Abstract
Angiostatin is a circulating inhibitor of angiogenesis generated by proteolytic cleavage of plasminogen. In this study we have used recombinant human and murine angiostatins (kringles 1-4) as well as native human angiostatin (prepared by elastase digestion of plasminogen [kringles 1-3] or by plasmin autocatalysis in the presence of a free sulfhydryl donor [kringles 1-4]). We report that angiostatin reduces endothelial cell number in a 4-day proliferation assay without affecting cell cycle progression into S-phase (as determined by bromodeoxyuridine labeling). This suggested that the reduction in cell number in the proliferation assay might in part be due to cytotoxicity. This was confirmed by the observation that ethidium homodimer incorporation (a measure of plasma membrane integrity) into endothelial cells was increased by angiostatin in a manner similar to that seen with tumor necrosis factor- (TNF-) and transforming growth factor-β1 (TGF-β1), both of which induce apoptosis in endothelial cells. In contrast to TNF- and TGF-β1, angiostatin did not induce cytotoxicity in human MRC-5 fibroblast, rat smooth muscle, canine MDCK epithelial, or murine B16-F10 melanoma cell lines. Angiostatin-induced apoptosis was confirmed by endothelial cell nuclear acridine orange incorporation as well as by annexin V and TUNEL staining. These in vitro findings point to endothelial cell apoptosis as a mechanism for the antiangiogenic effect of angiostatin in vivo.
ANGIOGENESIS IS THE formation of new capillary blood vessels by a process of sprouting from pre-existing vessels and occurs during development as well as in a number of physiological and pathological settings. Angiogenesis is necessary for tissue growth, wound healing, and female reproductive function and is a component of pathological processes such as hemangioma formation and ocular neovascularization (reviewed in Folkman1 and Pepper2). However, much of the longstanding interest in angiogenesis comes from the notion that for solid tumors to grow beyond a critical size, they must recruit endothelial cells from the surrounding stroma to form their own endogenous microcirculation.3 With respect to angiogenesis, two phases can be recognized in tumor progression: prevascular and vascular. The prevascular phase is characterized by an initial increase in tumor growth followed by a plateau in which the rate of tumor cell growth is balanced by an equivalent rate of cell death (apoptosis). During the vascular phase, which is characterized by exponential growth, tissue invasion, and the hematogenous spread of tumor cells, the rate of tumor cell proliferation is unaltered, and the increase in tumor growth is largely due to a decrease in the rate of tumor cell apoptosis.4 5 Pharmacological inhibition of angiogenesis is therefore envisaged as an important adjunct to current therapeutic strategies aimed at inhibiting tumor growth and the spread of metastases.
With the exception of angiogenesis that occurs in response to tissue injury or in female reproductive organs, endothelial cell turnover in the healthy adult organism is very low. The maintenance of endothelial quiescence is thought to be due to the presence of endogenous negative regulators, because positive regulators are frequently detected in adult tissues in which there is apparently no angiogenesis. The converse is also true, namely that positive and negative regulators often coexist in tissues in which endothelial cell turnover is increased. This has lead to the notion of the angiogenic switch, in which endothelial activation status is determined by a balance between positive and negative regulators: in activated (angiogenic) endothelium, positive regulators predominate, whereas endothelial quiescence is achieved and maintained by the dominance of negative regulators (reviewed in Bouck6 and Hanahan and Folkman7). Used initially in the context of tumor progression to describe the passage from a prevascular to a vascular phase, the notion of the switch can also be applied in the context of developmental, physiological, and pathological angiogenesis. The current working hypothesis is that the switch involves either the induction of a positive regulator and/or the loss of a negative regulator.
Positive regulators of angiogenesis include members of the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) families. However, although a role for VEGF in the development of the embryonic vasculature and in tumor angiogenesis has been unequivocally established, the precise role of bFGF in the endogenous regulation of angiogenesis remains to be clarified (reviewed in Dvorak et al,8 Pepper et al,9Christofori,10 Ferrara and Davis-Smyth,11 and Korpelainen and Alitalo12). An additional positive regulator is angiopoietin-1, one of the ligands for the Tie-2 tyrosine kinase receptor.13 Angiopoietin-2, recently identified as a second ligand for the Tie-2 receptor, competes with angiopoietin-1 for binding to Tie-2 without inducing receptor autophosphorylation.14 Endogenous negative regulators of angiogenesis include angiostatin, endostatin, thrombospondin, interferons, platelet factor IV, 16-kD N-terminal fragment of prolactin, interleukin-12 (IL-12), gro-β, proliferin-related protein, protease inhibitors, 2-methoxyestradiol, and soluble cytokine receptors (reviewed in Folkman1 and Pepper2).
Angiostatin is an internal proteolytically derived fragment of plasminogen spanning the first four kringle domains.15 In vivo, angiostatin inhibits experimental primary tumor growth as well as angiogenesis-dependent growth of metastases in mice.5,15-20In vitro, angiostatin inhibits endothelial cell migration and reduces cell number in assays of proliferation; this effect is specific for endothelial cells.15,17,18,21-23 In addition, angiostatin inhibits endothelial invasion and tube formation in three-dimensional in vitro angiogenesis systems.20,23,24 The in vitro activity of angiostatin in endothelial cell proliferation assays resides in kringles 1-3, with kringle 1 being the most potent inhibitor and kringle 4 being relatively ineffective.21 Using the same assay, kringle 5 was shown to have inhibitory activity that is at least as potent, if not more, than angiostatin.25 Recently, kringle 5 was also shown to specifically inhibit endothelial cell migration.26
It has been suggested that, depending on the type of tumor, either tumor cells themselves or tumor-associated macrophages express enzymatic activity that is capable of converting inactive plasminogen into the angiogenesis-inhibiting angiostatin. Thus, macrophage-derived metalloelastase,27 matrilysin (MMP-7), gelatinase B (MMP-9),28 and stromelysin-1 (MMP-3)29 are capable of hydrolyzing plasminogen to generate angiostatin. Angiostatin can also be generated by plasmin autodigestion. This reaction can be catalyzed either by a protein disulfide isomerase30 or occurs in the presence of a free sulfhydryl donor.22 31
In the present studies, we have (1) assessed whether angiostatin is cyotoxic for endothelial cells and have related this to cell cycle progression and the reduction in endothelial cell number seen in a 4-day proliferation assay; (2) quantitatively assessed the effect of multiple forms of angiostatin on the induction of endothelial cell apoptosis and determined the cell-type specificity of this effect.
MATERIALS AND METHODS
Materials.
Plasminogen was prepared from outdated human plasma.32Angiostatin was prepared from human plasma-derived plasminogen using two methods. In the first method, plasminogen was digested with pancreatic elastase and was purified to apparent homogeneity using lysine sepharose.15 Angiostatin prepared in this way was provided by Dr M.S. O’Reilly (Children’s Hospital, Harvard Medical School, Boston, MA) or was produced by TechnoClone G.m.b.H (Vienna, Austria) and provided by Dr W.-D. Schleuning (Schering AG, Berlin, Germany) or Dr B. Binder (University of Vienna, Vienna, Austria). In the second method, angiostatin was generated in a cell-free system by plasmin autocatalysis in the presence of a free sulfhydryl donor.31 Recombinant human angiostatin was produced inPichia pastoris17 or in CHO cells (MacDonald et al, manuscript in preparation) and was purified in a single step using lysine-Sepharose affinity chromatography. Recombinant mouse angiostatin was produced using baculovirus-infected insect cells18 and was purified using lysine-Sepharose chromatography followed by reverse-phase high-performance liquid chromatography (HPLC) on a C8 Aquapore column (Machery Nagel AG, Oensingen, Switzerland) equilibrated in 0.1% trifluoroacetic acid and eluted with a linear gradient of 0% to 100% acetonitrile. Recombinant human bFGF (155 amino acid form) was provided by Dr P. Sarmientos (Farmitalia Carlo Erba, Milan, Italy). Recombinant human tumor necrosis factor-α (TNF-α) was provided by Dr L. Fransen (Innogenetics, Ghent, Belgium) and had a specific activity of 2 × 107 IU/mg. Human platelet-derived transforming growth factor-β1 (TGF-β1) was purchased from R&D Systems Europe Ltd (Abdingdon, UK). Lipopolysaccharide (LPS) was purchased from DIFCO Laboratories (Detroit, MI). Actinomycin D, acridine orange, Hoechst 33258, and propidium iodide were obtained from Sigma (Buchs, Switzerland; Stockholm, Sweden; and St Louis, MO). LPS levels were determined using the Limulus Amebocyte Lysate (LAL) Kinetic-QCL kit (BioWhittaker Inc, Walkersville, MD).
Western blot analysis.
Plasminogen and angiostatin were electrophoresed in a 12% polyacrylamide gel under nonreducing conditions and transferred to nitrocellulose membranes, and nonspecific binding was blocked overnight in Tris-buffered saline (TBS) containing 3% dried milk. Mouse monoclonal antibodies (MoAbs) were added for 2 hours at 37°C. The membranes were washed 3 times for 10 minutes in TBS/0.1% Tween-20. This was followed by addition of a secondary horseradish peroxidase (HRP) goat antimouse antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) for 1 hour. The membranes were washed for 10 minutes in TBS/0.1% Tween-20, then for 60 minutes in TBS/0.1% Tween-20, and finally for 15 minutes in TBS. Protein bands were shown using the ECL detection system (Amersham International plc, Little Chalfont, Buckinghamshire, UK).
Mouse MoAbs used were (1) MoAb 36E6 directed against lysine binding site I (LBS I, kringles 1-3) of human plasminogen,33 which should recognize both angiostatin and plasminogen; and (2) MoAb 42B12 directed against kringle 5 of human plasminogen,29 34 which should recognize plasminogen and not angiostatin.
Cell culture.
Bovine adrenal cortex-derived microvascular endothelial (BME) cells35 (provided by Drs M.B. Furie and S.C. Silverstein, Columbia University, New York, NY) were grown in minimal essential medium, α modification (α-MEM; GIBCO AG, Basel, Switzerland) containing 15% heat-inactivated donor calf serum (DCS; GIBCO), penicillin (110 U/mL), and streptomycin (110 μg/mL). Bovine adrenal cortex-derived capillary endothelial (BCE) cells (provided by Dr J. Folkman, Children’s Hospital, Harvard Medical School, Boston, MA) were grown in Dulbecco’s modified minimal essential medium (DMEM; GIBCO) supplemented with L-glutamine, 100 μg/mL endothelial cell mitogen (R & D Systems, Minneapolis, MN), 10% DCS, and antibiotics. Bovine aortic endothelial (BAE) cells, isolated from scrapings of adult bovine thoracic aortas and cloned by limiting dilution,36were cultured in DMEM, 10% DCS, and antibiotics. Bovine lymphatic endothelial (BLE) cells, isolated from mesenteric lymphatic vessels and cloned by limiting dilution,37 were cultured in DMEM supplemented with 1 mmol/L sodium pyruvate, 10% DCS, and antibiotics. Human umbilical vein endothelial (HUVE) cells isolated by collagenase digestion (provided by Dr N. Maggiano, University of Rome, Rome, Italy) were grown in medium 199 (GIBCO) supplemented with L-glutamine, 30 μg/mL endothelial cell growth supplement (Collaborative Research, Waltham, MA), 100 μg/mL heparin (sodium salt, grade I, from porcine intestinal mucosa; Sigma), 20% DCS, and antibiotics. SV40 large T antigen-transformed human dermal microvascular endothelial cells (HMEC-138; provided by Drs T.J. Lawley [Department of Dermatology, Emory University School of Medicine, Atlanta, GA] and E.W. Ades [Biological Products Branch, CDC, Atlanta, GA]) were grown in Endothelial Cell Basal Medium (modified MCDB 131 medium; Clonetics, San Diego, CA) supplemented with 10 ng/mL epidermal growth factor (Boehringer Mannheim GmbH, Biochemica, Mannheim, Germany), 1 μg/mL hydrocortisone (Sigma), 10% fetal calf serum (FCS; GIBCO), and antibiotics. Human dermal microvascular endothelial (DMVE) cells (purchased from InvitroCyte Inc, Seattle, WA) were grown in α-MEM, 15% DCS, and antibiotics. All endothelial cell lines were maintained in 1.5% gelatin-coated tissue culture flasks (Falcon Labware, Becton Dickinson Co, Lincoln Park, NJ) and subcultured at a split ratio of 1/3 or 1/4.
MRC-5 fibroblasts (CCL-171) were purchased from the American Type Culture Collection (Rockville, MD) and grown in minimal essential medium (MEM; GIBCO), 10% FCS, and antibiotics. Smooth muscle cells from the normal rat aortic media (provided by Dr G. Gabbiani, University Medical Center, Geneva, Switzerland) were cultured in high glucose DMEM, 10% FCS, and antibiotics. MDCK cells (provided by Dr K. Simons, EMBL, Heidelberg, Germany) were cultured in MEM supplemented with Earl’s salts, L-glutamine, 5% FCS, and antibiotics. B16-F10 mouse melanoma cells (provided by Dr I.J. Fidler, University of Texas M.D. Anderson Cancer Center, Houston, TX) were grown in MEM supplemented with L-glutamine, nonessential amino acids, 10% FCS, and antibiotics.
Proliferation assay.
Endothelial cells were seeded into 23-mm wells of a 12-well plate (Costar, Cambridge, MA) at 10,000 cells per well in medium with 2% DCS. Two hours later, angiostatin or TGF-β1 was added to the wells at the indicated concentrations; this was followed 1 hour later by the addition of bFGF (10 ng/mL) to half of the wells. Medium and compounds were renewed after 2 days, and cells were counted after a further 2 days using a FACScan Analyser (Becton Dickinson, San José, CA). Results are shown as the mean of duplicate wells ± SD.
Bromo-deoxy uridine labeling and annexin V staining.
Low-density cultures of BME cells were grown for 72 hours in the presence of angiostatin in medium containing 5% serum. The number of cells in S-phase was assessed by a 5-hour labeling with bromo-deoxy Uridine (BrdU; Amersham International plc). The number of apoptotic cells was simultaneously assessed by annexin V staining (Boehringer Mannheim). To this end, cells were fixed in 2% paraformaldehyde for 10 minutes, washed in phosphate-buffered saline (PBS), and incubated with fluorescein isothiocyanate (FITC)-labeled annexin V for 30 minutes. Cells were then washed in PBS (3 × 5 minutes), incubated in 1.5 mol/L HCl, and washed again in PBS (3 × 5 minutes). Nonspecific staining was blocked with 10% horse serum for 15 minutes before the application of an anti-BrdU mouse MoAb (Dakopatts AB, Hägersten, Sweden) and incubation for 1 hour at room temperature. Positive signal was visualized with a secondary rhodamine-labeled antimouse antibody. Nuclei were counterstained with Hoechst 33258. Results were analyzed using a Leitz-DMRB fluorescence microscope (Leica, Heidelberg, Germany) equipped with a Hamamatsu 4800 CCD camera (Hamamatsu, Herrsching, Germany). Digital images were processed in Adobe Photoshop (Adobe Inc, San José, CA).
Ethidium homodimer incorporation assay.
An ethidium homodimer-1 incorporation assay was used to detect endothelial cell cytotoxicity. Ethidium homodimer-1 (Molecular Probes, Leiden, The Netherlands) is a high-affinity red fluorescent DNA dye that is only internalized through altered cell membranes. This assay has been shown to be more sensitive than the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-Tetrazoliumbromide (MTT)-incorporation assay when screening for TNF-α cytotoxicity39 and has previously been used to screen for TNF-α–induced cytotoxicity of microvascular endothelial cells.40 Briefly, cells grown in DMEM without phenol red (phenol red quenches the ethidium homodimer signal) were incubated under the various conditions described. Where indicated, angiostatin was preincubated for 2 hours at room temperature with mouse MoAbs 36E6 and 42B12 (described above) before addition to the cells. After the indicated times of exposure to angiostatin, cytokines, or LPS, cells were treated for 30 minutes at 37°C with 8 mmol/L ethidium homodimer solution. Controls included cells pretreated for 30 minutes at room temperature with 33% ethanol (positive control = 100% incorporation) or cells incubated in medium alone (negative control = 0% incorporation). The fluorescence signal was read in a CytoFluor II Fluorescence Multi-well Plate Reader (PerSeptive Biosystems, Framingham, MA) using an excitation wavelength of 530 nm and an emission wavelength of 620 nm. The percentage of cells incorporating ethidium homodimer was calculated using the following formula: ([sample value] − [negative control value])/([positive control value] − [negative control value]) × 100. Because it is not possible from this assay to determine baseline levels of ethidium homodimer incorporation, values represent the percentage of labeled cells above control values.
Apoptosis assays.
For acridine orange uptake,41 confluent monolayers of BME cells were grown in the presence of the various angiostatin preparations, TNF-α, TGF-β1, LPS, or plasminogen for 48 hours in gelatinized wells of 96 multiwell plates; cells were incubated with 10 μg/mL acridine orange for 5 minutes, rinsed with PBS, and fixed in 4% paraformaldehyde. Cellular morphology was assessed by fluorescence microscopy. Apoptosis was identified as nuclear chromatin condensation and cytoplasmic blebbing.42 Randomly selected microscopic fields were examined for each experimental condition, and the percentage of cells undergoing apoptosis in each field was determined. For propidium iodide and Terminal deoxyribonucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining,43 confluent monolayers of BCE or B16-F10 cells on gelatinized coverslips in 24 multiwell plates were exposed overnight to phAst. Cells were fixed in 2% paraformaldehyde in PBS and stained with propidium iodide and TUNEL using an Apoptag kit (Oncor, Gaithersburg, MD). To quantitate apoptosis, a minimum of 500 cells were analyzed per condition. Results are expressed as the mean percentage (±SEM) of cells with evidence of apoptosis.
RESULTS
Characterization of the angiostatin preparations used in this study.
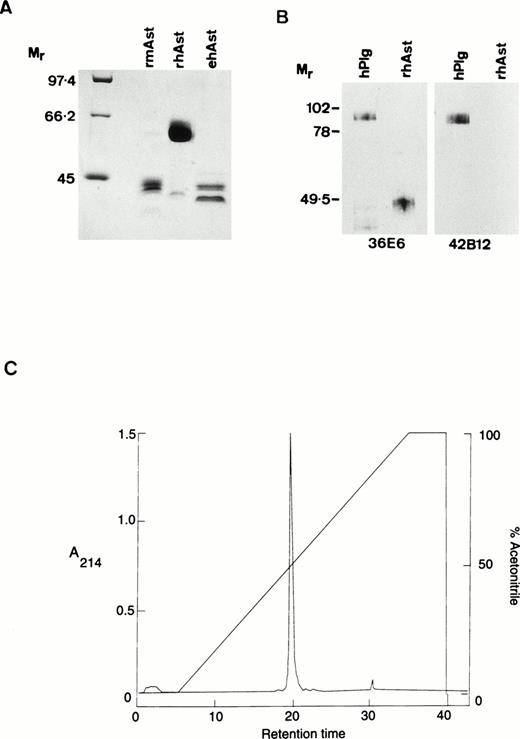
Angiostatin preparations used in the present study were designated as follows: rhAst: recombinant human angiostatin, CHO cells; rmAst: recombinant mouse angiostatin, baculovirus; ehAst: angiostatin derived from elastase cleavage of plasminogen; and phAst: angiostatin derived from plasmin in the presence of a free sulfhydryl donor. These preparations are listed in Table 1, together with their kringle numbers and amino acid residues. The carboxy terminus of phAst is currently being determined. LPS levels, as determined using the LAL assay (nanograms of LPS per milligram of protein), are also shown in Table 1. Figure1A shows a Coomassie blue-stained 9% polyacrylamide gel after electrophoresis of three of the angiostatin preparations (10 μg/lane) run under reducing conditions. Figure 1B shows a Western blot of hPlg and rhAst (100 ng/lane, run under nonreducing conditions) probed with antibodies to LBS I/kringles 1-3 (36E6, lanes 1 and 2) or kringle 5 (42B12, lanes 3 and 4). rhAst is recognized by the former but not by the latter. Figure 1C shows the elution profile of rmAst after preparative HPLC.
Angiostatin Preparations Used in This Study
| Species . | Abbr. . | Kringles . | Amino Acid Residues* . | Source . | Reference . | LPS Levels ng/mg Protein† . |
|---|---|---|---|---|---|---|
| Human | ehAst | K1-K3 | Tyr80-Val338 Tyr80-Val354 | Elastase-digested plasminogen | 59 | 4.5-9.2 |
| Human | K1-K4 | Leu74-Leu451 | Recombinant P pastoris | 17 | 0.40 | |
| Human | rhAst | K1-K4 | Leu74-Leu451 | Recombinant CHO cells | MacDonald et al (in preparation) | 0.43 |
| Human | phAst | K1-K4 + 2/3 of K5 | Lys78-? | Plasmin autocatalysis | 31 | <0.001 |
| Mouse | rmAst | NTP + K1-K4 | Asp1-Ser13; Arg78-Gly439 | Recombinant baculovirus | 18 | 0.27-1.74 |
| Human | hPlg | Plasminogen | Plasma | 0.02-0.09 |
| Species . | Abbr. . | Kringles . | Amino Acid Residues* . | Source . | Reference . | LPS Levels ng/mg Protein† . |
|---|---|---|---|---|---|---|
| Human | ehAst | K1-K3 | Tyr80-Val338 Tyr80-Val354 | Elastase-digested plasminogen | 59 | 4.5-9.2 |
| Human | K1-K4 | Leu74-Leu451 | Recombinant P pastoris | 17 | 0.40 | |
| Human | rhAst | K1-K4 | Leu74-Leu451 | Recombinant CHO cells | MacDonald et al (in preparation) | 0.43 |
| Human | phAst | K1-K4 + 2/3 of K5 | Lys78-? | Plasmin autocatalysis | 31 | <0.001 |
| Mouse | rmAst | NTP + K1-K4 | Asp1-Ser13; Arg78-Gly439 | Recombinant baculovirus | 18 | 0.27-1.74 |
| Human | hPlg | Plasminogen | Plasma | 0.02-0.09 |
Abbreviations: Abbr., abbreviation used in this study; K, kringle; NTP, N-terminal peptide.
Numbering is based on published sequences of human (791 residues)60 and mouse (793 residues)61plasminogens and begins at Glu1 and Asp1 in human and mouse, respectively (ie, excluding the 19 amino acid signal peptide that begins at Met-19).
LPS levels were determined using the LAL assay.
Characterization of angiostatin preparations used in this study. (A) Ten micrograms of rmAst, rhAst, and ehAst were run under reducing conditions in a 9% polyacrylamide gel and stained with Coomassie blue. Mr = relative molecular mass × 1,000. (B) hPlg and rhAst were analyzed by Western blotting under nonreducing conditions using mouse MoAbs 36E6 and 42B12 directed against kringles 1-3/LBSI and kringle 5, respectively.Mr = relative molecular mass × 1,000. (C) Elution profile of rmAst after purification by HPLC.
Characterization of angiostatin preparations used in this study. (A) Ten micrograms of rmAst, rhAst, and ehAst were run under reducing conditions in a 9% polyacrylamide gel and stained with Coomassie blue. Mr = relative molecular mass × 1,000. (B) hPlg and rhAst were analyzed by Western blotting under nonreducing conditions using mouse MoAbs 36E6 and 42B12 directed against kringles 1-3/LBSI and kringle 5, respectively.Mr = relative molecular mass × 1,000. (C) Elution profile of rmAst after purification by HPLC.
With regard to rmAst, when freshly purified this protein migrates as a single band around 52 kD under reducing conditions. However, under a variety of conditions (including freezing and thawing), it is degraded down to smaller species, including a doublet at around 42 kD, which retains angiostatin activity (Y. Shing, unpublished observation). The rmAst used in our study was purified by HPLC after lysine sepharose chromatography to remove contaminating LPS, which is likely to explain the reduction in apparentMr to around 42,000, as seen in Fig 1A. The single major peak in the HPLC profile shown in Fig 1C is compatible with the material seen in lane 2 of Fig 1A. rhAst runs at around 48 kD under nonreducing and at 62 kD under reducing conditions (Fig 1A and B). ehAst runs as at least a doublet at 42 to 44 kD under reducing conditions (Fig 1A). phAst runs as a doublet at 52 to 55 kD under nonreducing conditions and runs slightly slower under reducing conditions (Gately et al31 and data not shown). Additional minor bands are seen at 62 and 40 kD in the rmAst and at 43 kD in the rhAst preparations (Fig 1A).
Angiostatin reduces endothelial cell number without inhibiting S-phase progression.
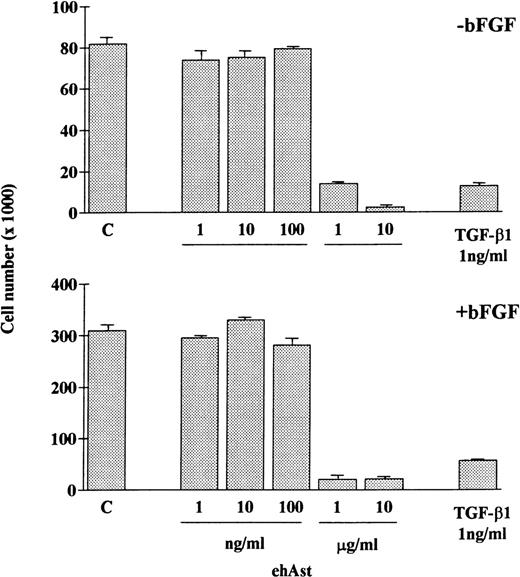
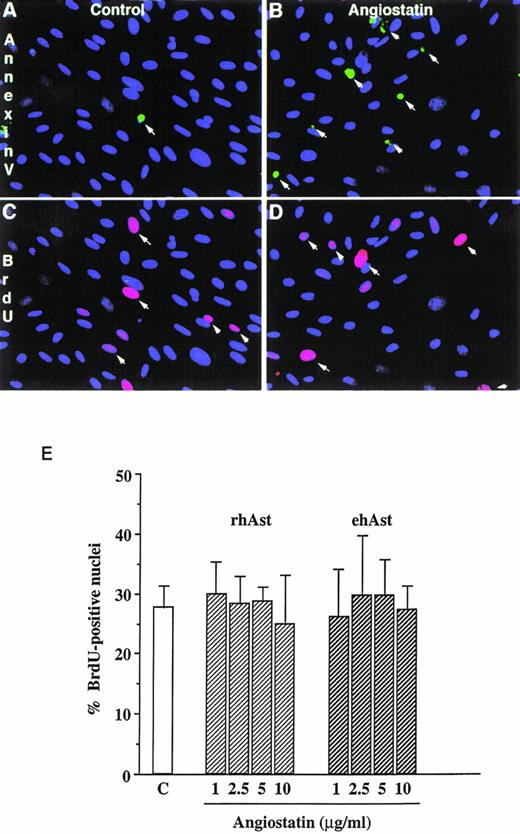
In accordance with previous observations, we observed that BAE and BME cell number was reduced by ehAst (1 and 10 μg/mL) in a 4-day proliferation assay, either in the presence or absence of bFGF (10 ng/mL; Fig 2 and data not shown). This experiment has been performed four times with minor variations, and similar results have been obtained using three different preparations of ehAst. When BME cell progression into S-phase was assessed by BrdU incorporation, this was found to be unaffected by ehAst (Fig 3A and B). Figure 3A shows BME cells double-labeled with annexin V and BrdU. From these results it appears that the two techniques label different cells, suggesting that it is not the proliferating cells that are targetted by angiostatin. These results raise the possibility that the reduction in endothelial cell number in the absence of altered cell cycle progression could in part be due to a cytotoxic effect.
Angiostatin reduces endothelial cell number. ehAst or TGF-β1 were added to BAE cells at the concentrations indicated, either in the presence or absence of bFGF over a 4-day proliferation assay. Results are shown as the mean cell number × 1,000 (±SD) from duplicate samples.
Angiostatin reduces endothelial cell number. ehAst or TGF-β1 were added to BAE cells at the concentrations indicated, either in the presence or absence of bFGF over a 4-day proliferation assay. Results are shown as the mean cell number × 1,000 (±SD) from duplicate samples.
Angiostatin induces endothelial cell apoptosis without affecting cell cycle progression. (A) Simultaneous detection of cell proliferation and apoptosis by BrdU labeling and annexin V staining. Low-density cultures of BME cells grown in 5% serum were treated either with vehicle alone (A and C) or with 5 μg/mL rhAst (B and D) for 72 hours. Apoptotic cells were detected by incubation with FITC-labeled annexin V (A and B, arrows). In the same field, proliferating cells were identified with a MoAb against BrdU (C and D, arrows). Original magnification × 80. (B) Lack of effect of angiostatin on endothelial cell S-phase entry. Low-density cultures of BME cells grown in medium containing 5% serum were treated with increasing amounts of angiostatin for 72 hours. The cells were pulsed for 5 hours with BrdU, and uptake was analyzed immunohistochemically as described in Materials and Methods. The percentage of positive nuclei was estimated by fluorescence microscopy. Results are shown as the mean of triplicate samples; error bars depict SEM.
Angiostatin induces endothelial cell apoptosis without affecting cell cycle progression. (A) Simultaneous detection of cell proliferation and apoptosis by BrdU labeling and annexin V staining. Low-density cultures of BME cells grown in 5% serum were treated either with vehicle alone (A and C) or with 5 μg/mL rhAst (B and D) for 72 hours. Apoptotic cells were detected by incubation with FITC-labeled annexin V (A and B, arrows). In the same field, proliferating cells were identified with a MoAb against BrdU (C and D, arrows). Original magnification × 80. (B) Lack of effect of angiostatin on endothelial cell S-phase entry. Low-density cultures of BME cells grown in medium containing 5% serum were treated with increasing amounts of angiostatin for 72 hours. The cells were pulsed for 5 hours with BrdU, and uptake was analyzed immunohistochemically as described in Materials and Methods. The percentage of positive nuclei was estimated by fluorescence microscopy. Results are shown as the mean of triplicate samples; error bars depict SEM.
Angiostatin induces cytotoxicity in endothelial cells.
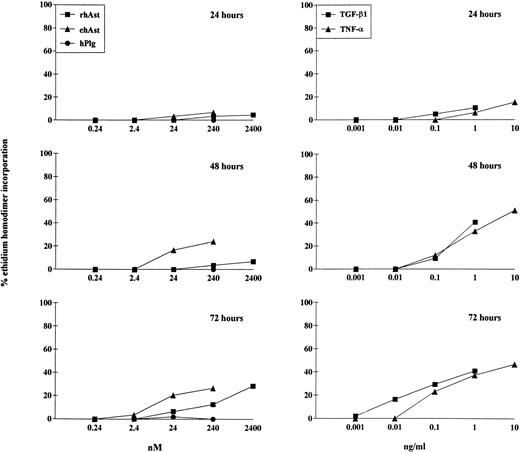
To quantitate the cytotoxic effect of angiostatin, endothelial cells were treated for 24, 48, and 72 hours with different doses of ehAst and rhAst, and the percentage of dying cells was assessed using the ethidium homodimer incorporation assay. Although this assay measures loss of plasma membrane integrity (ethidium homodimer-1 intercalates into DNA, which increases its fluorescence intensity 40-fold), it does not distinguish between apoptosis and necrosis. Figure 4 and Table 2 show that both angiostatins induce cytotoxicity in BME cell monolayers in a dose-dependent manner, with ehAst being more potent than rhAst. This effect becomes prominent from 48 hours on. rmAst and recombinant human angiostatin from P. pastoris also induced cytotoxicity in BME cells (Table 2). Plasminogen at equimolar concentrations had no effect (Fig 4 and Table2). The level of angiostatin cytotoxicity was slightly less than that seen with TNF-α and TGF-β1 (Fig 4 and Table 2). Cytotoxicity induced by ehAst was density-dependent: under subconfluent conditions, BME cells were less sensitive to the lytic effect of these agents than when they were confluent (data not shown). For ehAst, rhAst, and rmAst, cytotoxicity induced by different batches of the same angiostatin type was roughly equivalent (data not shown).
Kinetics of angiostatin-, hTNF-–, and TGF-β1–induced cytotoxicity in BME cells. BME cell monolayers were treated with ehAst, rhAst, or hPlg (left hand column) or with hTNF- or TGF-β1 (right hand column) for 24, 48, or 72 hours at the indicated concentrations. Results are shown as the mean of duplicate samples. Cytotoxicity was assessed using the ethidium homodimer incorporation assay. Although both ehAst and rhAst induce a dose-dependent cytotoxicity in BME cell monolayers (which becomes prominent from 48 hours), sensitivity to the former was greater than to the latter; plasminogen at equimolar concentrations had no effect (240 nmol/L = ∼10 μg/mL for rhAst and ehAst and 22 μg/mL for hPlg). Both hTNF- and TGF-β1 induced dose-dependent cytotoxicity in confluent BME cell monolayers, which became prominent from 48 hours.
Kinetics of angiostatin-, hTNF-–, and TGF-β1–induced cytotoxicity in BME cells. BME cell monolayers were treated with ehAst, rhAst, or hPlg (left hand column) or with hTNF- or TGF-β1 (right hand column) for 24, 48, or 72 hours at the indicated concentrations. Results are shown as the mean of duplicate samples. Cytotoxicity was assessed using the ethidium homodimer incorporation assay. Although both ehAst and rhAst induce a dose-dependent cytotoxicity in BME cell monolayers (which becomes prominent from 48 hours), sensitivity to the former was greater than to the latter; plasminogen at equimolar concentrations had no effect (240 nmol/L = ∼10 μg/mL for rhAst and ehAst and 22 μg/mL for hPlg). Both hTNF- and TGF-β1 induced dose-dependent cytotoxicity in confluent BME cell monolayers, which became prominent from 48 hours.
Cytotoxicity and Apoptosis in BME Cells
| . | % Ethidium Homodimer Incorporation Above Control Levels . | % Acridine Orange-Labeled Cells . |
|---|---|---|
| Control | 7.18 ± 0.68 (n = 20) | |
| hPlg | ||
| 2.2 μg/mL | 0.67 ± 0.67 (n = 3) | ND |
| 11.0 μg/mL | ND | 7.55 ± 1.23 (n = 5) |
| 22.0 μg/mL | 0 (n = 4) | 5.99 ± 0.70 (n = 8) |
| 100 μg/mL | 2.3 (n = 1) | ND |
| rhTNF-α | ||
| 10.0 ng/mL | 27.05 ± 21.15 (n = 2) | 49.18 ± 9.51 (n = 6) |
| rmTNF-α | ||
| 1.0 ng/mL | 22.04 ± 6.50 (n = 5) | ND |
| 10.0 ng/mL | 30.79 ± 5.78 (n = 5) | ND |
| 1.0 μg/mL | ND | 77.68 ± 7.14 (n = 9) |
| hTGF-β1 | ||
| 1 ng/mL | 22.60 ± 18.40 (n = 2) | 13.95 ± 1.17 (n = 8) |
| LPS | ||
| 1 μg/mL | 4.90 ± 1.20 (n = 2) | ND |
| 10 μg/mL | 16.30 ± 10.70 (n = 2) | ND |
| ehAst | ||
| 1 μg/mL | 14.45 ± 3.35 (n = 4) | 12.32 ± 0.98 (n = 2) |
| 10 μg/mL | 21.61 ± 3.65 (n = 8) | 19.69 ± 2.49 (n = 8) |
| rhAst (CHO) | ||
| 1 μg/mL | 4.27 ± 2.13 (n = 3) | 13.80 ± 1.44 (n = 8) |
| 10 μg/mL | 8.00 ± 2.06 (n = 4) | ND |
| 50 μg/mL | 24.10 (n = 1) | 17.39 ± 3.54 (n = 6) |
| rhAst (P.p.) | ||
| 1 μg/mL | 4.05 ± 4.05 (n = 2) | ND |
| 10 μg/mL | 13.10 ± 6.50 (n = 2) | ND |
| rmAst (Bac.) | ||
| 1 μg/mL | 4.35 ± 0.85 (n = 2) | ND |
| 10 μg/mL | 12.25 ± 2.05 (n = 2) | 7.16 ± 0.47 (n = 8) |
| 25 μg/mL | ND | 38.75 ± 4.26 (n = 5) |
| . | % Ethidium Homodimer Incorporation Above Control Levels . | % Acridine Orange-Labeled Cells . |
|---|---|---|
| Control | 7.18 ± 0.68 (n = 20) | |
| hPlg | ||
| 2.2 μg/mL | 0.67 ± 0.67 (n = 3) | ND |
| 11.0 μg/mL | ND | 7.55 ± 1.23 (n = 5) |
| 22.0 μg/mL | 0 (n = 4) | 5.99 ± 0.70 (n = 8) |
| 100 μg/mL | 2.3 (n = 1) | ND |
| rhTNF-α | ||
| 10.0 ng/mL | 27.05 ± 21.15 (n = 2) | 49.18 ± 9.51 (n = 6) |
| rmTNF-α | ||
| 1.0 ng/mL | 22.04 ± 6.50 (n = 5) | ND |
| 10.0 ng/mL | 30.79 ± 5.78 (n = 5) | ND |
| 1.0 μg/mL | ND | 77.68 ± 7.14 (n = 9) |
| hTGF-β1 | ||
| 1 ng/mL | 22.60 ± 18.40 (n = 2) | 13.95 ± 1.17 (n = 8) |
| LPS | ||
| 1 μg/mL | 4.90 ± 1.20 (n = 2) | ND |
| 10 μg/mL | 16.30 ± 10.70 (n = 2) | ND |
| ehAst | ||
| 1 μg/mL | 14.45 ± 3.35 (n = 4) | 12.32 ± 0.98 (n = 2) |
| 10 μg/mL | 21.61 ± 3.65 (n = 8) | 19.69 ± 2.49 (n = 8) |
| rhAst (CHO) | ||
| 1 μg/mL | 4.27 ± 2.13 (n = 3) | 13.80 ± 1.44 (n = 8) |
| 10 μg/mL | 8.00 ± 2.06 (n = 4) | ND |
| 50 μg/mL | 24.10 (n = 1) | 17.39 ± 3.54 (n = 6) |
| rhAst (P.p.) | ||
| 1 μg/mL | 4.05 ± 4.05 (n = 2) | ND |
| 10 μg/mL | 13.10 ± 6.50 (n = 2) | ND |
| rmAst (Bac.) | ||
| 1 μg/mL | 4.35 ± 0.85 (n = 2) | ND |
| 10 μg/mL | 12.25 ± 2.05 (n = 2) | 7.16 ± 0.47 (n = 8) |
| 25 μg/mL | ND | 38.75 ± 4.26 (n = 5) |
Confluent monolayers of BME cells in 96-well plates were exposed to the agents indicated above, and cytotoxicity (ethidium homodimer incorporation) and apoptosis (acridine orange labeling) were determined after 72 and 48 hours, respectively. Values are the mean ± SEM. For ethidium homodimer incorporation, n is the number of experiments. For acridine orange labelling, n is the number of photographic fields analyzed; results are pooled from 4 separate experiments and have been normalized to the mean of the mean control values of the four experiments.
Abbreviations: ND, not determined; CHO, Chinese hamster ovary cells;P.p., P. pastoris; Bac., baculovirus.
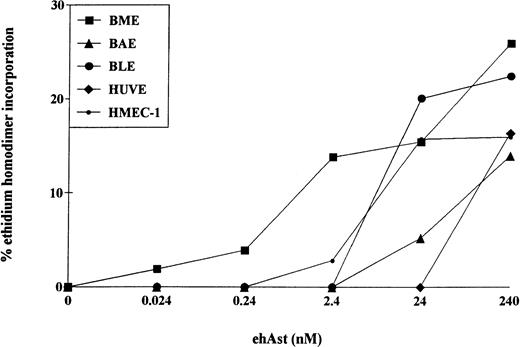
The cytotoxic effect of ehAst was not limited to BME cells. Cytotoxicity was also observed after 72 hours in BAE, BLE, HUVE, HMEC-1, and DMVE cells (Figs 5 and6); however, the greatest sensitivity was seen with BME cells. TNF-α also induced cytotoxicity in all six endothelial cell types tested (data not shown).
Angiostatin induces cytotoxicity in bovine and human endothelial cell types. Confluent monolayers of BME, BAE, BLE, HUVE, and HMEC-1 cells were treated with ehAst at the indicated concentrations, and endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay after 72 hours. Results are shown as the mean of duplicate samples.
Angiostatin induces cytotoxicity in bovine and human endothelial cell types. Confluent monolayers of BME, BAE, BLE, HUVE, and HMEC-1 cells were treated with ehAst at the indicated concentrations, and endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay after 72 hours. Results are shown as the mean of duplicate samples.
LPS-induced cytotoxicity in endothelial and smooth muscle cells. Confluent monolayers of BME, DMVE, or rat aortic smooth muscle (RASM) cells were treated with ehAst or LPS at the indicated concentrations, and endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay after 72 hours. Results are shown as the mean of duplicate samples.
LPS-induced cytotoxicity in endothelial and smooth muscle cells. Confluent monolayers of BME, DMVE, or rat aortic smooth muscle (RASM) cells were treated with ehAst or LPS at the indicated concentrations, and endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay after 72 hours. Results are shown as the mean of duplicate samples.
One of our major concerns has been to exclude contamination of the angiostatin preparations by LPS. Table 1 shows the levels of LPS detected in the various preparations using the LAL test. Table 2 and Fig 6 demonstrate that cytotoxicity is only seen upwards of 1 μg/mL of LPS. This was seen with BME, DMVE, and rat aortic smooth muscle cells. Because LPS levels never exceeded 10 ng per milligram of angiostatin, it is highly unlikely that LPS contributes to the cytotoxic effect of our angiostatin preparations. In experiments in which angiostatin and LPS were coadded, the cytotoxic effect was never greater than additive (data not shown).
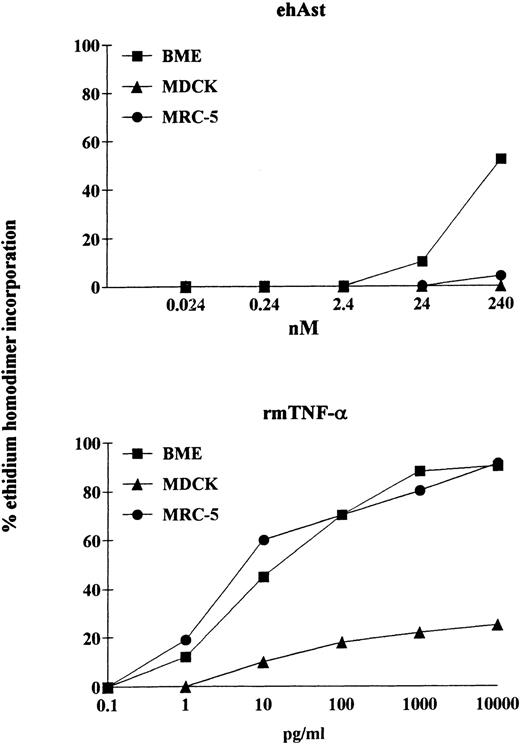
We have observed that angiostatin-induced cytotoxicity is specific for endothelial cells. It is well known that pretreatment of various cell types with the transcriptional inhibitor actinomycin D can either induce or increase sensitivity to TNF-α. We have observed that, in the presence of actinomycin D (1 μg/mL), ehAst induces significant cytotoxicity in BME cells after 24 hours (Fig 7). However, in contrast to BME cells, when exposed to ehAst for 24 hours in the presence of actinomycin D, neither MDCK epithelial cells nor MRC-5 fibroblasts were affected. However, all three cell types were efficiently lysed after exposure to TNF-α in the presence of actinomycin D for 24 hours (Fig 7). Actinomycin D alone was not cytotoxic for any of the cell lines used (data not shown). Unlike BME cells treated with ehAst alone, accelerated cytotoxicity in the presence of actinomycin D is not density dependent (data not shown).
The cytotoxic effect of angiostatin is endothelial cell-specific. In a 24-hour assay in the presence of actinomycin D, neither MDCK epithelial cells nor MRC-5 fibroblasts were lysed by ehAst (up to 10 μg/mL; 240 nmol/L), whereas, like BME cells, both nonendothelial cell types were lysed by rmTNF- in a dose-dependent manner. Cytotoxicity was assessed using the ethidium homodimer incorporation assay, and results are shown as the mean of duplicate samples.
The cytotoxic effect of angiostatin is endothelial cell-specific. In a 24-hour assay in the presence of actinomycin D, neither MDCK epithelial cells nor MRC-5 fibroblasts were lysed by ehAst (up to 10 μg/mL; 240 nmol/L), whereas, like BME cells, both nonendothelial cell types were lysed by rmTNF- in a dose-dependent manner. Cytotoxicity was assessed using the ethidium homodimer incorporation assay, and results are shown as the mean of duplicate samples.
Limited cytotoxicity was seen with ehAst in rat aortic smooth muscle cells (Fig 6). However, this was much lower than the cytotoxicity seen with angiostatin in the various endothelial cell types, as well as with LPS in smooth muscle cells.
To determine the specificity of angiostatin-induced cytotoxicity, ehAst was preincubated with mouse MoAbs that recognize kringles 1-3 (36E6) or kringle 5 (42B12) of human plasminogen before addition to BME cells. Figure 8 shows that cytoxicity could be completely inhibited with MoAb 36E6 and was unaffected by MoAb 42B12. These results demonstrate that cytoxicity is mediated by ehAst (kringles 1-3) and excludes the presence of contaminants that induce significant cytotoxicity.
Neutralization of angiostatin-induced cytotoxicity in BME cells. BME cell monolayers were incubated for 72 hours in the presence of ehAst that had been preincubated with mouse MoAbs to kringles 1-3 (36E6) or kringle 5 (42B12) of human plasminogen. At the end of the incubation, endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay. Results are shown as the mean of duplicate samples.
Neutralization of angiostatin-induced cytotoxicity in BME cells. BME cell monolayers were incubated for 72 hours in the presence of ehAst that had been preincubated with mouse MoAbs to kringles 1-3 (36E6) or kringle 5 (42B12) of human plasminogen. At the end of the incubation, endothelial cell cytotoxicity was assessed using the ethidium homodimer incorporation assay. Results are shown as the mean of duplicate samples.
Angiostatin induces apoptosis in endothelial cells.
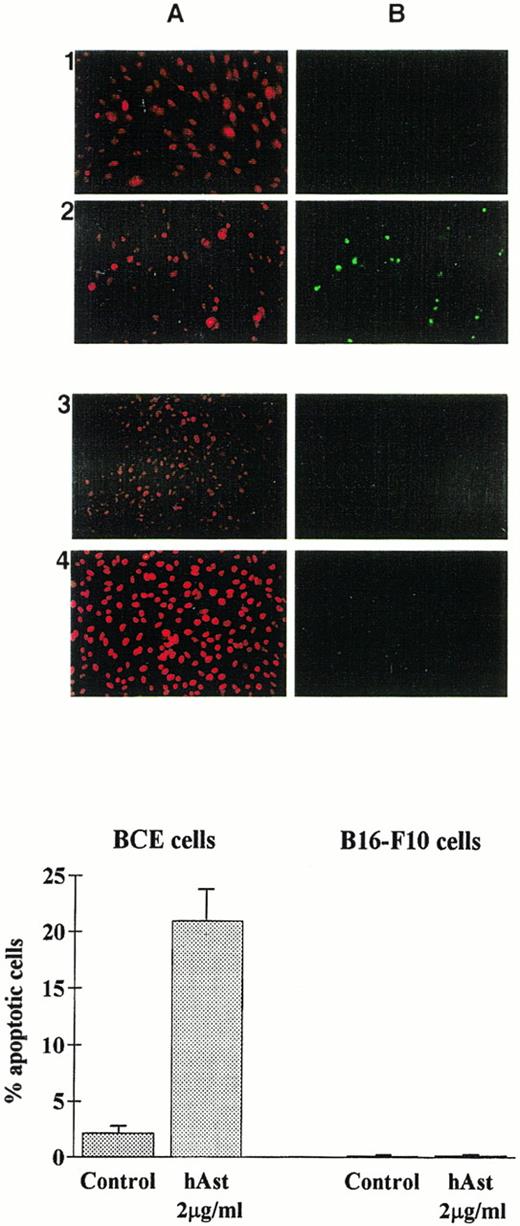
To determine whether angiostatin induces apoptosis in endothelial cells, BME or BCE cell monolayers were incubated in the presence of ehAst, rhAst, or phAst for 24 to 72 hours, and various assays for apoptosis were performed. Figure 3A and 3B show annexin V labeling of BME cell nuclei treated with rhAst (5 μg/mL) for 72 hours. Table 2shows levels of apoptosis as determined by acridine orange labeling of BME cells treated with cytokines, LPS or various preparations of angiostatin or plasminogen for 48 hours. Apoptosis was also induced in monolayers of BCE but not B16-F10 cells treated with phAst (2 μg/mL) for 15 hours, as determined by TUNEL staining (Fig 9).
Angiostatin induces apoptosis in endothelial cells. BCE (1 and 2) or B16-F10 (3 and 4) cell monolayers were exposed overnight to phAst (2 μg/mL) in 0.5% serum, and apoptosis was assessed (A) by propidium iodide or (B) by TUNEL staining. (Lower panel) Quantitation of apoptosis as determined by TUNEL staining; data are pooled from two experiments, and results are expressed as the mean percentage (±SEM) of cells with evidence of apoptosis. Original magnification in (A) × 40.
Angiostatin induces apoptosis in endothelial cells. BCE (1 and 2) or B16-F10 (3 and 4) cell monolayers were exposed overnight to phAst (2 μg/mL) in 0.5% serum, and apoptosis was assessed (A) by propidium iodide or (B) by TUNEL staining. (Lower panel) Quantitation of apoptosis as determined by TUNEL staining; data are pooled from two experiments, and results are expressed as the mean percentage (±SEM) of cells with evidence of apoptosis. Original magnification in (A) × 40.
DISCUSSION
The purpose of the studies reported here was first to assess the effect of angiostatin on endothelial cell proliferation and cell cycle progression. We found that, although angiostatin reduced cell number in a 4-day proliferation assay, this was not due to a reduction in entry into S-phase. These findings suggested that cell death might contribute to the reduction in cell number. Our second objective was therefore to quantitatively assess whether angiostatin is cytotoxic for endothelial cells. To this end, we used the ethidium homodimer incorporation assay that measures for loss of plasma membrane integrity.39 We found that angiostatin induced cytotoxicity in three bovine and three human endothelial cell lines in a time- and dose-dependent manner, with kinetics that were similar to those seen with TNF-α and TGF-β1, both of which have previously been shown to induce apoptosis in vascular endothelial cells.41 44-47 Angiostatin-induced cytotoxicity could be completely abolished by preincubating ehAst with an MoAb against kringles 1-3 (LBS I) but not by an antibody to kringle 5 (which is present in plasminogen but not in angiostatin). Angiostatin cytotoxicity was not observed in three nonendothelial cell lines, namely human MRC-5 fibroblasts, canine MDCK epithelial cells, and murine B16F10 melanoma cells, although minimal cytotoxicity was observed in smooth muscle cells from the rat aortic media. Based on the levels of LPS detected with the LAL assay in the various angiostatin preparations we have used, it is highly unlikely that contaminating LPS is responsible for the cytotoxicity we observed. We found that angiostatin increased annexin V, acridine orange, and TUNEL staining of microvascular endothelial cell nuclei, indicating that angiostatin induces apoptosis in these cells. Finally, we have assessed five different types of angiostatin in this study and have found that angiostatin derived by elastase digestion of human plasminogen (kringles 1-3) is the most potent.
Is there a causal relationship between endothelial cell apoptosis and inhibition of angiogenesis? A recent study using tetracycline-regulated VEGF expression in xenografted C6 glioma cells has shown that VEGF withdrawal results in tumor endothelial cell shedding and apoptosis and that this is accompanied by hemorrhagic tumor necrosis.48TNF-α–induced hemorrhagic tumor necrosis is likewise caused by the destruction of the tumor microvasculature,44,49 and this is mediated in part by a reduction in endothelial cell αvβ3 activation.50 In this report, we demonstrate that angiostatin, which inhibits angiogenesis and tumor growth in mice,5,15,17 induces endothelial cell apoptosis. Likewise, it has recently been reported that 2-methoxyestradiol, which inhibits angiogenesis and tumor growth,51 also induces apoptosis in endothelial cells.52 Finally, we have observed that deprivation of endogenous bFGF, which induces endothelial cell apoptosis,53 also inhibits VEGF-induced in vitro angiogenesis.47 Taken together, these observations suggest that endothelial cell apoptosis induced by a variety of mechanisms (including growth factor deprivation) may be responsible for inhibiting angiogenesis, thereby preventing the growth of primary tumors and their metastases.
Is the induction of apoptosis in endothelial cells the mechanism by which angiostatin inhibits tumor growth? Relatively little is known concerning the mechanisms of the antiangiogenic and antitumor effects of angiostatin. In vitro, angiostatin reduces endothelial cell number in assays of proliferation15,17,18,21 (this report). However, although one report demonstrated that adenovirus-derived angiostatin (kringles 1-3) selectively disrupts the G2/M transition in endothelial cells,20 others have found that rhAst has little effect on the distribution of endothelial cells in G0/G1, S, and G2/M.54 Similarly, ehAst does not affect growth factor-induced signal transduction or [3H]-thymidine uptake by endothelial cells.23The data presented here demonstrate that, (1) endothelial cell BrdU and [3H]-thymidine uptake are unaffected by either ehAst or rhAst; (2) proliferating cells are not targetted by angiostatin as assessed by annexin V and BrdU double-labeling; and (3) sensitivity to angiostatin increases as endothelial cells approach confluence (and therefore stop dividing). Our findings therefore suggest that the reduction in endothelial cell number seen in assays of proliferation may be due in part to the induction of apoptosis. It has recently been reported that angiostatin leads to an RGD-independent induction of the activity of focal adhesion kinase, and it was suggested that this may lead to the subversion of endothelial cell adhesion plaque formation.23 These investigators also reported that angiostatin induces apoptosis in endothelial cells. It is therefore possible that the antiangiogenic activity of angiostatin results from its ability to induce endothelial cell apoptosis as well as to inhibit endothelial cell migration22,23 (this report) and that these effects are the consequence of disrupted endothelial cell-extracellular matrix interactions. Finally, angiostatin upregulates E-selectin (but not P-selectin) in proliferating but not quiescent endothelial cells, and this increases adhesion of HL-60 promyelocytic leukemia cells to angiostatin-treated endothelium.54 It remains to be determined whether this effect may contribute to the anti-tumor activity of angiostatin in vivo.
The tumor microvasculature is an important target in strategies aimed at inhibiting tumor growth. It has been reported that human and mouse angiostatins do not induce systemic cytotoxicity when administered to tumor-bearing mice,5,15,18 which suggests that angiostatin’s antitumor effect is mediated by specific targeting of angiogenic endothelial cells. Furthermore, although loco-regional treatment of inoperable melanoma49 and soft tissue sarcoma55 has been successful with TNF-α, in view of its high systemic toxicity56 and metastasis-inducing capacity,57 58 this cytokine cannot be administered systemically to cancer patients. Therefore, the search for factors that interact specifically with the tumor microvasculature without inducing systemic toxicity is an important therapeutic objective. In this report, we show that, in contrast to TNF-α, angiostatin is specifically cytotoxic for endothelial cells in vitro. Because the effective doses of angiostatin required for inhibition of tumor growth do not appear to induce systemic toxicity in mice, angiostatin represents an important therapeutic option for the treatment of cancer.
ACKNOWLEDGMENT
The authors are grateful to Dr H.R. Lijnen for useful advice and for providing antiangiostatin and antiplasminogen antibodies, Drs M.B. Furie and S.C. Silverstein for providing the BME cells, Dr J. Folkman for the BCE cells, Dr N. Maggiano for the HUVE cells, Drs T.J. Lawley and E.W. Ades for the HMEC-1 cells, Dr G. Gabbiani for the smooth muscle cells, Dr K. Simons for the MDCK cells, Dr I.J. Fidler for the B16-F10 cells, Dr P. Sarmientos for recombinant human bFGF, Dr L. Fransen for human TNF-α, and Drs M.S. O’Reilly, W.-D. Schleuning, and B. Binder for human angiostatin. Technical assistance was provided by C. Di Sanza, Y.R.A. Donati, C. Giroud, and M. Quayzin and photographic work was performed by B. Favri.
Supported by a grant from the Swiss National Science Foundation (0031-43364.95; to M.S.P.), by the Swedish Cancer Society and the Åke Wiberg Foundation (to L.H.), by the National Institutes of Health (Grant No. CA 71875; to G.A.S.), and by a grant in aid from the Ligue Genevoise Contre le Cancer (to G.E.G.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M.S. Pepper, MD, PhD, Department of Morphology, University Medical Center, 1, rue Michel-Servet, 1211 Geneva 4, Switzerland; e-mail: michael.pepper@medecine.unige.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal