Abstract
Thrombotic events are life-threatening complications of human hemolytic anemias such as paroxysmal nocturnal hemoglobinuria, sickle cell disease, and thalassemia. It is not clear whether these events are solely influenced by aberrant hematopoietic cells or also involve aberrant nonhematopoietic cells. Spherocytosis mutant (Spna1sph/Spna1sph; for simplicity referred to as sph/sph) mice develop a severe hemolytic anemia postnatally due to deficiencies in -spectrin in erythroid and other as yet incompletely defined nonerythroid tissues. Thrombotic lesions occur in all adult sph/sph mice, thus providing a hematopoietically stressed model in which to assess putative causes of thrombus formation. To determine whether hematopoietic cells fromsph/sph mice are sufficient to initiate thrombi, bone marrow from sph/sph or +/+ mice was transplanted into mice with no hemolytic anemia. One set of recipients was lethally irradiated; the other set was genetically stem cell deficient. All mice implanted withsph/sph marrow, but not +/+ marrow, developed severe anemia and histopathology typical of sph/sph mice. Histological analyses of marrow recipients showed that thrombi were present in the recipients of sph/sph marrow, but not +/+ marrow. The results indicate that the -spectrin–deficient hematopoietic cells of sph/sph mice are the primary causative agents of the thrombotic events.
THROMBOSIS IS clinically relevant in humans with hematopoietic diseases, most notably paroxysmal nocturnal hemoglobinuria (PNH), sickle cell disease (SCD), and thalassemia.1-5 In patients with hereditary spherocytosis (HS), there is one report of thrombosis.6 However, because expression of cytoskeletal genes is not restricted to erythroid cells, it is not clear whether the thrombotic events are primarily due to aberrant cells of the hematopoietic lineage or if there are also contributions from nonhematopoietic tissues.
Deficiencies of α- and β-spectrin, ankyrin, and band 3, essential structural components of the red blood cell (RBC) cytoskeleton,7 are responsible for most cases of HS in humans.8,9 In the absence of any of these cytoskeletal components, a severe hemolytic anemia can arise due to increased osmotic fragility of the erythrocytes.10,11 The severity of disease in humans is quite variable, ranging from relatively mild to very severe clinical manifestations.8,9 Recently, hydrops fetalis has also been associated with HS due to α- or β-spectrin defects.12 13
Known HS syndromes in laboratory mice (Mus) can be attributed to autosomal recessive mutations at three separate loci. Mice with normoblastosis (Ank1nb/Ank1nb; for simplicity, referred to as nb/nb) are ankyrin deficient, jaundiced (Spnb1ja/Spnb1ja; for simplicity referred to as ja/ja) mice are β-spectrin deficient, and spherocytic (Spna1sph/Spna1sph; for simplicity referred to as sph/sph) mice are α-spectrin deficient.14-16 HS mice lacking RBC band 3 have been generated by disruption of the gene through homologous recombination.17,18 All mice with defective cytoskeletons develop a severe hemolytic anemia within hours of birth.19The spherocytic erythrocytes are extremely short-lived (1 day v48 days in normal mice), and reticulocytes comprise 75% to 95% of the peripheral hemoglobinized cells.14 Pathophysiologic effects of the hemolytic anemia include cardiomegaly, hepatomegaly, splenomegaly, deposition of iron in the liver and kidney, and premature death.20
HS disease manifestations in nonerythroid tissues may not be entirely due to secondary effects of the hemolytic anemia. Expression of erythroid cytoskeletal genes is not limited to RBCs. It is, in fact, not yet clear how widely distributed transcripts of any of these cytoskeletal components may be. Isoforms of both α- and β-spectrin have been reported in brain21-24 and eye.24 In addition, β-spectrin has been detected in skeletal and heart muscle,22,23 as well as in kidney.25 Ankyrin and band 3 have tissue distributions similar to β-spectrin.26-28 Deficiency of ankyrin in the cerebellum of nb/nb mice, and not the hemolytic anemia, causes latent Purkinje cell degeneration concomitant with the development of a neurologic phenotype.28
Recently, we detected thrombotic lesions in adult mice with α-spectrin, β-spectrin, and ankyrin deficiencies but not in any of their normal littermates.20 All adult sph/sphmice had thrombotic and embolic lesions in the heart and brain. By contrast, only 15% of ja/ja mice had heart lesions and 38% had brain lesions.20 Twenty percent of nb/nb mice had both heart and brain lesions (J.E. Barker, unpublished data). A high incidence of thrombosis has also been detected in mice homozygous for one of the band 3 knockouts.29 Mice with HS provide a model for thrombosis in the context of an underlying hemolytic anemia. Because erythroid cytoskeletal genes are expressed in nonhematopoietic tissues, we wished to determine whether thrombogenesis in sph/sph mice involved only aberrant hematopoietic cells. In the present report, we show thatsph/sph hematopoietic cells are sufficient to induce thrombus formation when transferred to hematopoietically ablated mice that have normal cytoskeletal gene expression in nonhematopoietically derived cell types.
MATERIALS AND METHODS
Mice used for bone marrow transplantation (BMT).
Normal and mutant mice were maintained on both the WB/Re (WB) and C57BL/6J (B6) backgrounds. F1 hybrid (WBB6F1) mutant mice were generated by mating WB-sph/+ × B6-sph/+ mice. Normal WBB6F1 mice were generated by mating WB-+/+ × B6-+/+ mice. Donors for BMT experiments were WBB6F1-sph/sph (henceforth referred to as sph/sph) and WBB6F1-+/+ (henceforth referred to as +/+) females. These mice were homozygous for the B isoform of the ubiquitously expressed enzyme glucose phosphate isomerase 1 (GPI1;Gpi1b/Gpi1b) and heterozygous for β-globin haplotype (Hbbd/Hbbs). Recipients were irradiated male and female WB(B6.CAST-Gpi1a)F1-+/+ (henceforth referred to as +/+a) and genetically stem cell-deficient WBB6F1-W/Wv (henceforth referred to asW/Wv) mice. The +/+a recipients were obtained by mating wild-type WB-Gpi1b/Gpi1b,Hbbd/Hbbd to congenic B6.CAST-Gpi1a/Gpi1a,Hbbs/Hbbs mice and were therefore heterozygous for both GPI1 (Gpi1a/Gpi1b) and β-globin haplotype (Hbbd/Hbbs). Donor and +/+ahost cells were easily differentiated through analyses of GPI1AB versus BB ratios in peripheral blood cells. One hundred percent GPI1BB was indicative of complete conversion to donor cells. TheW/Wv recipient mice had the same genetic markers at the Gpi1 and Hbb loci as the donors. Donor repopulation of the W/Wv hosts was assessed by the cure of the host macrocytic, normochromic anemia after implantation of +/+ cells and by the worsening of the anemia and assumption of typical sph /sph histopathology after implantation of sph/sph cells. Mice were housed and cared for according to AAALAC specifications.
To ensure recipient survival during the initial amplification of donor cells, two modifications were made to our existing protocols.30 First, the irradiation-myeloablated +/+a recipients were injected with donor marrow cells supplemented with peripheral RBCs from B6-+/+,Gpi1b/Gpi1b,Hbbs/Hbbs female mice. Second,W/Wv mice were used as recipients, because they survive long term without transplants but selectively expand donor cells due to their heritable stem cell deficiency.30 The use of W/Wv recipients, because they require no irradiation to provide sites for implantation of donor stem cells, also provided a control for the effects of irradiation on the host microenvironment.
Donor marrow extraction, washing, adjustment of cell concentration, and tail vein injection of the indicated number of cells (Table 1) in a volume of 0.1 mL were performed as described previously.30 Recipient +/+a mice were irradiated with a total dose of 10 Gy (BMTI) or 11.5 Gy (BMTII) by γ-rays generated from a137Cs source at a rate of 0.155 Gy/min. The protocol for BMT experiments I and II is summarized in Table 1.
Protocol for BMT Experiments
| Experiment . | Donor . | Recipient . | No. of Cells Injected* . | RBC Suppl. (+ or −)† . | Irradiation‡ . | Assessment1-153 . |
|---|---|---|---|---|---|---|
| BMTI | ||||||
| +/+ → +/+a | +/+ | +/+a | 3 × 106 | + | 10 Gy | GPI-1 conversion |
| sph/sph → +/+a | sph/sph | +/+a | 3 × 106 | + | 10 Gy | GPI-1 conversion |
| +/+ → W/Wv | +/+ | W/Wv | 3 × 106 | − | − | RBC counts |
| sph/sph → W/Wv | sph/sph | W/Wv | 3 × 106 | − | − | RBC counts |
| BMTII | ||||||
| +/+ → +/+a | +/+ | +/+a | 2.5 × 106 | + | 11.5 Gy | GPI-1 conversion |
| sph/sph → +/+a | sph/sph | +/+a | 2.5 × 106 | + | 11.5 Gy | GPI-1 conversion |
| +/+ → W/Wv | +/+ | W/Wv | 2.5 × 106 | − | − | RBC counts |
| sph/sph → W/Wv | sph/sph | W/Wv | 2.5 × 106 | − | − | RBC counts |
| Experiment . | Donor . | Recipient . | No. of Cells Injected* . | RBC Suppl. (+ or −)† . | Irradiation‡ . | Assessment1-153 . |
|---|---|---|---|---|---|---|
| BMTI | ||||||
| +/+ → +/+a | +/+ | +/+a | 3 × 106 | + | 10 Gy | GPI-1 conversion |
| sph/sph → +/+a | sph/sph | +/+a | 3 × 106 | + | 10 Gy | GPI-1 conversion |
| +/+ → W/Wv | +/+ | W/Wv | 3 × 106 | − | − | RBC counts |
| sph/sph → W/Wv | sph/sph | W/Wv | 3 × 106 | − | − | RBC counts |
| BMTII | ||||||
| +/+ → +/+a | +/+ | +/+a | 2.5 × 106 | + | 11.5 Gy | GPI-1 conversion |
| sph/sph → +/+a | sph/sph | +/+a | 2.5 × 106 | + | 11.5 Gy | GPI-1 conversion |
| +/+ → W/Wv | +/+ | W/Wv | 2.5 × 106 | − | − | RBC counts |
| sph/sph → W/Wv | sph/sph | W/Wv | 2.5 × 106 | − | − | RBC counts |
Number of bone marrow cells injected in a 0.1 mL volume.
Supplementation of bone marrow cells with peripheral RBCs from a B6 donor.
Total irradiation dose per mouse; “−” indicates no irradiation.
Method used to assess implantation of donor marrow.
Hbb and Gpi1 phenotypes.
The β-globin haplotype was determined as previously described.31 The percentages of s (single) and d (diffuse major and minor) hemoglobins were quantified on a Molecular Dynamics Densitometer (Sunnyvale, CA). RBCs retrieved from the packed pellet in a hematocrit tube were assessed for GPI1 phenotype, as described,32 and the concentration of each isoform was quantified as for β-globin. At several time points, the proportions of donor and host GPI1 were determined in enriched populations of RBCs, white blood cells, platelets, and lymphocytes.33 34
Measurements of blood parameters.
Recipient blood was removed from the retroorbital sinus in a 100-μL microhematocrit tube at 2 weeks posttransplantation and thereafter at monthly intervals. RBCs were counted on a Coulter Counter model ZBI (Hialeah, FL).
Histopathology.
Heart, brain, liver, kidney, and spleen were collected from mice perfused transcardially with 1× phosphate-buffered saline (PBS) followed by Bouin’s fixative. Body, spleen, and heart weights were determined postfixation. Tissues were embedded in paraffin, followed by routine staining with hematoxylin and eosin (H&E). Gomori’s stain was used to detect nonhemoglobin iron. Thrombi and emboli in heart and brain sections were distinguished from postmortem blood clots by the presence of fibrin filaments throughout the thrombus. Infarcted areas in both brain and heart sections were scored by the identification of necrotic cells within an area of tissue and by a clear boundary between affected and unaffected areas of tissue.
Statistics.
All statistical analyses were performed using the unpairedt-test or using the unpaired nonparametric t-test where noted, using the Instat program.
RESULTS
GPI1 assays indicate that donor cells repopulated the +/+arecipients.
Confirmation that the sph/sph and +/+ donor marrow cells implanted and subsequently amplified was a prerequisite for determining the effects of hematopoietic cells on thrombus formation. In the +/+a recipients, a genetic marker (Gpi1) was used to detect donor cells. The conversion from the host GPI1AB phenotype to the donor GPI1BB phenotype in the RBCs was monitored over time. Table 2 shows the number of mice in each experimental group that were 100% donor type at 2, 6, and 10 weeks posttransplantation. Repopulation with sph/sph RBCs lagged noticeably behind that of +/+ RBCs in BMTI. We hypothesized that the delay was fostered by insufficient irradiation of the recipients, generating a situation in which the sph/sph bone marrow was at a competitive disadvantage with surviving host cells. To address this possibility, the radiation dose per mouse was increased from 10 to 11.5 Gy in BMTII. Conversion to 100% donor GPI1 was accomplished more rapidly among all recipients when compared with BMTI but was still delayed in sph/sph marrow recipients when compared with +/+ marrow recipients (Table 2). The single recipient in BMTII that was not converted at 10 weeks posttransplantation (Table 2) was 100% GPI1BB by 15 weeks posttransplantation (not shown).
Proof of Bone Marrow Implantation in RBC Population
| Experimental Group . | Recipients (no./total) 100% Implanted* at 2 wk† . | Recipients (no./total) 100% Implanted* at 6 wk† . | Recipients (no./total) 100% Implanted* at 10 wk† . |
|---|---|---|---|
| BMTI | |||
| +/+ → +/+a (5 mice) | 1/5 | 5/5 | 5/5 |
| sph/sph → +/+a (22 mice) | 0/22 | 8/22 | 22/22 |
| BMTII | |||
| +/+ → +/+a (5 mice) | 5/5 | 5/5 | 5/5 |
| sph/sph → +/+a(22 mice) | 0/22 | 19/22 | 21/22 |
| Experimental Group . | Recipients (no./total) 100% Implanted* at 2 wk† . | Recipients (no./total) 100% Implanted* at 6 wk† . | Recipients (no./total) 100% Implanted* at 10 wk† . |
|---|---|---|---|
| BMTI | |||
| +/+ → +/+a (5 mice) | 1/5 | 5/5 | 5/5 |
| sph/sph → +/+a (22 mice) | 0/22 | 8/22 | 22/22 |
| BMTII | |||
| +/+ → +/+a (5 mice) | 5/5 | 5/5 | 5/5 |
| sph/sph → +/+a(22 mice) | 0/22 | 19/22 | 21/22 |
One hundred percent implanted with donor cells, as determined by GPI isotype assay.
Weeks posttransplantation.
All +/+→+/+a mice in both experiments and allsph/sph→+/+a mice in BMTII remained 100% donor type throughout the duration of the experiment. Three of the 22 sph/sph→+/+amice in BMTI showed a resurgence of the host GPI1AB RBCs at 15 weeks posttransplantation. One of these three maintained the host GPI type in the RBC population for the duration of the experiment; the other two returned to donor GPI1BB RBCs by 22 and 30 weeks, respectively. Unexpectedly, the GPI1BB cells in the +/+→+/+a but not in the sph/sph→+/+a mice from BMTII showed 100% conversion to the SS hemoglobin phenotype of the B6 RBCs used to ensure survival of the irradiated recipients (not shown). Regardless of the source of progenitor cells, the control mice in BMTII were completely repopulated with control (+/+) cells.
Peripheral blood cells in the +/+a recipients were enriched for platelets, lymphocytes, and granulocytes at 22 and 48 weeks posttransplantation in BMTI and at 15 and 42 weeks posttransplantation in BMTII. Repopulation of donor cells was not limited to RBCs. The GPI1 haplotype was 100% donor type (BB) in all transplant recipients, including those recipients that at the same time point were not 100% donor type in the RBC population (not shown).
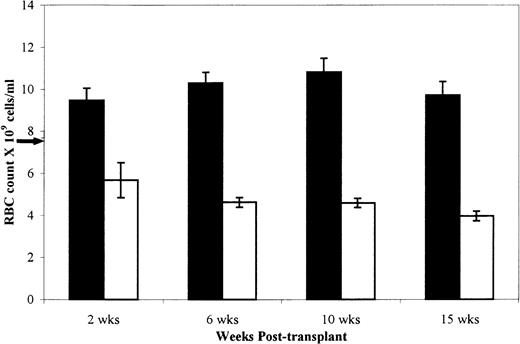
RBC counts show that donor cells implanted in the W/W v recipients.
All W/Wv recipients had a macrocytic, normochromic anemia with RBC counts of 7.47 ± 0.15 (standard error of the mean, SEM) × 109 cells/mL at the inception of the experiments. Alterations in the RBC counts were therefore used as a measure of successful donor cell implantation. Figure 1 depicts the RBC counts ± SEM in the W/Wv recipients at four time points posttransplantation. Results from BMTI and II were pooled, because the differences between values were not statistically significant. Injection of +/+ cells resulted in alleviation of the host anemia and near normal RBC counts (10.30 ± 0.51 × 109) by 6 weeks posttransplantation. Injection of sph/sph cells caused a further decrement in RBC counts to typical sph/sphlevels (3.74 ± 0.18 × 109) by 15 weeks posttransplantation. All of theW/Wv recipients of sph/sphmarrow became moribund by 28.5 weeks posttransplantation. RBC counts in the +/+→+/+a andsph/sph→+/+a mice mimicked the data for the W/Wv recipients and further confirmed the expansion of donor cells (data not shown).
Mean RBC count of W/Wv mice implanted with sph/sph or +/+ bone marrow at 2, 6, 10, and 15 weeks posttransplantation. Results are presented as mean ± SEM. (▪) +/+→W/Wv, n = 10, except n = 8 at 15 weeks. (□)sph/sph→W/Wv, n = 10, except n = 7 at 15 weeks. Arrow on the y axis indicates mean RBC count for unmanipulated W/Wv mice.
Mean RBC count of W/Wv mice implanted with sph/sph or +/+ bone marrow at 2, 6, 10, and 15 weeks posttransplantation. Results are presented as mean ± SEM. (▪) +/+→W/Wv, n = 10, except n = 8 at 15 weeks. (□)sph/sph→W/Wv, n = 10, except n = 7 at 15 weeks. Arrow on the y axis indicates mean RBC count for unmanipulated W/Wv mice.
Recipients injected with sph/sph or +/+ marrow assume the donor morphometry.
Comparisons were made at the time of autopsy to determine whether spleen and heart weights were dependent on the donor genotype. Mice that became moribund (those receiving sph/sph marrow) were immediately anesthetized and perfused. Healthy mice (all recipients of +/+ cells and 4 sph/sph→+/+a mice from BMTI) were anesthetized and perfused at 50 weeks (BMTI) and 46 weeks (BMTII). Values were not obtained from 4sph/sph→+/+a mice from each experiment that died unexpectedly and were unsuitable for autopsy when discovered.
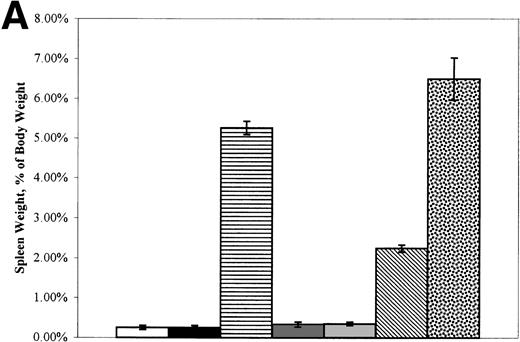
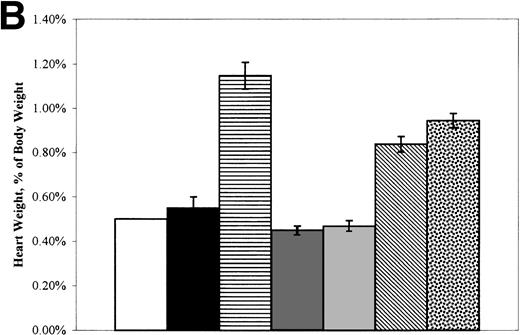
Figure 2A depicts spleen weight and Fig 2B depicts heart weight, both expressed as the percentage of total body weight. Because the data were similar for the experimental groups in BMTI and II regardless of the date of autopsy, results were pooled. Neither the spleen:body weight nor the heart:body weight ratios of +/+→+/+a or +/+→W/Wv mice were significantly different from those of the +/+ or W/Wv control mice. The spleen:body weight and heart:body weight ratios ofsph/sph→+/+a andsph/sph→W/Wv mice were significantly higher than those of +/+ or W/Wvcontrols (P ≤ .0005 for spleen and P ≤ .0028 for heart). The morphometry of the sph/sph marrow recipients was similar to that typically observed in unmanipulated sph/sphmice. There seemed to be a modulating effect of the +/+a, but not the W/Wv, host background on the donorsph/sph marrow-induced increase in spleen weight. This was not true of the increase in heart weight. We conclude that the host mice acquired morphometric changes typical of the marrow donors, further confirming donor cell repopulation.
(A) Mean (±SEM) spleen weight (expressed as the percentage of body weight) of control, W/Wv, sph/sph, and BMT recipients at the time of death. (□) +/+ control, n = 2. (▪) W/Wv control, n = 2. (▤) sph/sph control, n = 17. (▩) +/+→+/+a, n = 9. (░) +/+→W/Wv, n = 10. (▧)sph/sph→+/+a, n = 32. ()sph/sph→W/Wv, n = 10. (B) Mean (±SEM) heart weight (expressed as the percentage of body weight) of control, W/Wv, sph/sph, and BMT recipients at the time of death. Bars are as defined for (A); numbers in each group are as in (A), except forsph/sph→+/+a, in which n = 28.
(A) Mean (±SEM) spleen weight (expressed as the percentage of body weight) of control, W/Wv, sph/sph, and BMT recipients at the time of death. (□) +/+ control, n = 2. (▪) W/Wv control, n = 2. (▤) sph/sph control, n = 17. (▩) +/+→+/+a, n = 9. (░) +/+→W/Wv, n = 10. (▧)sph/sph→+/+a, n = 32. ()sph/sph→W/Wv, n = 10. (B) Mean (±SEM) heart weight (expressed as the percentage of body weight) of control, W/Wv, sph/sph, and BMT recipients at the time of death. Bars are as defined for (A); numbers in each group are as in (A), except forsph/sph→+/+a, in which n = 28.
Recipients of sph/sph but not of +/+ cells develop pathology typical of hemolytic anemia.
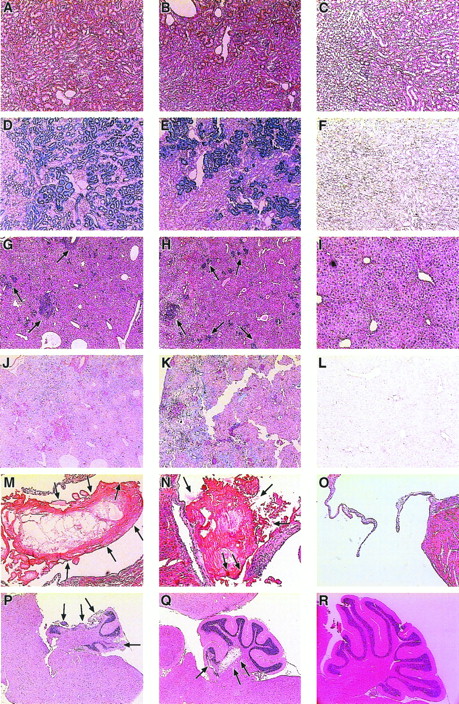
Histological observations ofsph/sph→+/+a mice showed cellular casts and debris in the proximal convoluted tubules of the kidneys, as well as glomerular nephritis and proliferation (Fig 3B). Both the casts and the renal tubular epithelium were laden with hemosiderotic iron (Fig 3E). These observations were similar to those made in sph/sph control mice (Fig 3A and D) and insph/sph→W/Wv mice (not shown). The +/+→+/+a (Fig 3C and F) and +/+→W/Wv (not shown) mice had apparently normal kidneys with no iron deposition.
Histological sections of kidney, liver, heart, and brain from sph/sph,sph/sph→+/+a, and +/+→+/+a mice. (A, D, G, J, M, and P) WBB6F1-sph/sph. (B, E, H, K, N, and Q)sph/sph→+/+a. (C, F, I, L, O, and R) +/+→+/+a. (A through C) Kidney sections stained with H&E. (D through F) Identical kidney sections as in (A) through (C) stained with Gomori’s iron stain, which stains nonhemoglobin iron blue (D and E). (G through I) Liver sections stained with H&E. Clusters of extramedullary hematopoiesis (arrows) stain purple (G and H). (J through L) Identical regions of liver as shown in (G) through (I) stained with Gomori’s iron stain. (M through O) Heart sections stained with H&E showing the large atrioventricular valve. Note the presence of thrombi (indicated by arrows) in the valves of (M) and (N), whereas the valve of (O) is unobstructed. (P through R) Sagittal brain sections stained with H&E showing the cerebellar region of the brain. Note the degeneration (arrows) of portions of the cerebellum in both (P), in which about one third of the cerebellum is missing due to infarction, and (Q), in which an interior portion of the cerebellum is infarcted. Note also the lack of degeneration in (R).
Histological sections of kidney, liver, heart, and brain from sph/sph,sph/sph→+/+a, and +/+→+/+a mice. (A, D, G, J, M, and P) WBB6F1-sph/sph. (B, E, H, K, N, and Q)sph/sph→+/+a. (C, F, I, L, O, and R) +/+→+/+a. (A through C) Kidney sections stained with H&E. (D through F) Identical kidney sections as in (A) through (C) stained with Gomori’s iron stain, which stains nonhemoglobin iron blue (D and E). (G through I) Liver sections stained with H&E. Clusters of extramedullary hematopoiesis (arrows) stain purple (G and H). (J through L) Identical regions of liver as shown in (G) through (I) stained with Gomori’s iron stain. (M through O) Heart sections stained with H&E showing the large atrioventricular valve. Note the presence of thrombi (indicated by arrows) in the valves of (M) and (N), whereas the valve of (O) is unobstructed. (P through R) Sagittal brain sections stained with H&E showing the cerebellar region of the brain. Note the degeneration (arrows) of portions of the cerebellum in both (P), in which about one third of the cerebellum is missing due to infarction, and (Q), in which an interior portion of the cerebellum is infarcted. Note also the lack of degeneration in (R).
Livers in the sph/sph controls (Fig 3G), thesph/sph→+/+a mice (Fig 3H), and thesph/sph→W/Wv mice (not shown) had sites of extramedullary hematopoiesis. Iron was present in the hepatocytes of the liver, but not in the regions of hematopoiesis (Fig3J and K). Recipients of +/+ marrow had histologically normal livers (Fig 3I and L). Spleen histology (not shown) fromsph/sph→+/+a andsph/sph→W/Wv mice showed an expanded red pulp but no evidence of iron deposition, consistent with observations previously made for sph/sph mice.20No expansion of red pulp was noted in the spleens of +/+→+/+a and +/+→W/Wvmice. The histological observations made in recipients ofsph/sph bone marrow are consistent with the pathology of a severe hemolytic anemia.
Thrombotic and embolic lesions are present in recipients ofsph/sph cells.
Histological examination of heart sections showed the presence of thrombotic lesions in the atria and/or atrioventricular valves of recipients of sph/sph marrow (Fig 3N). These thrombi were similar in appearance, although smaller than the thrombi seen in 100% of adult sph/sph mice (Fig 3M). In contrast to the kidney, liver, and spleen pathology noted in all recipients ofsph/sph marrow, thrombotic lesions were not detected in the heart of every sph/sph→+/+a orsph/sph→W/Wv mouse (Table 3). Thrombotic lesions were not detected in the heart of any of the recipients of +/+ marrow (Table 3and Fig 3O) or in unmanipulated +/+a andW/Wv controls (not shown).
Prevalence of Thrombotic or Embolic Lesions
| Experimental Group . | Heart . | Brain . |
|---|---|---|
| BMTI | ||
| +/+ → +/+a (5 mice) | 0% | 0% |
| sph/sph → +/+a (18 mice) | 72% | 44% |
| +/+ → W/Wv (5 mice) | 0% | 0% |
| sph/sph → W/Wv (5 mice) | 80% | 20% |
| BMTII | ||
| +/+ → +/+a (4 mice) | 0% | 0% |
| sph/sph → +/+a(18 mice) | 89% | 17% |
| +/+ → W/Wv (5 mice) | 0% | 0% |
| sph/sph → W/Wv (5 mice) | 60% | 0% |
| Totals | ||
| +/+ marrow recipients | 0% | 0% |
| sph/sph marrow recipients | 78% | 26% |
| Experimental Group . | Heart . | Brain . |
|---|---|---|
| BMTI | ||
| +/+ → +/+a (5 mice) | 0% | 0% |
| sph/sph → +/+a (18 mice) | 72% | 44% |
| +/+ → W/Wv (5 mice) | 0% | 0% |
| sph/sph → W/Wv (5 mice) | 80% | 20% |
| BMTII | ||
| +/+ → +/+a (4 mice) | 0% | 0% |
| sph/sph → +/+a(18 mice) | 89% | 17% |
| +/+ → W/Wv (5 mice) | 0% | 0% |
| sph/sph → W/Wv (5 mice) | 60% | 0% |
| Totals | ||
| +/+ marrow recipients | 0% | 0% |
| sph/sph marrow recipients | 78% | 26% |
Prevalence as defined in Materials and Methods.
In the cerebellum of sph/sph (Fig 3P),sph/sph→+/+a (Fig 3Q), andsph/sph→W/Wv (not shown) mice, areas of infarction were noted. Figure 3P and 3Q show clearance of necrotic tissue, leaving an area devoid of any cellular structure; this is typical of an insult that occurred some time previously. Infarcts, although more frequent in the cerebellum, were also seen in the hippocampus and surrounding brain tissue. Microthrombi in blood vessels were occasionally detected near the areas of infarction (not shown). Given the extent of cerebellar damage in some of these mice, it was surprising that only two mice showed any visible signs of neuromotor defects. Neither infarctions nor thrombi were present in brains from +/+→+/+a (Fig 3R), +/+→W/Wv, or +/+a andW/Wv control mice (not shown). The overall incidence of brain infarctions in thesph/sph→+/+a andsph/sph→W/Wv mice is shown in Table 3. No recipient of sph/sph marrow showed evidence of brain infarctions without also having thrombotic lesions in the heart (data not shown), suggesting that the brain lesions are due to emboli emanating from the heart thrombi.
DISCUSSION
The recent discovery of thrombotic lesions in all sph/sphmice20 provides an opportunity to assess the genesis of thrombus formation in a genetically homogeneous animal model that can be examined sequentially over time. In the current report, our hypothesis is that the thrombotic events are triggered by hematopoietic cells, not nonhematopoietic cells, that are deficient in α-spectrin. To test this theory, marrow transfers between affected and normal animals were performed. The results (Table 3) indicate that the thrombi are due to α-spectrin defects in cells derived from sph/sphbone marrow. The thrombi are not due to host irradiation or to the transplantation, because neither the +/+→irradiated +/+a nor the +/+→nonirradiatedW/Wv mice are affected.
A high proportion (78%) of the sph/sph bone marrow recipients in these experiments developed cardiac thrombi. It is possible, given their overall smaller size, that thrombi in some mice were either missed through selective examination of heart sections or dislodged from the heart by the perfusion process. The lack of brain lesions without concomitant heart thrombi in sph/sph marrow recipients is an exciting finding not previously reported forsph/sph mice. This suggests that the brain pathology in these mice is directly related to the presence of thrombi in the heart. The smaller cardiac thrombi seen in the mice in our experiments were probably less subject to the shear forces present in the heart and therefore less likely to produce emboli that would travel to the brain. The incidence of brain lesions (38%) is higher than the incidence of heart thrombi (15%) in β-spectrin–deficient (ja/ja) mice,20 suggesting that only some of the brain lesions result from emboli produced from heart thrombi. Alternatively, it is possible that, in some ja/ja mice, the entire heart thrombus has dislodged and traveled to the brain. Cardiac thrombosis has also been documented in a mouse homozygous for a knockout of the band 3 gene29 but has not been investigated in an independently generated band 3 knockout mouse.18 Thrombosis is a major factor contributing to morbidity and mortality in patients with SCD, PNH, and thalassemia.1-5 The use of magnetic resonance imaging technology has indicated that the incidence of silent thrombotic events in patients with SCD is much higher than originally thought.35,36 Thrombosis has also been reported in one patient with HS.6 The low incidence of thrombosis in human HS may reflect the fact that many patients with severe HS are splenectomized,9 removing a major source of the hemolysis that may trigger thrombotic events. Splenectomy is immediately lethal in mice with severe HS, probably because the spleen serves as a major site of murine erythropoiesis.
A possible explanation for the thrombotic lesions is that the donor hematopoietic cells create a toxic environment required for clot formation. All recipients of sph/sph cells have extensive pathological changes in the liver, spleen, and kidneys due to transfer of the hemolytic anemia. Such alterations may contribute to thrombotic events by increasing the concentration of certain serum proteins that are indicative of thrombotic risk in humans with SCD.37-40This fails to completely explain the thrombotic events in our mice, because recipients of sph/sph cells that develop thrombi in the heart do not show more extensive tissue pathology than those that do not have heart thrombi. In addition, our previous work has shown that the severity of hemolytic anemia does not correlate with the prevalence of thrombotic lesions in mice with HS.20Nevertheless, the possible contribution of hemolysis-initiated toxicity to thrombogenesis in sph/sph mice cannot at this point be excluded.
It is not possible to limit causation of thrombi to thesph/sph erythroid lineage, because, at least in the irradiated hosts injected with genetically marked donor cells, all circulating hematopoietic cells are donor type. Donor cells other than the erythroid population may also be deficient in α-spectrin. Alternatively, donor hematopoietic cells that do not express α-spectrin may interact with defective cells to stimulate thrombogenesis. Recent studies have suggested that thrombogenesis in SCD may be related to increased erythrocyte phosphatidylserine exposure and platelet-RBC adhesion.41,42 Investigation of phosphatidylserine exposure on RBCs from mice with HS and studies of RBC adhesion in sph/sph mice are currently under way. Platelets have a spectrin-based cytoskeleton, but it is not clear, due to the nature of the antibodies used, whether it is erythroid spectrin or nonerythroid fodrin.43 Phagocytic monocytes are not known to express α-spectrin, but are known to induce local fibrin deposition during oxygen deprivation,44 a likely side effect of the hypochromic, macrocytic anemia in the HS mice. The predisposition to thrombotic lesions in sph/sph mice provides a new model in which to assess factors that may be involved in the thrombogenic process. It is clear from our current experiments thatsph/sph hematopoietic cells are sufficient to induce thrombosis. Repopulation with sph/sph-derived lineages of selected hematopoietic lineages in host (+/+) mice will allow further definition of the sph/sph hematopoietic lineages involved in thrombogenesis in these mice. In addition, the continuing delineation of erythroid cytoskeletal gene expression in nonerythroid hematopoietic tissues in both humans and mice will also aid in the elucidation of the thrombotic process in hemolytic anemia.
ACKNOWLEDGMENT
The authors greatly appreciate the technical assistance of Susan Deveau and Nancy Hamblen. We thank Drs David Harrison and David Serreze for critical review of the manuscript. We thank the Biological Imaging and Graphics Services at the Jackson Laboratory for photographic assistance (partially funded by National Institutes of Health [NIH] Core Grant No. CA34196).
Supported by National Institutes of Health (NIH) NRSA F32 DK09482 (N.J.W.), NIH F32 DK09054 (T.M.K.), and NIH Grant No. R01 HL29305 (J.E.B.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Nancy J. Wandersee, PhD, The Jackson Laboratory, 600 Main St, Bar Harbor, ME 04609; e-mail:njw@aretha.jax.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal