To the Editor:
Several laboratories have proposed that glycoprotein (GP) Ib, the receptor for von Willebrand factor (vWF) on platelet external surfaces, is rapidly redistributed to internal membranes during cardiopulmonary bypass surgery, induction of bleeding time wounds, or exposure to thrombin in vitro (for review, see Nurden1). The recent article by van Zanten et al2 confirms the observation that GPIb decreases on the surfaces of activated platelets, but shows that a 50% reduction in the vWF receptor does not affect platelet adhesion under flow conditions. The findings contrast with those of earlier investigators who presumed that clearance of GPIb resulted in decreased adhesive capacity,3,4 but agree with reports showing that carriers of the Bernard-Soulier syndrome (BSS) with half the normal number of GPIb copies on their platelets do not bleed.5,6Yet, following the logic of previous investigations, BSS carrier platelets should also lose 60% to 80% of the half normal number of GPIb receptors present on their resting cells after activation in vitro or in vivo.1 Thus, the assumption that the data obtained in this study explain why carriers of BSS syndrome do not bleed seems open to question. A more inviting rationale regarding the lack of bleeding symptoms in carriers of BSS might be that the half normal number of GPIb receptors remain on exposed membranes of their activated platelets for interaction with damaged vascular surfaces.
Results of the ultrastructural studies performed by van Zanten et al2 are pertinent to that concern. Normal platelets treated with thrombin receptor activation peptide (TRAP) in suspension showed a marked reduction in immunogold particles on external membranes and increased frequency of particles associated with membranes of the open canalicular system (OCS) when compared with resting control cells. However, when TRAP-treated platelets were allowed to interact with collagen under conditions of high shear, the frequency of immunogold particles bound to GPIb on external and internal membrane surfaces was the same as that on resting control platelets. The investigators suggested that GPIb receptors cleared to the OCS after exposure to TRAP in suspension were rapidly returned to the exterior membrane during spreading on collagen.
An alternative explanation for the results obtained by van Zanten et al2 deserves consideration. There appears to be considerably more internal membrane in the TRAP-treated platelet shown in Fig 4B of their report than in the control cell shown in Fig 4A. The number of OCS channels does not increase after platelet activation in suspension, but due to shape change and internal transformation, OCS channels become dilated and indistinguishable from the surface membranes with which they are continuous.7
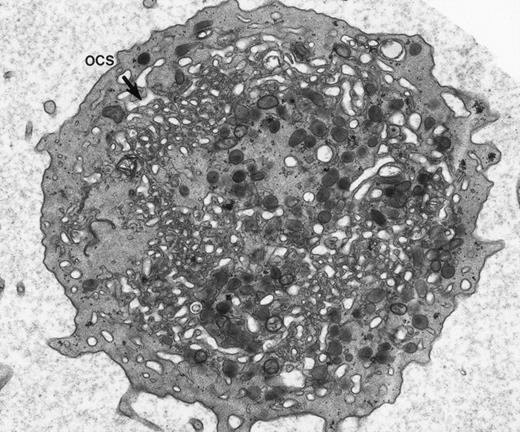
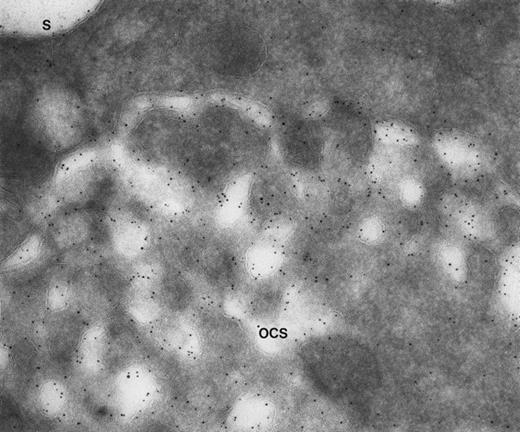
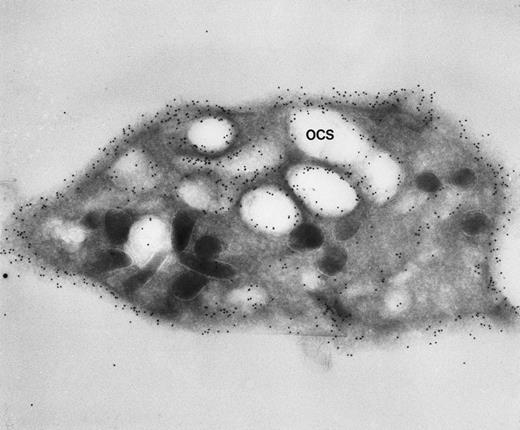
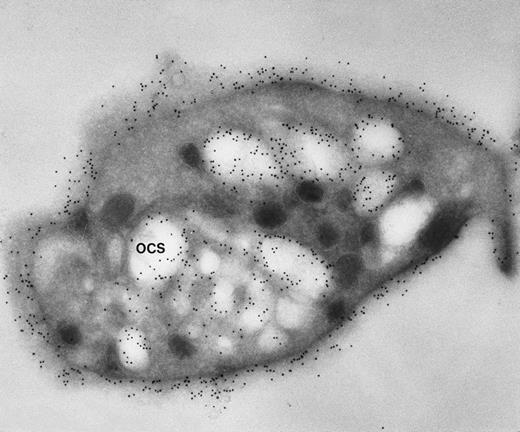
Our experience indicates that it is always easier to label the receptors of dilated channels than those on membranes of narrow canaliculi in well-preserved, discoid platelets.8 Others are beginning to make similar observations.1 As an example, we have noted the same distribution of GPIb on external and internal OCS membranes of platelets from patients with giant platelet disorders, other than the BSS, whose OCS channels are usually dilated (Figs 1 and2). Another confirmation is based on studies with cytochalasin B (CB), an agent that inhibits new actin filament assembly and is reputed to prevent translocation of GPIb. CB causes dilation of the OCS of resting cells. Immunogold staining for GPIb is as prominent on membranes of the OCS in CB-treated discoid platelets as on the exposed surface (Figs 3 and4).
Giant platelet from peripheral blood of a patient with May-Hegglin anomaly. Channels of the surface-connected OCS are usually dilated in these large cells. (Original magnification ×20,000.)
Giant platelet from peripheral blood of a patient with May-Hegglin anomaly. Channels of the surface-connected OCS are usually dilated in these large cells. (Original magnification ×20,000.)
Giant platelet from a patient with Epstein’s syndrome. A frozen thin section from one of the large platelets has been stained by the immunogold technique for GPIb/IX. Gold particles are 5 nm in diameter. Membranes lining OCS channels are stained by as many gold particles identifying GPIb/IX as are present on the exterior surface (S). (Original magnification ×104,000.)
Giant platelet from a patient with Epstein’s syndrome. A frozen thin section from one of the large platelets has been stained by the immunogold technique for GPIb/IX. Gold particles are 5 nm in diameter. Membranes lining OCS channels are stained by as many gold particles identifying GPIb/IX as are present on the exterior surface (S). (Original magnification ×104,000.)
Frozen thin section of a CB-treated normal discoid platelet stained by the immunogold technique for GPIb/IX. The gold particles coupled to anti-IgG are 10 nm in diameter. Dilated OCS channels are as heavily stained by gold particles indicating sites of GPIb/IX as are present on the exposed surface. (Original magnification ×44,000.)
Frozen thin section of a CB-treated normal discoid platelet stained by the immunogold technique for GPIb/IX. The gold particles coupled to anti-IgG are 10 nm in diameter. Dilated OCS channels are as heavily stained by gold particles indicating sites of GPIb/IX as are present on the exposed surface. (Original magnification ×44,000.)
Frozen thin section of another CB-treated resting platelet stained for GPIb/IX. Again, gold particles identifying sites of GPIb/IX are as frequent on membranes lining OCS channels as are on the exposed surface. (Original magnification ×44,000.)
Frozen thin section of another CB-treated resting platelet stained for GPIb/IX. Again, gold particles identifying sites of GPIb/IX are as frequent on membranes lining OCS channels as are on the exposed surface. (Original magnification ×44,000.)
Accepting the possibility that immunocytochemical techniques have limitations in their ability to quantitate antigens on narrow internal channels would lead to a second view of the morphometric data presented in Table 2 of the report by van Zanten et al.2 According to the data, gold labeling for GPIb in resting platelets is distributed 70% versus 25% (external v internal membranes). After TRAP activation, the distribution is modified to 35% versus 58% (externalv internal membranes).
Studies of giant platelets and CB-treated normal platelets described above would lead to the assumption that the frequency of GPIb/IX receptors on internal and external membranes of resting discoid platelets should be the same. Thus, a starting point of 47.5% versus 47.5% (external v internal membranes) would be expected. Current literature suggests that activation with TRAP should cause disappearance of 60% to 80% of GPIb/IX receptors from the platelet surface as a result of translocation to internal membranes.1 If that was the case, immunolabeling on TRAP-activated platelets should bring immunolabeling distributions close to 20% versus 75% (external v internal membranes). This would represent a real internalization of GPIb receptors. Interestingly, the results in Table 2 of van Zanten et al2show that the immunolabeling on the internal membranes of TRAP-activated platelets is still below that on external membranes of resting cells (57.9% v 69.0%; activated v resting). We believe that the proposed increase in the percentage of gold particles identifying GPIb on internal membranes of TRAP-treated platelets may be due to their increased availability for staining in dilated channels rather than to translocation.
Restoration of the percentages of gold particles marking GPIb on internal and external membranes of TRAP-treated platelets after spreading on collagen to values found on resting platelets in suspension is also of interest. During the course of surface interaction, channels of the OCS are evaginated and become part of the exposed surface, accounting for a greater than 400% increase in surface area on spread platelets.9 This should result in a decreased percentage of gold particles on internal membranes, because few OCS channels remain inside. It should also cause a marked increase in the relative percentage of particles on the spread cell surface for the same reason. It is unlikely that fully spread cells would have the same percentages of GPIb on internal and external membranes as found in suspended control cells. Evidence has been presented to support that point of view.10 Perhaps van Zanten et al2could respond to these concerns.
Response
We thank Drs Escolar and White for their interest in our study reporting that a 50% reduction of platelet surface GPIb expression does not affect platelet adhesion. When we activate platelets in vitro, a reduced expression of GPIb on the platelet membrane was found, both using semiquantitative immuno electron microscopy (IEM) as well as FACS analysis. We have much experience in immunogold cytochemistry and we are aware of the pitfalls in quantifying gold particles. Indeed, a dilated open canalicular system (OCS) is better accessible to luminal antibodies than a narrow OCS. However, limited access is generally not a problem for antigens present on the cell surface. Because we found comparable results with both EM and FACS techniques, we are convinced that in in vitro experiments GPIb expression on the surface is reduced. The impact of our article is that reduction of GPIb on the platelet surface does not influence platelet adhesion. It was not our aim to enter the endless discussion of whether GPIb expression on the cell surface is reduced or not.
At present, it is still unclear whether platelet activation in vivo results in diminished surface expression of GPIb. Unpublished studies performed in our laboratory during cardio-pulmonary bypass surgery did not show a reduced GPIb surface expression, which is in line with other studies that showed only a minor reduction. An interesting question is what happens with platelets of carriers of Bernard-Soulier syndrome when their platelets are activated and have lost 50% of the remaining GPIb on the cell surface. Obviously, the carriers do not bleed. There are two explanations for the lack of bleeding: the reduction of GPIb after activation only occurs in vitro and not in vivo or, as we have shown in our report, there is an immediate redistribution of GPIb after adhesion of the platelets, resulting in a restored GPIb expression on the platelet surface.
We agree that, in the case of fully spread platelets, most of the OCS membrane become part of the cell surface. The complete spreading of the platelets to which Drs Escolar and White refer is only achieved after 20 minutes of static adhesion to formvar and is never observed during a 5-minute perfusion over type III collagen, especially not when dRGDW is used to avoid platelet aggregation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal