Abstract
The objective of this study was to determine if vascular cell adhesion molecule (VCAM-1), E-selectin, and P-selectin could selectively recruit leukocyte subpopulations, and whether this was affected by shear force or adhesion molecule concentration. Cover slips coated with purified adhesion molecules were incorporated into laminar flow chambers. Whole human blood was perfused for 5 minutes over these cover slips at relative shear forces of 2 to 40 dynes/cm2. Chasing the whole blood with buffer permitted visualization of leukocyte-substratum interactions. Leukocytes were observed to roll on and adhere to VCAM-1 at shears between 2 and 15 dynes/cm2. As assessed by cover slip staining, the majority of these cells were lymphocytes, but eosinophils, monocytes, and, surprisingly, neutrophils were also recruited, events inhibitable by anti–4-integrin antibody (HP1/2). Neutrophils were effectively recruited onto the selectins, with interactions occurring at shears as high as 30 and 40 dynes/cm2 for E- and P-selectin respectively. Eosinophils had high affinity for P- but not E-selectin. Mononuclear cells did not have high affinity for either selectin, but interacted avidly with VCAM-1. Antibodies against P-selectin (G1) and E-selectin (ES-1) completely blocked interactions on these substrates. Reducing the concentration of adhesion molecules did not appreciably change recruitment patterns except for VCAM-1, where neutrophils were no longer recruited. The novel use of whole blood in flow chambers shows a partial selectivity of selectins and VCAM-1 for certain subpopulations of leukocytes under varying physiologic shear conditions.
IT IS WELL APPRECIATED that the selective recruitment of leukocytes occurs in various disease states and at various time points during the progression of a single disease but whether all leukocytes are indiscriminately recruited to the endothelial surface before initiation of the selection process or whether recruitment from the mainstream of blood is dependent on adhesive topography of the endothelial surface remains unknown. The process of leukocyte recruitment can be divided into four steps; initial contact (tethering), rolling, firm adhesion, and emigration, with each step being mediated by adhesion molecules expressed on the surface of activated endothelium. The leukocyte recruitment is designated as a cascade because interruption of the initial tethering and rolling prevents subsequent leukocyte adhesion and emigration. Therefore if selective, the initial capture of leukocytes would be a rate-limiting event to subsequent recruitment of that particular cell type and could potentially discriminate against other cell types.
This initial capture is known to be mediated by endothelial adhesion molecules including P-selectin, E-selectin, and vascular cell adhesion molecule (VCAM-1). P-selectin is found within membrane-bound vesicles termed Weibel Palade bodies and is rapidly (within minutes) expressed to the endothelial surface in response to histamine,1thrombin,1,2 and reactive oxygen metabolites.3Associated with the rapid expression of P-selectin is the early recruitment of neutrophils,4 raising the possibility of selective recruitment of neutrophils on this selectin. However, because isolated lymphocytes, monocytes, and eosinophils can also interact with P-selectin,5-7 the selectivity of this pathway, if any, remains completely unappreciated. By contrast, E-selectin expression requires de novo synthesis in response to inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor α (TNFα), and lipopolysaccharide (LPS), with maximal amounts occurring about 4 to 8 hours after stimulation.8 The expression of this selectin coincides with further recruitment of neutrophils and monocyte populations, however in vitro experiments show that this selectin can support rolling of eosinophils and lymphocytes,7,9 again providing little insight into selectivity for this adhesion molecule. VCAM-1 expression also requires de novo synthesis and may reach peak levels at 24 hours, slowly declining beyond that time point.10 This adhesion molecule supports capture of lymphocytes, monocytes, and eosinophils although these interactions typically occur at lower shear forces than observed for either of the selectins.11-13 However, despite selectivity for non-neutrophilic populations, there is some suggestion that even neutrophils may adhere to VCAM-1 if sufficiently activated.14
Generally, with in vitro approaches, the leukocytes are purified and in the case of the more scarce population of cells, concentrated. The adhesion molecules are coated onto the cover slip at concentrations that support adhesion and the shear forces are adjusted again so that the cell type under study can interact with the substratum. This makes direct comparison of efficiency of leukocyte recruitment between studies almost impossible. Additionally, it is now well appreciated that red blood cells enhance leukocyte-endothelial adhesion molecule interactions by pushing the larger leukocytes to the periphery,15 16 and therefore, certain interactions may be missed. Therefore, at present it is unclear whether selection of leukocytes for recruitment occurs after rolling is initiated or whether the initial capture of leukocytes by selectins or VCAM-1 is a discriminatory event favoring the recruitment of some but not other cell types.
This study was designed to systematically assess which leukocytes would be recruited from whole blood by a particular adhesion molecule. This was accomplished by perfusing whole blood through a standard laminar flow chamber for 5 minutes and allowing the cells to interact with a given substratum. The whole blood is then chased with physiological buffer for a brief period, which permits visualization of interacting leukocytes while all red blood cells and noninteracting leukocytes are rinsed away. At the end of the experiment the types of leukocytes interacting with the cover slips are identified. We report herein, that there is selectivity for different leukocyte populations dependent on the type of immobilized adhesion molecule on the cover slip as well as the shear force at which the leukocytes are perfused.
MATERIALS AND METHODS
Soluble adhesion molecules and antibodies.
Recombinant soluble P-selectin,17 monoclonal antibodies (MoAbs) G1 (blocking antibody for P-selectin 18), and S12 (nonblocking antibody for P-selectin 18) were generous gifts from Dr R.P. McEver (University of Oklahoma, Oklahoma City, OK). Soluble recombinant human VCAM-1 and MoAb HP1/2 (anti–α4-integrin) were generously supplied by Dr R. Lobb, Biogen Inc (Cambridge, MA).19 MoAb ES-1 (blocking antibody for E-selectin) was donated by Dr K.D. Patel, University of Calgary (Calgary, Alberta, Canada). Soluble E-selectin was purchased from R & D Systems (Minneapolis, MN).
Preparation of cover slips.
Glass cover slips (Fisher Scientific, Ottawa, Ontario, Canada) were coated with soluble adhesion molecules at various concentrations (1 to 10 mg/mL) and incubated at 4°C for 18 hours. To inhibit nonspecific interactions with the glass, cover slips were then incubated with 1% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) at 37°C for 2 hours. Whole blood for perfusion over protein-coated cover slips was collected from healthy donors and heparinized (30 units/mL) to prevent clotting.
Experimental protocol.
To study leukocyte recruitment from whole blood under shear conditions, a flow chamber assay was established as previously described.14,20 21 This model allows for observations of leukocyte interactions with various biologic substrata at defined shear forces. Adhesion molecule-coated cover slips were mounted into a polycarbonate chamber with parallel plate geometry. The flow chamber was placed onto the stage of an inverted microscope (Zeiss Canada, Don Mills, Ontario, Canada) and monolayers were visualized at 100× magnification using phase contrast imagery. The stage area was enclosed in a warm air cabinet and maintained at 37°C. Blood was maintained at 37°C using a water bath. A syringe pump (Harvard Apparatus, Canada) was used to draw the blood through the flow chamber at defined shear forces. Experiments were video recorded for later analysis via a CCD camera (Hitachi Denshi, Ltd, Tokyo, Japan) and a video cassette recorder (Panasonic, Secaucus, NJ) that were attached to the microscope.
Shear forces within the flow chamber are dependent on the dimensions of the chamber and on the flow rate and viscosity of the perfused fluid. The viscosity of whole blood is greater than that of isolated leukocytes in buffer, resulting in a higher shear force at equivalent flow rates for whole blood. The viscosity of whole blood can be derived from the hematocrit,22 which allowed for calculation of relative shear forces within the flow chamber according to the following formula21: R = (6μQ)/(B2W) in which μ is the relative viscosity of the blood, Q is the flow rate through the chamber, B is the chamber depth, and W is the chamber width.
Whole blood was perfused over adhesion molecule-coated cover slips for 5 minutes at different relative shear forces (2, 5, 10, 15, 20, 30, or 40 dynes/ cm2), followed by perfusion with Hanks’ balanced salt solution (HBSS) . Within 20 seconds of perfusion with buffer, interacting leukocytes could be visualized on the cover slips. Interacting cells were either rolling or adherent to the surface of the cover slip. A leukocyte was defined as adherent if it remained stationary for at least 10 seconds. To distinguish the types of leukocytes that were interacting with protein-coated cover slips after perfusion of whole blood, the cover slips were gently removed from the chambers and differentially stained (Wright-Giemsa stain). This procedure resulted in a minimal loss of interacting leukocytes as determined by comparing the number of leukocytes per mm2 on the cover slip before removal from the chamber with the number after the staining procedure.
Neutrophil isolation and fluorescent labelling.
Human neutrophils were harvested from citrate anticoagulated venous blood collected from healthy donors, as previously described,23 with minor modification. All isolation steps were performed at room temperature. Neutrophils were purified by dextran sedimentation (Dextran 250 000; Spectrum Chemicals, New Brunswick, NJ) followed by centrifugation through a density gradient (6.07% Ficoll Type 400; Sigma) with 10% Hypaque Sodium (Sterling-Winthrop, Markham, Ontario, Canada). Isolated neutrophils were resuspended in HBSS at a density of 2 × 107cells/mL. This yielded neutrophils that were 97% pure and 95% viable. Neutrophils were labelled with rhodamine 6G (100mg/mL) (Sigma) for 15 minutes and washed twice using centrifugation before being added back to whole blood (2 × 105 labelled neutrophils per 1 mL of blood).
RESULTS
Leukocytes are recruited from whole blood when perfused over immobilized VCAM-1.
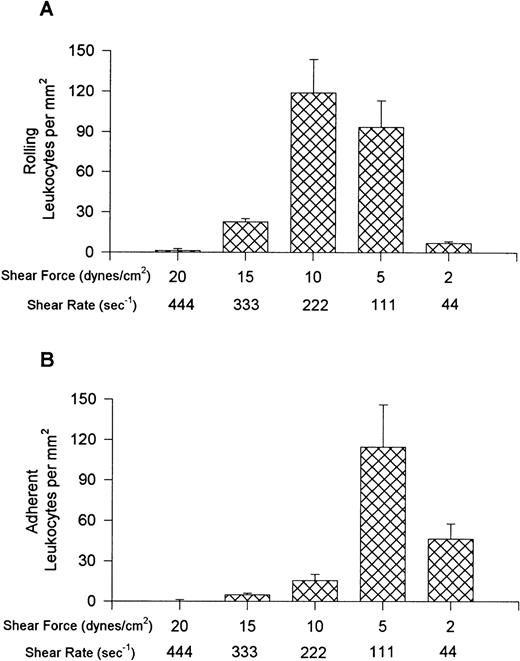
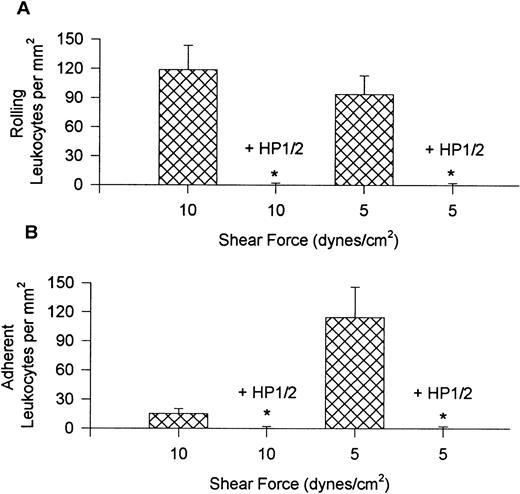
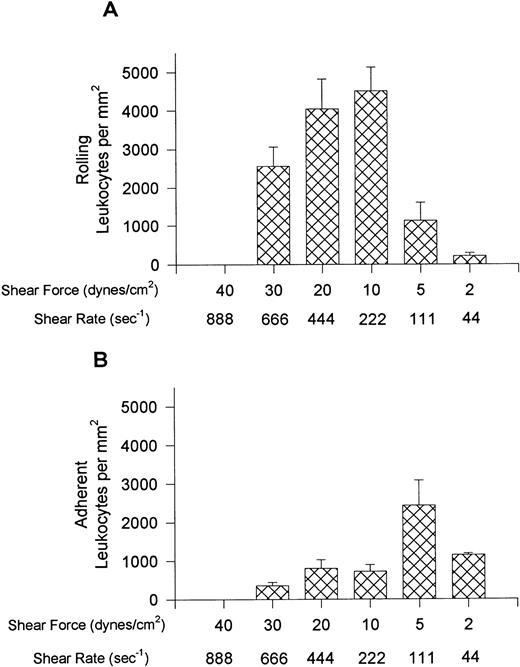
Figure 1 shows the rolling (A) and adhesion (B) data for leukocytes interacting with VCAM-1–coated cover slips. Notable rolling occurred at 15 dynes/cm2, with maximal rolling observed at 10 dynes/cm2 (A). At these shear forces, most interacting leukocytes were rolling, but in experiments where shear was 5 or 2 dynes/cm2, more of the cells were adherent, with adhesion being highest at 5 dynes/cm2 (B). At the lowest shear tested, fewer interactions occurred. Maximal total interactions with VCAM-1 were observed at 5 dynes/cm2. An important observation is that when the whole blood was pretreated with the anti-α4 antibody, HP1/2 (2 mg/mL), all leukocyte interactions were eliminated at all shear forces tested (shown for 10 and 5 dynes/cm2 in Fig 2), suggesting that these were specific α4-integrin/VCAM-1 interactions.
Leukocyte rolling (A) and adhesion (B) observed on soluble human VCAM-1–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Leukocyte rolling (A) and adhesion (B) observed on soluble human VCAM-1–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Rolling (A) and adhesion (B) of leukocytes recruited from whole blood onto VCAM-1 in the presence and absence of anti–4-antibody HP1/2 (2 μg/mL). * P < .05 relative to no antibody condition.
Rolling (A) and adhesion (B) of leukocytes recruited from whole blood onto VCAM-1 in the presence and absence of anti–4-antibody HP1/2 (2 μg/mL). * P < .05 relative to no antibody condition.
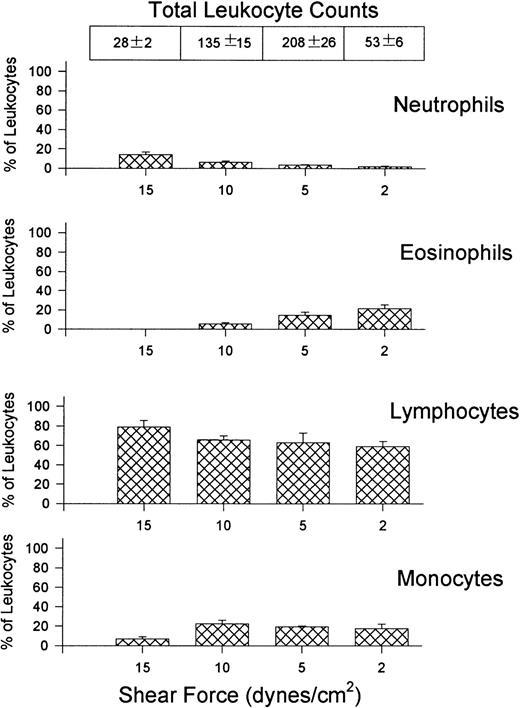
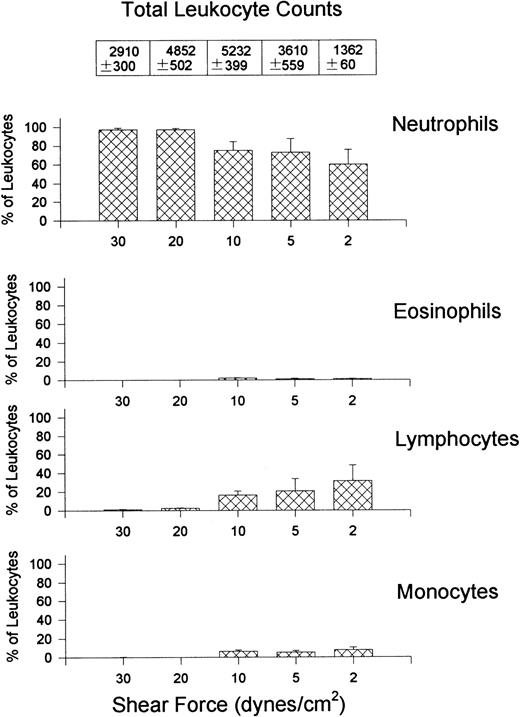
Figure 3 shows the percentage (bar graphs) and absolute numbers (top table) of neutrophils, eosinophils, lymphocytes, and monocytes recruited to VCAM-1 after whole blood perfusion at different shear forces. The ratio of recruited leukocytes varied depending on the shear force used in the experiment. Lymphocytes were always the most prevalent leukocyte regardless of the shear force, accounting for approximately 80% of leukocytes at the highest shear and 60% at the lowest shear tested, with the greatest number being recruited at 5 dynes/cm2. Monocytes were the second most notable leukocyte on VCAM-1, contributing about 10% to 20% of leukocytes recruited. Eosinophils were also able to attach to VCAM-1, but only at lower shear forces. At the highest shear force tested, no eosinophils were observed. Surprisingly, a small subset of neutrophils always adhered to the substratum. This was an α4-integrin–dependent event because HP1/2 entirely inhibited the interaction (Fig 2).
Quantitative assessment of the types of leukocytes found on VCAM-1–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Cover slips were differentially stained and analyzed for the presence of neutrophils, eosinophils, lymphocytes, and monocytes. Data is shown as the percentage of total leukocytes found on the cover slips. Total leukocyte counts are the actual number of leukocytes observed per mm2.
Quantitative assessment of the types of leukocytes found on VCAM-1–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Cover slips were differentially stained and analyzed for the presence of neutrophils, eosinophils, lymphocytes, and monocytes. Data is shown as the percentage of total leukocytes found on the cover slips. Total leukocyte counts are the actual number of leukocytes observed per mm2.
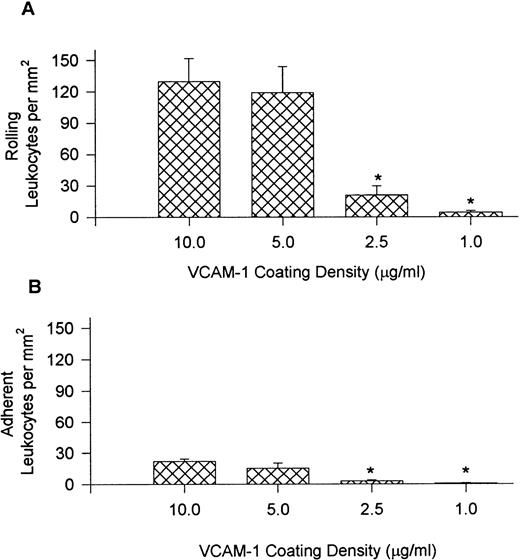
To establish further selectivity for the different leukocyte populations, the level of VCAM-1 densities was varied (10.0, 5.0, 2.5, and 1.0 μg/mL), and leukocyte recruitment from whole blood is presented in Fig 4 and Table 1. For these experiments, the shear force was maintained at 10 dynes/cm2. Fig 4 (A) illustrates that the number of rolling leukocytes was comparable at 10.0 and 5.0 μg/mL but the observable rolling decreased sharply at 2.5 μg/mL, and was minimal at 1.0 mg/mL. The adhesion to VCAM-1–coated cover slips followed a similar trend (B). Interestingly, the proportion of mononuclear cell types on VCAM-1 were remarkably similar regardless of the site density of VCAM-1 (Table 1). By contrast at 2.5 μg/mL of VCAM-1, neutrophils no longer accumulated appreciably. Cover slips coated at 1.0 μg/mL had very few leukocytes recruited from whole blood, and it was not possible to reliably determine leukocyte ratios.
Leukocyte rolling (A) and adhesion (B) observed on cover slips coated with different concentrations of VCAM-1 after perfusion of whole blood at 10 dynes/cm2. *P < .05 relative to 5.0 μg/mL
Leukocyte rolling (A) and adhesion (B) observed on cover slips coated with different concentrations of VCAM-1 after perfusion of whole blood at 10 dynes/cm2. *P < .05 relative to 5.0 μg/mL
Leukocyte Types Recruited Onto Cover Slips Coated With VCAM-1 at Varying Concentrations
| VCAM-1 . | Neutrophils (%) . | Eosinophils (%) . | Lymphocytes (%) . | Monocytes (%) . |
|---|---|---|---|---|
| 10 μg/mL | 6.4 ± 1.5 | 3.0 ± 0.6 | 66.0 ± 6.2 | 24.5 ± 4.4 |
| 5 μg/mL | 6.2 ± 1.3 | 5.4 ± 1.4 | 65.8 ± 3.9 | 22.6 ± 3.7 |
| 2.5 μg/mL | 0.9 ± 0.8* | 4.7 ± 0.5 | 67.7 ± 6.5 | 26.7 ± 5.9 |
| VCAM-1 . | Neutrophils (%) . | Eosinophils (%) . | Lymphocytes (%) . | Monocytes (%) . |
|---|---|---|---|---|
| 10 μg/mL | 6.4 ± 1.5 | 3.0 ± 0.6 | 66.0 ± 6.2 | 24.5 ± 4.4 |
| 5 μg/mL | 6.2 ± 1.3 | 5.4 ± 1.4 | 65.8 ± 3.9 | 22.6 ± 3.7 |
| 2.5 μg/mL | 0.9 ± 0.8* | 4.7 ± 0.5 | 67.7 ± 6.5 | 26.7 ± 5.9 |
P < .05 relative to 10 μg/mL.
Selective recruitment of leukocytes from whole blood onto E-selectin.
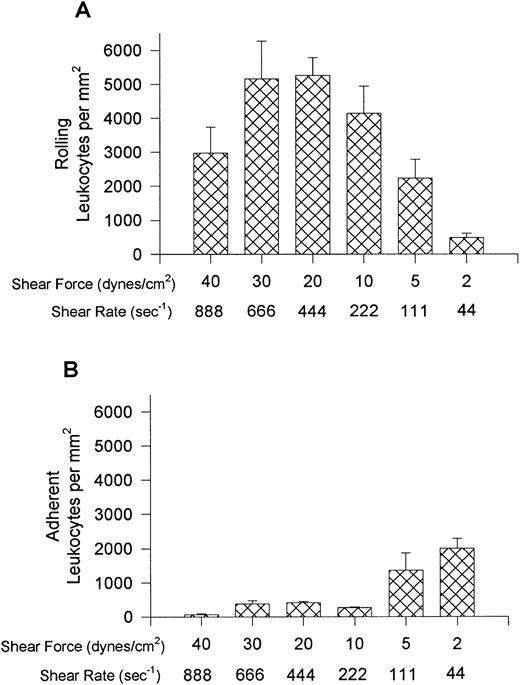
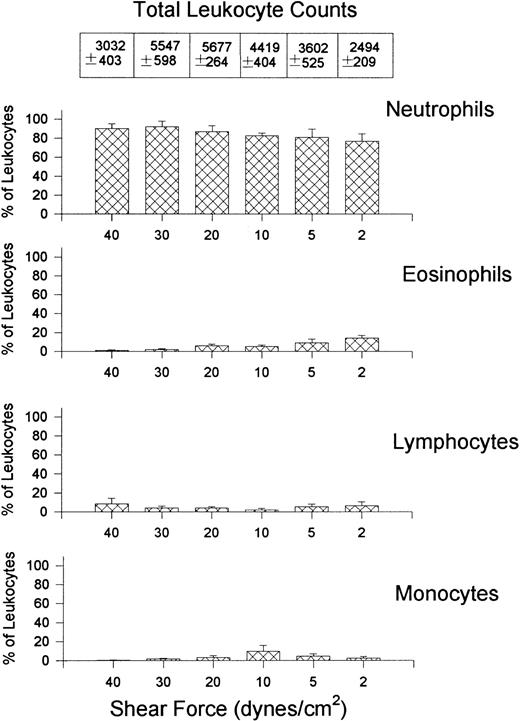
Leukocyte interactions with E-selectin occurred at higher relative shear forces (30 dynes/cm2) and were recruited in much larger numbers than that observed for VCAM-1 (Fig 5). Rolling was the predominant interaction observed at higher shear forces but cells began to arrest (without shape change) at relative shears less than 10 dynes/cm2 (panel B). No interactions were observed at 40 dynes/cm2 but the E-selectin was not removed by these high shears because leukocyte interactions occurred at 10 dynes/cm2 after initial perfusion at 40 dynes/cm2. At the highest shears neutrophils were essentially the only leukocyte type recruited onto E-selectin (Fig 6), whereas at lower shear forces lymphocytes were also recruited onto E-selectin, with the greatest number of lymphocytes being recruited at 10 dynes/cm2. A smaller number of monocytes and minimal numbers of eosinophils were observed at all shears tested. All leukocyte interactions were blocked with an anti–E-selectin antibody (ES-1, 5 μg/mL of whole blood), whereas an isotype-matched control antibody (S-12) had no effect (not shown).
Leukocyte rolling (A) and adhesion (B) observed on soluble human E-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Leukocyte rolling (A) and adhesion (B) observed on soluble human E-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Quantitative assessment of the types of leukocytes found on E-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Total leukocyte counts are the actual number of leukocytes observed per mm2.
Quantitative assessment of the types of leukocytes found on E-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Total leukocyte counts are the actual number of leukocytes observed per mm2.
Selective recruitment of leukocytes from whole blood onto P-selectin.
P-selectin was able to recruit leukocytes from whole blood at even higher shear forces than E-selectin (Fig7A). Again most cells rolled on P-selectin until shear was reduced to very low levels when arrest was apparent (Fig 7B). As observed for E-selectin, neutrophils were the primary leukocyte recruited onto P-selectin (Fig 8). By contrast, P-selectin also supported eosinophil recruitment as shear forces were decreased. Relative to neutrophils, a small number of lymphocytes and monocytes were recruited at all shear forces tested, being greatest at 40 dynes/cm2 for lymphocytes and 10 dynes/cm2 for monocytes. The P-selectin antibody (G1 at 2 μg/mL) completely blocked leukocyte interactions. An isotype-matched control antibody that binds to but does not block P-selectin (S-12) had no effect on leukocyte interactions on any of the substratum tested (not shown).
Leukocyte rolling (A) and adhesion (B) observed on soluble human P-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Leukocyte rolling (A) and adhesion (B) observed on soluble human P-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces.
Quantitative assessment of the types of leukocytes found on P-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Total leukocyte counts are the actual number of leukocytes observed per mm2.
Quantitative assessment of the types of leukocytes found on P-selectin–coated cover slips (5 μg/mL) after perfusion of whole blood at different shear forces. Total leukocyte counts are the actual number of leukocytes observed per mm2.
Selective recruitment is not due to gravity or change to buffer.
To determine if rolling cells remained on the cover slips when the chamber was disassembled during assay takedown, isolated, purified neutrophils were fluorescently labeled with rhodamine 6G and added back to whole blood before perfusion over VCAM-1, E-selectin, or P-selectin. Using fluorescent microscopy, labeled neutrophils were observed to tether, roll, and adhere at 10 dynes/cm2 in whole blood. The number of rolling and adherent neutrophils did not change during the transfer to buffer conditions. After the cover slips were removed and air dried, they were once again observed under fluorescence. The number of labeled cells per mm2 did not change as a result of manipulation of the cover slip indicating that rolling cells were not lost during chamber disassembly.
It is conceivable that differential rates of leukocyte sedimentation may influence the ratio of leukocytes recruited on cover slips, with denser populations of leukocytes showing preferential recruitment. To determine if gravity had an effect in our assay system, experiments were performed in which the flow chamber was inverted, such that blood cells would settle away from the cover slip. This resulted in a decrease (approximately 30%) in the total number of leukocytes recruited onto each of the adhesion molecules, but did not affect the relative ratios of the leukocyte types. Thus differential sedimentation was not a factor in this assay system.
The relative binding efficiencies of leukocytes at varying shear forces.
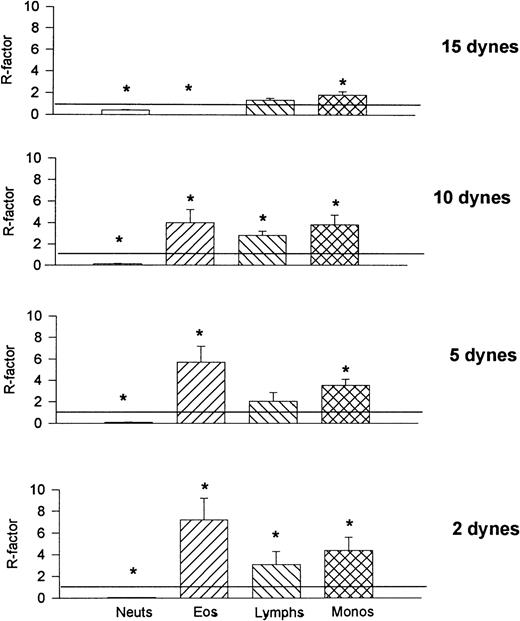
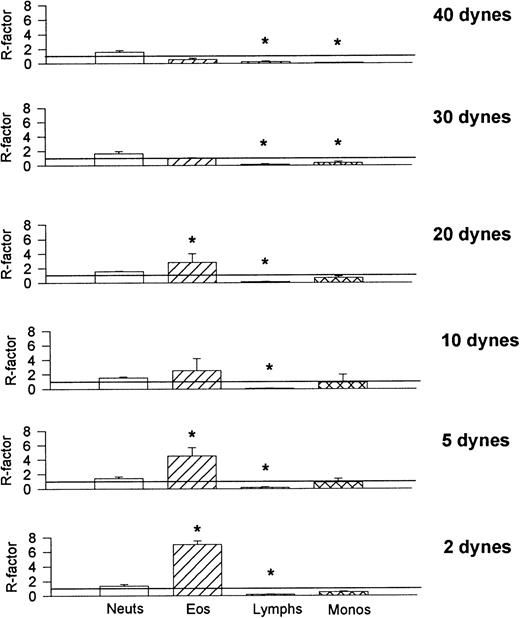
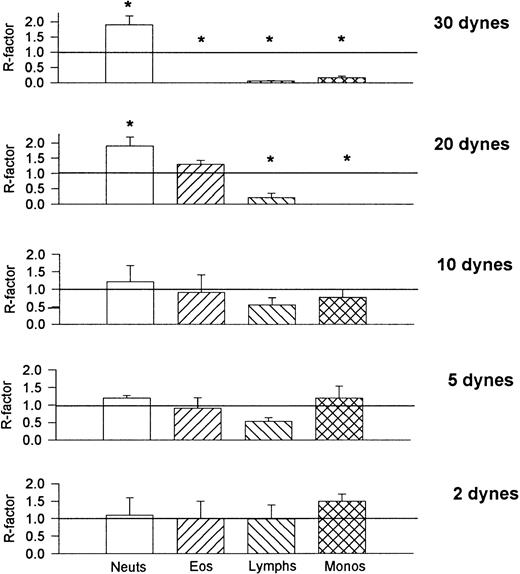
To compare the relative recruitment efficiencies of the various leukocytes onto cover slips, the ratio of leukocytes recruited onto the cover slips was compared with that found in the whole blood being perfused. The recruitment factor (R) was defined as R = (% of leukocytes on cover slip)/(% of leukocytes in blood) and provides a value for relative binding efficiency for a population of leukocytes. An R-factor of 1.0 indicates no preferential recruitment when compared with its presence in whole blood, whereas an R-factor greater than 1.0 suggests preference for that substrate. Figure 9 shows that at all shears neutrophils were less than preferentially recruited (R < 1.0) onto VCAM-1. Monocytes, lymphocytes, and eosinophils (except at the highest shear) were preferentially recruited onto VCAM-1. P-selectin provided an entirely different preferential profile (Fig 10); lymphocytes bound P-selectin with minimal efficacy at all shear forces and monocytes were also not very effective at binding P-selectin at the higher shears. Eosinophils were not effective at binding P-selectin at the highest shear but showed tremendous efficiency (R > 7.0) when the shears were lowered. Neutrophils bound to P-selectin just above an R-value of 1.0 under all shear conditions. Finally, E-selectin showed yet another preferential profile (Fig 11); at higher shear forces E-selectin preferentially recruited neutrophils, whereas all other cell types were less effective at binding the selectin. As the shear was reduced all leukocytes approached R = 1.0, suggesting a loss of preference. It should be noted that neutrophils can only increase to a maximum of 2.0 if 50% of leukocytes in blood are neutrophils, therefore an R-value of 1.90 on E-selectin suggests an almost exclusive recruitment of neutrophils at high shears. Although reducing selectin densities resulted in a decrease in the number of rolling and adherent leukocytes (Table 2), it did not alter the relative ratios of the types of leukocytes recruited (Table 3).
Recruitment factors (R-factors) for the various leukocytes found on VCAM-1–coated cover slips after perfusion of whole blood. The R-factor was defined as percentage of leukocytes present on the cover slip over the percentage found in the whole blood. An R-factor of 1 means that the percentage of a particular leukocyte found on the cover slip was equal to that found in the whole blood. *P < .05 relative to an R-factor value of 1.
Recruitment factors (R-factors) for the various leukocytes found on VCAM-1–coated cover slips after perfusion of whole blood. The R-factor was defined as percentage of leukocytes present on the cover slip over the percentage found in the whole blood. An R-factor of 1 means that the percentage of a particular leukocyte found on the cover slip was equal to that found in the whole blood. *P < .05 relative to an R-factor value of 1.
Recruitment factors (R-factors) for the various leukocytes found on P-selectin–coated cover slips after perfusion of whole blood. *P < .05 relative to an R-factor value of 1.
Recruitment factors (R-factors) for the various leukocytes found on P-selectin–coated cover slips after perfusion of whole blood. *P < .05 relative to an R-factor value of 1.
Recruitment factors (R-factors) for the various leukocytes found on E-selectin–coated cover slips after perfusion of whole blood. *P < .05 relative to an R-factor value of 1.
Recruitment factors (R-factors) for the various leukocytes found on E-selectin–coated cover slips after perfusion of whole blood. *P < .05 relative to an R-factor value of 1.
Rolling and Adherent Leukocytes Recruited Onto Cover Slips Coated With E-selectin and P-selectin at Varying Concentrations
| . | 5.0 μg/mL . | 2.5 μg/mL . | 1.0 μg/mL . |
|---|---|---|---|
| E-selectin | |||
| Rolling | 2129 ± 1107 | 1246 ± 543 | 508 ± 17* |
| Adhesion | 903 ± 330 | 641 ± 214 | 177 ± 163* |
| P-selectin | |||
| Rolling | 2602 ± 933 | 1811 ± 841 | 1310 ± 462 |
| Adhesion | 462 ± 174 | 431 ± 221 | 319 ± 211 |
| . | 5.0 μg/mL . | 2.5 μg/mL . | 1.0 μg/mL . |
|---|---|---|---|
| E-selectin | |||
| Rolling | 2129 ± 1107 | 1246 ± 543 | 508 ± 17* |
| Adhesion | 903 ± 330 | 641 ± 214 | 177 ± 163* |
| P-selectin | |||
| Rolling | 2602 ± 933 | 1811 ± 841 | 1310 ± 462 |
| Adhesion | 462 ± 174 | 431 ± 221 | 319 ± 211 |
The shear force was set at 10 dynes/cm2.
P < .05 relative to 5 μg/mL.
Leukocyte Types Recruited Onto Cover Slips Coated E-selectin and P-selectin at Varying Concentrations
| . | Neutrophils (%) . | Eosinophils (%) . | Lymphocytes (%) . | Monocytes (%) . |
|---|---|---|---|---|
| E-selectin | ||||
| 5.0 μg/mL | 97.8 ± 0.6 | 0 | 0.7 ± 0.2 | 1.5 ± 0.6 |
| 2.5 μg/mL | 96.0 ± 1.7 | 0 | 0.5 ± 0.2 | 3.5 ± 1.7 |
| 1.0 μg/mL | 97.5 ± 0.4 | 0 | 0.6 ± 0.3 | 1.9 ± 0.3 |
| P-selectin | ||||
| 5.0 μg/mL | 87.3 ± 4.2 | 3.2 ± 1.6 | 4.7 ± 1.1 | 4.9 ± 2.1 |
| 2.5 μg/mL | 88.1 ± 4.3 | 3.5 ± 0.9 | 4.4 ± 1.5 | 3.3 ± 1.6 |
| 1.0 μg/mL | 90.2 ± 3.7 | 3.0 ± 1.5 | 1.6 ± 1.3 | 2.4 ± 1.9 |
| . | Neutrophils (%) . | Eosinophils (%) . | Lymphocytes (%) . | Monocytes (%) . |
|---|---|---|---|---|
| E-selectin | ||||
| 5.0 μg/mL | 97.8 ± 0.6 | 0 | 0.7 ± 0.2 | 1.5 ± 0.6 |
| 2.5 μg/mL | 96.0 ± 1.7 | 0 | 0.5 ± 0.2 | 3.5 ± 1.7 |
| 1.0 μg/mL | 97.5 ± 0.4 | 0 | 0.6 ± 0.3 | 1.9 ± 0.3 |
| P-selectin | ||||
| 5.0 μg/mL | 87.3 ± 4.2 | 3.2 ± 1.6 | 4.7 ± 1.1 | 4.9 ± 2.1 |
| 2.5 μg/mL | 88.1 ± 4.3 | 3.5 ± 0.9 | 4.4 ± 1.5 | 3.3 ± 1.6 |
| 1.0 μg/mL | 90.2 ± 3.7 | 3.0 ± 1.5 | 1.6 ± 1.3 | 2.4 ± 1.9 |
The shear force was set at 10 dynes/cm2.
DISCUSSION
The capture of leukocytes to the endothelial cell surface is the initial critical event in leukocyte recruitment. Studies have shown that P-selectin, E-selectin, and VCAM-1 can capture leukocytes under flow conditions. We extend this work to provide evidence that the initial capture of leukocytes is in part a selective process, particularly at the higher shear forces tested. Neutrophils were less than preferentially recruited (R < 1.0) on VCAM-1 but favored recruitment on the two selectins. P-selectin clearly preferred neutrophils and eosinophils but had much less affinity for lymphocytes and monocytes. E-selectin showed little affinity for any of the mononuclear cells but preferred neutrophils at higher shear forces. There, however, appeared to be some residual overlap for all of the adhesion molecules suggesting that no adhesion molecule was entirely exclusive for any leukocyte population at any shear.
There is some suggestion that there is selectivity for some adhesion molecules by subpopulations of leukocytes. For example, eosinophils can use all of the selectins to form attachments under shear conditions, however, P-selectin is much better at mediating eosinophil recruitment than E-selectin in vitro,7 our own data clearly support this view. P-selectin but not E-selectin was extremely effective at capturing eosinophils from whole blood. Moreover, at a relative shear as high as 20 dynes/cm2 eosinophils were captured with about twice the efficiency of neutrophils. This observation is consistent with a previous report that eosinophils have at least twice the P-selectin ligand (PSGL-1), that neutrophils express.7Interestingly, the number of P-selectin ligands are not the only factor at the highest shears (40 dynes/cm2). P-selectin selectivity for eosinophils disappeared and neutrophils continued to be recruited with tremendous avidity. Physical characteristics of eosinophils may make this leukocyte less capable of being captured by P-selectin at the highest shears. Eosinophils were also captured very effectively onto VCAM-1. Alternatively, PSGL-1 may differ between neutrophils and eosinophils such that eosinophils bind P-selectin with greater efficacy at lower but not higher shear. Indeed, eosinophil PSGL-1 has a different peptide backbone and has less and different SLEX-containing molecules.24 25
Whether coexpression of P-selectin and VCAM-1 would further enhance eosinophil recruitment in an additive or even a synergistic manner remains to be determined. This increased selectivity for P-selectin and VCAM-1 may contribute to the fact that even though eosinophils represent a very minor fraction of circulating leukocytes, they are a major component of the cellular infiltrate found in allergic diseases such as asthma and late-phase cutaneous reactions in which both P-selectin and VCAM-1 have been shown to play a role.26-28This selective recruitment would be far more important in eosinophils than neutrophils, which are 50 to 100 times more abundant in blood, and neutrophils would therefore be recruited in sufficient quantities even without any selectivity (R=1).
It is intriguing that neither selectin recruited monocytes or lymphocytes as effectively at densities and shear forces that were conducive to recruitment of other cell types. VCAM-1 did preferentially recruit monocytes but not at the same shear as observed for the selectins. A potential explanation may be related to a role for the third selectin (L-selectin) and an as yet unidentified L-selectin ligand. Indeed Lucinskas et al6 reported that L-selectin was absolutely critical for monocyte recruitment, and that the other selectins played only a minor role. In those studies, P-selectin accounted for only 30% and E-selectin 0% of monocyte recruitment on TNFα-stimulated endothelium in spite of the fact that these were isolated concentrated monocyte populations. Our data would agree that these selectins are very inefficient at recruiting monocytes (R < 1). Once the L-selectin ligand in the periphery is identified it will be interesting to determine whether monocytes are preferentially recruited by L-selectin.
A surprising observation in this study was that unstimulated neutrophils could be recruited onto VCAM-1 via α4-integrin, a mechanism commonly thought to be restricted to mononuclear leukocytes. Although previous work has described α4-integrin–dependent recruitment of isolated neutrophils,14 this required maximal stimulation with dihydrocytochalasin B in combination with FMLP, and only occurred at shears below 1.5 dynes/cm2. In contrast, VCAM-1–dependent neutrophil recruitment from whole blood occurred without the need for exogenous stimulation and was greatest at 15.0 dynes/cm2(14% ± 2.6% of total leukocytes). This was not a nonspecific effect inasmuch as the α4-integrin antibody blocked these interactions. Although human neutrophils are generally thought not to express α4-integrin, neutrophil precursors in the bone marrow express α4-integrin, with that expression diminishing during terminal differentiation.29 It is conceivable that a small percentage of circulating neutrophils that are either not fully mature, still express α4-integrin, or that some neutrophils re-express α4-integrin in the circulation. Indeed, Issekutz et al30 has shown that α4-integrin can partially mediate neutrophil recruitment into inflamed joints and dermal inflammatory sites in the rat. It is, however, important to note that in the current study, neutrophil capture was reduced relative to other leukocytes at lower VCAM-1 densities, suggesting disproportionately high levels of VCAM-1 were required to recruit neutrophils. Nevertheless, the data suggests that in disease states when VCAM-1 is over-expressed, neutrophils could conceivably be recruited independent of selectins and perhaps even independent of β2-integrins.
Discussion of model.
Blood is a non-Newtonian fluid, making it difficult to assess actual values of the viscosity of whole blood. Therefore, based on the work of Fan et al,22 who provided viscosity data for whole blood at varying hematocrits, we provide relative shear stress values in our flow chamber. Of course, as one moves from the mainstream of blood to the vessel or chamber wall, viscosity drops markedly so that our shear forces almost certainly overestimate the true values at the leukocyte-substratum interface.21 Nevertheless, even if our viscosity values were considered to be those of water (0.01 centipoise) the leukocytes were tethering to P-selectin at 8 dynes/cm2, to E-selectin at 6 dynes/cm2, and to VCAM-1 at 3 dynes/cm2. These values are as much as fourfold higher than that previously reported for leukocyte tethering to any of the aforementioned substratum. This is consistent with the view that the smaller red blood cells tend to push the larger leukocytes from the axial flow to the vessel wall and thereby enhance leukocyte-substratum interactions. Indeed, Melder et al31 showed that addition of even 5% red blood cells enhanced leukocyte interactions under flow conditions.
Additional issues raised are the potential differential rates of leukocyte sedimentation, potential loss of rolling cells but not adhering cells during disassembly of the chamber, and the use of heparin, which may affect both primary and secondary interactions. Inverting the flow chamber recruited 30% fewer leukocytes but the proportions did not change. The addition of fluorescently labelled neutrophils to this system has in the first instance allowed us to observe these cells selectively in whole blood and to establish that the number of rolling and adherent cells visualized under flow conditions was equivalent to the total number of fluorescently labelled cells after disassembly of the chamber. This suggests that neither rolling nor adherent cells are lost during disassembly. Moreover, addition of heparin at the same concentration used in this study to neutrophils isolated from citrate-anticoagulated blood did not affect neutrophil-selectin interactions (unpublished observations). Therefore, it is unlikely that the concentration of heparin used was affecting leukocyte interactions in whole blood. Indeed, preliminary work from our laboratory suggests that addition of increasing concentrations of heparin does not affect the total number of primary or secondary interactions in whole blood (Mitchell, Reinhardt, and Kubes, unpublished observation).
This novel approach of studying leukocyte-endothelium and leukocyte-immobilized protein interactions has many advantages. First, the shear forces clearly better reflect shear forces observed in vivo. Second, this system incorporates red blood cells that, as already discussed, clearly impact on leukocytes tethering to substratum. Although it is well appreciated that initial flow chamber work of single concentrated populations of cells was critical to the understanding of the function of adhesion molecules, our approach may now extend those studies to establish which adhesion molecules and combinations of adhesion molecules will recruit selective populations of leukocytes. Moreover, the perfusion of whole blood over endothelium stimulated with various cytokines (for example IL-4 and TNFα will provide further insight into which cytokines recruit which type of leukocytes. This technology can also be used for patient blood to establish whether the pathology of a particular disease may be an inherent humoral problem either of enhanced adhesivity of leukocytes or enhanced activity of plasma. Indeed, preliminary work from our laboratory suggests a very dramatic increase in leukocyte-substratum interactions in septic patients (Ibbitson and Kubes, unpublished observations). Finally, this technique can be used to circumvent trying to isolate sufficient amounts of leukocytes from rats and mice; 1 mL can be perfused over a cover slip and leukocyte-substratum interactions can be observed. One limitation of this technique is the inability to identify the type of rolling versus adherent cells. Nevertheless, we show with this system that tethering/rolling of leukocytes from whole blood onto various adhesion molecules is a selective process.
ACKNOWLEDGMENT
We thank Danielle Davids for performing the E-selectin and P-selectin dose-response experiments.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal