Abstract
CD95 (Fas)-induced apoptosis plays a critical role in the elimination of activated lymphocytes and induction of peripheral tolerance. Defects in CD95/CD95L (Fas-Ligand)-apoptotic pathway have been recognized in autoimmune lymphoproliferative diseases (ALPS) and lpr or gld mice and attributed to CD95 and CD95L gene mutations, respectively. Large granular lymphocyte (LGL) leukemia is a chronic disease characterized by a proliferation of antigen-activated cytotoxic T lymphocytes. Autoimmune features such as hypergammaglobulinemia, rheumatoid factor, and circulating immune complexes are common features in LGL leukemia and ALPS. Therefore, we hypothesize that expansion of leukemic LGL may be secondary to a defective CD95 apoptotic pathway. In this study, we investigated expression of CD95 and CD95L in 11 patients with CD3+ LGL leukemia and explored the apoptotic response to agonistic CD95 monoclonal antibody (MoAb). We found that leukemic LGL from each patient expressed constitutively high levels of CD95/CD95L, similar to those seen in normal activated T cells. However, cells from 9 of these 11 patients were totally resistant to anti-CD95–induced apoptosis. Similarly, cells were resistant to anti-CD3-MoAb–triggered cell death. Lack of anti-CD95–induced apoptosis was not due to mutations in the CD95 antigen. Leukemic LGL were not intrinsically resistant to CD95-dependent death, because LGL from all but 1 patient underwent apoptosis after phytohemagglutinin/interleukin-2 activation. The patient whose leukemic LGL were intrinsically resistant to CD95 had an aggressive form of LGL leukemia that was resistant to combination chemotherapy. These findings that leukemic LGL are resistant to CD95-dependent apoptosis despite expressing high levels of CD95 are similar to observations made in CD95L transgenic mice. These data suggest that LGL leukemia may be a useful model of dysregulated apoptosis causing human malignancy and autoimmune disease.
LARGE GRANULAR lymphocyte (LGL) leukemia can be classified into CD3+ (T cell) and CD3− (natural killer [NK] cell) type depending on the cell lineage of the leukemic cells.1 Autoimmune manifestations are a prominent and characteristic feature of T-LGL leukemia. Serologic abnormalities are frequent, including autoantibodies such as rheumatoid factor and antinuclear antibody, as well as high levels of circulating immune complexes and polyclonal hypergammaglobulinemia.1-5 Autoimmune disease, particularly rheumatoid arthritis, also occurs frequently in LGL leukemia. Increased numbers of LGL could be explained either by stimulation of proliferation or by inhibition of apoptosis. Circulating leukemic LGL are in the Go/G1 phase of the cell cycle6; therefore, we hypothesize that extended cell survival may be secondary to defects in apoptosis.
Leukemic LGL show many characteristics of antigen-activated T cells.6 The physiological deletion of antigen-activated T cells occurs through apoptosis, mediated through CD95 antigen (Fas).7,8 CD95 is a transmembrane protein, belonging to the tumor necrosis receptor family.9 Ligation of CD95 by CD95 Ligand (FasL) or by anti-CD95 monoclonal antibody (MoAb) induces apoptosis of target cells bearing CD95.10-14CD95/CD95L-triggered apoptosis is involved in control of the immune response, induction of peripheral tolerance, and killing of viral-infected or malignant cells.8,15 16
A defect in CD95-dependent apoptosis is the underlying pathogenetic mechanism in animal models of lymphoproliferative disorders associated with autoimmune manifestations. Lpr/Lpr mice have mutations in CD95, whereas gld/gld mice have mutations in CD95L.17,18 Both animal models are characterized by hypergammaglobulinemia, rheumatoid factor, and circulating immune complexes, features similar to those observed in LGL leukemia.19,20 Dysregulation of CD95/CD95L is also seen in CD95L transgenic mice.21 In this model, high levels of CD95L result in the selection of a novel population of activated T cells that express high levels of CD95, but that are resistant to CD95-mediated apoptosis.
In this study, we examined expression of CD95 and CD95L by leukemic LGL and evaluated whether leukemic LGL were susceptible to CD95 and T-cell receptor (TCR)-induced apoptosis. We found that leukemic LGL expressed high levels of CD95 and CD95L, similar to levels seen on normal activated T cells. Despite high CD95 expression, leukemic LGL were resistant to CD95 or TCR-triggered cell death.
PATIENTS AND METHODS
Patients.
All patients met clinical criteria of T-LGL leukemia, with LGL counts ranging from 600 to 27,000/μL (normal, 223 ± 99/μL) and evidence of clonal TCR gene rearrangement.1 Clinical and laboratory features of these patients are shown in Table 1. Nine patients had chronic disease not requiring treatment. Patient no. 5 was receiving methotrexate for neutropenia and rheumatoid arthritis; on therapy, the neutrophil count increased into the normal range. Patient no. 10 had an aggressive form of LGL leukemia, refractory to methotrexate. He presented with massive, painful hepatomegaly with increased circulating LGL (16 × 109/L) and thrombocytopenia (20 to 30 × 109 platelets/L). This patient did not have γδ T-cell lymphoma, because the leukemic cells were αβ+ and γδ− as determined by MoAb staining. Four cycles of CHOP produced no response.
Clinical and Laboratory Features of LGL Leukemia Patients
| Patient No. . | Age/Sex . | Splenomegaly* . | RA . | WBC . | ANC . | ALC . | HCT† . | PLT . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29/F | Yes | No | 30,800 | 310 | 30,000 | 26 | 365,000 |
| 2 | 68/M | No | No | 3,800 | 1,600 | 1,630 | 34 | 158,000 |
| 3 | 79/M | No | No | 2,700 | 100 | 2,450 | 31 | 145,000 |
| 4 | 66/M | Yes | No | 15,400 | 8,800 | 5,100 | 31 | 368,000 |
| 5 | 58/M | Yes | Yes | 17,400 | 3,800 | 13,050 | 42 | 221,000 |
| 6 | 75/M | No | No | 15,700 | 1,700 | 13,700 | 27 | 386,000 |
| 7 | 67/M | No | No | 12,200 | 2,400 | 9,300 | 42 | 206,000 |
| 8 | 68/M | No | No | 4,500 | 720 | 3,375 | 24 | 95,000 |
| 9 | 60/M | No | Yes | 3,200 | 1,700 | 1,000 | 36 | 173,000 |
| 10 | 56/M | Yes | No | 15,800 | 1,100 | 11,500 | 30 | 96,000 |
| 11 | 63/F | No | No | 6,800 | 270 | 6,100 | 37 | 189,000 |
| Patient No. . | Age/Sex . | Splenomegaly* . | RA . | WBC . | ANC . | ALC . | HCT† . | PLT . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29/F | Yes | No | 30,800 | 310 | 30,000 | 26 | 365,000 |
| 2 | 68/M | No | No | 3,800 | 1,600 | 1,630 | 34 | 158,000 |
| 3 | 79/M | No | No | 2,700 | 100 | 2,450 | 31 | 145,000 |
| 4 | 66/M | Yes | No | 15,400 | 8,800 | 5,100 | 31 | 368,000 |
| 5 | 58/M | Yes | Yes | 17,400 | 3,800 | 13,050 | 42 | 221,000 |
| 6 | 75/M | No | No | 15,700 | 1,700 | 13,700 | 27 | 386,000 |
| 7 | 67/M | No | No | 12,200 | 2,400 | 9,300 | 42 | 206,000 |
| 8 | 68/M | No | No | 4,500 | 720 | 3,375 | 24 | 95,000 |
| 9 | 60/M | No | Yes | 3,200 | 1,700 | 1,000 | 36 | 173,000 |
| 10 | 56/M | Yes | No | 15,800 | 1,100 | 11,500 | 30 | 96,000 |
| 11 | 63/F | No | No | 6,800 | 270 | 6,100 | 37 | 189,000 |
All cell counts are per microliter and represent blood values at the time of laboratory studies.
Abbreviations: RA, rheumatoid arthritis; WBC, white blood cell counts; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; HCT, hematocrit (%); PLT, platelet count.
Patients no. 1 and 10 had prior splenectomies for symptomatic splenomegaly; patient no. 4 had splenectomy for ITP.
Patients no. 4, 6, and 8 were transfusion-dependent.
CD95 and CD95L expression.
Fresh peripheral blood mononuclear cells (PBMC) were obtained from the 11 LGL leukemia patients and from the buffy coat of healthy donors. PBMC were isolated by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation and then analyzed by flow cytometry (FacScan; Becton Dickinson, Mountain View, CA).
Analysis of CD95 surface expression was performed using phycoerythrin (PE)-conjugated UB2 MoAb (Kamiya Biomedical Corp, Tukwila, WA). To measure CD95 expression on LGL leukemic cells, the cells were incubated with UB2 MoAb and anti-CD57-fluorescein isothiocyanate (FITC) MoAb (Immunotech, Marseille, France). Negative isotype control MoAbs were IgG1-PE (Immunotech) and IgM-FITC (Dako, Copenhagen, Denmark). PBMC from healthy donors and leukemic LGL were cultured in RPMI medium (Fischer Scientific, Pittsburgh, PA) supplemented with 10% fetal calf serum and activated for 2 days with 1 μg/mL phytohemagglutinin (PHA; Sigma, St Louis, MO) and for 10 additional days with recombinant interleukin-2 (IL-2; 100 U/mL; Chiron, Emeryville, CA). CD95 expression was determined before and after activation. For determination of CD95 expression on normal CD57+ T cells or NK cells, three-color staining was performed using MoAbs: CD95-PE UB2, CD57-FITC, and CD3-Cy5 (Immunotech). CD3+/CD57+ and CD3−/CD57+ cells were gated for determination of CD95 expression.
Detection of CD95L expression required intracellular staining as previously described.22,23 PBMC were washed twice with phosphate-buffered saline (PBS) and fixed for 10 minutes at 4°C with 0.5% paraformaldehyde-PBS. After centrifugation, PBMC were resuspended in 0.1% Triton X-100–PBS for 3 minutes. PBMC were then washed and incubated with 2 μg anti-CD95L C-20 MoAb (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 minutes at 4°C and then rinsed three times in PBS containing 1% bovine serum albumin and 0.1% sodium azide. Nonspecific binding sites were blocked for 30 minutes with 20% normal swine serum. The cells were washed again and incubated with an FITC-conjugated swine antirabbit Ig MoAb (Dako) for 30 minutes at 4°C. Normal rabbit Ig (Dako) diluted to the same protein concentration as the primary antibody was used as a negative control. After washing, the cells were analyzed using flow cytometry.24 25
Apoptosis assay.
For the apoptosis assays, unactivated or activated PBMC were transferred to a 96-well culture plate at a concentration of 5 × 105/mL. The cells were then incubated with 1 μg/mL of anti-CD95 MoAb (CH 11; Kamiya Biomedical Corp) or 10 μg/mL of anti-CD3 MoAb (BC3; kindly provided by C. Anasetti, FHCRC, Seattle, WA) for 24 or 48 hours. The same conditions were used to induce cell death induced by 50 μmol/L of C2-ceramide (Sigma). Determination of apoptosis was performed by staining with 7-amino-actinomycin D (7-AAD; Calbiochem, San Diego, CA) and propidium iodide (PI; Molecular Probes, Inc, Eugene, OR), as described.24 25 For 7-AAD staining, cells were incubated with 7-AAD at a concentration of 20 μg/mL for 30 minutes at 4°C in the dark. The cells were then resuspended in PBS and analyzed using flow cytometry. For PI staining, cells were incubated with 50 μg/mL of PI for 30 minutes at room temperature, after fixation overnight with 70% ethanol. To ensure that apoptotic signal was related specifically to anti-CD95, the cells were preincubated with 500 ng/mL CD95 blocking ZB4 MoAb (Kamiya Biomedical Corp) for 60 minutes before treatment with the apoptosis-inducing MoAb CH11. T-cell leukemia CEM cell line was used as a positive control. CD95- or TCR-specific apoptosis was determined as follows: (% of apoptotic cells in the assay well − % of apoptotic cells in the control well)/(100 − % of apoptotic cells in the control well) × 100.
Cell sorting.
In experiments examining apoptosis of activated LGL, flow cytometry was used to isolate the leukemic CD57+ cells. Cells (12 to 15 × 106) were washed in PBS, resuspended in 300 μL of PBS, and incubated for 30 minutes at 4°C with CD57 FITC MoAb. CD57+ cells were then sorted on a FACStar (Becton Dickinson) cell sorter and directed to apoptosis assay as described above. The purity of enriched CD57+ cells was 93% to 96%.
Western blotting.
Cells were lysed in a buffer composed of 1% Nonidet P-40, 10 mmol/L Tris (pH 7.4), 0.1 mmol/L phenylmethylsulfonyl fluoride, EDTA, 10 mmol/L iodometacine, 1 μg/mL leupeptin, 1 μg/mL apoprotin, 0.4 mmol/L Na orthovanadate, and antipain for 45 minutes at 4°C. Equal amounts of protein were loaded on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. After blocking with PBS buffer containing 5% milk and 0.1% tween, the membranes were probed with N-20 anti-CD95L (Santa Cruz Biotechnology) for 2 hours at room temperature followed by horseradish peroxidase (HRP)-conjugated secondary antibody. Membranes were washed and developed using a chemoluminescent detection system (ECL; Amersham Life Science, Arlington Heights, IL).
Analysis of CD95 gene.
Sufficient material was available for mutational analyses of the CD95 coding sequence in 5 patients. These analyses were performed by reverse transcription-polymerase chain reaction (RT-PCR) single-stranded conformation polymorphism (SSCP) followed by cDNA sequencing. Total RNA was extracted from PBMC of LGL leukemia patients or normal subjects and transcribed to cDNA. The open reading frame of the CD95 cDNA was amplified with three overlapping sets of primers and analyzed by SSCP under previously established conditions.26 27 The cytoplasmic region, which encompasses the signal transducing death domain, was further examined for mutations by denaturing gradient gel electrophoresis (DGGE). The cDNAs showing mobility shifts were extracted from the gel and reamplified using the same primer set. PCR products were subcloned into the TA cloning vector pCR2.1 (Invitrogen, La Jolla, CA), and multiple clones were selected for bidirectional sequencing (ALF, Piscataway, NJ).
RESULTS
CD95/CD95L expression.
PBMC from all patients expressed constitutively high levels of surface CD95 and CD95L protein (Table 2). The mean percentage of CD95+ LGL cells was 88% ± 7%, similar to that of activated T cells (94% ± 3%) and much higher than that in normal PBMC, in which CD95 is expressed at a relatively low level (35% ± 11%). Almost 80% of leukemic CD57+ cells coexpressed CD95 (Fig 1), whereas in normal PBMC, only a minority of CD57+ cells expressed CD95 (32% ± 12%). Because normal CD57+ cells may be either CD3− or CD3+, we performed three-color analysis of normal PBMC to further delineate CD95 expression. We found that CD95 was expressed on 32% ± 10% of normal CD3+, CD57+ cells (n = 10). Therefore, there was a much higher frequency of CD95 expression on leukemic LGL compared with their normal CD3+, CD57+ counterparts. It is of interest that the percentage of normal CD57+ cells expressing CD95 after activation was similar to that seen constitutively in leukemic LGL. Figure 2 shows the results of flow cytometry detection of CD95L. As previously described, CD95L was expressed on normal PBMC only after activation. In contrast, we found constitutive expression of CD95L on PBMC from all LGL leukemia patients. The level of expression of CD95L on leukemic LGL appeared higher than that observed on activated normal T cells. The mean fluorescence intensity of CD95L was 3.9 (range, 1.7 to 7) in leukemic LGL, as compared with 1.5 (range, 1.43 to 1.6) in activated PBMC. Western blot analysis of whole cell lysates confirmed the elevated levels of CD95L in leukemic LGL compared with normal activated PBMC (not shown).
Expression of CD95 and CD95L on Leukemic LGL
| Samples . | Phenotype (%) . | |||
|---|---|---|---|---|
| CD95 . | CD57 . | CD57/CD95* . | CD95L . | |
| PBMC | 35 ± 11 | 6 ± 2 | 32 ± 12 | 2 ± 1 |
| PBMC post-PHA/IL-2 | 94 ± 3 | 9 ± 2 | 90 ± 3 | 42 ± 5 |
| CD3+ LGL leukemia (patient no.) | ||||
| 1 | 96 | 44 | 80 | 90 |
| 2 | 78 | 30 | 62 | 43 |
| 3 | 85 | 74 | 85 | ND |
| 4 | 95 | 65 | 84 | 44 |
| 5 | 95 | 52 | 93 | 90 |
| 6 | 78 | 33 | 77 | 79 |
| 7 | 84 | 76 | 84 | 35 |
| 8† | 96 | 15 | 72 | 80 |
| 9 | 91 | 41 | 82 | 50 |
| 10‡ | 87 | 12 | 64 | 89 |
| 11 | 82 | 38 | 63 | 88 |
| Mean ± SD | 88 ± 7 | 44 ± 22 | 77 ± 10 | 69 ± 23 |
| Samples . | Phenotype (%) . | |||
|---|---|---|---|---|
| CD95 . | CD57 . | CD57/CD95* . | CD95L . | |
| PBMC | 35 ± 11 | 6 ± 2 | 32 ± 12 | 2 ± 1 |
| PBMC post-PHA/IL-2 | 94 ± 3 | 9 ± 2 | 90 ± 3 | 42 ± 5 |
| CD3+ LGL leukemia (patient no.) | ||||
| 1 | 96 | 44 | 80 | 90 |
| 2 | 78 | 30 | 62 | 43 |
| 3 | 85 | 74 | 85 | ND |
| 4 | 95 | 65 | 84 | 44 |
| 5 | 95 | 52 | 93 | 90 |
| 6 | 78 | 33 | 77 | 79 |
| 7 | 84 | 76 | 84 | 35 |
| 8† | 96 | 15 | 72 | 80 |
| 9 | 91 | 41 | 82 | 50 |
| 10‡ | 87 | 12 | 64 | 89 |
| 11 | 82 | 38 | 63 | 88 |
| Mean ± SD | 88 ± 7 | 44 ± 22 | 77 ± 10 | 69 ± 23 |
Abbreviations: PBMC, peripheral blood mononuclear cells from normal controls, n = 6; ND, not determined.
Percentage of CD57+ cells expressing CD95 is indicated.
Sixty-one percent of the cells were CD16+.
Thirty-eight percent of the cells were CD56+.
Representative flow cytometry results showing coexpression of CD95 on CD57+ leukemic LGL from patient no. 9. Ninety-one percent and 41% of the cells express CD95 (graph on the left) and CD57 (graph on the center), respectively. In dual fluorescence, 82% of the CD57+ cells coexpress CD95 (graph on the right).
Representative flow cytometry results showing coexpression of CD95 on CD57+ leukemic LGL from patient no. 9. Ninety-one percent and 41% of the cells express CD95 (graph on the left) and CD57 (graph on the center), respectively. In dual fluorescence, 82% of the CD57+ cells coexpress CD95 (graph on the right).
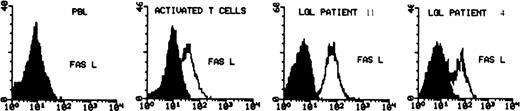
Determination of CD95L expression on normal PBMC (before and after PHA/IL-2 activation) and in 2 cases of freshly isolated LGL leukemia (cases no. 4 and 11). The cells were fixed and permeabilized and then stained with anti-CD95L MoAb (C-20) followed by secondary antirabbit-FITC MoAb. The shaded area represents the level of fluorescence obtained with an isotype control MoAb, the unshaded area represents the level with CD95L MoAb.
Determination of CD95L expression on normal PBMC (before and after PHA/IL-2 activation) and in 2 cases of freshly isolated LGL leukemia (cases no. 4 and 11). The cells were fixed and permeabilized and then stained with anti-CD95L MoAb (C-20) followed by secondary antirabbit-FITC MoAb. The shaded area represents the level of fluorescence obtained with an isotype control MoAb, the unshaded area represents the level with CD95L MoAb.
Results of apoptosis assay (Table3 ).
Freshly isolated PBMC from 10 of 11 patients showed no apoptosis after 24 hours of exposure to anti-CD95 MoAb (Fig3). After prolonged incubation with anti-CD95 MoAb for 48 hours, cells still remained resistant, except for patient no. 3. In this patient, 31% of the cells were apoptotic. Similar results were seen after 48 hours of anti-CD3 MoAb activation. Absence of apoptosis was observed in 9 of 10 patients, with only patient no. 3 showing slight susceptibility with 28% apoptotic cells (not shown). Ceramide-induced cell death was detected in all 8 cases studied and was comparable to normal PBMC (47% ± 13% v 42% ± 11%, respectively; not shown).
Results of CD95-Dependent Apoptosis in LGL Leukemia
| CD3+ LGL Leukemia (patient no.) . | % Specific Cell Death After Anti-CD95 MoAb Exposure . | |
|---|---|---|
| Freshly Isolated . | Post-PHA/IL-2 . | |
| 1 | 34 | 50 |
| 2 | <5 | 33 |
| 3 | <5 | 56 |
| 4 | <5 | 43 |
| 5 | <5 | 53 |
| 6 | <5 | 53 |
| 7 | <5 | 57 |
| 8 | <5 | 30 |
| 9 | <5 | 45 |
| 10 | <5 | <5 |
| 11 | <5 | 76 |
| CD3+ LGL Leukemia (patient no.) . | % Specific Cell Death After Anti-CD95 MoAb Exposure . | |
|---|---|---|
| Freshly Isolated . | Post-PHA/IL-2 . | |
| 1 | 34 | 50 |
| 2 | <5 | 33 |
| 3 | <5 | 56 |
| 4 | <5 | 43 |
| 5 | <5 | 53 |
| 6 | <5 | 53 |
| 7 | <5 | 57 |
| 8 | <5 | 30 |
| 9 | <5 | 45 |
| 10 | <5 | <5 |
| 11 | <5 | 76 |
Percentage of specific cell death was calculated as described in the Patients and Methods. Anti-CD95 MoAb was added for 24 hours. As positive controls for apoptosis assays, CEM cell line and normal activated T cells were used. For CEM, apoptosis was 60% ± 5% (n = 5). For normal activated T cells, 50% ± 15% cell death was observed (n = 5).
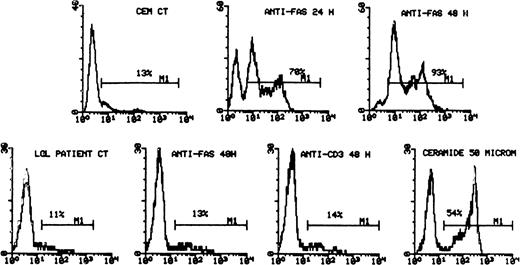
Freshly isolated LGL are resistant to CD95 and anti-CD3–mediated apoptosis. CEM-sensitive cell line was used as a positive control for CD95-induced apoptosis (top panel). The cells were incubated with media alone (histograms on the left) or with anti-CD95 (CH11, 1 μg/mL) for 24 and 48 hours, as shown. The cells were then stained with 7-AAD and analyzed by flow cytometry. The LGL leukemic cells are analyzed in the bottom panel. The percentage of apoptotic cells is indicated in each histogram. Although leukemic LGL were resistant to both anti-CD95– and anti-CD3–induced apoptosis (BC3, 10 μg/mL), they remained susceptible to ceramide.
Freshly isolated LGL are resistant to CD95 and anti-CD3–mediated apoptosis. CEM-sensitive cell line was used as a positive control for CD95-induced apoptosis (top panel). The cells were incubated with media alone (histograms on the left) or with anti-CD95 (CH11, 1 μg/mL) for 24 and 48 hours, as shown. The cells were then stained with 7-AAD and analyzed by flow cytometry. The LGL leukemic cells are analyzed in the bottom panel. The percentage of apoptotic cells is indicated in each histogram. Although leukemic LGL were resistant to both anti-CD95– and anti-CD3–induced apoptosis (BC3, 10 μg/mL), they remained susceptible to ceramide.
After activation with PHA and IL-2, PBMC from 10 of 11 patients showed susceptibility to anti-CD95, with a mean percentage of apoptotic cells of 50% (range, 30% to 76%). Likewise, exposure to anti-CD3 MoAb induced an apoptotic response in 10 of 11 patients, with a mean percentage of apoptotic cells of 45% (range, 29% to 63%). The apoptotic effect of anti-CD95 MoAb was specifically inhibited by ZB4 MoAb (Fig 4). To ensure that apoptosis was occurring in LGL leukemic cells rather than in normal cells expanded after 10 days of IL-2 activation, CD57+ cells from 3 patients were sorted after activation and exposed to anti-CD95 and anti-CD3 MoAb. Purity after cell sorting was greater than 93% CD57+ cells in each case. Figure 5 shows that 76% of the CD57+ cells underwent apoptosis after anti-CD95 MoAb in patient no. 4. Similar results were seen after activation with anti-CD3 MoAb (not shown). The same data were obtained with the CD57+-sorted cells from patients no. 5 and 7.
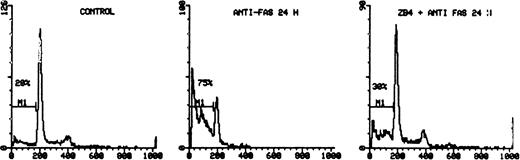
Protection of CD95-induced apoptosis by CD95 blocking MoAb ZB 4. The activated PBMC of patient no. 11 were preincubated with ZB 4 (500 ng/mL for 60 minutes) and then exposed to anti-CD95 for 48 hours. The cells were incubated with 50 μg/mL of PI for 30 minutes at room temperature, after fixation overnight with 70% ethanol, and the DNA content was analyzed using flow cytometry. Histograms on the left, center, and right represent the results after incubation with serum alone, anti-CD95, and ZB4 + anti-CD95, respectively. The percentage of apoptotic cells is indicated on the left side of each panel.
Protection of CD95-induced apoptosis by CD95 blocking MoAb ZB 4. The activated PBMC of patient no. 11 were preincubated with ZB 4 (500 ng/mL for 60 minutes) and then exposed to anti-CD95 for 48 hours. The cells were incubated with 50 μg/mL of PI for 30 minutes at room temperature, after fixation overnight with 70% ethanol, and the DNA content was analyzed using flow cytometry. Histograms on the left, center, and right represent the results after incubation with serum alone, anti-CD95, and ZB4 + anti-CD95, respectively. The percentage of apoptotic cells is indicated on the left side of each panel.
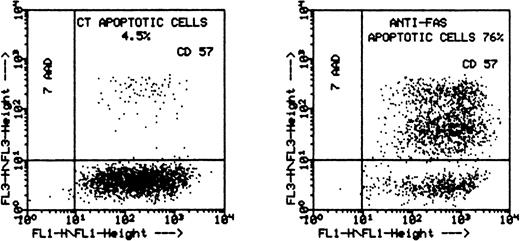
Flow cytometry results showing that leukemic LGL are susceptible to anti-CD95–induced apoptosis after activation. The PBMC of patient no. 4 were cultured initially with PHA (1 μg/mL) for 2 days and then with IL-2 (100 U/mL) for 10 more days. The cells were then stained with CD57+ and sorted on FacStar. The purified CD57+ cells (94%) were then incubated with anti-CD95 MoAb (CH11, 1 μg/mL) for 48 hours and stained with 7-AAD before analysis using flow cytometry. The graph on the left represents the control (media alone); the graph on the right represents the cells incubated with CH11. The percentage of apoptotic cells is shown on the upper-right quadrant.
Flow cytometry results showing that leukemic LGL are susceptible to anti-CD95–induced apoptosis after activation. The PBMC of patient no. 4 were cultured initially with PHA (1 μg/mL) for 2 days and then with IL-2 (100 U/mL) for 10 more days. The cells were then stained with CD57+ and sorted on FacStar. The purified CD57+ cells (94%) were then incubated with anti-CD95 MoAb (CH11, 1 μg/mL) for 48 hours and stained with 7-AAD before analysis using flow cytometry. The graph on the left represents the control (media alone); the graph on the right represents the cells incubated with CH11. The percentage of apoptotic cells is shown on the upper-right quadrant.
Analysis of CD95 gene mutation.
Function ablating mutations in the CD95 gene have been shown to be causative in some autoimmune diseases. Using RT-PCR SSCP and DGGE analysis, followed by cDNA sequencing of PCR products, we examined the CD95 coding sequence to determine if mutations might account for the failure of LGL leukemia cells to undergo apoptosis when exposed to anti-CD95 MoAb. No mutations were detected in the CD95 coding sequence of the LGL leukemia patients studied. Four of the five patients examined expressed a previously documented polymorphism at bp 836, which does not alter the amino acid sequence.
DISCUSSION
CD3+ LGL leukemia is a chronic clonal T-cell lymphoproliferative disorder recognized as a distinct entity using clinical, immunological, and molecular parameters.1,2,28The clonal expansion of leukemic LGL may require a multistep pathogenesis. Leukemic LGL have many characteristics of antigen-activated cytotoxic T lymphocytes (CTL): (1) They express a T-cell cytotoxic phenotype and can be activated via the CD3/CD16 pathway.6,29 (2) They constitutively express perforin and CD95L.30,31 (3) At least in some cases, they use a restricted Vβ repertoire, reinforcing the hypothesis of antigenic selection.32 Increased numbers of LGL could be explained either by a stimulation of proliferation or inhibition of programmed cell death. CD95/CD95L interactions play a major role in the induction of cell death after T-cell activation.8-11 CD95 is weakly expressed on the surface of resting T cells and is upregulated after antigen activation.9 14 The accumulation of peripheral T cells might result from a defect in removing antigen-activated T cells. Therefore, expansion of LGL leukemic cells could be due to a defect in the CD95 apoptotic pathway.
We studied the CD95/CD95L apoptotic pathway in 11 cases of T-LGL leukemia. We found a constitutively high surface expression of CD95 and CD95L in all 11 cases at levels similar to, if not greater than, those seen in normal activated T cells. LGL specifically expressed CD95, because almost 80% of CD57+ LGL coexpressed CD95. Despite this high level of CD95 expression and evidence of a constitutively activated T-cell phenotype, freshly isolated cells from 9 of 11 patients were completely resistant to anti-CD95–induced apoptosis. In the 2 remaining patients (patients no. 1 and 3), CD95-induced apoptosis was lower than levels seen in control cells, especially in patient no. 3, whose cells displayed a slight apoptotic response only after 2 days of anti-CD95 MoAb exposure. A similar pattern of resistance to TCR-triggered cell death was also observed. Normal T cells become sensitive to apoptosis after activation, when expression of high level of CD95 and CD95L is observed. Our observations that leukemic LGL is resistant to apoptosis despite expressing high levels of both CD95 and CD95L suggest that this apoptotic pathway is dysregulated in LGL leukemia.
These results of CD95 resistance are similar to findings observed in animal models of lymphoproliferation and autoimmune disease occurring in lpr and gld mice.19,20 The pathogenesis of CD95 resistance in these murine models is due to mutations in CD95 and CD95L, respectively.17,18 Recently, resistance to anti-CD95–induced apoptosis has been described in a human disease, termed autoimmune lymphoproliferative syndrome (ALPS).33-37The clinical and biological features of ALPS are very similar to those observed in LGL leukemia, including the following: (1) The majority of the uniquely expanded CD4−, CD8−cells in ALPS are CD57+.33 (2) The patients present with splenomegaly, hypergammaglobulinemia, and autoimmune hemolytic anemia, thrombocytopenia, or neutropenia. CD95 gene mutations primarily involving the death domain have been described in these patients. We previously reported the absence of mutations in the CD95 death domain in seven patients.31 However, some patients with ALPS have had mutations in regions of CD95 other than the death domain.34 For these reasons, we examined the entire CD95 antigen cDNA in the LGL leukemia patients. We found no evidence for CD95 mutation in each of the 5 patients studied. We have also found no evidence for CD95 mutation in 4 additional patients (unpublished observations). Therefore, dysregulated CD95-dependent apoptosis in LGL leukemia does not result from mutations in CD95. Recently, an autoimmune lymphoproliferative disease (ALD) has been described with generalized autoimmune manifestations but without expansion of dual CD4/CD8-negative T cells.38Interestingly, the CD95+ cells are resistant to anti-CD95–induced apoptosis but do not display any CD95 gene mutations. Ceramide is the second messenger produced by hydrolysis of sphingomyelin after a CD95 apoptotic signal.39 In ALD patients, ceramide induced cell death is deficient, suggesting a downstream alteration of the apoptosis pathway.38Ceramide-induced apoptosis was normal in LGL leukemia patients, suggesting that the cause of apoptotic resistance is different in LGL leukemia compared with ALD patients.
The mechanism resulting in resistance to CD95-induced apoptosis in LGL leukemia is not known. From our study we do know that resistance is not due to lack of CD95 surface expression or mutant CD95 protein, as seen in myeloma samples or myeloma cells lines.25,27 We also know that there is not an intrinsic defect in apoptosis in LGL leukemia, because leukemic cells from 10 of 11 patients underwent apoptosis after activation with IL-2. It is conceivable that lack of IL-2 in vivo might explain the apoptotic resistance. IL-2 predisposes peripheral T cells to CD95 and anti-CD3/TCR–induced cell death.14 IL-2–deficient mice (IL-2−/−) develop a fatal disease with lymphadenopathy, splenomegaly, and multiorgan T-cell infiltration.40 Activated T cells from these IL-2−/− mice display a CD95-resistant phenotype, although they express CD95 similarly to cells from their IL-2+/+ littermates. Although LGL leukemic cells constitutively express p75 IL-2 receptor, they do not produce IL-2 gene transcripts or secrete IL-2 even after anti-CD3 MoAb activation.41 42
Leukemic cells from 1 patient remained resistant to both CD95 and TCR triggered cell death even after activation with IL-2. No abnormalities were observed in CD95 gene, suggesting that the defect in apoptosis is distal to the receptor. This patient had an aggressive clinical course, presenting with pancytopenia and massive hepatomegaly, which was refractory to combination chemotherapy. We recently reported that chronic LGL leukemias express relatively high levels of multidrug resistance gene (MDR1) and that P-glycoprotein was functionally active in CD57+ leukemic LGL.43 It is of interest that leukemic LGL from this patient showed a high level of P-glycoprotein surface expression (not shown). It has been suggested that chemotherapeutic agents may induce apoptosis through the CD95/CD95L pathway.16 Anthracycline-resistant cell lines are also resistant to CD95-induced apoptosis.25 Therefore, elucidation of the mechanism of resistance to CD95-mediated apoptosis in this patient may also help delineate mechanisms involved in drug resistance.
Our data suggest that LGL leukemia can serve as a useful model of dysregulated apoptosis causing human malignancy and autoimmune disease. Our results showing that leukemic LGL express high levels of CD95 yet are resistant to CD95-mediated apoptosis are similar to findings observed in CD95L transgenic mice. In this animal model, expression of CD95L leads to disease manifestations. High levels of soluble CD95L have been found in sera from LGL leukemia patients.44 Growth of hematopoietic colonies in vitro is negatively regulated by activation of the CD95 pathway.45Mature neutrophils undergo apoptotic death through CD95 triggering.46 Taken together, these data suggest that secretion of CD95 ligand may be a mechanism leading to neutropenia in LGL leukemia. Studies investigating this hypothesis are ongoing in our laboratory.
Supported by the Veterans Administration. T.L. is a recipient of “Association pour la Recherche Contre le Cancer” and “Pharmacia” grants. The Flow Cytometry and Molecular Biology Core laboratories at H. Lee Moffitt Cancer Center and Research Institute were used in the course of this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Thomas P. Loughran, Jr, MD, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: Loughrat@moffitt.usf.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal