. Whereas progress has been made in defining human B-lineage developmental populations9-14and the consequences of inherited B-lineage genetic defects,4,15-18 the evolution of human B-lineage culture models analogous to those established using murine cells19-23 has been technically challenging.24Recently, important progress has been made in sustaining the growth of immature human B progenitors using primary human bone marrow stroma or using human or murine stromal cell lines.25-30 As part of these studies we have developed a stromal cell-dependent culture model that permits the generation and long-term growth of CD10+CD19+ human B progenitors derived from either CD34+CD38+ or more primitive CD34+CD38− multipotent hematopoietic stem cell progenitors.31-33
An ideal B-lineage culture model should support B-lymphoid commitment from multipotent input stem cells and both primary and secondary B-lymphoid development. A major limitation of the human long-term B-progenitor culture system we have described and of related murine B-lineage culture systems has been their limited capacity for expansion of surface Ig+ (sIg+) B cells and the generation of mature B-cell populations. Some of the key signals controlling mature B-lineage development have been modeled in vitro using fibroblast lines expressing the CD40 ligand (CD40L; reviewed in Banchereau et al49).34 35 CD40 activation in association with specific growth factors has been used to promote mature B-cell survival, proliferation, and differentiation. These observations suggested that a B-lineage culture system that combined sequentially the use of a bone marrow stromal cell line and CD40 activation signals might overcome some of the limitations of current in vitro culture models.
We describe here the establishment of a human B-lineage culture system that supports the major events in both primary and secondary B-lineage development. This system uses two in vitro culture stages: generation of pro-B–cell to immature B-cell populations from multipotent hematopoietic progenitors using stromal cell support; and polyclonal expansion and maturation of these immature IgM+IgD+/− B cells into Ig-producing B cells after CD40 cross-linking. This system provides a powerful model for use in a range of future studies of both normal and altered human B lymphopoiesis, including evaluation of the capacity for B-lineage development after gene transfer into normal or immunodeficient hematopoietic stem cells.
MATERIALS AND METHODS
Cell sources, cytokines, antibodies, and flow cytometry.
Umbilical cord blood, obtained according to the guidelines approved by the UCLA or Childrens Hospital Los Angeles Committees on Clinical Investigation (IRB), was placed in sterile tubes containing heparin (10 U/mL), stored at room temperature, and processed within 24 hours. The murine stromal cell line S17 (kindly provided by Dr K. Dorshkind, University of California, Los Angeles, CA)36 and murine fibroblasts stably transfected with a human CD40 ligand (CD40L) cDNA (kindly provided by Dr J. Banchereau, Schering-Plough, Dardilly, France)37 38 were maintained as previously described. Human recombinant cytokines were used at the following concentrations: interleukin-10 (IL-10; 100 ng/mL) and IL-4 (100 ng/mL) (generous gift from Dr S. Narula, Schering-Plough, Kenilworth, NJ), Flt3 ligand (FL; 50 U/mL), and IL-2 (100 ng/mL; a gift from Amgen Inc, Thousand Oaks, CA).
Antibodies, including fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD19 (Leu 12), PE-conjugated anti-CD20 (Leu 19), FITC-conjugated anti-CD10 (Calla), tricolor-conjugated anti-CD38 (OKT10), Simultest anti-κ/λ, FITC-conjugated anti-major histocompatibility complex (MHC) class II DR (Becton Dickinson Immunocytometry Systems, San Jose, CA), FITC-or PE-conjugated anti-IgM F(ab)′2, PE-conjugated anti-IgD, and FITC-conjugated antimouse IgM (Southern Biotechnology, Birmingham, AL) were used for phenotypic studies as recommended by the manufacturers. The biotinylated anti-VpreB monoclonal antibody (MoAb; MAD688) was used as previously described.39 Two-color flow cytometry analysis of cultured cells was performed as previously described,31,32 and all samples were simultaneously analyzed using isotype control antibodies. For multicolor staining using indirect staining with the anti-VpreB MoAb, cells were successively incubated with biotinylated anti-VpreB and spectral-Red conjugated streptavidin before incubation with other antibodies or cell permeabilization. For intracellular analysis of μ heavy chains, cells were permeabilized and fixed by 30 minutes of incubation at room temperature in Permeafix solution as recommended by the manufacturer (Ortho Diagnostics Systems, Raritan, NJ). Gating on MAD688-positive cell populations was determined using analysis with control Ig (or spectral-Red–conjugated streptavidin) versus anti-κ/λ or anti-IgM. Control stains were evaluated using for both cell surface analysis and in analysis of permeabilized cells using VpreB-positive and -negative control cell lines and CD19+ progenitors. Cell cycle analysis was determined by measurement of the incorporation of the DNA fluorochrome propidium iodide (32 μmol/L) as described.40
Enrichment of CD34+ cord blood cells and establishment of human B-progenitor long-term cultures (HB-LTC).
Low-density mononuclear cells (MNC) from umbilical cord blood were separated by centrifugation on Ficoll-Hypaque (d = 1.077; Amersham Pharmacia, Piscataway, NJ) and CD34+ progenitor cells were isolated using the MACS CD34 Isolation Kit (Miltenyi Biotechnology, Auburn, CA) as recommended by the manufacturer. Collected adsorbed cells were typically greater than 95% CD34+ by flow cytometry. Highly purified CD34+CD38−cord blood cells were defined strictly as those with high CD34 expression and anti-CD38 PE fluorescence less than one half of the maximum PE fluorescence of the isotype control and were isolated by cell sorting as previously described.32 S17 stromal cells, resuspended in RPMI1640 (Irvine Sciences, Tustin, CA) supplemented with 2 mmol/L glutamine, 50 mmol/L 2-mercaptoethanol, and 3% defined fetal calf serum (Hyclone Laboratories, Logan, UT) were plated in tissue culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) to obtain 50% confluent monolayers within 24 to 48 hours. Cord blood stem cell progenitors were plated on the 50% to 60% confluent S17 monolayers as previously described.31,32Briefly, CD34+ enriched cells were plated at a density of 0.5 to 2 × 106 cells per 10-cm dish and sorted CD34+CD38− cells were plated at a density of 0.5 to 5 × 103 cells/well. Cultures were fed biweekly, once with the addition of fresh medium and once by aspiration of 80% of the medium in the culture well and addition of fresh medium. Some cultures were supplemented with recombinant soluble FL at a final concentration of 50 U/mL as described previously.32
Separation of the CD19+ cells from long-term S17 culture and culture on CD40L fibroblasts.
Long-term cultured cells were harvested with or without the adherent cell monolayer and passed through a 40-μm nylon cell strainer (Falcon). Dead cells and debris (mostly from the S17 monolayer) were removed by centrifugation of the single-cell suspension over a Ficoll-Hypaque cushion and CD19+ B cells were isolated by using the MiniMacs cell separation system as recommended by the manufacturer (Miltenyi Biotech). Briefly, cells were incubated with a anti-CD19 antibody coupled to super-paramagnetic beads before being passed through a positive selection column (MACS column, or MACS MS when stromal cells were harvested in association with hematopoietic cells). Collected adsorbed cells were used for staining and/or for secondary cultures. Secondary cultures were prepared using either a newly plated 60% confluent S17 monolayer or CD40L fibroblasts as previously described.37,38 41 CD40L fibroblasts were irradiated with 8,000 rad before plating in 96- or 48-well plates at 5 × 103 or 2 × 104 cells/well, respectively. CD19+ cells were added to CD40L fibroblasts (0.05 to 5 × 105 cells/well) with cytokines as indicated in Results. CD19+ cells were transferred to freshly irradiated CD40L fibroblasts every 5 to 7 days for the duration of the experiments described.
Ig secretion assay.
Purified CD19+ cells were seeded on CD40L L cells at 5 × 105 cells/mL (or at other cell densities as noted) in 96-well plates in a final volume of 100 μL with the indicated cytokines. At days 8 and 15, cells were transferred to freshly irradiated CD40L fibroblasts with fresh cytokines (final volume, 200 μL) and cultured for an additional 7-day period. Culture supernatant (180 μL) was collected at day 7, 14, or 21; spun to remove cell debris; and frozen for further analysis. Enzyme-linked immunoabsorbtion assay (ELISA) for IgM production was performed in microtiter plates coated overnight with 1/5,000 dilution of goat antihuman IgM (μ specific; Southern Biotechnology) in carbonate buffer. Plates were incubated with samples or standards (human Ig standard; ICN Pharmaceuticals, Aurora, OH) for 4 hours at 4°C and bound antibody was detected using an alkaline phosphatase-conjugated rabbit antihuman IgM (1:5,000 dilution; Jackson Laboratories, West Grove, PA) followed by p-nitrophenyl phosphate as substrate. Plates were scanned at 409 nm in a multiwell reader (Molecular Devices, Sunnyvale, CA) and analyzed using Soft Max software (Molecular Devices). For IgG determinations, goat antihuman IgG (1:5,000 dilution; Southern Biotechnology) was used as capture antibody, followed by detection using biotinylated goat antihuman Ig (H +L chains; 1:5,000 dilution; Southern Biotechnology), streptavidin-alkaline phosphatase (1:1,000 dilution; Sigma, St Louis, MO), and substrate. Limits of detection were 2 ng/mL (IgM) and 0.5 ng/mL (IgG). Results are presented as the average of ≥3 determinations. The coefficient of variation between samples was always less than 10%. Notably, the addition of 50 μmol/L 2-mercaptoethanol supported cell survival and was associated with an approximately threefold to fivefold increase in detectable Ig in cultures established with HB-LTC CD19+ progenitors.
cDNA preparation and polymerase chain reaction (PCR).
Cells were collected and RNA extracted using RNAzol (Biotecx, Houston, TX) or, alternatively, limited numbers of fluorescence-activated cell sorting (FACS)-sorted cells (103, 102, 101, or single cells) were resuspended in 5 μL of phosphate-buffered saline (PBS). Samples were heated for 2 minutes at 65°C and then chilled on ice before the addition of the reverse transcriptase (RT) reaction mix (Superscript GIBCO; GIBCO, Grand Island, NY; Ghia et al12). The RT reaction was performed by incubation for 1 hour at 37°C, followed by inactivation of the enzyme for 10 minutes at 95°C. RT-PCR amplification of VpreB, μ, CD79b (B29), κ light chain, terminal deoxynucleotidyl transferase (TdT), and recombination associated gene (Rag) transcripts were performed in one or two rounds using primer pairs specific for VpreB, μ, CD79b, κ, TdT, Rag 1, and G3PDH (complete primer sequences available from D.J.R. upon request).
RT-PCR amplification of VH families was performed using primers described previously.42 After two rounds of PCR, products of the expected size were evaluated by ethidium bromide staining after separation by agarose gel electrophoresis. Comparison of the reaction products using decreasing numbers of input CD19+ cells (103, 102, and 101 cells, respectively) were used to semiquantitatively evaluate the relative expression of the individual VHfamilies. The limits for the detection of VH messages by ethidium staining was 100 to 1,000 cells from HB-LTC and 10 to 100 cells from CD40L cultures. This was consistent with the expansion of IgM+ B cells in the CD40L system. Each PCR reaction consisted of denaturation cycle at 94°C for 2 minutes, followed by 30 cycles each of 94°C for 30 seconds, 55°C for 40 seconds, 72°C for 60 seconds, and a final extension cycle at 72°C for 7 minutes. The reaction mix for the first round PCR included all the VH family primers. Second-round PCR was performed using individual primer pairs and 1 μL of the first-round product.
RESULTS
Multiple early B-lineage transcripts are detectable in HB-LTCs.
Long-term culture of human cord blood CD34+ input cells on the S17 murine bone marrow stromal cell line results in the emergence of a highly enriched population of CD34−CD19+CD10+ B-lineage progenitors (HB-LTC).31 32 In the current study, we sought to determine if these cultures supported B-lineage subpopulations representative of those present in vivo in normal human bone marrow and, if so, whether these populations retained the capacity for mature B-lineage development.
HB-LTC progenitors were first evaluated for the expression of key developmentally regulated B-lineage–specific transcripts, including TdT, the recombination-activating genes (Rag-1 and -2), surrogate light chain (VpreB), μ heavy chain, and κ or λ light chains. Analysis of the expression pattern of these transcripts and their protein products has previously been used to order the pro-B–cell to immature B-cell developmental sequence in normal human bone marrow.12 CD19+ B-progenitor cells were collected from long-term cultures at 8 weeks for RNA extraction and RT-PCR analysis. Messages for TdT and Rag-1 and -2 (Fig 1) were clearly detected, consistent with their expression by an early B-progenitor population. Transcripts encoding VpreB, μ heavy chain, and κ or λ light chains were also detectable by RT-PCR analysis (Fig 1). The results obtained evaluating bulk CD19+ populations were confirmed by additional analysis of FACS-purified HB-LTC subpopulations (data not shown). Together, these results suggested that HB-LTC might support several distinct B-lineage developmental subpopulations, including early TdT+ B progenitors, VpreB+ cells, and, possibly, IgM+ immature B cells.
B progenitors from HB-LTC express mRNA for TdT, Rag, VpreB, μ, and light chain. RT-PCR was performed using the primer pairs as described in Materials and Methods with (lane 1) RNA from nonadherent cells of 8-week-old HB-LTC; (lane 2) RNA from S17 cells grown in the same conditions without addition of the CD34+ progenitors; (lane 3) no added RNA; (N) RNA from the human pre-B–cell line, Nalm-6; or (R) RNA from the human IgM+ immature B-cell line, Ramos. Lane M contains DNA size standards. Results are representative of at least three independent experiments using unrelated HB-LTCs.
B progenitors from HB-LTC express mRNA for TdT, Rag, VpreB, μ, and light chain. RT-PCR was performed using the primer pairs as described in Materials and Methods with (lane 1) RNA from nonadherent cells of 8-week-old HB-LTC; (lane 2) RNA from S17 cells grown in the same conditions without addition of the CD34+ progenitors; (lane 3) no added RNA; (N) RNA from the human pre-B–cell line, Nalm-6; or (R) RNA from the human IgM+ immature B-cell line, Ramos. Lane M contains DNA size standards. Results are representative of at least three independent experiments using unrelated HB-LTCs.
HB-LTC supports the expansion of human pro-B cells, pre-B cells, and immature IgM+ B cells.
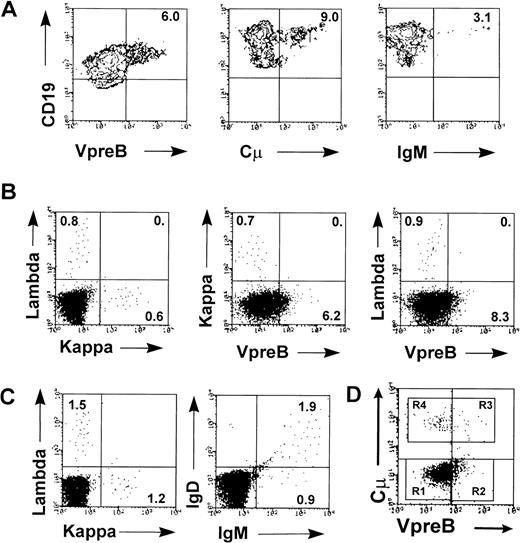
To further characterize the B-progenitor subsets in HB-LTC, nonadherent cells from multiple 4- to 10-week-old cultures were analyzed by flow cytometry for expression of CD19 with either the VpreB surrogate light chain or intracytoplasmic or surface Ig μ chains (cμ and IgM; Fig 2). The MoAb, MAD688, recognizes both VpreB+cμ− and VpreB+cμ+ B progenitors and has been used to characterize these populations in human cell lines and bone marrow.12 39 VpreB expression was detected as early as 4 weeks in HB-LTC, and 5% to 15% of the CD19+ cells expressed VpreB by 6 to 8 weeks (Fig 2A).
Multiple B-progenitor cell subsets are represented in HB-LTC. Nonadherent cells from HB-LTC were collected at 6 to 8 weeks for FACS analysis. (A) Two-color analysis demonstrating the presence of HB-LTC CD19+ progenitors expressing surrogate light chain (VpreB), cytoplasmic μ (Cμ), or surface IgM. (B) Analysis demonstrating independent expression κ versus λ light chains and of VpreB versus κ or λ light chain. (C) Analysis demonstrating coexpression of IgM and IgD within HB-LTC immature IgM+B-cell population. (D) Example of three-color analysis of purified HB-LTC CD19+ progenitors showing staining of VpreB versus cμ. This analysis showed VpreB−cμ−IgM− (R1) progenitors; VpreB+cμ−IgM−(R2) and VpreB+cμ+IgM− (R3) subsets; and VpreB−cμ+IgM−and VpreB−cμ+IgM+ subsets (R4; IgM data not shown). Results are representative from more than eight independent experiments. Numbers indicate the percentage of cells in each quadrant.
Multiple B-progenitor cell subsets are represented in HB-LTC. Nonadherent cells from HB-LTC were collected at 6 to 8 weeks for FACS analysis. (A) Two-color analysis demonstrating the presence of HB-LTC CD19+ progenitors expressing surrogate light chain (VpreB), cytoplasmic μ (Cμ), or surface IgM. (B) Analysis demonstrating independent expression κ versus λ light chains and of VpreB versus κ or λ light chain. (C) Analysis demonstrating coexpression of IgM and IgD within HB-LTC immature IgM+B-cell population. (D) Example of three-color analysis of purified HB-LTC CD19+ progenitors showing staining of VpreB versus cμ. This analysis showed VpreB−cμ−IgM− (R1) progenitors; VpreB+cμ−IgM−(R2) and VpreB+cμ+IgM− (R3) subsets; and VpreB−cμ+IgM−and VpreB−cμ+IgM+ subsets (R4; IgM data not shown). Results are representative from more than eight independent experiments. Numbers indicate the percentage of cells in each quadrant.
Rearrangement of the μ heavy chain locus leads initially to intracytoplasmic expression of μ in early B-cell precursors. Expression of cμ was found in 8% to 18% of the CD19+cells (Fig 2A). Successful rearrangements of the κ or λ light chain genes in cμ+ cells leads to surface IgM expression. IgM expression was detected in 3% to 10% of the CD19+CD10+ progenitors (3% in Fig 2A). Double staining demonstrated coexpression of either κ or λ light chains with surface IgM (data not shown). The CD19+ cells expressing either κ or λ light chain did not react with the anti-VpreB antibody (Fig 2B). Notably, approximately 60% of the IgM+ cells also coexpressed IgD (Fig 2C). Overall, the frequency of VpreB+, cμ+, and IgM+ progenitor cells varied relatively little between cultures or within individual cultures during weeks 6 through 8. In six independent cultures, an average of 6% (±2%) VpreB+cells, 10% (±2.5%) cμ+ cells, and 3.3% (±0.9%) sμ+ cells were detected.
We used three-color FACS analysis for VpreB, cμ, and surface IgM chain expression to further delineate the CD19+subpopulations from several HB-LTCs. Analysis of purified CD19+ progenitors (>95% CD19+) confirmed the presence of several distinct B-lineage subpopulations, including a predominant population of VpreB−Cμ−IgM−cells (74% in this example; Fig 2D and data not shown); smaller numbers of VpreB+ cells consisting of VpreB+Cμ−IgM− and VpreB+Cμ+IgM− subsets (18%); and a limited number of both VpreB−Cμ+IgM− and VpreB−Cμ+IgM+ cells (8%).
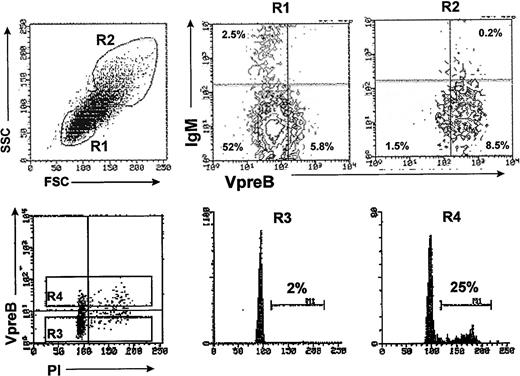
Because pre-B cells comprise the major cycling population in murine and human bone marrow,12 43 we evaluated the relative size and cell cycle status of the VpreB+ progenitors present in HB-LTCs. Analysis according to cell size showed that the VpreB+ population consisted predominantly of larger cells (Fig 3A, R2 gate), whereas the VpreB−IgM− and IgM+populations were composed of very small cells (R1 gate). In addition, analysis of DNA content indicated that the VpreB+ cells contained the majority of CD19+ cells in cell cycle (25% of VpreB+ were in S/G2/M v 2 % for VpreB− cell population; Fig 3B).
The HB-LTC CD19+ VpreB+population is composed of predominantly large, cycling cells. B-lineage cells were separated from 8-week-old HB-LTC by positive selection using anti-CD19 magnetic beads and stained with anti-VpreB and anti-IgM antibodies and analyzed by flow cytometry. (A) (Left) Forward and side scatter analysis of total cell population showing gated R1 (smallest) or R2 (largest) cell populations. (Right) VpreB+ and VpreB− populations in gates R1 or R2, with numbers indicating the percentages of the total CD19+ cell population in each quadrant. (B) Cell cycle analysis of CD19+VpreB+ cells. CD19+ were stained with the anti-VpreB antibody before permeabilization and staining with propidium iodide for FACS analysis of DNA content versus surface VpreB expression (R3 = VpreB− and R4 = VpreB+ populations, respectively). Numbers indicate the relative percentage of cells in S/G2/M.
The HB-LTC CD19+ VpreB+population is composed of predominantly large, cycling cells. B-lineage cells were separated from 8-week-old HB-LTC by positive selection using anti-CD19 magnetic beads and stained with anti-VpreB and anti-IgM antibodies and analyzed by flow cytometry. (A) (Left) Forward and side scatter analysis of total cell population showing gated R1 (smallest) or R2 (largest) cell populations. (Right) VpreB+ and VpreB− populations in gates R1 or R2, with numbers indicating the percentages of the total CD19+ cell population in each quadrant. (B) Cell cycle analysis of CD19+VpreB+ cells. CD19+ were stained with the anti-VpreB antibody before permeabilization and staining with propidium iodide for FACS analysis of DNA content versus surface VpreB expression (R3 = VpreB− and R4 = VpreB+ populations, respectively). Numbers indicate the relative percentage of cells in S/G2/M.
Together, these results demonstrate that HB-LTC results in the expansion of multiple stages of early B-cell development, including a majority population of pro-B cells (CD19+VpreB−Cμ−IgM−); large, cycling pre-B cells (VpreB+Cμ−IgM− and VpreB+Cμ+IgM− cells); and a limited number of relatively quiescent, immature B cells (VpreB−Cμ+IgM+IgD+/−). The multiparameter analysis described above also suggested the presence of transitional subpopulations within these major developmental stages. As previously reported, CD34+ pro-B cells were not detected in HB-LTC.31
The continuum of B-lineage differentiation in HB-LTC stops at the stage of immature IgM+ B cells.
IgM+ cells were undetectable by FACS analysis before 5 weeks of culture on S17 stroma. The relative frequency of IgM+ cells increased slowly beginning at 5 weeks and reached a maximum of 3% to 10% between weeks 6 and 8. Extended culture for up to 12 weeks resulted in no significant further increase in the fraction of IgM+ B cells (data not shown). Consistent with these observations, analysis of cell size and cell cycle demonstrated that the κ- or λ-expressing cells were predominantly within the smaller, quiescent CD19+population (data not shown). Notably, all the HB-LTC–derived CD19+ cells, including the IgM+, IgD+ population, coexpressed both CD10 and CD38 (data not shown and Fig 4C). This phenotype contrasts with that of circulating naive human peripheral B cells, which are predominantly composed of IgM+IgD+CD10−CD38−cells. Together, this relatively immature surface phenotype and the limited expansion of IgM+ cells suggested that the S17 stromal line was unable to provide the appropriate developmental signals required for the expansion and subsequent maturation of the IgM+ immature B cells generated in these HB-LTCs.
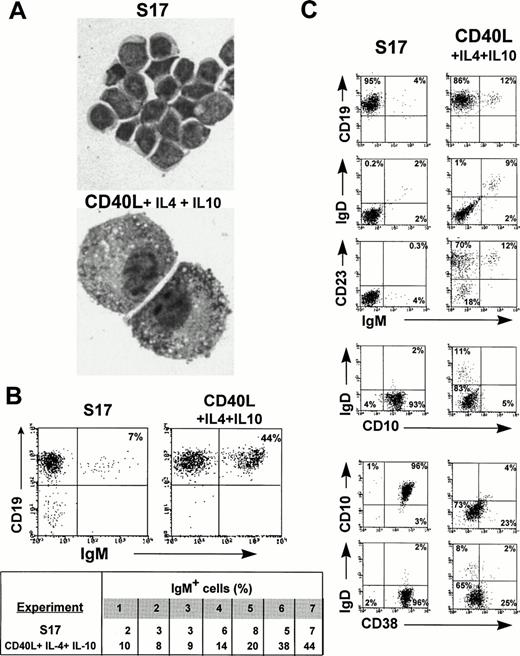
CD40 ligand stimulation promotes the expansion and maturation of long-term cultured IgM+ B cells. Purified CD19+ progenitors from 8-week-old HB-LTC were transferred onto freshly plated S17 stroma or onto irradiated CD40L fibroblasts in medium supplemented by IL-4 and IL-10 and collected after 8 days in culture. (A) Wright-Giemsa staining of CD19+ cells (original magnification × 1,000) demonstrating plamacytoid appeareance of CD19+ cells in the CD40L system. (B) (Upper panel) FACS analysis demonstrating increase in IgM+ B cells after transfer to the CD40L system. (Lower panel) Results of anti-IgM staining after transfer to S17 versus the CD40L system using CD19+ cells from 7 independent HB-LTCs. (C) FACS analysis demonstrating the increase in IgM+, IgM+IgD+ populations, the increase in CD23 expression, and the decrease CD10 and CD38 expression in CD19+ progenitors after transfer to the CD40L system.
CD40 ligand stimulation promotes the expansion and maturation of long-term cultured IgM+ B cells. Purified CD19+ progenitors from 8-week-old HB-LTC were transferred onto freshly plated S17 stroma or onto irradiated CD40L fibroblasts in medium supplemented by IL-4 and IL-10 and collected after 8 days in culture. (A) Wright-Giemsa staining of CD19+ cells (original magnification × 1,000) demonstrating plamacytoid appeareance of CD19+ cells in the CD40L system. (B) (Upper panel) FACS analysis demonstrating increase in IgM+ B cells after transfer to the CD40L system. (Lower panel) Results of anti-IgM staining after transfer to S17 versus the CD40L system using CD19+ cells from 7 independent HB-LTCs. (C) FACS analysis demonstrating the increase in IgM+, IgM+IgD+ populations, the increase in CD23 expression, and the decrease CD10 and CD38 expression in CD19+ progenitors after transfer to the CD40L system.
CD40 ligand stimulation promotes the expansion and maturation of long-term cultured IgM+ B cells.
Analysis of HB-LTC–derived CD19+ progenitors clearly demonstrated cell surface expression of CD40 (data not shown). Both fetal BM-derived CD34+CD19− and CD19+IgM+/− progenitors express CD40 and proliferate in response to CD40 activation.44-46 CD40 activation is also critical for activation and proliferation of mature human B cells.47-49 We therefore evaluated whether a combination of CD40L activation in association with cytokines would promote the expansion and/or the maturation of HB-LTC B progenitors. CD19+ cells were isolated from 8-week-old cultures and transferred onto either irradiated mouse fibroblasts stably expressing human CD40L37 38 or to fresh S17 stroma, each in the presence of IL-4 and IL-10 (100 ng/mL). Strikingly, large, loose aggregates of activated blast-like CD19+ cells in close association with the CD40L fibroblasts were evident within 5 to 8 days after cell transfer. The majority of these CD19+ cells exhibited eccentric nuclei, a low nuclear to cytoplasmic ratio, and increased mitotic figures (Fig 4A). Within 14 days of culture on CD40L fibroblasts, a predominant population of large plasmacytoid cells were present. In contrast, cells transferred to S17 with or without cytokines contained only small lymphoid cells, with a high nuclear to cytoplasmic ratio without evidence of cellular activation.
FACS analysis showed significant alterations in the surface phenotype of the CD40L-cultured B cells (Fig 4B and C). In seven independent cultures established with cord blood progenitors, the frequency IgM+ B cells increased to an average of greater than fourfold within 7 to 14 days. This included an increase in the frequency of both IgM+IgD− and IgM+IgD+ B cells. In the representative experiment presented in Fig 4C, the total IgM+ population increased from 4% to 12% and the IgM+IgD+population increased from 2% to 9%, respectively. IgM− and naive IgM+ B cells upregulate CD23 expression, and naive B cells also downmodulate CD10 and CD38 expression in response to CD40 cross-linking.45 49-51 The majority of HB-LTC–derived CD19+ cells, including all the IgM+ cells, acquired CD23 expression after culture with CD40L fibroblasts. CD10 and CD38 expression also decreased significantly in both the IgD− and IgD+populations (Fig 4C). In contrast, CD19+ cells transferred onto S17 with IL-4 and IL-10 showed no alteration in phenotype, with the exception of a slight increase in CD23 expression.
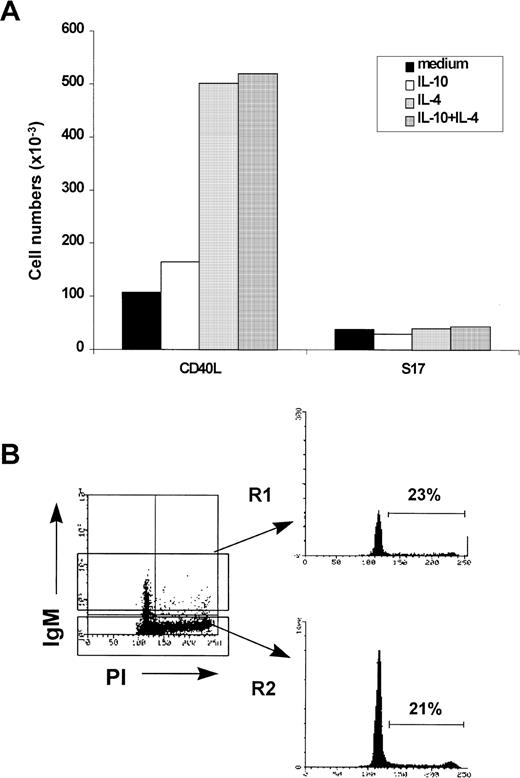
We also determined the proliferative capacity of HB-LTC–derived CD19+ cells after transfer to the CD40L system. CD19+ cells isolated from 8-week-old HB-LTCs were cultured with CD40L or S17 stroma with or without added cytokines (Fig 5A). Whereas culture on CD40L fibroblasts without added cytokines maintained input CD19+cell numbers, the addition of IL-4 increased cell recovery fivefold. IL-10 had no significant effect on total cell recovery. The addition of IL-4 and/or IL-10 to S17 stromal culture did not appreciably affect viable cell recovery. Analysis of thymidine incorporation in CD19+ cells under these culture conditions yielded similar results (data not shown).
CD40L culture enhances the proliferation of HB-LTC–derived CD19+ progenitors. A representative experiment in which CD19+ progenitors from an 8-week-old HB-LTC were seeded in microwells (105 cells/well) containing S17 stromal cells or CD40L fibroblasts in medium containing no added cytokines, IL-4, IL-10, or both cytokines. (A) Cell recovery was determined by trypan blue dye exclusion and expressed as the mean number of viable cells. (B) Cell cycle analysis of CD19+cells removed from the CD40L system. (Left) Dot plot of IgM versus DNA content analyzed by propidium iodide staining. (Right) Histogram illustrating DNA content in the IgM+ (R1) versus the IgM− (R2) populations. Numbers indicate the percentage of cells in S/G2/M.
CD40L culture enhances the proliferation of HB-LTC–derived CD19+ progenitors. A representative experiment in which CD19+ progenitors from an 8-week-old HB-LTC were seeded in microwells (105 cells/well) containing S17 stromal cells or CD40L fibroblasts in medium containing no added cytokines, IL-4, IL-10, or both cytokines. (A) Cell recovery was determined by trypan blue dye exclusion and expressed as the mean number of viable cells. (B) Cell cycle analysis of CD19+cells removed from the CD40L system. (Left) Dot plot of IgM versus DNA content analyzed by propidium iodide staining. (Right) Histogram illustrating DNA content in the IgM+ (R1) versus the IgM− (R2) populations. Numbers indicate the percentage of cells in S/G2/M.
Consistent with these observations, the combination of CD40 activation and IL-4 supported entry of the B-lineage cells into cell cycle. By day 8, 15% to 35% of cells under these conditions were in S/G2/M phase (∼25% in the experiment shown in Fig 5B). Double staining for surface IgM and DNA content indicated that both the IgM− and IgM+ CD19+progenitors were in cell cycle at this time point (with 21% and 23% of cells in cycle, respectively). By transferring the cells weekly onto newly irradiated fibroblasts with IL-4 and IL-10, CD19+cell expansion could be maintained for more than 4 weeks.
HB-LTC–derived IgM+ B cells secrete Ig and undergo isotype switch in response to CD40 cross-linking and cytokines.
Although our data indicated that both the IgM− and IgM+ HB-LTC populations proliferated and modulated their surface phenotype in response to CD40 activation, it remained unclear if these populations had the capacity for more mature B-lineage functions, including Ig secretion. We therefore evaluated if culture in the CD40L system lead to Ig secretion. Table 1 shows a representative experiment in which CD19+ cells isolated from a 6-week-old HB-LTC were transferred to CD40L fibroblasts with cytokines including IL-2, IL-4, and/or IL-10. Strikingly, IgM production was detected by ELISA within 7 to 14 days in supernatants of cultures supplemented with both IL-4 and IL-10. Secretion of IgM was detected using CD19+ progenitors derived from 6 of 6 independent HB-LTCs cultures after transfer onto CD40L-expressing fibroblasts supplemented with both IL-4 and IL-10 (data not shown). IgM production peaked after 21 days and subsequently decreased (Table 1 and data not shown). Using limiting dilution, IgM production (2 to 5 ng/mL) was readily detectable with as few as 103 of CD19+ input cells. More limited IgM secretion was also detected in cultures supplemented with only IL-4, with only IL-10, or with both IL-2 and IL-10.
CD40L Culture Supports Differentiation of the HB-LTC–Derived CD19+ Cells Into IgM-Producing B Cells and Leads to Ig Isotype Switching
| Culture Conditions . | Ig Production (ng/mL) . | |||
|---|---|---|---|---|
| Day 14 . | Day 21 . | |||
| IgM . | IgG . | IgM . | IgG . | |
| CD34+ | ||||
| Medium | <0.5 | <0.1 | 0.9 | <0.1 |
| IL-2 | <0.5 | <0.1 | 0.6 | 0.3 |
| IL-4 | 5.0 | 0.2 | 54 | 7.3 |
| IL-10 | 14 | 0.9 | 22 | 8.1 |
| IL-2/IL-10 | 66 | 0.8 | 60 | 0.8 |
| IL-4/IL-10 | 111 | 9.7 | 176 | 78 |
| CD34+CD38− | ||||
| IL-4/IL-10 | 3.5 ± 2.2 | 23.1 ± 2.8 | ND | ND |
| Culture Conditions . | Ig Production (ng/mL) . | |||
|---|---|---|---|---|
| Day 14 . | Day 21 . | |||
| IgM . | IgG . | IgM . | IgG . | |
| CD34+ | ||||
| Medium | <0.5 | <0.1 | 0.9 | <0.1 |
| IL-2 | <0.5 | <0.1 | 0.6 | 0.3 |
| IL-4 | 5.0 | 0.2 | 54 | 7.3 |
| IL-10 | 14 | 0.9 | 22 | 8.1 |
| IL-2/IL-10 | 66 | 0.8 | 60 | 0.8 |
| IL-4/IL-10 | 111 | 9.7 | 176 | 78 |
| CD34+CD38− | ||||
| IL-4/IL-10 | 3.5 ± 2.2 | 23.1 ± 2.8 | ND | ND |
Representative experiment evaluating the time course of IgM production after transfer of HB-LTC CD19+ cells onto CD40L fibroblasts under alternative cytokine conditions. Secretion of IgM and IgG derived from CD34+CD38+ (top, 1 × 105 input CD19+ cells) and CD34+CD38− progenitors (bottom, 5 × 103 input CD19+ cells).
Abbreviation: ND, not determined.
Engagement of CD40 on naive B cells activates signals required for Ig isotype switching and this response is modulated by specific cytokines.49 We evaluated whether culture of HB-LTC CD19+ cells in the CD40L system could support the events leading to IgG secretion by this cell population (Table 1). Soluble IgG was clearly detected in supernatants from cells cultured in IL-4 and IL-10 after 14 days. IgG secretion was significantly less than the production of IgM (9.7 v 111 ng/mL). IgG production increased eightfold by day 21 and the relative amount of IgG versus IgM also increased (78 v 176 ng/mL). Limited IgG production (∼10-fold less than in cultures containing both cytokines) was also detected using either IL-4 or IL-10 alone.
The concentration of IgM produced in the CD40L system correlated with the relative percentage of IgM+ cells in the input cell population (data not shown). To determine the CD19+population responsible for the majority of Ig production, identical numbers of FACS sorted CD19+IgD− and more mature CD19+IgM+IgD+ cells from a 7-week-old culture were cultured in the CD40L system. Cell expansion was equivalent for both populations. A small subpopulation of IgM+ IgD+ cells (5%) was detectable within 8 days in cultures initiated with IgD− cells (data not shown). However, only cultures initiated with the IgD+cells produced significant amounts of soluble IgM within that time period (>300 ng/mL; data not shown). These data support the conclusion that expansion and differentiation of the IgM+B-cell subpopulation from HB-LTCs is responsible for the majority of Ig production after transfer to the CD40L system.
HB-LTC B progenitors exhibit polyclonal VH family gene usage before and after transfer to the CD40L system.
The cell expansion observed in the HB-LTC or the CD40L stages of this culture model could have resulted from either expansion of a limited number of independent B-cell clones with a high proliferative capacity or by expansion of multiple independent clones. To broadly distinguish between these possibilities, we evaluated whether the emergence of IgM+ B cells in HB-LTC and/or the subsequent expansion of these cells after transfer to the CD40L system was associated with the presence of oligoclonal B-cell populations. VH family gene usage in CD19+ cells from 8-week-old HB-LTC was determined by RT-PCR analysis using primers specific for the six major human VH families (VH1 to VH6).42 A portion of the CD19+ cells from each HB-LTC were also transferred in CD40L fibroblasts with IL-4 and IL-10 and reevaluated by RT-PCR after 8 days in culture (Table 2).
HB-LTC B Progenitors Exhibit Polyclonal VHFamily Gene Usage Before and After Transfer to the CD40L System
| VH Family . | 103 Cells . | 102 Cells . | 10 Cells . | |||
|---|---|---|---|---|---|---|
| HB-LTC . | CD40L IL-10 + IL-4 . | HB-LTC . | CD40L IL-10 + IL-4 . | HB-LTC . | CD40L IL-10 + IL-4 . | |
| VH1 | +++ | +++ | + | ++ | +/− | + |
| VH2 | + | + | +/− | ++ | − | − |
| VH3 | ++ | ++ | + | + | − | + |
| VH4 | ++ | + | + | + | − | +/− |
| VH5 | + | − | − | − | − | − |
| VH 6 | ++ | ++ | ++ | + | ++ | − |
| VH Family . | 103 Cells . | 102 Cells . | 10 Cells . | |||
|---|---|---|---|---|---|---|
| HB-LTC . | CD40L IL-10 + IL-4 . | HB-LTC . | CD40L IL-10 + IL-4 . | HB-LTC . | CD40L IL-10 + IL-4 . | |
| VH1 | +++ | +++ | + | ++ | +/− | + |
| VH2 | + | + | +/− | ++ | − | − |
| VH3 | ++ | ++ | + | + | − | + |
| VH4 | ++ | + | + | + | − | +/− |
| VH5 | + | − | − | − | − | − |
| VH 6 | ++ | ++ | ++ | + | ++ | − |
Semiquantitative PCR results of a representative experiment using decreasing numbers of CD19+ input cells before and after transfer onto CD40L fibroblasts are shown. VH family gene usage was determined by RT-PCR analysis as described in Materials and Methods. Results shown are representative of three independent experiments using unrelated HB-LTCs.
VH transcripts for all six families (with predominant use of the VH1, VH3, VH4, and VH6 families) were detected in HB-LTC B progenitors from five independent cultures (Table 2). Transfer of these same HB-LTC CD19+ populations onto CD40L fibroblasts with IL-4 and IL-10 (and the associated proliferation of IgM+ B cells) did not significantly alter the relative pattern of VH gene family usage. Thus, rather than supporting the outgrowth of a limited subset of oligoclonal B-lineage progenitors, these data strongly suggest that the HB-LTC system and culture of HB-LTC–derived B progenitors on CD40L support the expansion and subsequent activation of polyclonal B-cell populations expressing a diverse repertoire of VH family genes.
Generation of Ig-secreting IgM+ B cells from highly purified CD34+CD38− hematopoietic stem cells.
We evaluated whether HB-LTCs initiated with a highly enriched hematopoietic stem cell population (composed of CD34+CD38− cells) were also capable of generating IgM+ B cells and, if so, whether these cells could differentiate in the CD40L system. A small subpopulation of IgM+ cells coexpressing either λ or κ light chains were detectable by 12 weeks in cultures initiated with highly purified CD34+CD38− cord blood cells (Fig 6A). The generation IgM+cells was relatively delayed in cultures initiated with CD34+CD38− cells compared with cultures containing CD34+CD38+ input cells (10 to 12 weeks v 5 to 6 weeks). These findings were consistent with our previous studies demonstrating delayed expansion of this relatively quiescent population in long-term cultures.32,52 As previously reported for CD19+ B-progenitor outgrowth,32 53 addition of FL throughout the culture period increased the total production of B-lineage progenitors, including immature IgM+ B cells (data not shown).
HB-LTCs initiated with CD34+CD38− stem cells lead to production of IgM+ B cells capable of secreting of Ig after transfer to the CD40L system. HB-LTCs were established in microwells using FACS-sorted CD34+CD38− cord blood cells. (A) Total cultured cell populations (adherent and nonadherent cells) from independent wells were collected at weeks 12 and 13 and evaluated by FACS after gating on the lymphoid-sized population. (B) Decreasing numbers CD19+ cells from cultures initiated with either CD34+CD38+ (0.125 to 2 × 104 CD19+ cells/well) or CD34+CD38− input cells (5 × 103 CD19+ CD19+ cells/well; 4 independent wells shown) were transferred to CD40L system with IL-4 and IL-10. Production of IgM was evaluated by ELISA after 14 days. Results shown are representative of three independent experiments.
HB-LTCs initiated with CD34+CD38− stem cells lead to production of IgM+ B cells capable of secreting of Ig after transfer to the CD40L system. HB-LTCs were established in microwells using FACS-sorted CD34+CD38− cord blood cells. (A) Total cultured cell populations (adherent and nonadherent cells) from independent wells were collected at weeks 12 and 13 and evaluated by FACS after gating on the lymphoid-sized population. (B) Decreasing numbers CD19+ cells from cultures initiated with either CD34+CD38+ (0.125 to 2 × 104 CD19+ cells/well) or CD34+CD38− input cells (5 × 103 CD19+ CD19+ cells/well; 4 independent wells shown) were transferred to CD40L system with IL-4 and IL-10. Production of IgM was evaluated by ELISA after 14 days. Results shown are representative of three independent experiments.
CD19+ B progenitors from HB-LTC initiated with CD34+CD38− were isolated by FACS sorting and transferred onto CD40L fibroblasts with IL-4 and IL-10 (5 × 103 cells/well). After 15 days, culture supernatants were evaluated by ELISA for the presence of Ig. Replicate wells containing CD19+ cells from three independent CD34+CD38− cord blood cultures contained 3 to 6 ng/mL of IgM (Fig 6B). This level of IgM production was comparable to the levels produced using identical numbers of CD19+ cells isolated from cultures initiated with CD34+CD38+ progenitors. Notably, IgG production was also clearly detectable in cultures initiated with CD34+CD38− cells (20 to 25 ng/mL; Table1). Interestingly, in this limited set of experiments, the relative production of IgG versus IgM was significantly higher than in cultures initiated with CD34+CD38+ input cells. Together, these observations strongly support the conclusion that this culture model supports both the events regulating commitment of hematopoietic stem cells to B-lymphoid development and the sequential B-lineage developmental events culminating in production of mature Ig-secreting B cells.
DISCUSSION
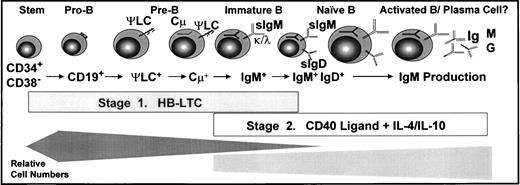
The culture system described in this work meets several key criteria of an optimal in vitro human B-lineage developmental model (Fig 7). First, this model supports the growth of B-lineage progenitor populations with cell surface phenotypes consistent with most of the major B-lineage developmental subpopulations present in normal human bone marrow, including pro-B cells, cycling pre-B cells, and IgM+IgD+/− immature and naive B cells. Notably, because the signals generated by the human pre-BCR remain very poorly defined, the capacity for this culture model to support a cycling pre-B–cell population may facilitate future studies regarding this receptor system. Most notably, activation of these long-term cultured CD19+ progenitors by CD40 cross-linking lead to mature B-lineage developmental events, including Ig production and isotype switching. Determination of the expression pattern of developmentally regulated B-lineage gene products (including VDJ gene rearrangement status, Rag and TdT expression, and mature B-lineage marker expression) within individual FACS-sorted cells is essential to fully characterize these populations. However, the demonstration of mature B-lineage function strongly supports the conclusion that this model represents a physiologically relevant continuum of human B lymphopoiesis.
In vitro culture model of human B lymphopoiesis. B-lineage developmental subpopulations supported by the two-stage culture system: HB-LTC followed transfer to CD40L fibroblasts. (Bottom) Bars approximate the relative cell expansion of the subpopulations supported in each culture stage.
In vitro culture model of human B lymphopoiesis. B-lineage developmental subpopulations supported by the two-stage culture system: HB-LTC followed transfer to CD40L fibroblasts. (Bottom) Bars approximate the relative cell expansion of the subpopulations supported in each culture stage.
Second, this model supports both the commitment of multipotent stem cells to B-lineage development and the progression of these committed progenitors into functionally mature B cells. This conclusion is supported by analysis of HB-LTCs initiated with highly purified CD34+CD38− cells, a rare, quiescent, primitive progenitor cell population capable of myeloid, B- and T-lymphoid, erythroid, and megakaryocitic differentiation.30,32,33,54-56 The mature B cells generated in these cultures are very unlikely to be derived from contaminating IgM+ input cells for several reasons, including the lack of CD19 expression in the purified CD34+CD38− input population32; the demonstrated capacity of single CD34+CD38− cells to generate both myeloid and B-lymphoid progenitors in S17 stroma-supported culture33; the delayed kinetic of CD19+IgM+/− cell outgrowth; and the inability of S17 stromal cultures to support long-term expansion of isolated cord blood IgM+ B cells (Fluckiger and Rawlings; unpublished data).
Third, a key objective of in vitro culture models is that they support efficient expansion of polyclonal cell populations representative of those present in vivo. Analysis of the repertoire of Ig VHgene expression demonstrated that this model supports expansion and activation of multiple independent B-lineage clones. In addition, B-lineage subpopulations are reproducibly generated in sufficient numbers for relatively extensive phenotypic, molecular, biochemical, and/or functional analyses. For example, a typical 6-week-old culture established using CD34+ progenitors yielded approximately 1 × 108 CD19+progenitors (including 0.5 to 1 × 106CD19+IgM+ B cells) and sustained this production level for several weeks.
Fourth, several groups have used SCID/Hu and related in vivo models to maintain either immature human B progenitors derived from multipotent hematopoietic progenitors or mature IgM+ B cells.57-60 However, the continuum of human B lymphopoiesis present in the culture model described in this work has not yet been possible with SCID/Hu systems. This in vitro model uses two well-characterized cell lines, recombinant cytokines, and has been established with a relatively broad group of serum sources. It should therefore be reproducible in many laboratories. These features are likely to permit studies that are not readily achievable using a more complex SCID/Hu system.
The most significant development described in this work is the combination of HB-LTC with signals leading to mature B-lineage development. This study and previous studies have suggested that bone marrow stroma-supported cultures do not provide the signals required for maturation of IgM+ immature B cells.29,61,62 We demonstrate that a combination of CD40 activation in association with IL-4 and IL-10 is sufficient to promote the expansion and maturation of CD19+ progenitors derived from multipotent postfetal hematopoietic progenitors. These signals lead to modulation of cell surface receptor expression, polyclonal expansion of IgM+ B cells (including generation of IgM+IgD+ cells from IgM+IgD− cells), Ig production, and Ig isotype switching. The HB-LTC–derived IgM+IgD+/− B cells were responsible for the vast majority of Ig production under these culture conditions. Transfer to CD40L fibroblasts also led to expansion of the CD19+IgM− population and supported limited differentiation of this population into IgM+IgD+ cells. Our observations are consistent with previous work demonstrating that short-term culture with an activated CD4+ T-cell line and IL-4 led to generation of Ig-producing B cells from human fetal bone marrow-derived pre-B cells.63 The activated T-cell line could be replaced using a membrane preparation from the same cells. Our data suggest that the key costimulatory signal in those studies was also most likely provided by CD40L.
Culture of HB-LTC CD19+ cells in the CD40L system leads to generation of a population of CD19+CD10dim/−CD38dim/−IgM+IgD+/−B cells phenotypically similar to naive tonsillar B cells. The response of these cells in the CD40L system also closely paralleled the response of naive B cells under similar conditions.49 Both HB-LTC CD19+ cells and naive B cells proliferated in response to IL-4 and secreted significant amounts of IgM and IgG with the addition of IL-10.41,47,48 The addition of IL-10 also resulted in generation of activated B cells with plamacytoid cell morphology from HB-LTC–derived CD19+ cells, as previously reported using naive B cells.64 Finally, adjusting for relative numbers of IgM+IgD+/− B cells, HB-LTC–derived B cells secreted similar amounts of total IgM as naive B cells in response to IL-10 and IL-4 in the presence of CD40 cross-linking (∼0.05 to 0.1 ng secreted IgM per IgM+input cell). Together, these observations indicate that the HB-LTC–derived immature B-cell population functions analogously to in vivo postnatally derived naive B cells, further supporting the conclusion that this model reproduces key events in mature B-cell development.
In summary, this culture system provides a unique model for the study and genetic manipulation of the major transition points in human B ontogeny. Because this model uses postfetal tissues (including both cord blood and bone marrow), it should also facilitate future analyses using hematopoietic tissues derived from individuals with disorders leading to altered B lymphopoiesis as well as normal individuals. Use of this model should allow experiments not previously possible with other human cell culture systems, including, eg, the in vitro analysis of the B-lineage developmental consequences of oncogenic protein expression and the mechanisms regulating the B-cell antigen receptor repertoire. Finally, optimization of this model should ultimately permit the generation of Ig-producing B cells from single multipotent hematopoietic progenitors.
ACKNOWLEDGMENT
The authors thank J. Shimaoka and J. White for assistance with manuscript preparation; B. Ank, the obstetrical nursing staffs of Santa Monica-UCLA, St. John’s, and Sunset Kaiser Permanente Hospitals, and J. Fraser and members of the UCLA Cord Blood Bank for assistance in obtaining cord blood; and K. Dorshkind and E. Montecino for helpful discussion and critical reading of the manuscript.
Supported in part by NIH Grants No. CA12800, AR01912, HL54850, and NCI 2CA-14089 and the facilities of the UCLA Jonsson Comprehensive Cancer Center. A.-C.F. was supported by a Human Frontier Science Program fellowship. E.S. was recipient of short-term fellowship from the Comunidad Autonoma de Madrid and was supported in part by Grants No. SAF96-2010CO2-01 and CAM98-96. G.M.C. is the recipient of a Translational Research grant from the Leukemia Society of America. O.N.W. is an Investigator of the Howard Hughes Medical Institute. D.J.R is recipient of a National Institutes of Health (NIH) Physician Scientist Award, a McDonnell Scholar Award, and UCLA Child Health Research, HHMI, and Pennington Scholar Awards.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David J. Rawlings, MD, Division of Pediatric Immunology/Rheumatology, UCLA School of Medicine, 22-387 MDCC, 10833 Le Conte Ave, Los Angeles, CA 90095-1752; e-mail:Drawling@pediatrics.medsch.ucla.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal