Abstract
Chemotherapeutic agents exert their antitumor effects by inducing apoptosis. The microculture kinetic (MiCK) assay provides an automated, continuous means of monitoring apoptosis in a cell population. We used the MiCK assay to determine the chemosensitivities of the human promyelocytic HL-60 and lymphoblastic CEM cell lines and leukemia cells freshly isolated from patients with acute nonlymphocytic (ANLL) or acute lymphocytic (ALL) leukemias. Continuous monitoring of apoptosis in the MiCK assay permits determination of the time to the maximum apoptosis (Tm) and its two components which are initiation time (Ti) and development time (Td). Duration of the three timing components of apoptosis varies from hours to days depending on the drug, drug concentration, and type of target cells. In the MiCK assay, the extent of apoptosis is reported in kinetic units of apoptosis. Kinetic units are determined by the slope of the curve created when optical density caused by cell blebbing is plotted as a function of time. Using the leukemia cell lines, we define the relationship between kinetic units determined by the MiCK assay and the percentage of morphologically apoptotic cells in the culture. Flow cytometry analysis of apoptosis in Annexin-V-fluorescein isothiocyanate–labeled preparations of HL-60 and CEM cells was also used to compare with data obtained by the MiCK assay. The feasibility of the MiCK assay of apoptosis as a chemosensitivity test was confirmed by its comparison with a 3H-thymidine incorporation assay. We show that samples from 10 ANLL and ALL patients patients tested for sensitivity to various doses of idarubicin (IDR), daunorubicin (DNR), or mitoxantrone (MTA) gave the same percentages of apoptotic cells when calculated by the MiCK assay as when determined by morphological analysis. The MiCK assay was used for dose-response analyses of the sensitivities to IDR, DNR, and MTA of leukemia cells from 4 other patients (2 ANLL and 2 ALL). The results from both cell lines and patient samples indicate that ANLL cells are more sensitive than ALL cells to all three of these chemotherapeutic agents. However, for individual patients the chemosensitivities varied significantly among the three chemotherapeutic agents. These varying responses to IDR, DNR, and MTA indicate that the MiCK assay results can be of potential use in designing a treatment regimen for a specific patient with acute leukemia. Among several drugs of presumed similar efficacy, the MiCK assay can permit the selection of the specific chemotherapeutic agent that causes the most apoptosis in the patient's leukemic cells.
© 1998 by The American Society of Hematology.
LABORATORY TESTS for chemosensitivity of leukemia cells can be separated into two major groups: (1) long-term assays based on clonal growth of the leukemic stem cells, and (2) short-term assays of the total cell population relying on loss of cell viability.1-6 Technical and theoretical limitations of these methods have been reviewed by several investigators7-10 with the common conclusion that development of new chemosensitivity assays based on alternative methodologies is needed.
Apoptosis is a distinct mode of cell death that occurs under physiological conditions and yet can be induced in normal and malignant cells by low doses of chemical and physical factors. Because chemotherapeutic agents exert their in vivo antitumor activity by triggering apoptosis in susceptible tumor cells,11-14 the in vitro measurement of drug-induced apoptosis should provide a mechanism-based test for the chemosensitivity of the tumor cells isolated from an individual patient. When malignant cells are exposed to a chemotherapeutic agent in vitro, apoptosis in the population can be determined at a specific time by microscopic examination, electrophoretic separation of DNA fragments,15 or flow cytometry.16 However, apoptotic cells exist in vitro for a limited time, after which they promptly disintegrate.17 18Thus, apoptosis in vitro occurs in a relatively narrow temporal window such that an accurate determination of apoptosis in a population of cells requires either frequent determinations or continuous monitoring. The above-mentioned tests for apoptosis require multiple steps and yet they permit only one time point per sample to be examined. These drawbacks make current assays of apoptosis cumbersome and impractical for clinical use.
We have recently described a microculture kinetic (MiCK) assay for determining apoptosis in a population of cells.19 In this method, the cells are cultured in a 96-well microtiter plate and serial measurements of optical density (OD) are made automatically every 5 minutes over a period of hours to days. By plotting OD as a function of time, both the extent of apoptosis and its precise timing can be determined in the cell population. Among the various methods for determining apoptosis, the MiCK assay is unique for three reasons. First, the MiCK assay is based on the appearance of the characteristic membrane distortions termed blebs that precede DNA breakdown, which is most commonly used to quantitate apoptosis.17 19 Second, the MiCK assay uses prolonged monitoring of a cell population by frequent, repeated measurements without the removal, destruction, or fixation of any part or the whole of the cell population being examined. Third, once the cultures are initiated, the MiCK assay is completely automated such that no further procedures, technician time, or instruments are required. These unique qualities of the MiCK assay for apoptosis make it especially useful for determining the in vitro sensitivity of a leukemia cell population to specific chemotherapeutic agents.
In the present study, the MiCK assay is used to determine the in vitro chemosensitivities for (1) the human promyelocytic HL-60 and lymphoblastic CEM leukemia cell lines, and (2) leukemia cells isolated from the blood or bone marrow (BM) of patients with acute nonlymphocytic (ANLL) or lymphocytic (ALL) leukemia. We introduce a unit for measurement of apoptosis in the MiCK assay, the kinetic unit (KU) of apoptosis, which is correlated with the percentage of morphologically apoptotic cells within the leukemic population. We demonstrate that the leukemia cells of the same patient can show differential sensitivities to drugs with similar mechanisms of action, ie, the anthracyclines, daunorubicin and idarubicin, and the synthetic anthracenedione, mitoxantrone. Because these chemotherapeutic agents are used clinically for the treatment of acute leukemias, the information provided by the MiCK assay may help in the clinical decisions about which specific chemotherapeutic agents are most likely to induce remission in an individual patient.
MATERIALS AND METHODS
Cell lines.
Human HL-60 acute promyelocytic leukemia cells (purchased from American Type Culture Collection, Rockville, MD) and lymphoblastic CEM cells (a gift of Dr W.T. Beck, St Jude Children's Research Hospital, Memphis, TN) were maintained in RPMI-1640 medium without phenol red and supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin (complete medium) in completely humidified air with 5% CO2at 37°C. The cultures were diluted every third day to a concentration of 5 × 105 cells/mL. Before use, exponentially growing cells were obtained, washed with prewarmed RPMI-1640 medium, and resuspended at various concentrations in complete medium. Cell counts and viability were determined using a hemocytometer and trypan blue dye exclusion.
Patient leukemia samples.
Venous blood samples or BM aspirates were obtained with informed consent from 8 patients with a diagnosis of primary ANLL, 3 patients with a diagnosis of primary B-lineage ALL, 1 patient with a diagnosis of T-cell ALL, and 2 patients with biphenotypic acute leukemia before treatment. Profiles of the patients are summarized in Table 1. The heparinized samples were diluted twofold with RPMI-1640 medium and the mononuclear cell fractions were collected after centrifugation on a Ficoll-Hypaque gradient (Nicomed Pharma AS, Oslo, Norway). T lymphocytes and monocytes were removed from the mononuclear fractions using magnetic beads conjugated with monoclonal antibodies (MoAbs) to CD2 and CD14, respectively (Dynal, Inc, Lake Success, NY). The resulting leukemia cell populations contained more than 95% blast cells and had viabilities exceeding 90%.
Patient Characteristics
| Patient No. . | Age/Sex . | Diagnosis . | Presentation . | WBC (×109/L) . | Cytogenetics . |
|---|---|---|---|---|---|
| 1 | 20/M | ALL-early B | Relapse | 830 | t(4;11)(q21;q23) |
| t(der4;11)(q21;p13) | |||||
| 2 | 24/M | ALL-T cell | Relapse | 77.8 | Normal |
| 3 | 70/M | AML/RAEBit | Relapse | 21.3 | ND |
| 4 | 19/M | ALL-early B | De novo | 3.0 | Complex, including −8,−13,der(14), t(8;14)(q11;q31) |
| 5 | 48/F | AML (M2) | De novo | 21.9 | Normal |
| 6 | 61/M | MBC/CGL | De novo | 2.4 | t(9;22)(q34;q11) |
| 7 | 15/M | AML/ALL | De novo | 302 | Normal |
| 8 | 17/M | ALL-early B | Relapse | 31.2 | +21 |
| 9 | 12/M | AML/ALL | De novo | 66.6 | del(9) |
| 10 | 31/M | APL (M3) | Relapse | 50.5 | ND |
| 11 | 78/M | AML (NOS) | De novo | 30.1 | Normal |
| 12 | 30/F | AML (M4) | Relapse | 16.7 | +8 |
| 13 | 3/M | AMMoL (M5a) | De novo | 159 | Normal |
| 14 | 46/F | AML (M2) | Relapse | 6.0 | −7 |
| Patient No. . | Age/Sex . | Diagnosis . | Presentation . | WBC (×109/L) . | Cytogenetics . |
|---|---|---|---|---|---|
| 1 | 20/M | ALL-early B | Relapse | 830 | t(4;11)(q21;q23) |
| t(der4;11)(q21;p13) | |||||
| 2 | 24/M | ALL-T cell | Relapse | 77.8 | Normal |
| 3 | 70/M | AML/RAEBit | Relapse | 21.3 | ND |
| 4 | 19/M | ALL-early B | De novo | 3.0 | Complex, including −8,−13,der(14), t(8;14)(q11;q31) |
| 5 | 48/F | AML (M2) | De novo | 21.9 | Normal |
| 6 | 61/M | MBC/CGL | De novo | 2.4 | t(9;22)(q34;q11) |
| 7 | 15/M | AML/ALL | De novo | 302 | Normal |
| 8 | 17/M | ALL-early B | Relapse | 31.2 | +21 |
| 9 | 12/M | AML/ALL | De novo | 66.6 | del(9) |
| 10 | 31/M | APL (M3) | Relapse | 50.5 | ND |
| 11 | 78/M | AML (NOS) | De novo | 30.1 | Normal |
| 12 | 30/F | AML (M4) | Relapse | 16.7 | +8 |
| 13 | 3/M | AMMoL (M5a) | De novo | 159 | Normal |
| 14 | 46/F | AML (M2) | Relapse | 6.0 | −7 |
Abbreviations: AML, acute myelocytic leukemia; RAEBit, refractory anemia with excess blasts in transformation; MBC, myeloid blast crisis; CGL, chronic granulocytic leukemia; AML/ALL, biphenotypic acute leukemia; APL, acute promyelocytic leukemia; AMMoL, acute myelomonocytic leukemia; NOS, not otherwise specified; ND, not done.
MiCK assay for apoptosis.
The MiCK assay for apoptosis was performed as described previously19 with minor modifications. Cells were suspended in complete medium and plated in 240-μL aliquots in 96-well microtiter plates (Corning-Costar, Cambridge, MA). Seeding cell concentrations were determined in preliminary experiments and varied depending on the size of the cells. Appropriate dilutions of daunorubicin (DNR), idarubicin (IDR), or mitoxantrone (MTA) were added to wells in 10-μL aliquots to yield final concentrations of 0.05, 0.1, 0.5, 1, 2.5, 5, 10, and 20 μmol/L. The microtiter plates were incubated at 37°C for 30 minutes in a completely humidified atmosphere of 5% CO2 in air. Next, 50 μL of sterile mineral oil (Sigma, St Louis, MO) was layered on the top of each microculture. The microtiter plate was placed in the incubated chamber of a spectrophotometer (SPECTRAmax 340; Molecular Devices Corp, Sunnyvale, CA), incubated at 37°C, and the OD at 600 nm was read every 5 minutes for a period of 24 to 48 hours. The reader was calibrated to zero absorbance using wells containing only complete medium without cells. The AUTOmix feature of the reader was used to shake the microtiter plate before each OD reading.
Data processing.
During the assay, the OD readings are plotted against time providing a kinetic representation of the drug responses. Apoptosis is graphically indicated by an “apoptotic curve” with a characteristic portion showing a steep increase of the OD of the culture (Fig 1). The slope of this steep increase in OD is called the maximum kinetic rate (Vmax) and can be calculated automatically by the computer software (SOFTmax Pro; Molecular Devices) provided with the microtiter plate reader. We have previously shown that the value of the Vmax of the apoptotic curve is correlated directly with the percentage of morphologically apoptotic cells in the culture.19 This automatic determination of the Vmax rate of the apoptotic curve provides an objective and convenient measure of the extent of apoptosis in cultures. Our observations with a variety of myeloid and lymphoid leukemia cells have shown 3 hours as the minimal time that elapses between the initiation of the steep rising portion of the apoptotic curve and its maximum. Based on these data, the Vmax of the steep rising portion of the apoptotic curve was always determined over a 3-hour period. This determination was made by setting the SOFTmaxPro software to detect Vmax over 36 consecutive OD points which were taken with a periodicity of 5 minutes. With this setting, the slopes of the multiple lines derived from the 36 consecutive OD measurements were calculated over the entire period of the assay and the steepest one was displayed by the software as Vmax.
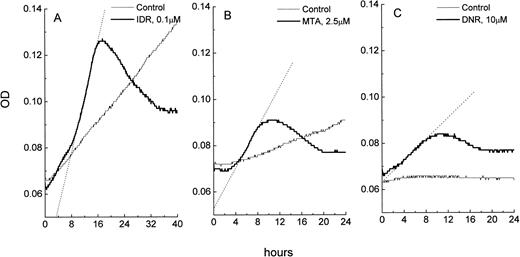
Changes in OD as a function of time in microcultures of leukemic cells: control and drug-treated HL-60 cells (A), freshly isolated ANLL cells (B), and ALL cells (C). The OD data were imported from SOFTmaxPro software to a spreadsheet program (MicroCal Origin) and plotted against time. Exponentially growing control cultures of HL-60 cells display a consistent OD increase over time which is due to an increase in cell number in the microculture. OD versus time curve from control ANLL cells shows a pattern of a slow autonomous cell growth while control ALL cells do not grow, resulting in a flat OD versus time curve. In all three cell types, exposure to IDR, MTA, or DNR causes apoptosis which is indicated by steep increases of the OD in cultures. Extent of apoptosis in HL-60, ANLL, and ALL cells varies and is proportional to the slope of the best fit line (shown as dotted lines) of the apoptotic curves. OD in the blank wells was 0.045 ± 0.001.
Changes in OD as a function of time in microcultures of leukemic cells: control and drug-treated HL-60 cells (A), freshly isolated ANLL cells (B), and ALL cells (C). The OD data were imported from SOFTmaxPro software to a spreadsheet program (MicroCal Origin) and plotted against time. Exponentially growing control cultures of HL-60 cells display a consistent OD increase over time which is due to an increase in cell number in the microculture. OD versus time curve from control ANLL cells shows a pattern of a slow autonomous cell growth while control ALL cells do not grow, resulting in a flat OD versus time curve. In all three cell types, exposure to IDR, MTA, or DNR causes apoptosis which is indicated by steep increases of the OD in cultures. Extent of apoptosis in HL-60, ANLL, and ALL cells varies and is proportional to the slope of the best fit line (shown as dotted lines) of the apoptotic curves. OD in the blank wells was 0.045 ± 0.001.
Quantitation of apoptosis in cell cultures.
During 48 hours of culture, varying proportions of leukemic cells die by spontaneous apoptosis. Also, in proliferating cultures, cell number may increase slightly over the time when the steep OD increase caused by apoptosis is developed (usually this period lasts from 3 to 10 hours). These spontaneous apoptosis-related changes in cell morphology and slight increase in cell number can contribute to the rate of the OD increases of both the drug-treated and control cells. To distinguish these background OD changes from OD changes due to drug-induced apoptosis, the Vmax was determined in the control wells (cells in complete medium with no chemotherapeutic agents added) over the same period of culture when the steep OD increase was observed in the wells containing drugs. This Vmax value for the control wells was subtracted from the Vmax values of the apoptotic curves for each of the drug-treated wells:
Resulting values of VmaxApoptosis were used to quantitate extent of apoptosis in cell cultures.
Cell morphology.
Percentages of cells with morphological evidence of apoptosis were counted on Giemsa-stained cytospin preparation of control and drug-treated cultures. A total of 200 cells was counted on each preparation. Apoptotic cells were identified by plasma membrane protrusions, aggregated chromatin, fragmented nuclei, and condensed basophilic cytoplasm.
Fluorescein-conjugated Annexin V (Annexin V-FITC) binding assay.
Labeling of cells with Annexin-V-FITC conjugate was performed using an Apoptosis Detection Kit (R&D Systems, Minneapolis, MN) in which Annexin V-FITC, propidium iodide (PI), and binding buffer were included as standard reagents.20 Flow cytometry was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) with excitation at 488 nm. FITC fluorescence was measured at 515-545 nm and fluorescence of DNA-PI complexes at 565-606 nm. Cell debris was excluded from analysis by appropriate forward light scatter threshold setting. Compensation was used wherever necessary.
3H-thymidine (3H-TdR) incorporation assay.
The short-term assay by Dosik et al21 was used to study effects of chemotherapeutic agents on proliferating activity of HL-60 and CEM cells. Chemotherapeutic agents were added to cultures in a 96-well microtiter plate that was incubated in a CO2-incubator at 37°C for various periods of time. During the final 2 hours of incubation, 10-μL aliquots of3H-TdR (specific activity of 6.7 Ci/mmol; NEN Life Science Products, Inc, Boston, MA) were added to each well for a final concentration of 2.5 μCi/mL. Cells were collected on a filter paper using an automated cell harvester (Scatron Instruments Inc, Sterling, VA). 3H-TdR incorporation was measured using a liquid scintillation analyzer (Beckman Instruments Inc, Irvine, CA) and results were expressed as counts per minute (cpm) per 103 cells. Data obtained from control and treated microcultures were used to calculate the percentage of3H-thymidine incorporation inhibition.
Statistics and graphics.
Polynomial and linear regression analyses of correlations between KU of apoptosis and percentages of morphologically apoptotic cells in cultures as well as all graphics and other statistics were performed using Origin Scientific Software (MicroCal Software, Inc, Northampton, MA).
RESULTS
Sensitivity limits and optimal cell concentrations.
The MiCK assay monitors OD changes in microcultures caused by the apoptosis-associated modifications in cell morphology. We have shown that membrane blebbing is the main determinant of the steep OD increases in apoptotic cultures.19 In principle, the MiCK assay should be able to monitor apoptosis in a single cell. In practice, however, the sensitivity of the MiCK assay is limited by the technical capabilities of commercially available spectrophotometers which require a certain number of cells undergoing apoptosis to produce a collective signal that can be detected. An initial cell density that ensured both consistent growth of immortalized cell lines and maintenance of slowly dividing or nondividing cells for 48 to 72 hours and yet provided an adequate signal due to apoptosis-related morphological changes in the cells is referred to here as an optimal cell concentration. It follows from our observations that, in the range of the optimal cell concentrations, a minimum of 5% of cells must undergo apoptosis to produce a signal which could be “visible” for the reader. We determined empirically the optimal cell concentrations for some human myeloid and lymphocytic leukemia cell lines (HL-60, U-937, AML-2, AML-3, CEM, Raji) and for freshly isolated human leukemic myeloblasts, lymphoblasts, B-cell chronic lymphocytic leukemia (CLL), and T-cell CLL cells. The optimal initial cell concentrations varied fivefold from 3 × 105cells/mL for HL-60 cells to 1.5 × 106 cells/mL for B-CLL cells and depended on cell size such that larger cells required lower initial cell concentrations than smaller cells. The common criterion used to determine the optimal cell concentration at the initiation of the culture was the difference between the initial OD of the control microcultures and the OD of a blank well containing only complete medium (ODcell − ODblank). The best results of the MiCK assay in terms of its sensitivity and reproducibility were always achieved when this difference was 0.03 ± 0.01 OD. Before a previously untested cell type was studied in the MiCK assay for apoptosis, serial dilutions of the cells were prepared and their ODs compared with the OD of a blank well in single point measurements at 600 nm. Once a cell concentration producing ODcell − ODblank of 0.03 was established, it was used for all experiments. In practice, because of unavoidable inaccuracies of both cell counting and diluting procedures, the value of ODcell − ODblank may deviate somewhat from 0.03. This may affect the maximum rate of the OD change that occurs as a result of apoptosis: a greater starting OD indicates that more cells are present and that a higher Vmax rate can be achieved during apoptosis. Therefore, the MiCK assay results were always normalized against the standard value of the ODcell − ODblank by multiplying the Vmax value of the apoptotic curve by a standard cell density coefficient (SCDC), which is calculated as follows:
Normalizing the results of the MiCK assay to a standard OD at the initiation of the culture enables direct comparisons between apoptotic responses of different types of immortalized cell lines as well as between fresh leukemia cells isolated from different patients.
KU of apoptosis.
The manufacturer's software (SOFTmaxPro; Molecular Devices) is preset to display Vmax for kinetic reactions in milli-optical density units per minute (mOD/min). This often results in extremely small numbers that are cumbersome and inconvenient for analyses. For convenience of presentation, we multiplied the software-determined value of Vmax by 60, thus converting data from mOD/min to mOD/h. Taking into account the Vmax value of the control cultures and normalizing the data with SCDC, the equation for determining the extent of apoptosis in the MiCK assay is:
The units of measurement of the resulting number is mOD/h. To emphasize that the MiCK assay provides real time monitoring of apoptosis, we have designated mOD/h as KU of apoptosis. Thus, hereafter, the results of the MiCK assay for apoptosis will be expressed in KU.
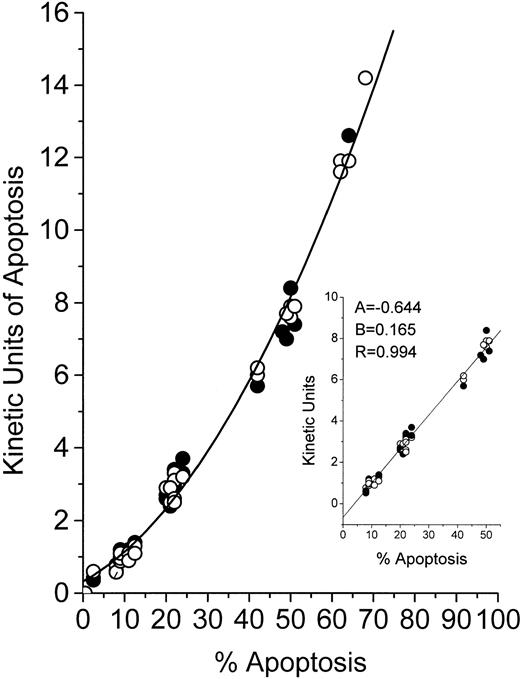
Because the MiCK assay relies on morphological changes in cells undergoing apoptosis, we compared results of the MiCK assay expressed in KU to results of the measurement of apoptosis in percentage of morphologically apoptotic cells. In Fig 2, the results of the MiCK assay expressed in KU are related to percentage of apoptotic cells as calculated in Giemsa-stained preparations of the same cultures. As can be seen, the relationship between KU and the percentage of apoptosis for both HL-60 and CEM cells is described by a polynomial regression (Y = 0.321 + 0.063x + 0.002x2) with a resulting curve that can serve as a nomogram. In cultures containing from 9% to 51% of morphologically apoptotic cells, there is an approximate linearity (correlation coefficient = .994) between value of KU and percent apoptosis (Fig 2, inset). Thus, when the MiCK assay results are in a range from 1.0 to 8 KU, the percentage of apoptosis in the cultures can easily be determined as follows:
whereKU are kinetic units of apoptosis, (−) 0.644 is the y-intercept, and 0.165 is the slope of the correlation curve (Fig 2, inset). Although this equation was obtained with myeloid and lymphoblastic leukemia cell lines, it can also be used when fresh leukemic cells are studied in the MiCK assay. This was shown when cells from 10 patients with various forms of leukemia were cultured with various doses of DNR, IDR, or MTA (Table 2). Apoptosis was determined by the MiCK assay and by counting morphologically apoptotic cells in Giemsa-stained cytospin preparations of the same cultures at times where the apoptotic curves reached their OD maxima. Similar percentages of apoptotic cells were calculated from the MiCK assay results and observed in Giemsa-stained preparations (Table 2).
Relationship between KU of apoptosis and percentage of cells with apoptotic morphology. HL-60 (○) or CEM (•) cells were exposed to the drugs and distributed in two 96-well microtiter plates. One plate was placed into an incubated spectrophotometer and apoptosis was studied in the MiCK assay as described in Materials and Methods. The second plate was incubated in a humidified CO2-incubator. At the time points when the MiCK assay reported maximum apoptosis, cytospin preparations were made from cell aliquots from appropriate wells of the second plate. Apoptosis was measured with the MiCK assay in KU and determined microscopically in Giemsa-stained preparations as described in Materials and Methods. A total of 50 cell cultures exposed to various inducers of apoptosis were studied.
Relationship between KU of apoptosis and percentage of cells with apoptotic morphology. HL-60 (○) or CEM (•) cells were exposed to the drugs and distributed in two 96-well microtiter plates. One plate was placed into an incubated spectrophotometer and apoptosis was studied in the MiCK assay as described in Materials and Methods. The second plate was incubated in a humidified CO2-incubator. At the time points when the MiCK assay reported maximum apoptosis, cytospin preparations were made from cell aliquots from appropriate wells of the second plate. Apoptosis was measured with the MiCK assay in KU and determined microscopically in Giemsa-stained preparations as described in Materials and Methods. A total of 50 cell cultures exposed to various inducers of apoptosis were studied.
Percentages of Apoptotic Cells as Calculated From the MiCK Assay and as Measured by Light Microscopy
| Patient No.* . | Leukemia Cell Type . | Drugs (μmol/L) . | KU of Apoptosis . | % of Apoptotic Cells . | |
|---|---|---|---|---|---|
| Calculated . | Observed . | ||||
| 3 | AML | MTA, 0.1 | 0.5 | 7.1 | 8 |
| 6 | MBC/CGL | DNR, 0.5 | 1.23 | 11.4 | 11 |
| 9 | AML/ALL | IDR, 0.5 | 1.25 | 11.5 | 12 |
| 1 | ALL | DNR, 1.0 | 1.3 | 11.8 | 11 |
| 2 | ALL | IDR, 5.0 | 2.31 | 17.9 | 14 |
| 10 | APL | MTA, 2.5 | 3.2 | 23.2 | 21.5 |
| 11 | AML | IDR, 5.0 | 4.25 | 29.5 | 28 |
| 7 | AML/ALL | IDR, 10.0 | 6.5 | 42.9 | 40.5 |
| 12 | AML | MTA, 5.0 | 7.1 | 46.9 | 45.2 |
| 13 | AMMoL | DNR, 20.0 | 8.2 | 53.6 | 51.9 |
| Patient No.* . | Leukemia Cell Type . | Drugs (μmol/L) . | KU of Apoptosis . | % of Apoptotic Cells . | |
|---|---|---|---|---|---|
| Calculated . | Observed . | ||||
| 3 | AML | MTA, 0.1 | 0.5 | 7.1 | 8 |
| 6 | MBC/CGL | DNR, 0.5 | 1.23 | 11.4 | 11 |
| 9 | AML/ALL | IDR, 0.5 | 1.25 | 11.5 | 12 |
| 1 | ALL | DNR, 1.0 | 1.3 | 11.8 | 11 |
| 2 | ALL | IDR, 5.0 | 2.31 | 17.9 | 14 |
| 10 | APL | MTA, 2.5 | 3.2 | 23.2 | 21.5 |
| 11 | AML | IDR, 5.0 | 4.25 | 29.5 | 28 |
| 7 | AML/ALL | IDR, 10.0 | 6.5 | 42.9 | 40.5 |
| 12 | AML | MTA, 5.0 | 7.1 | 46.9 | 45.2 |
| 13 | AMMoL | DNR, 20.0 | 8.2 | 53.6 | 51.9 |
Cells from 7 ANLL, 2 ALL, and 1 AML/ALL patients were exposed to DNR, IDR, or MTA and distributed in two 96-well microtiter plates. One plate was subjected to the MiCK assay while the other was incubated in a CO2-incubator. Apoptosis was measured in KU and determined in cytospin preparations as described in the legend to Fig2. Calculated percentages of apoptotic cells were determined using the equation: Apoptosis (%) = (KU + 0.644)/0.165 [see text].
Patient numbers as they were assigned in Table 1.
MiCK assay for apoptosis in HL-60 and CEM cells.
HL-60 and CEM cells were exposed to a wide range of concentrations of DNR, IDR, or MTA and studied in the MiCK assay for 48 hours. The maximum concentration of each drug was based on reported peak plasma concentrations in leukemia patients during induction therapy.22-26 The KU obtained for each drug was plotted against drug concentration resulting in dose-response curves (Fig 3A and B). The extent of apoptosis varied significantly depending on the drug, the drug concentration, and the cell line tested. In HL-60 cells, IDR and MTA caused maximum apoptosis at 0.5 μmol/L and 2.5 μmol/L (14.2 and 14.9 KU, respectively; Fig 3A). This extent of apoptosis corresponded to approximately 80% morphologically apoptotic cells (Fig2). Further increases in doses of both drugs were followed by a decrease of apoptosis in the MiCK assay. A different dose-response curve was obtained for HL-60 cells exposed to DNR. Here, two maximum apoptotic responses were seen at 0.1 μmol/L (9.4 KU) and 10 μmol/L (9.5 KU). These may reflect a cell-cycle phase-related heterogeneity in sensitivities of the subsets of the cell population to DNR such that more susceptible cells undergo apoptosis at 0.1 μmol/L DNR while for more resistant cells higher doses of DNR were required to initiate apoptosis.
Effects of DNR, IDR, and MTA on apoptosis in cultures of HL-60 (A) and CEM (B) cells. Control and drug-treated cells were distributed in a 96-well microtiter plate at 0.75 × 105(HL-60) or 1.25 × 105 (CEM) cells per 250 μL complete medium and studied for 48 hours in the MiCK assay. Each point represents the mean (±SD) of three independent experiments.
Effects of DNR, IDR, and MTA on apoptosis in cultures of HL-60 (A) and CEM (B) cells. Control and drug-treated cells were distributed in a 96-well microtiter plate at 0.75 × 105(HL-60) or 1.25 × 105 (CEM) cells per 250 μL complete medium and studied for 48 hours in the MiCK assay. Each point represents the mean (±SD) of three independent experiments.
Compared with HL-60 cells, lymphoblastic CEM cells showed less apoptosis upon exposure to the same doses of the drugs (Fig 3B). At 0.05 μmol/L only DNR but not IDR or MTA induced apoptosis in CEM cells during 48 hours of culture. Maximum apoptotic responses of 7.5, 6.3, and 4.7 KU were caused by 5 μmol/L IDR, 2.5 μmol/L DNR, and 5 μmol/L MTA, respectively. These maxima corresponded to morphological apoptosis ranging from 35% to 47% (Fig 2). Higher doses of IDR and DNR resulted in decreased apoptosis (Fig 3B) whereas increasing of the MTA concentrations from 0.05 to 5 μmol/L was always accompanied by an increase of apoptosis.
Analysis of Giemsa-stained cytospin preparations of both HL-60 and CEM cells showed that along with nonaffected and apoptotic cells, a varying proportion of necrotic cells was present in cultures exposed to all concentrations of the drugs. Comparisons between results of the MiCK assay and those of microscopic studies have shown that decreases in the proportion of apoptotic cells observed after a specific maximum were always accompanied by increases in the proportion of necrotic cells in the cultures. Figure 4 gives an example of these changes in proportion of necrotic cells. Most of HL-60 cells exposed to 0.05 μmol/L IDR (Fig 4A and B) displayed either nonaffected or apoptotic morphology; however, a small proportion of necrotic cells was also seen. At 10 μmol/L IDR (Fig 4C and D), only apoptotic or necrotic cells were observed with the proportion of necrotic cells being increased significantly.
Morphological changes in HL-60 cells at low or high IDR doses. Cells were exposed to 0.05 μmol/L IDR for 24 hours (A and B) or 10 μmol/L IDR for 6 hours (C and D). At these time points, maximum apoptosis was reported by the MiCK assay in respective cultures (see Fig 8). Giemsa-stained cytospin preparations show three distinctive morphological types of cells: (1) Cells displaying morphological features of interphase or mitotic promyelocytes with no visible changes in cell structure; two large cells in (A) and predominant cells in (B); (2) cells in the early stage of apoptosis (ap, in [A] and [C]) with aggregated chromatin abutting the nuclear membrane, and condensed, basophilic cytoplasm; (3) necrotic cells (nec, in [A] and [C]) with faintly stained nuclear “ghosts,” indistinct vacuolated cytoplasm which appears light due to loss of basophilia, and ruptured plasma membranes; predominant cells in (D). Low magnification (B and D) is used to show proportions of apoptotic and necrotic cells in cultures. Scale marker indicates 10 μm for (A) and (C) and 50 μm for (B) and (D).
Morphological changes in HL-60 cells at low or high IDR doses. Cells were exposed to 0.05 μmol/L IDR for 24 hours (A and B) or 10 μmol/L IDR for 6 hours (C and D). At these time points, maximum apoptosis was reported by the MiCK assay in respective cultures (see Fig 8). Giemsa-stained cytospin preparations show three distinctive morphological types of cells: (1) Cells displaying morphological features of interphase or mitotic promyelocytes with no visible changes in cell structure; two large cells in (A) and predominant cells in (B); (2) cells in the early stage of apoptosis (ap, in [A] and [C]) with aggregated chromatin abutting the nuclear membrane, and condensed, basophilic cytoplasm; (3) necrotic cells (nec, in [A] and [C]) with faintly stained nuclear “ghosts,” indistinct vacuolated cytoplasm which appears light due to loss of basophilia, and ruptured plasma membranes; predominant cells in (D). Low magnification (B and D) is used to show proportions of apoptotic and necrotic cells in cultures. Scale marker indicates 10 μm for (A) and (C) and 50 μm for (B) and (D).
Annexin V binding and 3H-TdR incorporation assays.
HL-60 and CEM cells were exposed to the drug concentrations which in the MiCK assay were found to induce maximum apoptosis in the cells (Fig3). The time of the drug exposure corresponded to the time at which the MiCK assay reported a maximum extent of apoptosis in each cell type (see Fig 9A and B). At the end of the incubation, cells were double labeled with Annexin V-FITC and propidium iodide (PI) to distinguish the early apoptotic cells, or studied with the3H-TdR incorporation assay for antiproliferative effects of the drugs. Simultaneously, cells were subjected to the MiCK assay of apoptosis.
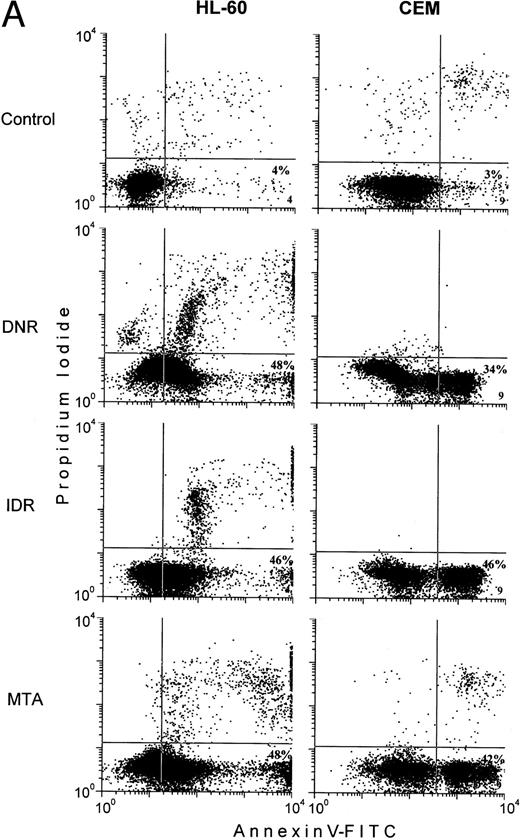
In control HL-60 and CEM cells, less than 5% of the early apoptotic cells, Annexin V+PI−, were usually detected (Fig 5A, lower right quadrants). Exposure of the cells to the chemotherapeutic agents was followed by more than 10-fold increases in subpopulations of the early apoptotic cells (Fig5). Nonetheless, in drug-treated HL-60 cells, the proportion of the early apoptotic cells measured by the Annexin V-FITC binding assay was from 10% to 25% lower than the extent of apoptosis determined by the MiCK assay (Fig 5A and C). In CEM cells exposed to DNR, IDR, and MTA, the percentages of the early apoptotic cells determined with the Annexin V-FITC binding assay (Fig 5A) were found to correlate well with the extent of apoptosis as measured in the MiCK assay (Fig 5C).
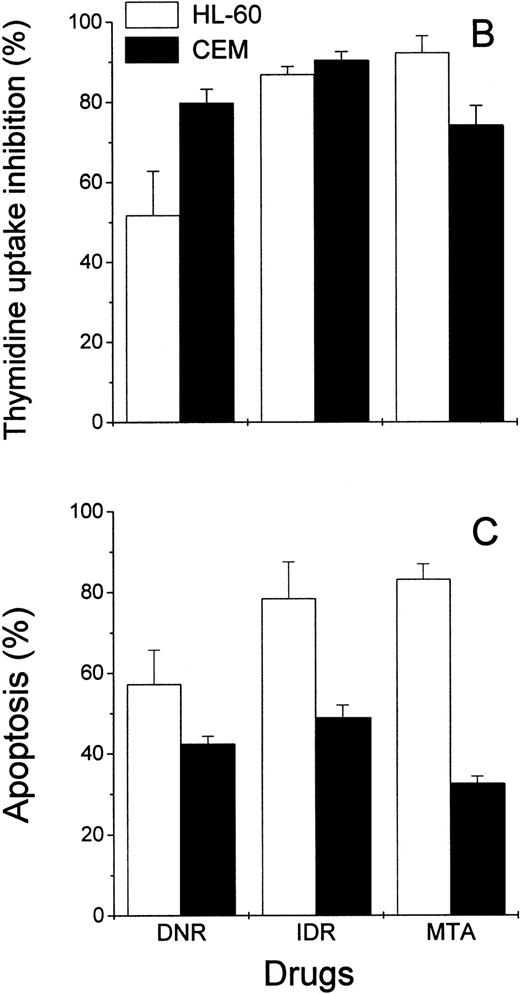
Effects of DNR, IDR, and MTA on HL-60 and CEM cells as measured with the Annexin V binding assay, the 3H-TdR incorporation assay, and the MiCK assay of apoptosis. HL-60 cells were exposed to 0.1 mm DNR for 36 hours and to 0.5 μmol/L IDR or 2.5 μmol/L MTA for 6 hours. CEM cells were exposed to 2.5 μmol/L DNR for 16 hours and to 5 μmol/L IDR or 5 μmol/L MTA for 7 hours. (A) Flow cytometry analyses of Annexin V-FITC/PI stained cells; 5,000 cells were analyzed in each condition. Quadrants were set using negative controls. The lower left quadrants of the cytograms show viable, An−PI−, cells. Numbers in the lower right quadrants refer to the percentage of apoptotic cells with preserved plasma membrane integrity (An+PI−, early apoptotic cells). Upper right quadrants show cells that have lost their plasma membrane integrity and became An+P+. The results of one of three independent experiments are shown. (B) Percentages of drug-induced inhibition of 3H-thymidine incorporation in HL-60 and CEM cells. Mean (±SD) of three independent experiments, each performed in three replicates. (C) Extent of apoptosis in drug-treated cells as determined with the MiCK assay of apoptosis. Results of the MiCK assay in KU were converted into percentages of apoptotic cells. Mean (±SD) of three independent experiments.
Effects of DNR, IDR, and MTA on HL-60 and CEM cells as measured with the Annexin V binding assay, the 3H-TdR incorporation assay, and the MiCK assay of apoptosis. HL-60 cells were exposed to 0.1 mm DNR for 36 hours and to 0.5 μmol/L IDR or 2.5 μmol/L MTA for 6 hours. CEM cells were exposed to 2.5 μmol/L DNR for 16 hours and to 5 μmol/L IDR or 5 μmol/L MTA for 7 hours. (A) Flow cytometry analyses of Annexin V-FITC/PI stained cells; 5,000 cells were analyzed in each condition. Quadrants were set using negative controls. The lower left quadrants of the cytograms show viable, An−PI−, cells. Numbers in the lower right quadrants refer to the percentage of apoptotic cells with preserved plasma membrane integrity (An+PI−, early apoptotic cells). Upper right quadrants show cells that have lost their plasma membrane integrity and became An+P+. The results of one of three independent experiments are shown. (B) Percentages of drug-induced inhibition of 3H-thymidine incorporation in HL-60 and CEM cells. Mean (±SD) of three independent experiments, each performed in three replicates. (C) Extent of apoptosis in drug-treated cells as determined with the MiCK assay of apoptosis. Results of the MiCK assay in KU were converted into percentages of apoptotic cells. Mean (±SD) of three independent experiments.
To confirm that the measurement of drug-induced apoptosis may reflect chemosensitivity of leukemia cells, the MiCK assay was compared with an3H-TdR incorporation assay, a long-standing standard chemosensitivity test.8 The antiproliferative effects of the drugs were expressed as the percentages of the 3H-TdR incorporation inhibition (Fig 5B). In drug-treated HL-60 cells, the degree of the 3H-TdR incorporation inhibition varied from 51% to 93% (Fig 5B), which correlated well with the proportion of apoptotic cells in the cultures (Fig 5C). In CEM cells, inhibition of3H-TdR uptake varied from 74% to 91% (Fig 5B) whereas the proportion of apoptotic cells in the same cultures was in the range of 40% to 52% (Fig 5C). Thus, in HL-60 cells, drug-induced decreases of3H-TdR incorporation were mainly caused by the cell death via apoptosis. In CEM cells, apoptotic cell death accounted for more than half of the decrease in 3H-TdR incorporation.
MiCK assay for apoptosis in human ANLL and ALL cells.
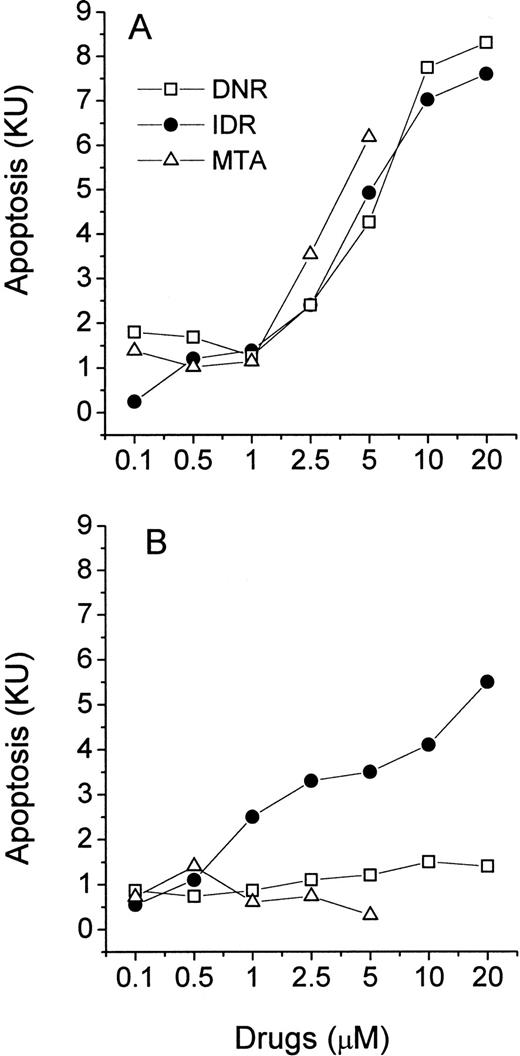
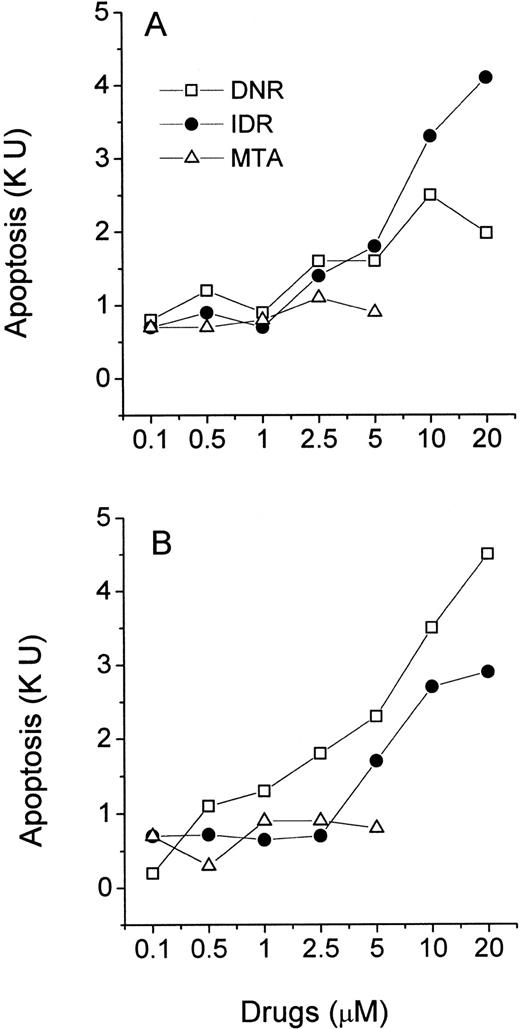
Freshly isolated leukemic myeloblasts and lymphoblasts were exposed to increasing concentrations of DNR, IDR, and MTA in a 40-hour MiCK assay. Apoptotic responses were determined and plotted against drug concentrations yielding drug-response curves (Figs 6 and 7). All three agents induced apoptosis in cells of ANLL patient no. 5 while only IDR was effective with cells of ANLL patient no. 14 (Fig 6A and B). The greatest apoptotic responses were seen in cells of patient no. 5 and patient no. 14 at 20 μmol/L IDR (8.2 KU or 53% of apoptotic cells and 5.6 KU, or 37.6% of apoptotic cells, respectively). By comparison, in myeloid HL-60 cells a similar extent of apoptosis could be induced by 0.1 μmol/L of any of the three drugs (Fig 3A). This indicates that freshly isolated myeloblasts from these two patients were less sensitive to chemotherapeutic drugs than HL-60 cells.
Effects of DNR, IDR, and MTA on apoptosis in cultures of ANLL leukemia cells. Purified leukemia cells from patient no. 5 (A) and patient no. 14 (B) were distributed in a 96-well microtiter plate at 2.5 × 105 cells per 250 μL complete medium and studied for 40 hours in the MiCK assay. Patients numbered according to Table 1. Each point represents apoptotic response measured in one experiment.
Effects of DNR, IDR, and MTA on apoptosis in cultures of ANLL leukemia cells. Purified leukemia cells from patient no. 5 (A) and patient no. 14 (B) were distributed in a 96-well microtiter plate at 2.5 × 105 cells per 250 μL complete medium and studied for 40 hours in the MiCK assay. Patients numbered according to Table 1. Each point represents apoptotic response measured in one experiment.
Effects of DNR, IDR, and MTA on apoptosis in cultures of ALL leukemia cells. Purified leukemia cells from patient no. 4 (A) and patient no. 8 (B) were distributed in wells of a 96-well microtiter plate at 2.5 × 105 cells per 250 μL complete medium and studied for 40 hours in the MiCK assay. Patients numbered according to Table 1. Each point represents apoptotic response measured in one experiment.
Effects of DNR, IDR, and MTA on apoptosis in cultures of ALL leukemia cells. Purified leukemia cells from patient no. 4 (A) and patient no. 8 (B) were distributed in wells of a 96-well microtiter plate at 2.5 × 105 cells per 250 μL complete medium and studied for 40 hours in the MiCK assay. Patients numbered according to Table 1. Each point represents apoptotic response measured in one experiment.
In cells from two ALL patients, the three agents induced less apoptosis compared with cells of the above described ANLL patients (Fig 7). The greatest apoptotic responses were seen in cells of ALL patient no. 4 at 20 μmol/L IDR (4.3 KU or 29.7% of apoptotic cells, Fig 7A) and in cells of ALL patient no. 8 at 20 μmol/L DNR (4.6 KU, or 31.5% of apoptotic cells, Fig 7B). MTA was unable to induce significant apoptosis in cells of either ALL patient. Similarly, the decreased effectiveness of MTA compared with DNR and IDR in the induction of apoptosis was observed in lymphoblastic CEM cells (Fig 3B).
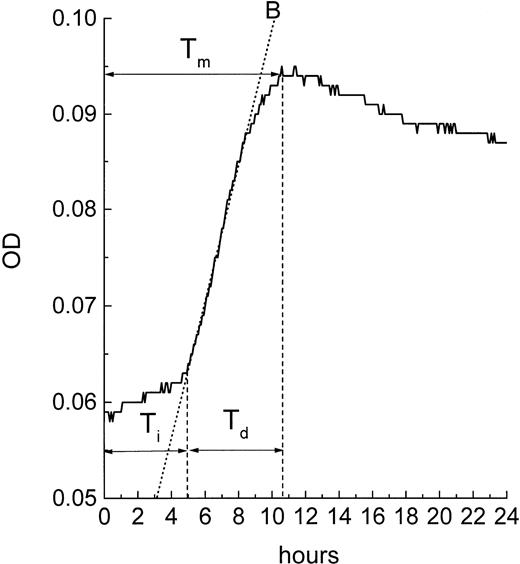
Timing of apoptosis.
Because the MiCK assay measures apoptosis continuously, it provides a unique opportunity for timing of the apoptotic process. A typical apoptotic curve from the MiCK assay of apoptosis is shown (see Fig 9). The maximum point of the apoptotic curve corresponds to the time when the greatest percentage of cells form membrane blebs,19 ie, undergo the initial stage of apoptosis. We have designated the period of time from the exposure of the cells to an apoptotic stimulus until the maximum apoptotic OD as the time to maximum apoptotic response (Tm). If apoptotic stimuli are added to cells shortly before initiation of the MiCK assay, Tm would correspond to the time from onset of the assay until the maximum OD. As is seen in Figure 8, Tm consists of two components. The first component is the initiation time (Ti) which is the period between exposure of the cells to an apoptotic stimulus and the beginning of the steep rising portion of the curve. The second component is the development time (Td), which is a period between the beginning of the steep rising portion of the curve and its apoptotic OD maximum. Figure 9shows that increases in concentrations of DNR, IDR, and MTA were always accompanied by shortening of Tm in both leukemia cell lines and freshly isolated leukemic cells. These shortenings of Tm resulted from shortening of both Ti and Td (data not shown). Although Ti did not have a definite lower limit, Td was never less than 3 hours. A relationship was seen between the degree of sensitivity of cells to a drug and the difference between Tm at the minimal and maximal drug concentrations. In cells from both ANLL and ALL patients exposed to IDR, significant differences between duration of Tm at minimal and maximal drug concentrations are evident (Fig 9C through F). This progressive shortening of Tm with increasing IDR dose corresponds to the effectiveness of IDR in the induction of apoptosis in cells of both groups of patients (Figs 6 and7). Conversely, an increase in concentration of MTA had little effect on Tm in cultures of ANLL patient no. 14 (Fig 9D) and both ALL patients (Fig 9E and F). The absence of significant changes of the Tm with increasing MTA dose corresponds to the low apoptotic response of the cells of these three patients to all tested concentrations of MTA (Figs 6B and 7A and B).
Timing of apoptosis in the MiCK assay. Typical plot of the OD versus time changes obtained from a microculture of cells exposed to an inducer of apoptosis is shown. Interval Tm(time to the maximum response) corresponds to the time when the maximum apoptotic response is achieved in the culture. Interval Ti(initiation time) corresponds to the time between adding an apoptosis inducer to the culture and the beginning of the steep rising portion of the curve. Interval Td (development time) corresponds to the time between the beginning of the steep rising portion of the curve and its maximum. B is the best fit line of the steep rising portion of the curve; earliest point of intersection between the B line and the OD versus time curve is used as a uniform indicator for the beginning of the steep rising, apoptotic portion of the curve.
Timing of apoptosis in the MiCK assay. Typical plot of the OD versus time changes obtained from a microculture of cells exposed to an inducer of apoptosis is shown. Interval Tm(time to the maximum response) corresponds to the time when the maximum apoptotic response is achieved in the culture. Interval Ti(initiation time) corresponds to the time between adding an apoptosis inducer to the culture and the beginning of the steep rising portion of the curve. Interval Td (development time) corresponds to the time between the beginning of the steep rising portion of the curve and its maximum. B is the best fit line of the steep rising portion of the curve; earliest point of intersection between the B line and the OD versus time curve is used as a uniform indicator for the beginning of the steep rising, apoptotic portion of the curve.
Relationship of drug dose and time to the maximum drug-induced apoptosis (Tm). (A) HL-60, (B) CEM, (C) ANLL patient no. 5, (D) ANLL patient no. 14, (E) ALL patient no. 4, and (F) ALL patient no. 8 leukemic cells were tested with varying doses of DNR, IDR, or MTA. For HL-60 and CEM cells, each point represents the mean (±SD) Tm of three independent experiments; for patient's cells, each point shows Tm determined in a single experiment.
Relationship of drug dose and time to the maximum drug-induced apoptosis (Tm). (A) HL-60, (B) CEM, (C) ANLL patient no. 5, (D) ANLL patient no. 14, (E) ALL patient no. 4, and (F) ALL patient no. 8 leukemic cells were tested with varying doses of DNR, IDR, or MTA. For HL-60 and CEM cells, each point represents the mean (±SD) Tm of three independent experiments; for patient's cells, each point shows Tm determined in a single experiment.
DISCUSSION
This study examined the applicability of the MiCK assay of apoptosis for determining sensitivities of myeloid and lymphoid leukemia cells to chemotherapeutic agents. The use of apoptosis as an indicator of drug effectiveness is based on the concept that activation of cellular self-destructive machinery is the main mechanism by which antitumor agents exert their therapeutic effects.11-14 27-29
In the MiCK assay, apoptosis is indicated by a steep increase of the OD. We have shown that membrane blebbing is the major determinant of these steep OD increases in cells undergoing apoptosis.19Formation of membrane protrusions, or blebs, is termed zeiosis and is considered to be an obligatory and early stage of apoptosis for most types of cells.30-32 Zeiosis causes dramatic changes of the cell shape that, in turn, result in an increased side scattering of light by the cells. The increased side light scattering is detected by a spectrophotometer as an increase of the OD of the cultures. Because the plasma membrane blebbing is a rapidly developing stage of the apoptotic process, the associated OD increase is very steep and can easily be distinguished from the gradual OD increase due to cell growth.19,33 When the steep rising portion of the apoptotic curve reaches its maximum, the greatest proportion of cells display membrane blebbing. The stage of membrane blebbing coincides with the initial stages of the nuclear morphological changes of apoptosis and may precede the internucleosomal DNA cleavage by several hours.17,19,32,34 It has been suggested that zeiosis results from severe changes in molecular structure of the cell membrane which include alteration of both the membrane phospholipid packing and distribution of the phospholipids between the two leaflets of the cell membrane.35,36 These and other molecular changes affecting the cell membrane not only contribute to blebbing but also facilitate recognition and phagocytosis of the apoptotic cells by macrophages.37 Sufficiency of the early membrane changes to prime the apoptotic cell for elimination suggests that membrane blebbing occurs after the “point of no return” in the sequence of events in apoptosis. To our knowledge, zeiosis has never been reported in cells undergoing necrosis. Therefore, zeiosis can serve as an early, reliable, and specific hallmark of the apoptotic process. Although zeiosis had long been known as a classical element in the sequence of morphological changes of apoptosis,30 31 it has never been used for quantitation or timing of apoptosis. The MiCK assay of apoptosis uses the cell membrane blebbing as a specific marker of apoptosis and provides an integrative analysis for the multiple occurrences of single-cell zeiosis over the culture period. All cells that ever entered apoptosis are scored by the MiCK assay and thus contribute to the final quantitation of the apoptotic response of the cell population.
Various apoptosis detection kits are available commercially. These kits are mostly based on detecting products of internucleosomal DNA fragmentation by either light or fluorescent microscopy or by flow cytometry. These tests are widely used for research purposes; however, their application for testing apoptosis in clinical samples is impractical for several reasons. First, these apoptosis tests are expensive, multistep procedures requiring substantial labor and time. Second, the cells under study must be either fixed or destroyed. As a result, each of these tests must be used as an endpoint assay that allows detection of apoptosis only at one specific time during a drug-cell interaction. The timing of apoptosis induced by a drug can vary greatly depending on the drug concentration and the type, metabolic status, and cell-cycle phase of the target cells. Thus, the choice of the time point at which cells are tested for drug-induced apoptosis is critical for its accurate detection. Nonetheless, with any of the endpoint tests for apoptosis it must be chosen arbitrarily. When these tests are used with a well-characterized, established cell line, this problem can partly be overcome by performing multiple experiments that help to determine the timing of apoptosis. However, this approach is unsuitable for studying drug-induced apoptosis in cell samples obtained directly from a patient because such cells comprise a unique population of limited size that differs from cells obtained from other patients. Therefore, the timing of apoptosis in samples of one patient may only be of minimal help when apoptosis is tested in cells of another patient.
In addition to the above-mentioned technical drawbacks of the assays relying on DNA cleavage, there are also theoretical limitations for their use with patient's samples. It is still not clear whether double-stranded cleavage of DNA is the critical event in apoptosis. A growing body of evidence shows that apoptosis can be observed without internucleosomal DNA breakage and can even be induced in enucleated cells.38-43 It follows that if drug-induced apoptosis develops in the patient's cells without internucleosomal DNA breakage, a falsely negative result would be reported by a test based on DNA breakage. Another problem stems from the fact that cell death via necrosis is accompanied by random fragmentation of DNA by multiple endonucleases. Degradation of DNA in necrotic cells can result in formation of 180- to 200-bp-length fragments, thus suggesting that internucleosomal DNA cleavage is not an exclusive prerequisite for apoptosis.39 44-46 This makes the biochemical discrimination between apoptosis and necrosis problematic. For example, commercial cell death kits using the TdT-mediated dUTP nick end labeling (TUNEL assay) cannot distinguish between necrotic and apoptotic cells. Thus, the TUNEL assay may be accurate for apoptosis only in those rare cases in which no necrotic deaths occur in culture. However, in vitro testing of a patient's sample for chemosensitivity requires the use of a wide range of drug concentrations to distinguish between those doses of drugs which are ineffective, those which induce apoptosis, and those which induce necrosis. As doses of drugs are increased, progressively larger proportions of necrotic cells are found in cultures (see Fig 4). In such cases, results of apoptosis tests relying on the DNA cleavage would be falsely positive.
It is generally agreed that a characteristic sequence of morphological alterations is the sine qua non of apoptosis.30-32,38,39 In both our previous publication19 and in the present study, the MiCK assay results were correlated with morphological evidence of apoptosis in Giemsa-stained cell preparations. To further validate the MiCK assay of apoptosis, we compared it with an Annexin V binding assay for detection of apoptosis.20 Annexin V is a calcium-dependent phospholipid-binding protein with high affinity for phosphatidylserine. It has been shown that cells become capable of binding Annexin V early in the course of apoptosis due to loss of the plasma membrane phospholipid asymmetry with exposure of phosphatidylserine on the outer plasma membrane leaflet.20 35 Use of the two-color flow cytometry analysis of Annexin V-FITC and PI-labeled cells makes the Annexin V binding assay a convenient test for distinguishing between nonaffected cells (An−PI−), apoptotic cells with preserved plasma membrane integrity (An+PI−), and cells that have lost their membrane integrity (An+PI+). We believe that the Annexin V binding assay is the most appropriate test to be compared with the MiCK assay of apoptosis because translocation of phosphatidylserine in the cell membrane which is detected with the Annexin V binding assay may precede or coincide with the cell membrane blebbing—the event which is monitored in the MiCK assay.
When apoptosis was measured in CEM cells, a good correlation was found between results of both assays (Fig 5A and C). With HL-60 cells, the percentage of apoptotic cells was 10% to 25% lower when measured with the Annexin V binding assay as compared with the MiCK assay (Fig 5A and C). One of the possible explanations of these observations is that drug-affected CEM cells preserve their plasma membrane integrity longer while HL-60 cells promptly proceed to the final steps of the apoptosis, lose their cell membrane integrity, and become An+PI+. Indeed, substantially lower proportions of An+PI+ cells were always observed in drug-treated CEM cells as compared with HL-60 cells (Fig 5A, upper right quadrants). Unfortunately, the proportion of the late apoptotic cells in the fraction of An+PI+ cells cannot be determined with the Annexin V-FITC binding assay because it does not distinguish between the late apoptotic and necrotic cells that both have lost membrane integrity and become An+PI+. Subpopulations of An+PI+cells have been variously referred to as late apoptotic,20nonviable/necrotic,47 secondary necrotic,48 or advanced apoptotic and necrotic.49 Because of this unclear nature of An+PI+ cells and in order to avoid confusion between late apoptotic and necrotic cells, we decided to exclude An+PI+ cells from our analyses. It should also be recalled that the endpoint Annexin V binding assay determines the proportion of the apoptotic cells with preserved plasma membrane integrity at a certain, single time point of culture and, thus, it may not measure the total number of apoptotic deaths over the entire culture period.
The use of apoptosis to measure drug sensitivity has been previously discussed in the literature.11,27-29 In our study, results of the MiCK assay of apoptosis were correlated with the antiproliferative effects of the drugs as measured with a standard chemosensitivity test, the 3H-thymidine incorporation assay. In HL-60 cells, similar results on drug sensitivity were obtained with both assays. In CEM cells, the antiproliferative effects of each drug was greater when compared with the percentages of apoptotic cells (Fig 5B and C). It has been shown that apoptosis-inducing concentrations of chemotherapeutic agents may exceed significantly (up to 25 times) doses that are required to inhibit cell proliferation.50 Thus, at least in some malignant cell types, tests relying on cell proliferation, eg,3H-thymidine incorporation or clonogenic assays, cannot differentiate between arrested cell proliferation that can be potentially reversed and cell death that cannot be reversed. In such cases, the extent of apoptosis, which indicates irreversible cell loss due to clinically relevant doses of chemotherapeutics, provides more reliable measure of drug effectiveness.
The MiCK assay automatically reports the maximum extent of apoptosis whenever it is achieved during the culture, thus standardizing the measurements of apoptosis under different conditions. Changes in the OD of each microculture are recorded by the computer and presented at the end of the assay in a graphic form. Analysis of the OD versus time curves from wells with cells undergoing apoptosis permits a unique opportunity for accurate determination of the time to maximum apoptotic response (Tm) and analysis of its components—the initiation time (Ti) and development time (Td). We suggest that during Ti, an active drug metabolite is reaching a threshold of intracellular concentration that is required to trigger the molecular mechanism of apoptosis. Ti depends on the rate at which the drug is taken up by the cell and is converted into its active form. Ti can be reduced significantly by increasing drug concentration. However, we found substantial differences in Ti between myeloid and lymphoid cells exposed to the same concentration of a therapeutic agent. This finding may reflect inherent differences between the two types of cells in both the rates of the drug uptake and the mechanisms of the intracellular metabolism of the same drugs.
Td is the period required for all the susceptible cells of the culture to undergo apoptosis. Two points about Td can be made. First, its duration depends on the rate at which cells are triggered into an apoptotic response and, therefore, Td may reflect the uniformity of the drug sensitivity of the cell population. Second, Td cannot be shorter than a minimum time that is required to complete the apoptosis program in a single cell. For both myeloid and lymphoid cells, the shortest development time observed was about 3 hours.
Determining the utility of the MiCK assay of apoptosis in predicting chemosensitivity of any individual leukemia will require the study of a large number of patients and comparisons of MiCK assay results with standardized treatment outcomes. Nevertheless, our preliminary data have shown the potential utility of the MiCK assay in making clinical decisions. In Fig 6, only for the leukemia of ANLL patient no. 5 did all three therapeutic agents show similar effectiveness in the MiCK assay (Fig 6A). The leukemia of ANLL patient no. 14 showed sensitivity to IDR but was resistant to both DNR and MTA (Fig 6B). In this latter case, the use of IDR rather than DNR or MTA might be beneficial in the patient's induction and consolidation chemotherapy. The leukemia of ALL patient no. 4 showed increased sensitivity to IDR compared with DNR (Fig 7A) while the leukemia of ALL patient no. 8 was more sensitive to DNR compared with IDR (Fig 7B). Thus, the use of IDR in the chemotherapeutic regimens of ALL patient no. 4 and DNR in the chemotherapeutic regimens of ALL patient no. 8 would provide the treatment that was found to be most effective in the MiCK assay. A study comparing the chemosensitivities of specific leukemias as determined by the MiCK assay and the clinical outcomes of the respective patients after treatment with standard chemotherapy regimens is now in progress.
ACKNOWLEDGMENT
The authors thank Gail Hermann for her assistance in procurement of clinical samples, Dr Jim Price for assistance with the Annexin V-FITC binding assay, and Prof Maurice Bondurant for critical review of the manuscript.
Supported by a Leukemia Society of America Translational Research Grant to M.J.K. Partial support for this research was provided by a gift from the Immunex Corporation.
Address reprint requests to Vladimir D. Kravtsov, MD, PhD, 547 MRB-II, Department of Medicine, Division of Hematology, Vanderbilt University Medical Center, 2220 Pierce Ave, Nashville, TN 37232-6305.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 4. Morphological changes in HL-60 cells at low or high IDR doses. Cells were exposed to 0.05 μmol/L IDR for 24 hours (A and B) or 10 μmol/L IDR for 6 hours (C and D). At these time points, maximum apoptosis was reported by the MiCK assay in respective cultures (see Fig 8). Giemsa-stained cytospin preparations show three distinctive morphological types of cells: (1) Cells displaying morphological features of interphase or mitotic promyelocytes with no visible changes in cell structure; two large cells in (A) and predominant cells in (B); (2) cells in the early stage of apoptosis (ap, in [A] and [C]) with aggregated chromatin abutting the nuclear membrane, and condensed, basophilic cytoplasm; (3) necrotic cells (nec, in [A] and [C]) with faintly stained nuclear “ghosts,” indistinct vacuolated cytoplasm which appears light due to loss of basophilia, and ruptured plasma membranes; predominant cells in (D). Low magnification (B and D) is used to show proportions of apoptotic and necrotic cells in cultures. Scale marker indicates 10 μm for (A) and (C) and 50 μm for (B) and (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.968/5/m_blod41527004y.jpeg?Expires=1765051120&Signature=aEqkGQqLOdafZHI8~9wneXXkp6uj8QiMJkdVXw7z9CLVujuOCJrBL7OcaGsNEgxQQbgvyp2PuRLRSXHmEPRid~PSlG30y5CDzggXmokauwV8bgaJK4qNUukwRZqdcuKVmw3S583Ww9e7Y0kKa81-I3zfQlYy4RWjcJW58vmZSWpWeAhRKiUEsWunDRho80WwtaCMqfa0-wR0Rl5Skq6BDerDdAti54sAjy3xghFEhFQjKDZULrq3H4r~sh3II7cAyWj5TUZagBBC9n-yeBKqHBZccFXDsj31JdOycCRVuUUEabEjTMGyZadwgKkXnMFlFlwemo6afesJmHw3sFz9bA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal