Abstract
Very late antigen-4 (VLA-4)/vascular cell adhesion molecule-1 (VCAM-1) are a pair of adhesion molecules mediating cell-cell interaction. The binding activity of each depends on its surface expression, yet integrin activity can also be modulated through inside-out signaling. However, the specific intracellular molecules involved in modulating integrin VLA-4 activation via inside-out signaling or in regulating VCAM-1 expression are poorly understood. We show here that constitutive coexpression of cyclin C and c-Myc in hematopoietic BAF-B03 cells induces homotypic cell adhesion, which results from enhanced VLA-4 ligand-binding activity and induced expression of VCAM-1. Furthermore, regulation of cell adhesion appears to be a feature unique to cyclin C, but not other G1 cyclins, E and D3, and its regulatory function is independent of CDK8 kinase activity. Our results provide a novel role for cyclin C and c-Myc in the regulation of cell adhesion through distinct mechanisms.
CELL-CELL INTERACTION via very late antigen-4 (VLA-4; α4β1) and its counterreceptor vascular cell adhesion molecule-1 (VCAM-1) has been implicated in numerous physiologic and pathologic processes.1 Although adhesion of VCAM-1 to its ligand depends fundamentally on the molecule numbers expressed on the cell surface, integrin-mediated cell adhesion can be regulated either through altering the repertoire of integrin expression, by clustering on the cell surface, or by modulating the affinity of the integrins for their ligands without changes in surface expression through an inside-out signaling mechanism.2 3
The precise molecular mechanism of integrin activation modulated by inside-out signaling remains largely unknown. It has been proposed that conformational changes in integrins are required for their activation. Studies using several unique monoclonal antibodies (MoAbs) demonstrate that these MoAbs recognize integrins in their conformation in which the expression of neoepitopes or ligand-induced binding sites are induced on the integrin.4-6 The cytoplasmic domains of both α and β subunits have been shown to be essential for the regulation of inside-out signaling.7,8 For instance, a membrane-proximal GFFKR sequence within the α subunit may possess α helical structure and has been proposed to function as a component of a hinge that connects the cytoplasmic and the transmembrane domains. Deletions in GFFKR result in a default high-affinity integrin that may be caused by locking the hinge in an irreversible high-affinity state. Several intracellular signaling molecule(s), such as calreticulin,9,10 ILK (Integrin-LinkedKinase),11 and cytohesin-1,12 have been identified as associating with the cytoplasmic domain of integrins. Such interactions may induce changes in the spatial relationships or conformations of the cytoplasmic tails of the integrin α and β subnuits and may participate in the regulation of both inside-out and outside-in signalings.
The activities of both protein kinases and phosphatases have been implicated in the inside-out signaling regulation.13-16 For instance, it has been shown that modulation of the ligand-binding affinity of β1 and β2 by phorbol ester (PMA) stimulation depends on protein kinase C (PKC) activation, and the cytoplasmic domain of the α6A subunit has been proven to be a substrate for PKC.17 Participation of oncogenic proteins in integrin activation has also been reported. Bcr/Abl exhibits positive effects on the function of VLA-4 and VLA-5 integrins,18 whereas pp60v-src was suggested to function as a negative regulator to reduce the binding capacity of β1 for its ligands via tyrosine phosphorylation.19,20 Intriguingly, recent studies demonstrate that members of the small GTPase family such as R-Ras and H-Ras are involved in this regulation, albeit these two members exhibit opposing effects.21,22 In addition, it has been shown that cytokine stimuli as well as elevation of intracellular Ca2+or cyclic AMP (cAMP) levels activate various integrin molecules.14,23 24
VCAM-1 is an Ig-superfamily protein and is primarily distributed on activated endothelium. Although it can also be expressed on many other cells, its expression on hematopoietic cells has not been reported. The ligand-binding activity of VCAM-1 is generally regulated at the level of its expression. Cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), IL-4, and lipopolysaccharide are known to be able to induce VCAM-1 expression.25,26 Two cell-type–specific NF-κB sites in the promoter region of VCAM-1 have been shown to be responsible for cytokine-induced expression of VCAM-1.27,28 In addition, two GATA elements28and an element 3′ to the TATA-box29 may also be important for the regulation of VCAM-1 expression.
During our study aimed at understanding the function of cyclin C in the regulation of cell cycle, we made an interesting observation that constitutive coexpression of cyclin C and c-Myc in hematopoietic progenitor BAF-B03 cells, which normally exists as a suspension of single cells, induces a homotypic cell adhesion. We show here that this homotypic aggregation is mediated by the interaction between activated α4 integrin and inducibly expressed VCAM-1. Cyclin C, which possesses an important function in the promotion of cell cycle progression,30 also plays a critical role in the regulation of cell adhesion in cooperation with c-Myc. Such a cell adhesive regulatory function is unique to cyclin C and is not seen with cyclin E and D3, the other two G1 cyclins. The involvement of both cyclin C and c-Myc in either the activation of integrin or regulation of VCAM-1 expression has not previously been defined. Our results provide not only a novel function for cyclin C and c-Myc, but also an interesting notion that a set of intracellular molecules is capable of regulating each partner of a ligand-receptor pair in a hematopoietic cell line through distinct mechanisms.
MATERIALS AND METHODS
Antibodies.
MoAbs used in this study are as follows: PS/2 (anti-α4),31 HMα5-1 (anti-α5),32 KBA (anti-αL),33 Mac-1 (anti-αM),34 RMV-7 (anti-αv),35 HMβ1-1 (anti-β1),36 M18/2 (anti-β2),34 HMβ3-1 (anti-β3),37 M293 (anti-β7),38 KAT-1 (anti–intercellular adhesion molecule-1 [ICAM-1]),39 and M/K-2 (anti–VCAM-1).40 Anti–Thy-1 MoAb was provided by Dr E. Shevach (NIH, Bethesda, MD). Fluorescein isothiocyanate (FITC)-conjugated goat-antirat and goat-antihamster IgG were purchased from Cappel Laboratories (Malvern, PA).
Cells and cell culture.
BAF-B03, a subclone of the Ba/F3 cell line, is a bone marrow-derived murine IL-3–dependent pro-B–cell line.41 BER2 cells were obtained by transfecting human epidermal growth factor (EGF) receptor gene into BAF-B03 cells42; BC and BEC cells were established by transfecting a human cyclin C expression plasmid (Rc-cycC43; kindly provided by R.A. Weinberg, MIT, Cambridge, MA) into BAF-B03 or BER2 cells, respectively; BM and BEM cells were obtained by transfection of the human c-mycexpression plasmid, pN-LTR-myc44 into BAF-B03 or BER2 cells, respectively; and BMC and BEMC clones were established by transfecting a human cyclin C expression plasmid into pooled BM or BEM cells, respectively. BMC-K8AMG cells were obtained by transfection of a catalytically inactive mutant of CDK8, in which the Asp (D) residue in the DMG motif within kinase subdomain VII was replaced by Ala (A). BMC, BMC-K8AMG, and BMC-K8WT (for BMC-K8AMG and BMC-K8WT, drug selection was performed in the presence of IL-3) were maintained in RPMI 1640 (Nissui, Tokyo, Japan) medium supplemented with 10% (vol/vol) fetal calf serum (FCS; JRH Biosciences, Lexena, KS); other cells were cultured in the same medium containing 10% (vol/vol) WEHI-3B culture supernatant as a source of IL-3.
DNA transfection.
Plasmid DNAs were transfected into cells by an electroporation procedure as described previously.45 Selection was initiated 24 hours after DNA transfection, using 2 mg/mL G418 for BC and BMC; 1 mg/mL hygromycin for BM, BEM, and BEC cells; or 0.75 μg/mL puromycin for BEMC, BEME, BEMD3, BMC-K8WT, and BMC-K8AMG cells. Drug-resistant clones were either pooled or subsequently cloned by limiting dilution, as described previously.46
Flow cytometry.
Cell surface expression of adhesion molecules was analyzed by immunofluorescence using MoAbs against the respective molecules as described previously.47 For each sample, a total of 1 × 106 cells were treated with respective MoAb for 30 minutes at 4°C. After washing, cells were stained with FITC-conjugated goat antirat or antihamster antibodies. The stained cells were analyzed by a Coulter Epics XL-MCL Flow Cytometer (Coulter K.K., Miami, FL).
Conjugation assay.
Formation of conjugates was measured using a two-color flow cytometer essentially as described.48 Briefly, cells for conjugate formations assay were divided into two portions. The one portion was labeled with sulfofluorescein diacetate to be fluorescent green and another portion was labeled with hydroethidine to be fluorescent red; these portions were mixed at a 4:1 ratio and allowed to settle for 1 hour at 4°C. The cells were then incubated for 6 minutes at 37°C, vortexed, and transferred into medium at 4°C. Conjugates were enumerated as a percentage of red cells in the conjugates. Because 5,000 events were analyzed, the counting errors are less than 2% and differences between samples of 2% to 3% conjugates are generally reproducible. In all experiments, the background due to coincident detection of red and green cells in a cell mix lacking conjugate (ie, a green to red mix analyzed immediately after mixing in suspension) was determined, and this background was subtracted from experimental values, which normally range from 2% to 5%. In assays of MoAb inhibition, saturating concentration of MoAb and human intact globulin to block Fc-binding sites were added before setting and were present continuously thereafter.
Antibody-blocking assay.
Aggregated BMC cells were mechanically separated. For each sample, 5 × 105 cells were replated in a 24-well plate (1 mL/well) along with various MoAbs at a saturating concentration of 10 μg/mL, which was shown in previous studies to produce a maximum inhibition of the relevant adhesive interaction.49Efficiency of MoAb on cell aggregation was evaluated by observation of photomicrographs after 2 to 8 hours of incubation.
Cell adhesion assay.
Adhesion assay of BAF-B03, BM, BC, and BMC cells to fibronectin (Fn) was performed essentially as previously described.50 Fn (5 μg/well; Seikagaku, Tokyo, Japan) or control 3% human serum albumin (HSA; Green-Cross, Osaka, Japan) was applied to a 48-well plate in phosphate-buffered saline (PBS) at 4°C overnight. Wells were subsequently blocked with Ca2+/Mg2+-free PBS/3% HSA for 2 hours at 37°C to reduce nonspecific attachment. The plates were washed three times with PBS before the addition of BAF-B03 or different transfectants. BAF-B03 (2 × 105) or transfectants were labeled with 51Cr (Dupont NEN, Wilmington, DE) in RPMI 1640 with 1% HSA and were added to the wells with or without blocking MoAb (10 μg/mL) in the presence or absence of PMA (10 ng/mL; Sigma). After settling phase for 30 minutes at 4°C, which also allowed MoAb binding, the plates were rapidly warmed to 37°C for 30 minutes, then gently washed twice with RPMI-1640 at room temperature to completely remove nonadherent cells. The contents of each well containing adherent cells were lysed with 250 μL of 1% Triton X-100 (Sigma), and the 51Cr radioactivity was measured using a γ-counter. Data were expressed as the mean percentage of the binding of indicated cells from a representative experiment.
Cell growth assay.
For the cell growth assay, factor-independent cells were cultured at a density of 5 × 105 cells/mL in RPMI 1640 supplemented with 10% FCS. Culture medium was changed every other day. Viable cell numbers were determined by trypan blue exclusion assay as described.51
RESULTS
Constitutive coexpression of cyclin C and c-Myc induces a homotypic cell adhesion.
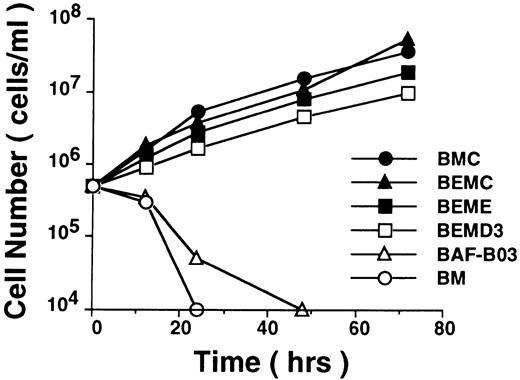
As an attempt to elucidate the role of cyclin C in the regulation of cell cycle, we investigated whether cyclin C by itself or in cooperation with cytokine-responsive immediately early gene products such as c-Myc was able to promote cell cycle progression of cytokine-dependent hematopoietic cells via substitution for growth factor-induced proliferative signals and therefore to be able to render these cells able to proliferate in a cytokine-independent manner. To this end, a human cyclin C gene (Rc-cycC), either singly or combined with a human c-myc expression plasmid (pN-LTR-myc), was transfected into BAF-B03 cell, an IL-3–dependent murine hematopoietic progenitor cell line and the BAF-B03–derived BER2 cell line42 (abbreviated here as BE cells), which expresses human epidermal growth factor receptor (EGFR), respectively. It was found that, although both BAF-B03 cells and BER2 cells ectopically expressing cyclin C (termed BC and BEC cells) or c-Myc (named BM and BEM cells) alone were unable to proliferate in the absence of growth factor, cells coexpressing cyclin C and c-Myc become able to proliferate in a growth factor-independent manner (Fig 1, termed BMC and BEMC cells).30 Interestingly, we also found that, like cyclin C, the other two G1 cyclins, cyclin E and cyclin D3, were also capable of inducing cytokine-independence in BAF-B03–derived cells in cooperation with c-Myc (Fig 1), despite the fact that expression of these cyclins by themselves failed to do so. The transfectants that coexpress cyclin E (Rc-cycE) with c-Myc or cyclin D3 (Rc-cycD3) with c-Myc in BER2 cells were named BEME or BEMD3 (Liu et al, manuscript in preparation), respectively. These results indicated that cyclin C plays a critical role in the promotion of cell cycle progression and that cyclin C may share a similar function(s) with cyclins E and D3 in the regulation of cell cycle progression.
Proliferation profiles for BAF-B03–derived cells. For growth assay, IL-3–dependent BAF-B03 and BM cells as well as IL-3–independent BMC, BEMC, BEME, and BEMD3 cells (pooled transfectants) were plated at 5 × 105 cells/mL in the absence of IL-3 after washing with PBS. The concentration of viable cells was counted at various times after plating and represented on a logarithmic scale.
Proliferation profiles for BAF-B03–derived cells. For growth assay, IL-3–dependent BAF-B03 and BM cells as well as IL-3–independent BMC, BEMC, BEME, and BEMD3 cells (pooled transfectants) were plated at 5 × 105 cells/mL in the absence of IL-3 after washing with PBS. The concentration of viable cells was counted at various times after plating and represented on a logarithmic scale.
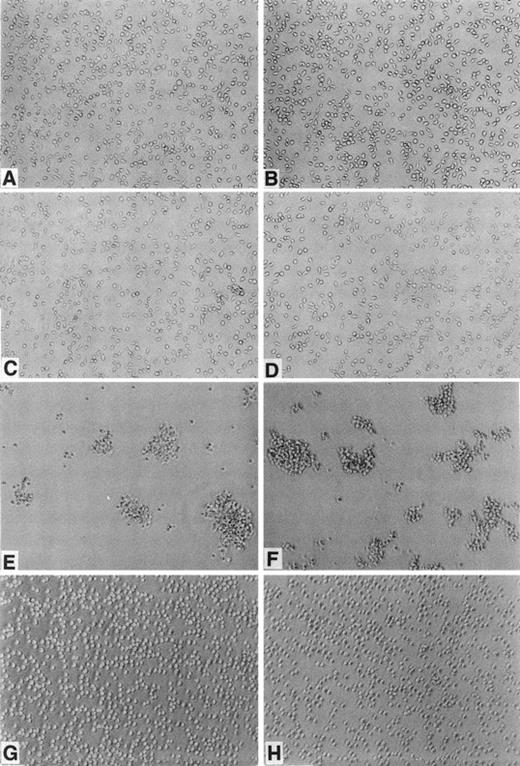
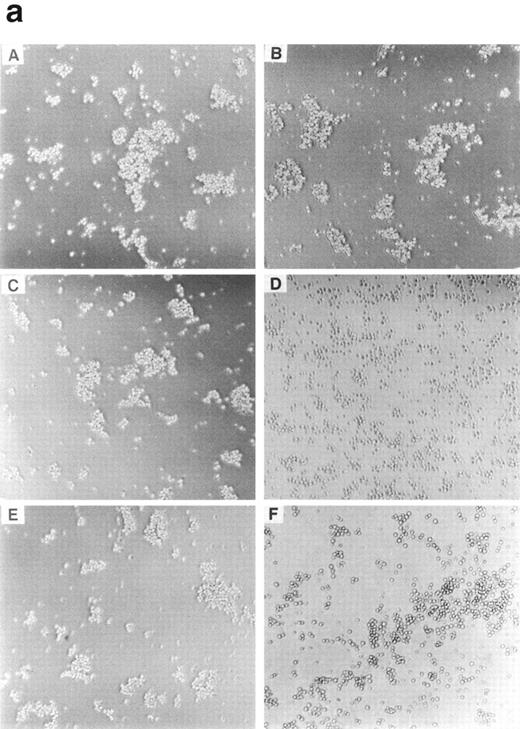
We noted that both BAF-B03 and BER2 cells, which grow as isolated cells in suspension (Fig 2A and B), form cell aggregates when cyclin C and c-Myc were ectopically coexpressed (Fig 2E and F). Cells expressing cyclin C (BC cells; Fig2C) or c-Myc (BM cells, Fig 2D) alone did not form such aggregates (BEC and BEM, data not shown), indicating that cooperation between cyclin C and c-Myc is required for homotypic cell adhesion. Interestingly, unlike BMC and BEMC cells, neither BEME (Fig 2G) nor BEMD3 cells (Fig2H) formed aggregates, suggesting that, among the G1 cyclins examined, cyclin C is required selectively for this observed cell adhesion. Consistent with the above-noted results, F-actin polymerization was apparent only in BMC and BEMC cells but not in BAF-B03, BER2, BEME, BEMD3, BC, BEC, BM, or BEM cells (data not shown), even in the presence of IL-3. Furthermore, it was found that homotypic cell adhesion of BMC cells requires divalent cations, because cell aggregation was blocked by the addition of EDTA (data not shown). Taken together, these results suggested that the cyclin C– and c-Myc–induced homotypic cell adhesion may be mediated by cell surface adhesion molecules, such as members of the integrin family.
Morphological properties of BAF-B03–derived cells. BAF-B03 (A), BER2 (B), BC (C), and BM (D) cells were cultured in the presence of IL-3, whereas BMC (E), BEMC (F) , BEME (G), and BEMD3 (H) cells were cultured in the absence of IL-3. The addition of IL-3 does not alter the cell cluster-forming properties of BMC, BEMC, BEME, and BEMD3 cells.
Morphological properties of BAF-B03–derived cells. BAF-B03 (A), BER2 (B), BC (C), and BM (D) cells were cultured in the presence of IL-3, whereas BMC (E), BEMC (F) , BEME (G), and BEMD3 (H) cells were cultured in the absence of IL-3. The addition of IL-3 does not alter the cell cluster-forming properties of BMC, BEMC, BEME, and BEMD3 cells.
The homotypic cell adhesion observed in both BMC and BEMC cells could occur even in the absence of IL-3, and restimulation with IL-3 did not alter their adhesive properties. To determine whether BMC cell adhesion results from stimulation by soluble factors that may be secreted as a result of ectopic coexpression of cyclin C and c-Myc, we harvested the supernants from growing BMC and BEMC cells; used these to culture either parental BAF-B03, BM, BC, BEME, or BEMD3 cells; and found that these cells do not form clusters, indicating that homotypic cell adhesion is not due to an indirect cytokine stimulation.
It should be noted that the exogenous human EGFR responds only to human EGF rather than any other factor that existed in the medium. In fact, ectopically expressed EGFR was not phosphorylated under our culture conditions, and BMC and BEMC exhibited almost similar degrees of both cytokine-independent growth and homotypic cell adhesion (data not shown). Thus, the difference between BMC or BEMC and BEME or BEMD3 cells primarily reflects a unique function of cyclin C; therefore, we used BMC cells in subsequent experiments.
Function of cyclin C in regulating cell adhesion is independent of CDK8 kinase activity.
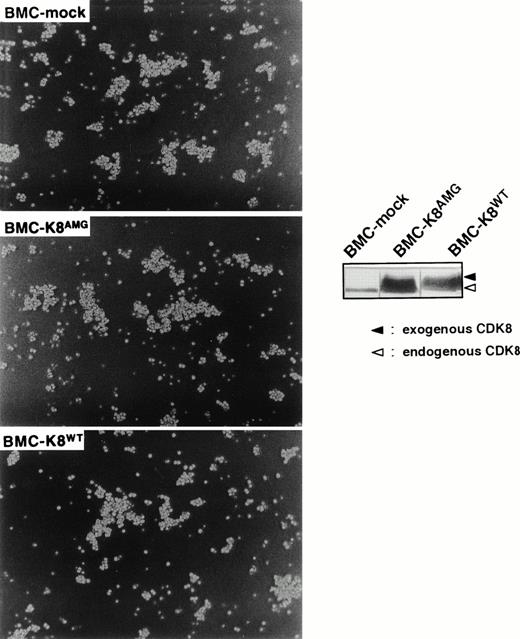
Because regulation of cell adhesion appears to be a unique characteristic of cyclin C and a recent work has demonstrated that cyclin C can associate with a novel cyclin-dependent kinase, CDK8,52 it is important to address whether the role of cyclin C in the regulation of cell adhesion is dependent on CDK8. To this end, we transfected a catalytically inactive mutant of CDK8 (kindly provided by Dr E. Nigg, Swiss Inst. Exp. Cancer Research, ISREL, Switzerland), in which an Asp (D) residue in the DMG motif within the kinase subdomain VII was mutated to Ala (A), into BMC cells and the resultant clones were designated BMC-k8AMG. It is noteworthy that this mutant form of CDK8 is still able to form a complex with cyclin C in vitro.30 As a control, wild-type CDK8 was also used in our transfection experiments and the resultant cells were termed BMC-k8WT. To avoid the possibility that expression of a catalytically inactive mutant of CDK8 may affect the role of cyclin C in cell proliferation and may induce cell death in BMC cells, drug selection was performed in the presence of IL-3. For each kind of transfectants, at least three independent clones were obtained and the results from a representative clone are presented. As shown in Fig 3 (inset panels), expression levels of exogenous CDK8 were about sixfold higher than those of endogenous CDK8. It was found that BMC cell aggregation was not affected by ectopic expression of either the catalytically inactive mutant or wild-type CDK8 (Fig 3), suggesting that the function of cyclin C in the regulation of cell adhesion is independent of CDK8 kinase activity. Furthermore, the activation and expression of the adhesion molecules, which are involved in homotypic adhesion of BMC cells (see below), were not altered by ectopic expression of either the catalytically inactive mutant or wild-type CDK8 in BMC cells, confirming that CDK8 kinase activity is not required for the function of cyclin C in the regulation of cell adhesion.
Effect of enforced expression of a catalytically inactive mutant of CDK8 on the homotypic cell adhesion of BMC cells. Morphological properties of BMC-mock, BMC-k8AMG, and BMC-k8WT are shown. Expression of CDK8 was detected by anti-CDK8 antibody (inset panels). Upper and lower bands indicate Myc-tagged exogenous CDK8 and endogenous CDK8.
Effect of enforced expression of a catalytically inactive mutant of CDK8 on the homotypic cell adhesion of BMC cells. Morphological properties of BMC-mock, BMC-k8AMG, and BMC-k8WT are shown. Expression of CDK8 was detected by anti-CDK8 antibody (inset panels). Upper and lower bands indicate Myc-tagged exogenous CDK8 and endogenous CDK8.
Expression of VCAM-1 is induced in BMC cells.
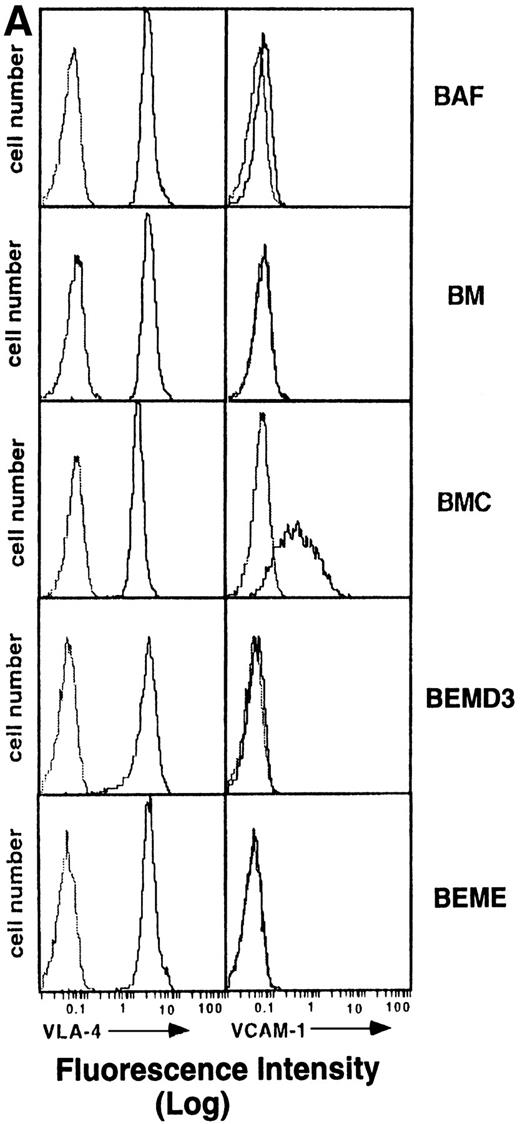
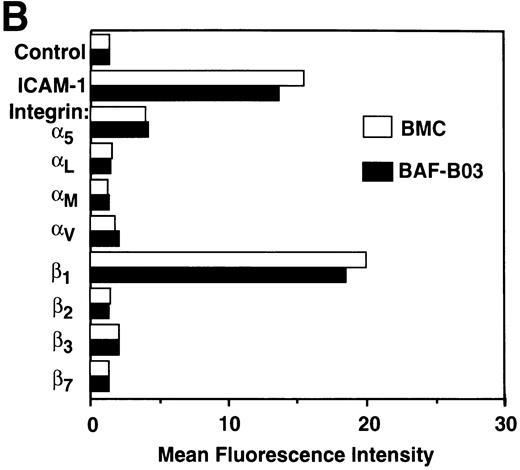
To investigate whether BMC cell adhesion is induced through increasing the cell surface expression of adhesion molecules such as integrins or Ig-superfamily proteins, flow cytometric analysis was performed using MoAbs against integrin α4, α5, αL, αM, αv, β1, β2, β3, β7 chain, VCAM-1, and ICAM-1. We found that the integrin α4(Fig 4A), α5, and β1 chains as well as ICAM-1 (Fig 4B) are expressed on BMC cells, but at levels comparable with those on the parental BAF-B03 cells. The αL, αM, αv, β2, β3, and β7 chains were not detectable on either BAF-B03 or BMC cells (Fig 4B). Noticeably, VCAM-1 is not expressed on parental BAF-B03 cells but is expressed substantially on the surface of BMC cells (Fig 4A). Induced VCAM-1 expression was not detectable on either BC or BM cells (data not shown). Furthermore, expression of VCAM-1 was not detectable on the surface of BEME and BEMD3 cells, indicating that, among the G1 cyclins examined, cyclin C is unique for the induced expression of VCAM-1 in conjunction with c-Myc (Fig 4A). Some other integrin subunits, such as α1 and α2, which are not generally involved in homotypic cell adhesion, were also examined and were undetectable on either BAF-B03 cells or transfectants (data not shown). It should be noted that expression of α4 integrin, the counterreceptor for VCAM-1, is not affected by ectopic coexpression of cyclin C and c-Myc (Fig 4A). Taken together, these results suggested that the cooperative effect of cyclin C and c-Myc on homotypic cell adhesion of BMC cells can be at least partly mediated by the induced expression of VCAM-1.
(A) Flow cytometric analysis of 4 integrin and VCAM-1 expression in BAF-B03 cells and transfectants. The various cells were unstained (dotted line) or stained with anti-4 integrin and anti–VCAM-1 MoAbs (solid line), followed by staining with FITC-labeled secondary antibodies and a flow cytometric analysis, as described in Materials and Methods. (B) Flow cytometric analysis of expression of various integrin subunits and ICAM-1 on BAF-B03 cells (□) and BMC cells (▪). The mean fluorescence intensity values are shown.
(A) Flow cytometric analysis of 4 integrin and VCAM-1 expression in BAF-B03 cells and transfectants. The various cells were unstained (dotted line) or stained with anti-4 integrin and anti–VCAM-1 MoAbs (solid line), followed by staining with FITC-labeled secondary antibodies and a flow cytometric analysis, as described in Materials and Methods. (B) Flow cytometric analysis of expression of various integrin subunits and ICAM-1 on BAF-B03 cells (□) and BMC cells (▪). The mean fluorescence intensity values are shown.
VLA-4 and VCAM-1 are involved in homotypic aggregation of BMC cells.
To characterize further the nature of BMC cell aggregation, we used several function-blocking MoAbs to identify the candidate adhesion molecules involved in mediating homotypic aggregation of BMC cells. It was found that homotypic aggregation of BMC cells was almost completely inhibited by anti-α4 MoAb (PS/2), whereas anti-αL (KBA), anti–ΙCΑΜ-1(KAT-1), anti-αv (RMV-7), or a control nonblocking anti-β2 (M18) MoAb failed to affect homotypic cell adhesion of BMC cells (Fig 5). Thus, the results suggested that α4 integrin is involved in homotypic cell adhesion of BMC cells and may be activated in these cells. On the other hand, anti–VCAM-1 MoAb, M/K-2, which has been demonstrated to inhibit VCAM-1–mediated cell adhesion,53exhibited an incompletely blocking effect on homotypic adhesion of BMC cells. This partial blocking effect of anti–VCAM-1 MoAb (M/K-2) might be due to the fact that this MoAb can bind to only one of the α4 integrin binding sites of VCAM-1, the D1 domain, but not to the other α4 integrin binding site, D4 domain.54 The D4 domain, which remains unoccupied by M/K-2, may still be able to partially mediate the association with α4 integrin. Alternatively, the homotypic aggregation of BMC cells may be partly mediated by the bridge of serum Fn in the medium.
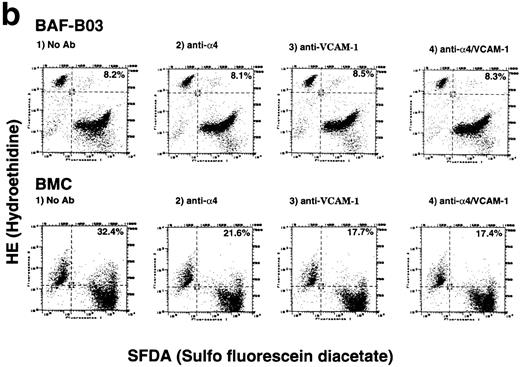
(a) Antibody-blocking analysis of homotypic cell adhesion of BMC cells. Morphological properties of BMC after treatment with a control nonblocking anti-β2 MoAb (A), anti-L (B), anti-v (C), anti-4 (D), as well as anti–ΙCΑΜ-1 (E) and anti–VCAM-1 MoAb (F) are shown. (b) Effects of anti-4integrin and anti–VCAM-1 MoAbs on early phase of homotypic aggregation of BMC cells. The cells are pretreated by 10 μg/mL of human Ig, divided into effector cells (labeled with hydroethidine) and target cells (labeled with sulfofluorescein diacetate) at a 4:1 ratio, added to 1 μg/106 cells of indicated MoAbs, and enumerated using a flow cytometric analysis. Data are reported as the percentage of total targets presented as conjugates. The conjugated percentage is shown in the top, right-hand corner of each panel.
(a) Antibody-blocking analysis of homotypic cell adhesion of BMC cells. Morphological properties of BMC after treatment with a control nonblocking anti-β2 MoAb (A), anti-L (B), anti-v (C), anti-4 (D), as well as anti–ΙCΑΜ-1 (E) and anti–VCAM-1 MoAb (F) are shown. (b) Effects of anti-4integrin and anti–VCAM-1 MoAbs on early phase of homotypic aggregation of BMC cells. The cells are pretreated by 10 μg/mL of human Ig, divided into effector cells (labeled with hydroethidine) and target cells (labeled with sulfofluorescein diacetate) at a 4:1 ratio, added to 1 μg/106 cells of indicated MoAbs, and enumerated using a flow cytometric analysis. Data are reported as the percentage of total targets presented as conjugates. The conjugated percentage is shown in the top, right-hand corner of each panel.
We also tested the abilities of anti-α4 integrin and anti–VCAM-1 MoAbs to inhibit homotypic aggregation of BMC cells at the very early phase of the conjugate formation. Homotypic conjugates were observed on BMC cells but not parental BAF-B03 cells within only 6 minutes after the culture at 37°C. As shown in Fig 5b, inhibition of the BMC cell conjugates was consistently observed with either antibodies against α4 integrin (PS/2) or VCAM-1 (M/K-2). Combination of these two MoAbs did not further increase their blocking efficiency. Taken together, these data indicated that α4integrin and VCAM-1 are involved in mediating homotypic aggregation of BMC cells.
The α4 subunit is capable of associating with either β1(α4β1, also termed VLA-4) or an alternative subunit, β7.55 Because β7subunit is not detectable on both BAF-B03 and BMC cells, it is indicated that α4 associates with β1 to form heterodimer (VLA-4) in these cells. Noticeably, an anti-β1 MoAb, Hmβ1, which has been demonstrated to be able to block the binding of α4β1 to Fn,36 failed to inhibit homotypic aggregation of BMC cells in our hand (up to 50 μg/mL; data not shown). Perhaps this is simply due to the fact that Hmβ1 does not recogize the epitope on the β1, which is required for interaction with VCAM-1, because it was known that the site(s) involved in the ligation of VLA-4/VCAM-1 and VLA-4/Fn is different.56 To test whether β1 is indeed involved in mediating cell aggregation of BMC cells, we used another antimouse β1 MoAb, 9EG7,57 which can recognize either activated or ligand-binding related epitopes of integrin β1, to perform a flow cytometric analysis. The analysis showed that expression of activated or ligand-binding related epitopes of integrin β1 is induced on BMC at a higher level compared with BAF-B03 cells (data not shown). Hence, VLA-4 is primarily responsible for the homotypic aggregation of BMC cells.
VLA-4 is activated on BMC cells.
VLA-4 is known to be able to bind to either the cellular counter-receptor VCAM-1 or extracellular matrix Fn. Because the activities of the integrins can be modulated via inside-out signaling, it is of importance to determine whether VLA-4 expressed on BMC cells is indeed activated as a result of the functional cooperation between cyclin C and c-Myc. For this purpose, we have examined the abilities of BAF-B03, BMC, and other BAF-B03–derived transfectants to attach to Fn coated on the plates. It was found that BMC cells bind efficiently to Fn-coated plates, but not to control HSA-coated plates (Fig 6A). About a fourfold increase in specific attachment to Fn was observed for BMC cells over that seen in BAF-B03, BM, BC, BEME, and BEMD3 cells (Fig 6A and data not shown). Furthermore, adhesion of BMC cells to Fn was selectively inhibited by anti-α4 MoAb, PS/2, but not by an irrelevant anti–Thy-1 MoAb. However, inhibition by PS/2 was incomplete and its blocking efficiency is approximately 50%. Probably another integrin(s), such as α5β1, may also be activated on BMC cells. Phorbol ester PMA is able to activate the integrins through a PKC-mediated inside-out signaling mechanism.58 Treatment with PMA drastically increased attachment of BAF-B03, BM, and BC as well as BEME and BEMD3 cells to Fn, whereas it had a marginal effect on BMC cells. Increased adhesion of BAF-B03, BM, and BC as well as on BEME and BEMD3 cells to Fn after PMA treatment was also inhibited by anti-α4MoAb, PS/2 (Fig 6B). Collectively, these results suggest that the VLA-4 expressed on the surface of BMC cells has already been activated by the cooperative effect of cyclin C and c-Myc before PMA stimulation.
(A) Adhesion of BAF-B03 cells and transfectants to Fn-coated or HSA-coated plates. Mean percentage of indicated cells binding to Fn (▪) or HSA (□) is shown. (B) Effect of anti-4 blocking MoAb (PS/2) and PMA treatment on the adhesion of BAF-B03 cells and transfectants to Fn. The cells indicated were untreated or treated with anti-4 integrin MoAb in the presence or absence of PMA. The mean percentage of binding is shown.
(A) Adhesion of BAF-B03 cells and transfectants to Fn-coated or HSA-coated plates. Mean percentage of indicated cells binding to Fn (▪) or HSA (□) is shown. (B) Effect of anti-4 blocking MoAb (PS/2) and PMA treatment on the adhesion of BAF-B03 cells and transfectants to Fn. The cells indicated were untreated or treated with anti-4 integrin MoAb in the presence or absence of PMA. The mean percentage of binding is shown.
DISCUSSION
A salient feature of the function of certain integrins is that their activities can be modulated through intracellular signaling without changing levels of expression. In contrast, the function of VCAM-1 depends primarily on its surface expression. It was originally supposed that these two processes are regulated by distinct mechanisms in different cells, because the cellular and tissue distribution of VLA-4 and VCAM-1 is quite different and VLA-4/VCAM-1 generally mediates heterotypic cell-cell interactions, such as those between leukocytes and endothelial cells. Also, it remains unclear whether the VLA-4/VCAM-1 pair can be regulated by the same intracellular molecule(s). In this study, we investigated the cooperative effect of cyclin C and c-Myc on hematopoietic cellular behavior and found that interaction of VLA-4 and VCAM-1 occurred on the same cells with VCAM-1 being induced, whereas VLA-4 is activated without alteration of its expression. Our studies provide the first example in which these two partners are regulated by a set of intracellular molecules but through distinct mechanisms in a given hematopoietic cell line.
Concomitant activation of VLA-4 and VCAM-1 in BMC cells appears not to be a result of mutual stimulation between VLA-4 and VCAM-1. Activation of VLA-4 by PMA in BAF-B03, BM, or BC cells does not induce homotypic aggregation of these cells (data not shown), suggesting that VCAM-1 is not simultanously induced. Thus, activation of VLA-4 by itself is not likely to be responsible for the induction of VCAM-1 expression. Likewise, VCAM-1 expression seems not to be responsible for the activation of VLA-4. The observation that coculture of BAF-B03, BM, or BC cells with COS cells expressing VCAM-1 does not result in apparent heterotypic cell-cell interactions (data not shown) supports this possibility. Further study will be required to confirm such a possibility.
Regulation of VLA-4/VCAM-1–mediated homotypic aggregation seems to be a unique character of cyclin C, because neither cyclin E nor cyclin D3 can induce cell adhesion or enhance cell attachment to Fn in cooperation with c-Myc, albeit the respective G1 cyclins in cooperation with c-Myc are sufficient to render BAF-B03 cells able to proliferate in a cytokine-independent fashion. Cyclin C was originally isolated through its ability to complement a Saccharomyces cerevisiaestrain lacking the G1 cyclin CLN1-3.59-61 Our very recent study demonstrated a critical role for cyclin C in the regulation of cell cycle transition by cooperation with c-Myc.30 Moreover, it has recently been reported that cyclin C is capable of associating with a unique cyclin-dependent protein kinase, CDK8, and that the cyclin C/CDK8 complex may regulate transcription via phoshorylation of RNA polymerase II.62Thus, it appears that cyclin C may play different roles in the regulation of cell cycle progression, transcription, and cell adhesion. However, the relationship between cyclin C-regulated cell cycle progression, transcription, and cell adhesion is not yet well understood. First, it remains unclear whether homotypic cell adhesion induced by cyclin C and c-Myc is a prerequisite for cell cycle progression mediated by these molecules. It seems that cytokine-independent BMC cell proliferation driven by the cooperative effect of cyclin C and c-Myc is not tightly dependent on homotypic cell aggregation, because BMC cells exhibit drastically decreased cell adhesion properties after treatment with anti-α4 integrin blocking MoAb, PS/2, but do not undergo apoptosis and can still proliferate well in the absence of cytokine (data not shown). In addition, PS/2 MoAb itself failed to inhibit apoptosis or promote proliferation of BAF-B03 cells (date not shown). These data suggest that cyclin C may function directly to promote cell cycle progression rather than via regulation of cell adhesion, which can supply survival signals to cooperate with c-Myc. This is likely because BAF-B03 cells are not anchorage-dependent and both BEME and BEMD3 cells can proliferate without cell adhesion. Thus, these observations suggest that cytokine-independent BMC cell proliferation is at least partly independent of cell-cell interaction that may provide adhesion-mediated proliferative signals. Second, kinase activity of CDK8, which has been implicated in transcription control, is not required for the cyclin C and c-Myc–mediated cell proliferation and cell adhesion. The observation that amounts of CDK8 remain constant throughout the cell cycle62 and that CDK8 kinase activity was not upregulated in BMC cells30 suggest that the function of exogenous cyclin C in promoting cell proliferation is not mediated by CDK8. Furthermore, it was found that enforced expression of a catalytically inactive mutant of CDK8 fails to reverse or alleviate homotypic aggregation of BMC cells or their attachment to Fn, indicating that CDK8 kinase activity is not required for cyclin C-dependent VLA-4/VCAM-1 activation. Collectively, these data implicate that cyclin C is a multifunctional molecule.
On the other hand, c-Myc is also required for the regulation of both VLA-4 activation and VCAM-1 expression. Involvement of c-Myc in the regulation of both integrin and VCAM-1 is poorly understood, except for the finding that c-Myc expression can downregulate LFA-1.63Our observation of the participation of c-Myc in the activation of VLA-4 integrin and induction of VCAM-1 is the first example of a novel role for c-Myc in regulating cell adhesion. However, how c-Myc cooperates with cyclin C is unclear. At least ectopic expression of c-Myc by itself does not upregulate cyclin C expression and vice versa (data not shown). It is unlikely that c-Myc can directly regulate VCAM-1 expression through its activity as a transcription factor, because c-Myc binding sequences have not been reported within the promoter region of VCAM-1. One possible mechanism for the induction of VCAM-1 is that c-Myc and cyclin C may induce the expression of cytokine(s), such as TNF-α, IL-1α, and IL-4, that are known to be able to induce VCAM-1 expression.25,26,64 However, our finding that the culture supernatants from growing BMC cells fail to induce cell cluster formation in PMA-stimulated BAF-B03, BM, or BC cells, on which VLA-4 is activated, suggests that VCAM-1 expression is not induced on these cells under these conditions (data not shown). Likewise, supernatants from growing BMC cells do not enhance the attachment of BAF-B03, BM, or BC cells to Fn (data not shown), excluding the possibility that activation of VLA-4 is mediated by such indirect cytokine stimulation, which is known to be able to activate certain integrins.14 It will be of interest to examine whether the signals of those cytokines in regulating cell adhesion are transmitted through activation of cyclin C and c-Myc. Because bothcyclin C and c-myc can be induced in hematopoietic cells by IL-3 stimulation,30,42 our findings may represent a novel mechanism responsible for cytokine-induced hematopoietic cell adhesiveness14 in which activation of VLA-4 is involved. Interestingly, dose-dependent and transient cell adhesiveness of hematopoietic cells, including BAF-B03 cells, is induced upon cytokine stimulation of factor-deprived cells, whereas activation of VLA-4 and induction of VCAM-1 observed in our experimental system are somehow constitutive. This may simply reflect a difference in the magnitude and duration of expression of these genes, because induction of these genes by cytokine stimulation is normally of a transient nature, whereas cyclin C and c-Myc are constitutively overexpressed in BMC cells.
Adhesion-induced outside-in signals, in particular in certain anchorage-dependent cells, have been known to integrate into the cell cycle machinery.65 Conversely, little is known about whether and how the cell cycle machinery is involved in the regulation of cell adhesion. It has been assumed that the intrinsic cell cycle machinery may play an important role(s) in the regulation of cell adhesion. For example, many types of cells become rounded and appear to lose adhesion during mitosis. In addition, it was found that ectopic expression of cyclin A in anchorage-dependent NRK fibroblasts renders cells able to proliferate in suspension,66 suggesting that cyclin A is involved in the control of cell adhesion. Our findings provide direct evidence of a critical role for cyclin C in the regulation of cell adhesion in cooperation with c-Myc. However, the exact mechanism of the cooperative effect of cyclin C and c-Myc on integrin activation remains unclear. Because neither c-Myc nor cyclin C possess any enzymatic activity, it is likely that any modulation of the cytoplasmic tails of integrins is brought about through an indirect regulatory mechanism. The cooperative effect of cyclin C and c-Myc on integrin activation may represent a novel signaling pathway, yet it will also be of interest to examine whether the function of cyclin C and c-Myc is mediated via regulation of several previously identified signaling pathways, such as R-Ras, PKC, protein tyrosine kinases, and intracellular Ca2+ or cAMP, etc. Alternatively, it may be also interesting to test whether cyclin C and c-Myc are targets of those signaling pathways. Treatment of BMC cells with either wortmannin (100 ng/mL) or rapamycin (10 ng/mL) exhibits no apparent effect on homotypic aggregation of BMC cells (data not shown), suggesting that the cooperative effect of cyclin C and c-Myc on VLA-4 activation may not be mediated by activation of phosphoinositide 3-OH kinase or the S6 kinase pathway. Further study will be required to elucidate the precise mechanism by which cyclin C and c-Myc cooperatively activate intergin and induce VCAM-1 expression.
We demonstrate here that the enhancement of BMC cell binding to VCAM-1 or Fn induced by the cooperative effects of cyclin C and c-Myc is a result of functional modulation of VLA-4 already present on the cell surface, because increased VCAM-1 ligation and Fn attachment occurred without alteration in the surface expression of this integrin. Hence, we conclude that functional cooperation of cyclin C and c-Myc is able to activate integrin, at least partly, by increasing integrin ligand-binding affinity via an inside-out signaling. However, our results do not exclude possible avidity changes that might result from integrin clustering, a postligand binding event that can also enhance ligand-binding ability. It should also be noted that we have examined only a limited selection of adhesion molecules in this study. It will be of interest to investigate whether cadherins, or other members of the integrin family, such as VLA-5, are also modulated. In addition, our results indicate that the VLA-4/VCAM-1 pair is primarily responsible for mediating homotypic aggregation of BMC cells; however, we do not exclude the possibility that BMC cell aggregation can be partly mediated by a VLA-4-VLA-4 interaction. In fact, it has been reported that the α4 integrin is a ligand for α4β1 and α4β7.67 It is also possible that serum Fn may also contribute to this cell adhesion through VLA-4-Fn-VLA-4 interactions.
The physiological and pathological significance of regulating functional properties of VLA-4 and VCAM-1 on hematopoietic cells remains to be determined. Thus far, the only example of a VLA-4/VCAM-1 interaction occuring between the same type of cells is an observation made by Rosen et al,68 in which they found that VCAM-1 and VLA-4 were expressed concomitantly on myoblasts. This VLA-4/VCAM-1 interaction has been suggested to be important for myogenesis. Activation of a VLA-4/VCAM-1 pair on the hematopoietic progenitors may be of importance for the regulation of hematopoiesis. Adhesive interactions of VLA-4 with VCAM-1 on stromal cells or with extracellular matrix retain hematopoietic progenitor cells in close proximity to the components of bone marrow microenvironment that is essential for the regulation of normal hematopoiesis. The importance of VLA-4 in hematopoiesis has been demonstrated by Miyake et al,31 who reported that the addition of anti-VLA-4 MoAb to long-term born marrow cultures abrogated lymphopoiesis and retarded myelopoiesis. VLA-4–specific antibodies have also been shown to abrogate stroma-dependent erythropoiesis.32 VCAM-1 may also help to promote lymphopoiesis and myelopoiesis.53,69,70 In addition, VLA-4/VCAM-1–mediated cell adhesion has been suggested to be involved in the migration of leukocytes71,72 and circulating malignant cells,73 74 which is a critical step in the process of inflammation and metastasis.
In summary, we show in this study that (1) cooperation of cyclin C and c-Myc induces homotypic aggregation of a hematopoietic progenitor, BAF-B03 cells, via activation of VLA-4 and induction of VCAM-1; (2) regulation of cell adhesion represents a novel function for cyclin C, and CDK8 kinase activity is not required for this function; (3) activation of VLA-4 by cyclin C and c-Myc is at least partly due to an increase in its ligand-binding activity through inside-out signaling; and (4) VCAM-1, which is not normally expressed on hematopoietic cells, can be induced on the BAF-B03 cells. These results clearly demonstrate a novel role for cyclin C and c-Myc in the regulation of cell adhesion. The elucidation of cyclin C and c-Myc–mediated signal transduction pathways governing cell adhesion will enable us to gain further important insights into mechanisms regulating multiple biological processes in which the VLA-4/VCAM-1 pair plays essential roles.
ACKNOWLEDGMENT
The authors are grateful to Dr R.A. Weinberg for human cyclin C, E, and D3 expression plasmids (Rc-cycC, Rc-cycE, and Rc-cycD3) and to Dr E.A. Nigg for pCMV-cdk8WT and pCMV-cdk8AMG plasmids. We also thank Dr A. Kukula for critical reading of the manuscript.
Supported by a Grant-in-Aid for Scientific Research on Priority Areas provided by the Ministry of Education, Science, Sports and Culture, Japan; by Nippon Boehringer Ingelheim Co, Ltd, Kawanishi Pharma Research Institute; by the Kato Memorial Bioscience Foundation; by The Naito Foundation; and by the Kanae Foundation For Life & Socio-Medical Science. Z.-J.L. was supported by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yasuhiro Minami, MD, Department of Biochemistry, Kobe University, School of Medicine, 7-5-1, Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan; e-mail:minami@kobe-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal