Abstract
The cDNA for eosinophil granule major basic protein (MBP) encodes a prepromolecule with a total length of 222 amino acids (preproMBP). PreproMBP includes a secretory leader of 15 amino acids, an acidic propiece of 90 amino acids, and a basic MBP portion of 117 amino acids. The function of the propiece, which has a predicted pI of 3.9, is unknown, but it gives proMBP an overall acidic charge. Because proMBP is not found in mature eosinophils, we analyzed eosinophil differentiation in interleukin-5 (IL-5)–stimulated umbilical cord stem cells cultured for 24 days. By immunofluorescence, proMBP appeared by day 6 and peaked on day 18, whereas MBP was prominent at days 12 to 24. By day 6, Western blots detected heterogeneous glycosylated 33-kD proMBP; its peak expression occurred on day 12. Western blots showed sequential processing of 33-kD proMBP to an 18-kD intermediate form and finally to 14-kD MBP. By dual label immunoelectron microscopy, proMBP was localized primarily to large uncondensed eosinophil granules, whereas MBP was localized to granules containing a condensed central area. Thus, proMBP is likely expressed and processed as the granule condenses in a multistep process to 14-kD MBP in differentiating eosinophils.
UNDER THE INFLUENCE of key hematopoietins, eosinophils develop from stem cells in the bone marrow and, from there, are released into the peripheral circulation.1-6 Once in the periphery, they are believed to play a key role in defense against invading helminths and serve as an important mediator cell in hypersensitivity diseases.7-9Eosinophil granules contain four principal cationic proteins8; their avidity for and binding of the acidic dye eosin imparts a characteristic orange-red coloration. Among these cationic proteins is the 14-kD major basic protein (MBP).8Named for its relative abundance and extreme cationicity (pI of 11), MBP forms the electron-dense crystalline core of the specific granules of mature peripheral blood eosinophils.8,10 11
Eosinophil granule MBP is a known toxin,8,9 and application of physiological amounts of MBP to tumor cells,12,13parasites,9 and bacteria14 causes direct toxicity. MBP may mediate effects on targets by its ability to react with acidic lipid bilayers,15 or alternatively, MBP may stimulate cells by binding to a specific receptor on the target. For example, application of physiological amounts of MBP to neutrophils caused both activation and degranulation, and this may involve G proteins as critical mediators.16,17 Application of MBP to platelets causes activation and aggregation,18 whereas application of MBP to basophils causes histamine release.17,19 MBP is deposited directly on affected tissue in allergic diseases, including asthma,7-9 eosinophilic gastroenteritis,20 and conjunctivitis.21Therefore, in areas of inflammation where eosinophil degranulation occurs, MBP most likely exerts an effect that is both toxic and proinflammatory in nature.
Therapeutic intervention aimed at halting inimical effects of MBP might include inhibition of the conversion of the proform of MBP (proMBP) to MBP. However, we have been unable to detect proMBP in mature eosinophils.22 Therefore, to study the processing of proMBP to MBP, we analyzed differentiating eosinophils in vitro by stimulating umbilical cord stem cells (UCC) with interleukin-5 (IL-5).23-25 By using a combination of antibodies to proMBP and MBP,22,26-28 we determined the kinetics of proMBP and MBP expression in developing eosinophils and localized the site of proMBP to MBP conversion. Our results show that conversion of proMBP to MBP occurs in the granule and lend support to the hypothesis that the function of the propiece of proMBP is to protect maturing eosinophils from toxic MBP during granule processing.12,22 28-30
MATERIALS AND METHODS
Preparation and evaluation of UCC cultures.
Cord blood cells were collected in heparin and layered over Histopaque 1077 (Sigma Chemical Co, St Louis, MO). After centrifugation at 400g for 30 minutes, mononuclear fractions were harvested. The cells were washed in phosphate-buffered saline (PBS) and incubated at 1 × 106 cells/mL in RPMI 1640 with 10% fetal calf serum (FCS; HyClone, Logan, UT) with or without recombinant human IL-5 (a generous gift from Schering-Plough Research Institute, Kenilworth, NJ) at 0 to 10 ng/mL for 0 to 24 days. Every 6 days, half of the media was harvested and replaced. At this time, 1 to 2 mL of cells were harvested, and cell counts and viabilities were determined. Cytospins of cell suspensions were prepared by centrifugation at 400g for 5 minutes onto clean glass slides that had been prespun for 2 minutes with RPMI containing 10% FCS. The slides were allowed to air dry and were stained with Wright-Giemsa. Poststaining, the slides were briefly destained in 95% ethanol for approximately 30 seconds, examined under oil with a ×100 objective on a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany), and photographed with Kodak Ektachrome 64 slide film (Eastman Kodak, Rochester, NY).
Specificity of antibodies to proMBP and MBP.
One antibody to proMBP and two antibodies to MBP were used. First, a murine monoclonal antibody, J163-15E10, was used for detection of proMBP in the immunofluorescence, Western blot, and immunoelectron microscopy analyses. J163-15E10 reacts with proMBP, but not MBP, by Western blot and immunoassay.26-28 Second, a murine monoclonal antibody for MBP, J6-8A4, was used for the detection of MBP in the Western blot analyses. J6-8A4 reacts intensely with 14 kD MBP and faintly with proMBP by Western blotting.22,26-28 Third, a polyclonal rabbit antibody, rabbit #14, was used for detection of MBP in the immunofluorescence and electron microscopy analyses. The specificity of rabbit #14 anti-MBP for 14 kD MBP was analyzed by radioimmunoassay (RIA) and by Western blotting. A competitive binding RIA was performed as described previously31 with modifications. Briefly, native proMBP and reduced and alkylated MBP standards (final concentrations of 0 to 128 ng/mL) were incubated with a 1:5000 dilution of rabbit #14 anti-MBP. 125I-radiolabeled reduced and alkylated MBP (1 ng/sample) was subsequently added, and the resulting immune complexes were precipitated by addition of normal rabbit serum and burro antirabbit IgG. After the samples were centrifuged, supernatants were discarded and sediments were counted. For Western blotting, proMBP and purified MBP (1.25 μg/lane) were analyzed as described below (Western blot analyses) by using a 1:200 dilution of rabbit #14 anti-MBP.
Immunofluorescence.
Cytospins obtained as described were fixed in 100% methanol for 10 minutes and air dried. The immunofluorescence assays for MBP and proMBP were performed as described previously7 with modifications. The slides were blocked overnight at 4°C in PBS containing 10% normal goat serum. After blocking, the slides were incubated with the primary antibody, either mouse monoclonal antihuman proMBP (J163-15E10),28 normal mouse Ig (control for J163-15E10), polyclonal rabbit #14 antihuman MBP, or normal rabbit serum (control for rabbit #14). Both the antibody to proMBP and the normal mouse Ig were purified by protein-A affinity chromatography (Pierce Chemical Co, Rockford, IL) according to the manufacturer's specifications and used at 20 μg/mL, and the antibody to MBP and the normal rabbit serum were used at a 1:40 dilution. After incubation in the primary antibody for 1 hour at 37°C in a humidified atmosphere, the slides were washed in PBS and incubated with a solution of 1% chromotrope 2R (Sigma) in distilled water for 1 hour. After the chromotrope 2R treatment, the slides were incubated with the secondary antibody, either goat antimouse Ig or goat antirabbit Ig conjugated to fluorescein isothiocyanate (Cappel, Durham, NC), also diluted 1:40, for 1 hour at 37°C. After incubation, slides were washed in PBS, mounted in glycerol containing 0.1% p-phenylenediamine, and coverslipped. Slides were examined and photographed as described above with Kodak Ektachrome 200 slide film. Replicates of a minimum of two cultures, and in most cases three or more cultures, were scored for the number of cells positive (minimum of 100 cells counted) at each time point and dose.
Western blot analysis.
Cells (1.0 × 106) induced with IL-5, 5 ng/mL, from each time point were harvested, pelleted, and resuspended in 100 μL 1 × sodium dodecyl sulfate (SDS) sample buffer followed by denaturation at 100°C for 3 to 5 minutes. Twenty-five microliters of each denatured sample, corresponding to 250,000 cells, was loaded onto a 15% Laemmli gel and electrophoresed. Postelectrophoresis, gels were transblotted onto Millipore Immobilon-P membranes (Millipore, Bedford, MA). Posttransfer, blots were blocked in Blotto/0.3% Tween25 for 1 hour at room temperature and incubated overnight with a 1:1,000 dilution of protein-A purified primary antibody against either proMBP (J163-15E10) or MBP (J6-8A4).22,26-28 Blots were then washed with Blotto/0.3% Tween followed by incubation for a minimum of 2 hours at room temperature with a 1:1,000 dilution of sheep antimouse Ig conjugated to alkaline phosphatase (Sigma). After washing, development was as described.28
Amino acid sequence analyses.
UCC lysates suspended in 1 × SDS sample buffer were electrophoresed on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to ProBlot (Applied Biosystems, Foster City, CA). The blots were stained and destained according to the manufacturer's specifications and bands for analysis were excised. N-terminal amino acid sequencing was performed on a Porton 2090E Integrated Microsequencing System (Porton Instruments Inc, Tarzana, CA).
Immunoelectron microscopy.
UCC induced with 5 ng/mL IL-5 for 24 days were harvested and fixed overnight in Trump's fixative.32 After fixation, cells were pelleted, rinsed in 0.1 mol/L phosphate buffer, dehydrated in graded acetone, embedded in Quetol 651, and polymerized at 42°C. Thin (0.12 μm) sections for labeling were mounted on nickel grids and dried at 40 to 50°C. Sections were etched in NaIO4,33 rinsed with water, and blocked in Tris buffered saline containing 0.1% Tween 20 and 1% bovine serum albumin (TBST). As primary antibodies, protein A purified antibodies to proMBP (J163-15E10) and MBP (rabbit #14) were diluted in TBST and applied to sections for 4 hours at 22°C at the following working dilutions: mouse anti-proMBP or normal mouse IgG at 20 μg/mL and rabbit anti-MBP or normal rabbit IgG at 17 μg/mL. After incubation in the primary antibodies, sections were rinsed in TBST and incubated in a secondary antibody (either goat antimouse or goat antirabbit IgG) conjugated to 15 nm colloidal gold (Amersham, Arlington Heights, IL). Sections were thoroughly rinsed in TBST, followed by water, and stained with uranyl acetate and lead citrate.
For double labeling, the sections were labeled first with anti-proMBP and then with anti-MBP. Between the two labeling procedures, the sections were fixed in Trump's so that the first primary antibody could no longer react with the protein A gold Fc binding receptors.34 Additionally, after rinsing in TBST, sections were treated with TBST/1% bovine serum albumin/0.2 mol/L glycine to block free aldehydes, which may have been generated by the fixation procedure. The proMBP label was localized with a 15-nm gold conjugate, and the MBP with a 30-nm gold conjugate. After labeling, sections were rinsed thoroughly with TBST and water, stained with uranyl acetate and lead citrate, and examined by transmission electron microscopy. Micrographs were taken on a JEOL 1200 EXII (Japanese Electronic Optical Laboratories, Peabody, MA) operating at 60 KV.
RESULTS
Morphologic evaluation of IL-5 stimulated UCC.
UCC were placed in complete medium for 24 days with and without IL-5. Morphological observation of cells from these cultures stained with Wright-Giemsa confirmed that cells characteristic of eosinophil lineage developed only in the presence of IL-5 (results not shown). Eosinophils at all phases of maturation were present, including eosinophil myelocytes, metamyelocytes, and even mature bilobed forms. In contrast, only partially degenerated mononuclear cells usually persisted in cultures maintained without IL-5.
Specificity of antibodies to proMBP and MBP.
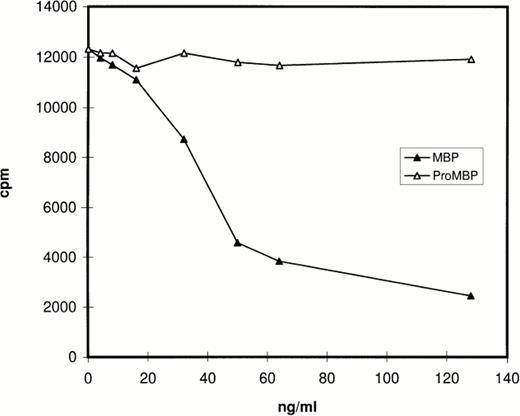
Prior studies by Western blotting had shown that the monoclonal antibody for proMBP (J163-15E10) reacts specifically with proMBP, but not MBP, and that the monoclonal antibody for MBP (J6-8A4) reacts intensely with MBP and weakly with proMBP.22 26-28 The specificity of rabbit #14 was analyzed by RIA and by Western blotting. The RIA results (Fig 1) showed that MBP, but not proMBP, inhibits the binding of rabbit #14 antibody to radiolabeled MBP, showing that rabbit #14 antibody is specific for MBP under nondenaturing conditions. The Western blotting experiment (data not shown) showed that rabbit #14 anti-MBP has approximately equal reactivity with both proMBP and MBP under denaturing conditions.
Analysis of the specificity of rabbit #14 anti-MBP by RIA. Native proMBP and reduced and alkylated MBP standards (0 to 128 ng/mL) were incubated with a 1:5000 dilution of rabbit #14 anti-MBP, followed by addition of 125I-radiolabeled MBP. Immune complexes were precipitated with burro antirabbit IgG and normal rabbit serum, and radioactivity in the precipitates was counted.
Analysis of the specificity of rabbit #14 anti-MBP by RIA. Native proMBP and reduced and alkylated MBP standards (0 to 128 ng/mL) were incubated with a 1:5000 dilution of rabbit #14 anti-MBP, followed by addition of 125I-radiolabeled MBP. Immune complexes were precipitated with burro antirabbit IgG and normal rabbit serum, and radioactivity in the precipitates was counted.
Immunofluorescence.
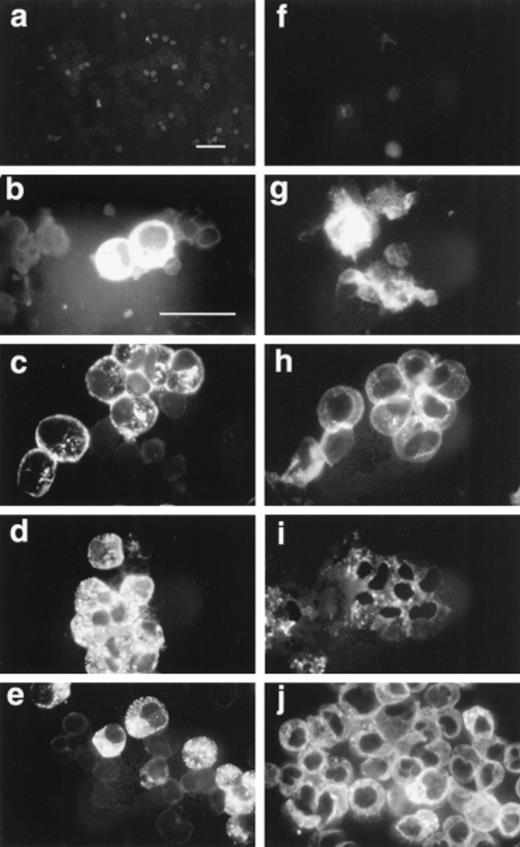
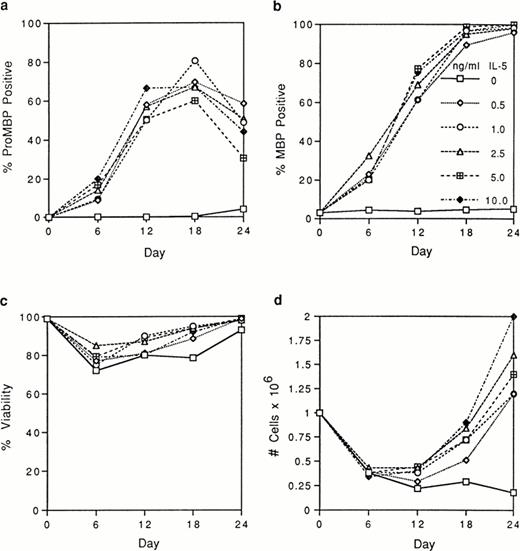
We tested by immunofluorescence the ability of varying IL-5 concentrations to induce proMBP production during eosinophil maturation. Representative photomicrographs of UCC cultured in the presence of 5 ng/mL IL-5 and stained with anti-proMBP (Fig 2a-e) or anti-MBP (Fig 2f-j) on culture days 0, 6, 12, 18, and 24 are shown. At each time point and dose, the absolute cell number and overall viability were also calculated (Fig 3). At every IL-5 concentration tested (0.5 to 10 ng/mL), both proMBP and MBP appeared by day 6 (Figs 2b, 2g, 3a, and 3b). In the presence of IL-5, the percentage of cells positive for proMBP increased until day 18, but by day 24 declined to approximately half of that seen at the day-18 maximum (Figs 2a-e and 3a). In contrast, the percentages of cells positive for MBP rose to nearly 100% by day 18 and remained at 100% throughout the remaining culture period (Figs 2f-j and 3b). When control UCC were cultured without IL-5, proMBP and MBP were not detected (Fig 3a and 3b) and absolute cell numbers fell by days 18 and 24 (Fig 3d). Even in the presence of IL-5, an initial decrease in absolute cell number occurred on days 0 to 12 (Fig 3d). This was followed by an absolute increase in the number of cells on days 12 to 24 with a final approximate doubling time of 6 days. Interestingly, overall cell viability did not change appreciably even in the absence of IL-5 (Fig 3c).
Immunofluorescent localization of proMBP and MBP in IL-5 induced UCC. UCC were stimulated for 0 to 24 days with 5 ng/mL IL-5. Panels (a to e) show examples of proMBP immunofluorescence on days 0, 6, 12, 18, and 24, respectively. Panels (f to j) show examples of MBP immunofluorescence on days 0, 6, 12, 18, and 24, respectively. Bar (a and f) = 10 μm; bar (b to e) and (g to j) = 10 μm.
Immunofluorescent localization of proMBP and MBP in IL-5 induced UCC. UCC were stimulated for 0 to 24 days with 5 ng/mL IL-5. Panels (a to e) show examples of proMBP immunofluorescence on days 0, 6, 12, 18, and 24, respectively. Panels (f to j) show examples of MBP immunofluorescence on days 0, 6, 12, 18, and 24, respectively. Bar (a and f) = 10 μm; bar (b to e) and (g to j) = 10 μm.
Analyses of UCC stimulated for 0 to 24 days with 0 to 10 ng/mL of IL-5. Cells from different donors at days 0, 6, 12, 18, and 24 were analyzed, and the mean for each separate point was determined. Each point represents the mean from at least two separate donors. (a) The percentage of immunofluorescent cells positive for proMBP determined by staining with anti-proMBP J163-15E10. (b) The percentage of cells positive for MBP determined by staining with rabbit #14 anti-MBP. (c) The percentage of viable cells determined by trypan blue uptake. (d) The absolute number of cells ×106/mL.
Analyses of UCC stimulated for 0 to 24 days with 0 to 10 ng/mL of IL-5. Cells from different donors at days 0, 6, 12, 18, and 24 were analyzed, and the mean for each separate point was determined. Each point represents the mean from at least two separate donors. (a) The percentage of immunofluorescent cells positive for proMBP determined by staining with anti-proMBP J163-15E10. (b) The percentage of cells positive for MBP determined by staining with rabbit #14 anti-MBP. (c) The percentage of viable cells determined by trypan blue uptake. (d) The absolute number of cells ×106/mL.
Western blot analysis.
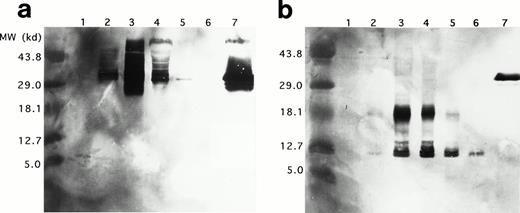
Western blots of duplicate gels containing equivalent UCC lysates from days 0, 6, 12, 18, and 24 and a lysate of purified mature eosinophils were analyzed with two murine monoclonal antibodies, J163-15E10 (specific for proMBP)22,26-28 and J6-8A4 (strongly reactive with 14 kD MBP and weakly reactive with proMBP).22 27Analyses with anti-proMBP (J163-15E10) showed that proMBP reactivity is maximal on day 12 (Fig 4a) and that UCC-derived proMBP is highly heterogeneous, with a spectrum of molecular weights from approximately 29 kD to greater than 43.8 kD. However, one predominant form with a band at an estimated 33 kD was consistently present. No proMBP was present in the purified mature eosinophil lysate. In contrast, analyses with anti-MBP (J6-8A4) showed that MBP reactivity was prominent at days 12, 18, and 24 (Fig 4b). Both an intermediate form of proMBP migrating at approximately 18 kD and mature MBP migrating at 14 kD were detected. Similar to the 33-kD unprocessed proMBP, the level of intermediate 18-kD proMBP peaked on day 12 and decreased until day 24. To verify these observations, the N-terminal sequences of UCC-derived 18-kD intermediate proMBP and the 14-kD mature MBP were obtained. Figure 5shows the amino acid sequences of UCC-derived preproMBP, the 18-kD intermediate, and 14-kD MBP.
Western blotting of proMBP and MBP in IL-5–induced UCC and peripheral blood eosinophils. (a) ProMBP with a major band at 33 kD detected with an anti-proMBP specific monoclonal antibody, J163-15E10. (b) MBP and a processed form of proMBP at 18 kD detected with the anti-MBP specific monoclonal antibody, J6-8A4. Lanes 1 to 5 were loaded with equivalent cell lysates from UCC at days 0, 6, 12, 18, and 24, respectively. Lane 6 was loaded with an equivalent cell lysate from purified peripheral eosinophils, and lane 7 contained 5 μg recombinant proMBP.28
Western blotting of proMBP and MBP in IL-5–induced UCC and peripheral blood eosinophils. (a) ProMBP with a major band at 33 kD detected with an anti-proMBP specific monoclonal antibody, J163-15E10. (b) MBP and a processed form of proMBP at 18 kD detected with the anti-MBP specific monoclonal antibody, J6-8A4. Lanes 1 to 5 were loaded with equivalent cell lysates from UCC at days 0, 6, 12, 18, and 24, respectively. Lane 6 was loaded with an equivalent cell lysate from purified peripheral eosinophils, and lane 7 contained 5 μg recombinant proMBP.28
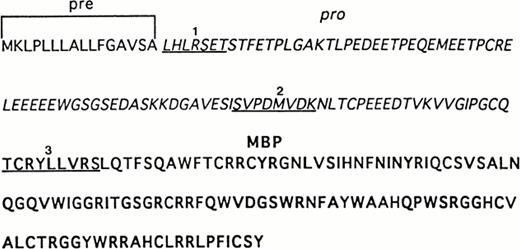
Sequence analysis of recombinant and UCC–derived proMBP. The complete amino acid sequence of preproMBP28-30 is shown. N-terminal sequences were obtained as described in Materials and Methods. Underlined sequences correspond to (1) the N-terminus of purified 33-kD recombinant proMBP, (2) the N-terminus of the corresponding 18-kD intermediate form of proMBP in day-12–induced UCC, and (3) the N-terminus of 14-kD MBP in day-24–induced UCC. Amino acid residues corresponding to the leader sequence are in plain font, the propiece residues are in italics, and mature MBP residues are in bold.
Sequence analysis of recombinant and UCC–derived proMBP. The complete amino acid sequence of preproMBP28-30 is shown. N-terminal sequences were obtained as described in Materials and Methods. Underlined sequences correspond to (1) the N-terminus of purified 33-kD recombinant proMBP, (2) the N-terminus of the corresponding 18-kD intermediate form of proMBP in day-12–induced UCC, and (3) the N-terminus of 14-kD MBP in day-24–induced UCC. Amino acid residues corresponding to the leader sequence are in plain font, the propiece residues are in italics, and mature MBP residues are in bold.
Immunoelectron microscopy.
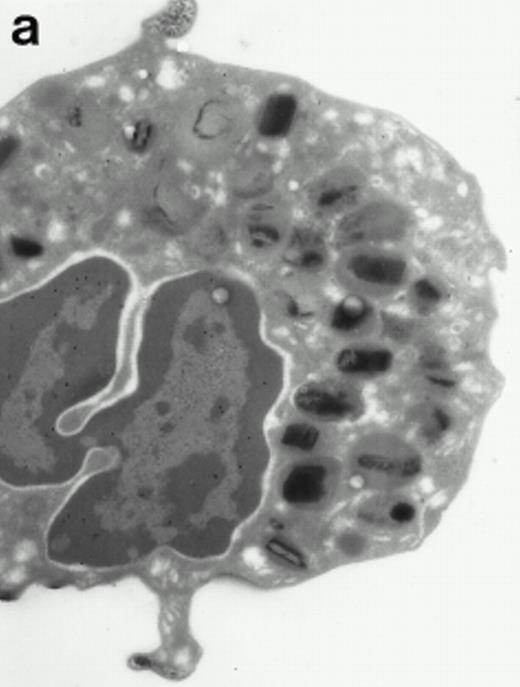
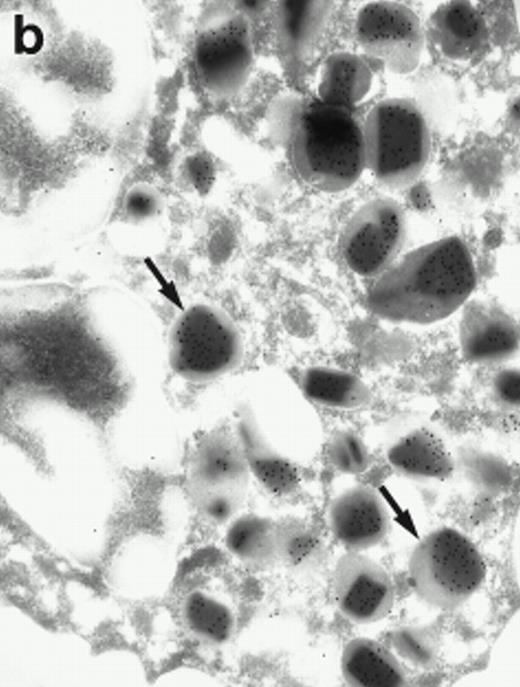
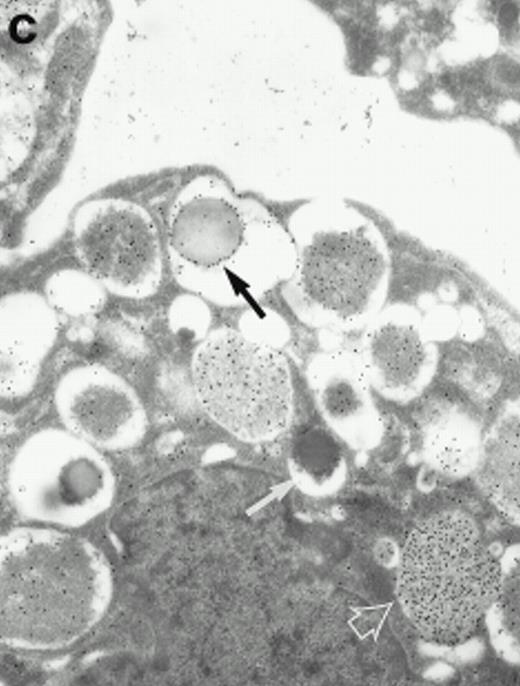
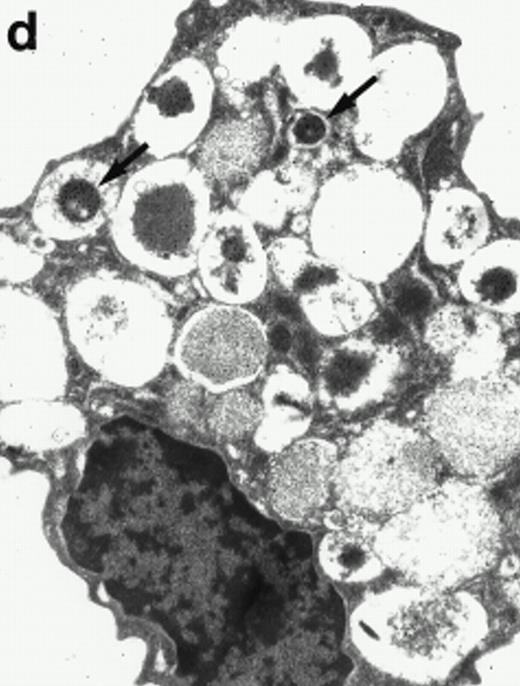
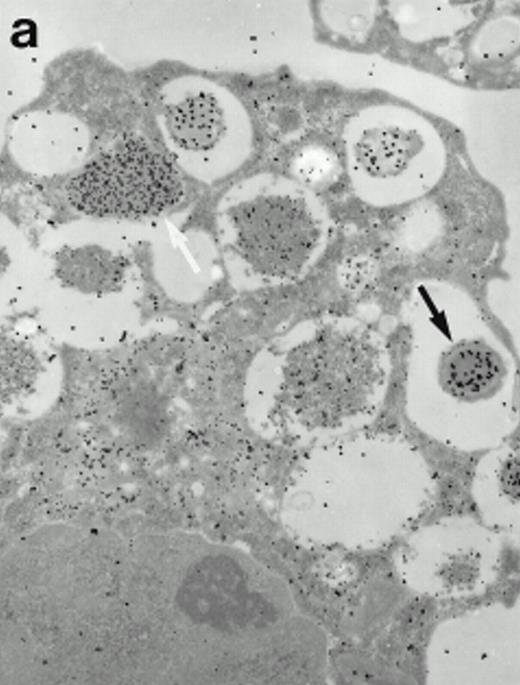
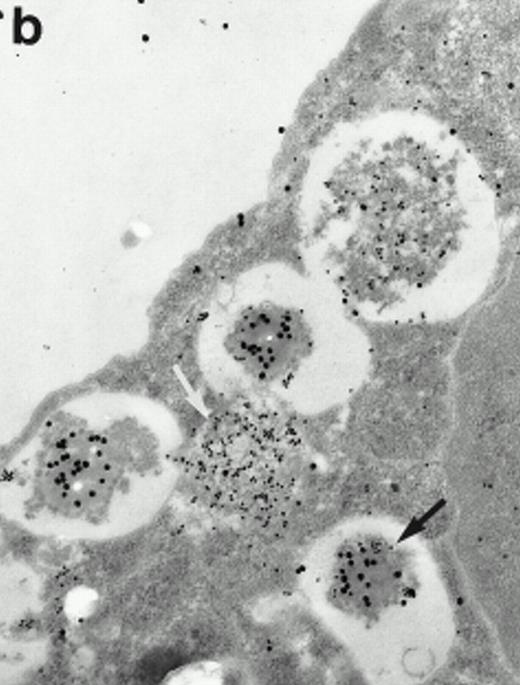
Morphologic analyses of both mature eosinophils and day-24 IL-5–induced UCC by immunoelectron microscopy were performed with proMBP-specific monoclonal antibody (J163-15E10) and with polyclonal antibody to MBP (rabbit #14). No proMBP staining was observed in peripheral blood eosinophils (Fig 6a); in contrast, MBP was localized specifically to the granule crystalline core (Fig 6b). Day-24 IL-5–induced UCC showed marked proMBP labeling of large uncondensed granules and minimal labeling of small condensed granules (Fig 6c); remarkably, proMBP was often seen as a ring around what appeared to be a granule in the process of condensing (black arrow). MBP labeling was confined primarily to condensing granules (Fig6d). Figure 7 shows results from double immunogold labeling of both proMBP (small gold) and MBP (large gold). ProMBP was concentrated in large uncondensed granules, and MBP was concentrated in condensed granules. Some granules (black arrow in Fig7b) contained labeling for MBP (primarily in the center) and proMBP (at the periphery). Controls using either normal mouse immunoglobulin or normal rabbit immunoglobulin as the primary label were negative (results not shown).
Localization of proMBP and MBP by immunoelectron microscopy. (a) A peripheral blood eosinophil labeled with anti-proMBP J163-15E10. Note the absence of specific labeling. (b) A peripheral blood eosinophil labeled with rabbit anti-MBP. Note that MBP is specifically localized to the granule crystalline core (black arrows). (c) Day-24 IL-5–induced UCC labeled with anti-proMBP. The black arrow shows localization of proMBP predominantly to the periphery of a condensing granule; very little proMBP is present in the interior of that granule. The white arrow points to a condensed granule, which lacks proMBP label. Also, note the presence of a large uncondensed granule densely labeled with proMBP proximal to the nucleus (open white arrowhead). (d) Day-24 IL-5–induced UCC labeled with anti-MBP. MBP is localized primarily to the interior of condensing granules (black arrows). (a, c, and d) Original magnification ×11,000; (b) ×15,000.
Localization of proMBP and MBP by immunoelectron microscopy. (a) A peripheral blood eosinophil labeled with anti-proMBP J163-15E10. Note the absence of specific labeling. (b) A peripheral blood eosinophil labeled with rabbit anti-MBP. Note that MBP is specifically localized to the granule crystalline core (black arrows). (c) Day-24 IL-5–induced UCC labeled with anti-proMBP. The black arrow shows localization of proMBP predominantly to the periphery of a condensing granule; very little proMBP is present in the interior of that granule. The white arrow points to a condensed granule, which lacks proMBP label. Also, note the presence of a large uncondensed granule densely labeled with proMBP proximal to the nucleus (open white arrowhead). (d) Day-24 IL-5–induced UCC labeled with anti-MBP. MBP is localized primarily to the interior of condensing granules (black arrows). (a, c, and d) Original magnification ×11,000; (b) ×15,000.
Localization of proMBP and MBP in day-24 IL-5–induced UCC by immunoelectron microscopy using double labeling. ProMBP was localized with 15-nm colloidal gold particles and MBP with 30-nm gold particles. (a) Although proMBP labeling (small gold) is seen throughout the Golgi, it is concentrated predominantly in the large uncondensed granules (white arrow); only minimal MBP labeling (large gold) is evident in the large granules. In contrast, the small condensed granules show marked MBP labeling in the interior and minimal proMBP labeling at the periphery (black arrow). (b) At higher magnification, dense labeling for proMBP (small gold) and minimal labeling for MBP (large gold) is evident in a large uncondensed granule (white arrow). The black arrow identifies a small condensed granule that contains predominantly MBP, along with minimal proMBP at the periphery. (a) Original magnification ×11,000; (b) ×20,000.
Localization of proMBP and MBP in day-24 IL-5–induced UCC by immunoelectron microscopy using double labeling. ProMBP was localized with 15-nm colloidal gold particles and MBP with 30-nm gold particles. (a) Although proMBP labeling (small gold) is seen throughout the Golgi, it is concentrated predominantly in the large uncondensed granules (white arrow); only minimal MBP labeling (large gold) is evident in the large granules. In contrast, the small condensed granules show marked MBP labeling in the interior and minimal proMBP labeling at the periphery (black arrow). (b) At higher magnification, dense labeling for proMBP (small gold) and minimal labeling for MBP (large gold) is evident in a large uncondensed granule (white arrow). The black arrow identifies a small condensed granule that contains predominantly MBP, along with minimal proMBP at the periphery. (a) Original magnification ×11,000; (b) ×20,000.
DISCUSSION
Before this report, the existence of proMBP in developing eosinophils had only been deduced from cDNA analysis.29,30 Here, with the proMBP-specific monoclonal antibody, J163-15E10, we show by immunofluorescence, Western blot analyses, and immunoelectron microscopy that proMBP is developmentally regulated in eosinophils and that processing to its mature MBP form occurs in condensing eosinophil granules. We found that the addition of recombinant human IL-5 to human UCC is sufficient not only to cause production of proMBP, but also results in the formation of mature MBP as well. By Western blot analyses, we showed that both recombinant Chinese hamster ovary (CHO) cell–derived proMBP and eosinophil-derived UCC proMBP are similarly heterogeneous with molecular masses ranging from approximately 33 to 84 kD.28 This marked heterogeneity is likely accounted for by differential carbohydrate addition. By sequence analysis, the propiece of proMBP contains one potential N-linked (Asn-X-Thr), one potential glycosaminoglycan (Gly-Ser-Gly), and 15 potential O-linked (Ser or Thr) attachment sites. Analyses of placentally derived proMBP, which has a similar molecular weight profile, indicate that the extreme molecular weight heterogeneity is accounted for by differential carbohydrate addition onto the propiece.27 35
By immunoelectron microscopy, proMBP was expressed in promyelocytes, myelocytes, and metamyelocytes of the eosinophilic lineage that were actively forming specific granules. Consistent with the electron microscopic description by Bainton and Farquhar1 of peroxidase, arylsulfatase, and acid phosphatase processing in the specific granules of eosinophil myelocytes, heavy proMBP labeling was seen throughout the Golgi, in secretory vesicles, and in uncondensed granules. Double immunostaining of proMBP and MBP showed that granules in the process of condensing have proMBP around the periphery of the central area and MBP in the central area, consistent with the view that conversion of proMBP to MBP is occurring in the granule.
Detailed examination by immunoelectron microscopy of cultured UCC labeled singly for proMBP or dually for both proMBP and MBP indicates that one or both of these forms are present in every granule. This is in accordance with the model for eosinophil development proposed by Hardin and Spicer36 and Parmley et al37; namely, that primary or azurophilic granules do not exist separately from mature specific granules. We extend their work by proposing that azurophilically or metachromatically staining “primary granules” are either immature granules containing unprocessed proMBP or condensing granules containing both proMBP and MBP.1,36Binding of acidic proMBP by the blue cationic elements of the dye would result in the characteristic azurophilic and metachromatic staining. Prior studies indicated that immature differentiating eosinophils contain granules with large amounts of “sulfated acid muco-substance,”36 which in retrospect may be proMBP. After immature proMBP-containing granules have undergone proteolytic processing and condensation, cationic MBP would become available, and the maturing granules would take up the acidic dye eosin. This, plus the contribution of dye binding by the other three cationic proteins in the granular matrix (eosinophil peroxidase, eosinophil cationic protein, and eosinophil-derived neurotoxin),8 11 would result in the appearance of bright red granules characteristic of mature eosinophils.
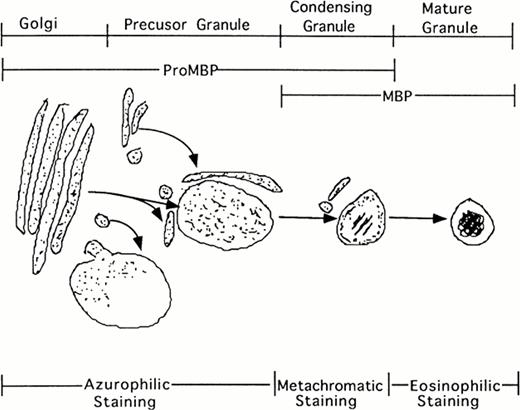
Additionally, our studies describing the localization of proMBP to immature granules parallel the description by Parmley et al37 of chondroitin sulfate localization and traffickingin differentiating eosinophils derived from bone marrow. Initially, staining for chondroitin sulfate was present in the Golgi. In the next phase, staining was present in small transfer vesicles that appeared to deposit their contents into large immature granules. In the following phase of granule maturation, somewhat distal from the Golgi, chondroitin sulfates formed rings around the periphery of what appeared to be granules in the process of condensing. The final phase of granule maturation was characterized by the total disappearance of chondroitin sulfate moieties and the appearance of condensed granules containing crystalline cores. Our studies on proMBP closely parallel their findings. We found that proMBP was initially present in large uncondensed granules. As granule maturation and eosinophil differentiation proceeded, proMBP ringed the periphery of condensing granules. During the final phase of granule condensation, peripheral reactivity for proMBP was lost and reactivity for mature MBP was gained (summarized in Fig 8). What remains enigmatic in our system, but is characteristic of eosinophils processed in cell culture2 is the observation that MBP fails to form crystalline cores in induced UCC.
Schematic representation of eosinophil granule maturation with proposed staining patterns. ProMBP is deposited into large vacuoles just proximal to the Golgi. From here, proMBP containing vacuoles appear to move toward the periphery where granule condensation begins to occur. As the granule condenses, proMBP can be seen around the periphery, and MBP can be seen in the interior. During the final stage of granule maturation, proMBP reactivity is lost, and only MBP reactivity remains.
Schematic representation of eosinophil granule maturation with proposed staining patterns. ProMBP is deposited into large vacuoles just proximal to the Golgi. From here, proMBP containing vacuoles appear to move toward the periphery where granule condensation begins to occur. As the granule condenses, proMBP can be seen around the periphery, and MBP can be seen in the interior. During the final stage of granule maturation, proMBP reactivity is lost, and only MBP reactivity remains.
The signal or trigger that mediates the cleavage of proMBP to MBP is unknown. It may be that a certain proMBP concentration has to be reached or that the converting enzyme must translocate to the granule before processing can occur. Palade38 has suggested that secretory granule condensation occurs as a function of sulfated polyanion aggregation and that sulfated polyanions bind to cationic proteins. Binding would result in an osmotic loss of water from the granule, thereby causing granule condensation. We have recently shown by isoelectric focusing and Western blotting that recombinant proMBP derived from bulk CHO supernatants, despite its predicted slightly acid pI of 6.2,29 is in reality quite anionic.22,28The predominant 33-kD form of proMBP possessed a pI of approximately 4.5, and higher molecular mass forms of proMBP (presumably more heavily glycosylated) possessed progressively more anionic isoelectric points.22,28 Also, by Western blot analyses, proMBP in IL-5–induced UCC appears electrophoretically identical to unprocessed recombinant proMBP secreted into CHO supernatants.22,28These observations suggest that eosinophil-derived proMBP, like recombinant proMBP, has excess anionic charge available for binding and aggregation. A test of this concept showed that proMBP was able to neutralize up to a twofold molar excess of 14-kD MBP.28Therefore, as suggested by Palade,38 the process of sulfated polyanionic aggregation with subsequent cationic protein binding may play an important role in eosinophil granule condensation, except that the acidic proportion of proMBP would substitute for a sulfated peptidoglycan.
In summary, to better understand how eosinophils develop and how granule processing of proMBP to MBP occurs, we studied differentiating eosinophils by the induction of UCC with IL-5. IL-5, first described as B-cell growth factor II,39,40 is now known to play a pivotal role in both eosinophil differentiation and activation.2,6,23,41 Our finding that IL-5 is sufficient to drive eosinophil differentiation is in contrast with the report by Warren and Moore,41 which cited the necessary role of additional cytokines in inducing eosinophil differentiation, but is in agreement with reports by Clutterbuck et al2 and Saito et al.23 However, Saito et al describe the decrease of absolute cell numbers and maturation only to myelocytes by week 3 of culture,23 whereas we report that precursor cells are still actively dividing and that maturation to bilobed forms has occurred by day 24. Finally, we propose that IL-5 induction of eosinophil differentiation can be used as a model for the study of protein processing in the pathway for stored secretory granule products.
ACKNOWLEDGMENT
We thank Lester Harris, PhD, from the Abbott Northwestern Cancer Research Laboratory for proofreading of the manuscript and helpful suggestions; Diane Squillace, BS, for technical assistance; Cheryl Adolphson, MS, for editorial assistance; and Linda H. Arneson for secretarial assistance from the Department of Immunology, Mayo Clinic and Foundation.
Received July 15, 1997; accepted March 18, 1998.
Supported by grants from the National Institutes of Health, AI 09728 and AI 07047, and by Mayo Foundation.
Address reprint requests to Gerald J. Gleich, MD, Department of Immunology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal