Abstract
Familial essential thrombocythemia (ET) is inherited in an autosomal-dominant manner. This finding implies that familial ET may arise as a consequence of a mutation(s) that activates platelet production. In 1994, the thrombopoietin (TPO) gene was isolated and cloned. The TPO-TPO receptor, encoded for by thec-mpl gene, are essential regulators of thrombopoiesis. Alterations of TPO or c-Mpl thus may constitute a pathogenic event leading to familial ET. In a case of familial ET presented in our institute, serum TPO levels were significantly elevated in affected members of the family as compared with nonaffected members. Moreover, we identified a one-base deletion in the 5′-untranslated region of theTPO gene in affected but not in nonaffected family members. In vitro experiments showed that the identified mutation increased TPO production. Based on our findings, we propose that this region of theTPO gene may play a crucial role in regulating TPO expression. Our results strongly suggest that the identified mutation leads to familial ET.
© 1998 by The American Society of Hematology.
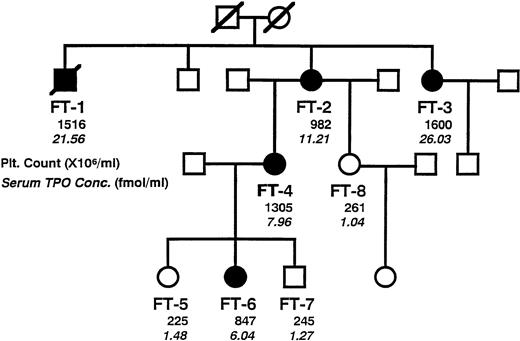
ESSENTIAL thrombocythemia (ET) belongs to the entity of chronic myeloproliferative disorders. ET patients present with an increased platelet count in peripheral blood accompanied by proliferation of megakaryocytes in the bone marrow. ET generally occurs in both males and females in middle age. So far, no common genetic alteration(s) causing the disease has been identified. To date, a few cases of familial occurrence of ET have been reported, which are generally inherited in an autosomal-dominant fashion.1-3Thus, familial ET is likely to be a consequence of mutations of a gene that presumably is a critical regulator of platelet production. A case of familial ET presented in our institution. The disease occurred in three successive generations, possibly by autosomal-dominant inheritance (Fig 1). FT-2, the propositus, occasionally presented with thrombocythemia. Her family history showed that thrombocythemia occurred in several members of the family, and the pedigree suggested that the disease was inherited in an autosomal-dominant manner. Detailed clinical examination did not detect any underlying disease suitable to cause secondary thrombocythemia. Thus, the affected members in this family were diagnosed as ET. During the clinical courses, none of the affected members developed any thrombotic/hemorrhagic symptoms.
A pedigree of the studied family, showing typical autosomal-dominant inheritance of disease penetration. Members of the family, designated as FT-1 through FT-8, were clinically examined to certify affected or not. Solid symbols indicate affected members. Circles denote female family members, squares male family members, and symbols with a slash deceased family members. Plain numerals indicate peripheral platelet count, and italic numerals indicate serum concentration of TPO.
A pedigree of the studied family, showing typical autosomal-dominant inheritance of disease penetration. Members of the family, designated as FT-1 through FT-8, were clinically examined to certify affected or not. Solid symbols indicate affected members. Circles denote female family members, squares male family members, and symbols with a slash deceased family members. Plain numerals indicate peripheral platelet count, and italic numerals indicate serum concentration of TPO.
The thrombopoietin (TPO) gene was isolated in 1994. TPO is believed to be a main regulator of platelet production.4-11 Serum concentration of TPO in the affected family members was higher than in nonaffected members. After polymerase chain reaction (PCR)-based amplification of the TPO gene, hetero-duplex formation, and sequence analysis, one-base deletion in the 5′-untranslated region (UTR) of the TPO gene was discovered. We propose that this mutation can raise the expression level of TPO, resulting in a familial occurrence of ET.
MATERIALS AND METHODS
All studies described were performed after obtaining informed consent of all family members and healthy volunteers.
Serum levels of TPO.
Serum concentration of TPO was measured by a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) as described by Tahara et al.12
Nested PCR amplification of the TPO genome.
Genomic DNA samples were extracted from peripheral leukocytes of the family members, except FT-1, and from one healthy volunteer. The structure of TPO genome has been reported by four groups.10,11,13,14 Three of them claim that the TPOgene consists of six exons and five introns,10,11,13whereas one group reported seven exons and six introns.14We refer here to the report by Sohma et al10 in which the initiation codon is located in exon 2. All exons of the TPOgene were amplified by nested PCR with specific primers, which were designed to amplify all exons of the TPOgenome in overlapping 7 fragments (Fig2A). The PCR reaction was performed in a reaction mixture containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 2 mmol/L MgCl2, 0.01% (wt/vol) gelatin, 250 μmol/L of dNTPs, 0.2 μmol/L each of 5′- and 3′-PCR primers, 0.25 U Taq DNA polymerase (TaKaRa Ex Taq; Takara Shuzo, Shiga, Japan), and genomic DNA in a volume of 50 μL. The samples were denatured at 94°C for 2 minutes, followed by 30 cycles of the amplification process, which consisted of 2 minutes of denaturation at 94°C, 1 minute of annealing at 55°C, and 2 minutes of extension at 72°C. The second PCR was performed with 1 μL of the first PCR product as the template. After the second PCR amplification, the reaction mixtures were inactivated by the addition of 1 μL of 0.5 mol/L EDTA. Segment 2 of the TPOgene was amplified with a set of the first PCR primers (5′-CAGGCTGGTCAGCATCTCAA, 5′-TACCAGTTACGCGGATAAAG) and a set of the second PCR primers (5′-CCAGGCAGTCTCTTCCTAGA, 5′-GGGATAATGTTGGGAGTTCT).
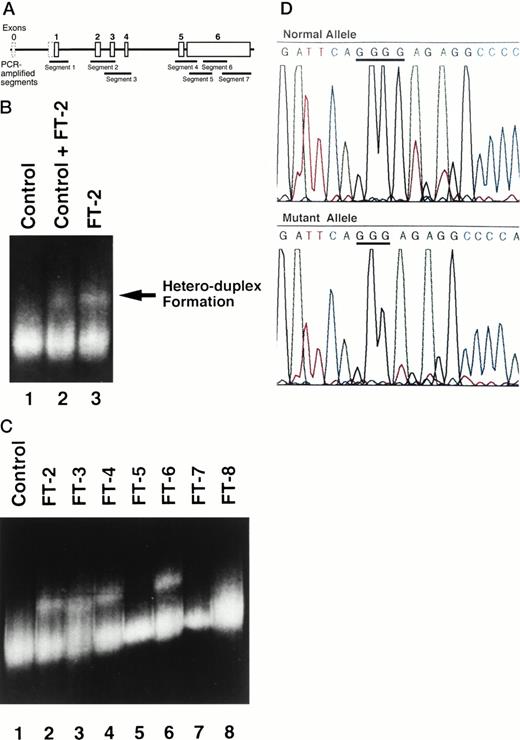
(A) A scheme of the genomic structure of TPO.According to GenBank accession no. D32046.10 Exon 0 (dashed box) corresponds to exon 1 of Chang et al.14In this reference, the initiation codon is located in exon 2. TheTPO genome was divided and amplified by nested PCR. Each PCR segment spans as described below: segment 1, from nucleotide (nt) 1240 to nt 1600; segment 2, from nt 3100 to nt 3696; segment 3, from nt 3626 to nt 4066; segment 4, from nt 5959 to nt 6440; segment 5, from nt 6390 to nt 6778; segment 6 from nt 6682 to nt 7071; and segment 7, from nt 6954 to nt 7545. (B) Hetero-duplex formation by amplified segment 2 PCR products. Hetero-duplex bands are indicated by an arrow. Lane 1, PCR products from a healthy volunteer alone as a control; lane 2, mixture of PCR products from FT-2 and control; and lane 3, PCR products from FT-2 alone. (C) Hetero-duplex analysis of segment 2 of all family members, except for FT-1. All of the ET-affected members display the hetero-duplex bands (FT-2, 3, 4, and 6). However, none of the healthy family members showed hetero-duplex formation in segment 2 (FT-5, 7, and 8). (D) Identification of the mutation in the segment 2 ofTPO gene. The upper panel shows the DNA sequence of wild-type allele, and the lower panel shows the mutant allele.
(A) A scheme of the genomic structure of TPO.According to GenBank accession no. D32046.10 Exon 0 (dashed box) corresponds to exon 1 of Chang et al.14In this reference, the initiation codon is located in exon 2. TheTPO genome was divided and amplified by nested PCR. Each PCR segment spans as described below: segment 1, from nucleotide (nt) 1240 to nt 1600; segment 2, from nt 3100 to nt 3696; segment 3, from nt 3626 to nt 4066; segment 4, from nt 5959 to nt 6440; segment 5, from nt 6390 to nt 6778; segment 6 from nt 6682 to nt 7071; and segment 7, from nt 6954 to nt 7545. (B) Hetero-duplex formation by amplified segment 2 PCR products. Hetero-duplex bands are indicated by an arrow. Lane 1, PCR products from a healthy volunteer alone as a control; lane 2, mixture of PCR products from FT-2 and control; and lane 3, PCR products from FT-2 alone. (C) Hetero-duplex analysis of segment 2 of all family members, except for FT-1. All of the ET-affected members display the hetero-duplex bands (FT-2, 3, 4, and 6). However, none of the healthy family members showed hetero-duplex formation in segment 2 (FT-5, 7, and 8). (D) Identification of the mutation in the segment 2 ofTPO gene. The upper panel shows the DNA sequence of wild-type allele, and the lower panel shows the mutant allele.
Hetero-duplex analysis and identification of a mutation.
The PCR products from FT-2 were mixed with an equal volume of corresponding PCR products from a normal control. These mixtures and each PCR product from either FT-2 or the normal control were used for hetero-duplex analysis. The samples were denatured for 3 minutes at 96°C and then kept at room temperature for 1 hour to allow duplex formation. The samples were resolved by electrophoresis in Mutation Detection Enhancement (MDE) gel (Hydrolink; AT Biochem, Malvern, PA) for 22 hours at 800 V, and bands were visualized by staining with ethidium bromide. Genomic samples of other family members were examined accordingly. When a region containing a mutation was detected with in the amplified PCR products, the respective PCR products were cloned into pT7-Blue(R) (Novagen, Madison, WI). Clones were used as templates for PCR and the products were then analyzed by MDE gel electrophoresis after mixing with equal amounts of corresponding wild-type PCR fragments. Clones that manifested hetero-duplex formation were sequenced by dideoxy chain termination using the Taq dye terminator cycle sequencing kit and the Model 370A DNA sequencer (Applied Biosystems, Foster City, CA) to determine the mutation.
Construction of plasmids.
Human TPO cDNA was obtained by reverse transcription-PCR (RT-PCR). A human liver sample previously obtained from a patient with liver disease by biopsy for diagnostic purpose was concomitantly used to amplify human TPO cDNA. One microgram of total RNA was added into a reaction mixture containing 100 pmol random primer (GIBCO-BRL, Grand Island, NY) in a volume of 10.5 μL. The mixture was heated at 96°C for 3 minutes, chilled on ice, and supplemented with 20 U of RNasin, 4 μL of 5× RT buffer, 2 μL of 0.1 mol/L dithiothreitol (DTT), 2 μL of 10 mmol/L each of dNTPs, and 100 U of Superscript reverse transcriptase (GIBCO-BRL) in a volume of 20 μL and then incubated at 42°C for 2 hours. The RT reaction product was then incubated at 90°C for 5 minutes and chilled on ice, and 40 μL of distilled water was added. The TPO cDNA was amplified by PCR with a set of primers (5′-CCAAGCTTAGAGGGCTGCCTGCTGTGCA and 5′-GATGTCGGCAGTGTCTGAGA). PCR products were cloned into pT7-Blue(R) named as pT7-hTPO. pT7-hTPO was partially digested with HindIII and BamHI. TheHindIII-BamHI fragment containing the 5′-UTR and entire coding region of the TPO cDNA was ligated intoHindIII-BamHI backbone fragment from pBluescript II KS(−) (Stratagene, La Jolla, CA). The HindIII-Xba I fragment of human TPO cDNA from this vector was ligated intoHindIII-Xba I backbone of pActNPM,15 which was designated as pAct-wTPO. To construct the TPO expression vector bearing the identified mutation, the Nco I-Eae I segment of the TPO cDNA was substituted for by the corresponding cDNA fragment obtained from FT-2, generating pAct-mTPO. To construct the TPO-luciferase fusion genes, either wild-type or mutant partial TPO cDNA was amplified by PCR with a set of primers (5′-CCAAGCTTAGAGGGCTGCCTGCTGTGCA and 5′-GAATCATGACCACGAGGA). These primers contained either the HindIII or BspHI site, respectively. The HindIII-BspHI fragments of the amplified cDNAs were ligated into the HindIII-Nco I backbone of PGV-C2 (Nippon Gene, Toyama, Japan). In these vectors, expression of the TPO-luciferase fusion gene was driven by the SV40 late promoter. The vector bearing wild-type partial TPO cDNA was named pWTP-luc, and the vector bearing the mutation was named pMTP-luc, respectively.
DNA transfection.
L929 cells (5 × 105 cells per 6 cm dish), a mouse-derived fibroblast cell line, were transfected with 10 μg of pActC, pAct-wTPO, or pAct-mTPO, respectively, by calcium-phosphate method, as described before.15 pActC is a control plasmid devoid of the cDNA insert. After 16 hours of incubation in 3 mL of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, the media were exchanged to 3 mL of DMEM supplemented with 1% fetal bovine serum. After 72 hours, both cells and conditioned media were collected. RNA blotting analysis was performed as described before.15 TPO concentrations in the conditioned media were assayed by ELISA. For luciferase assay, each 3 μg of pWTP-luc or pMTP-luc was transfected into L929 cells by calcium-phosphate method, and luciferase assay was performed as described before.15
RESULTS
Serum concentration of TPO.
TPO is believed to be a main cytokine regulating platelet production. Therefore, it is a primary candidate to contribute to the pathogenesis of familial ET. We evaluated serum concentrations of TPO in each of the family members (Fig 1). The nonaffected members manifested normal levels of TPO,12 whereas the affected members presented almost 6 to 20 times higher TPO levels.
Hetero-duplex analysis of TPO genome.
We suspected that the elevated TPO levels might be the pathogenic event leading to familial ET and subsequently examined the integrity of theTPO gene. To determine whether ET-affected patients harbor sequence alterations within the TPO gene, we first performed hetero-duplex analysis of genomic DNA. All exons of the TPOgene were amplified as described in Materials and Methods. A DNA sample from FT-2 was amplified by specific primers, and a DNA sample from a healthy volunteer was concomitantly amplified as a normal control. PCR products of segment 2 of the TPO genome formed hetero-duplexes (Fig 2B), but no hetero-duplex bands were detected in other segments (data not shown). Moreover, hetero-duplex bands were observed not only when PCR products of FT-2 and a healthy volunteer were mixed (Fig 2B, lane 2), but also in a sample of FT-2 alone (Fig 2B, lane 3). These results indicate that the TPO gene of FT-2 may be heterozygous, with one allele being wild-type and the other mutated. To elucidate whether hetero-duplex formation was linked to the occurrence of thrombocytosis, we examined the status of segment 2 in all family members, except for FT-1. Hetero-duplex bands were observed only in ET-affected family members (Fig 2C).
Identification of a TPO gene mutation.
Sequence analysis of amplified DNA showed one-base deletion in the 5′-UTR of the TPO gene. On the mutated allele, a guanine at nucleotide 325210 was deleted (Fig 2D). This guanine is located 47 bases upstream of the authentic initiation codon. This mutation was common in all the affected family members, but was not present in unaffected healthy family members.
Increased TPO production by mutant TPO cDNA.
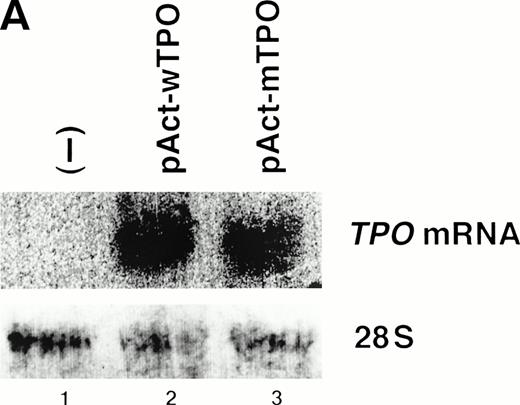
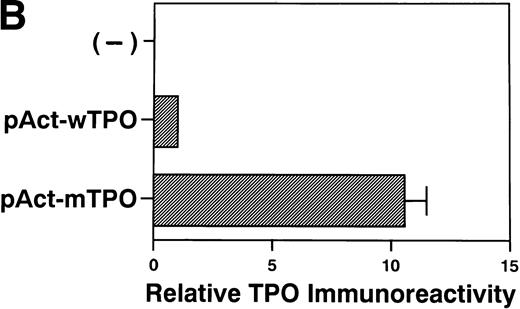
We speculated that the identified mutation might increase TPO gene expression, because the identified mutation resided in the 5′-UTR of the TPO cDNA and did not alter the coding sequence ofTPO gene. Thus, we constructed TPO expression vectors with theTPO cDNA downstream of the β-actin promoter. The vector bearing wild-type TPO cDNA was named pAct-wTPO, and the vector bearing the mutation was named pAct-mTPO. After transient transfection of these vectors into L929 cells, TPO levels in the culture media were analyzed by ELISA. The level of TPO mRNA was similar in the cells transfected with pAct-wTPO or with pAct-mTPO (Fig3A). However, TPO levels in the media were elevated almost 10 times in pAct-mTPO–transfected cells as compared with pAct-wTPO–transfected cells (Fig 3B). These findings support the notion that the identified mutation may lead to enhanced TPO expression. In addition, this elevation seems to result from a posttranscriptional regulatory mechanism. To further elucidate the mechanism, we performed in vitro transcription-translation assay ofTPO cDNA in rabbit reticulocyte lysates. As a result, we observed no significant difference in the amount of final TPO products between wild-type and mutated-type TPO cDNA (data not shown).
(A) Mutant-type TPO manifested the same level ofTPO mRNA as wild-type. L929 cells were transfected with 10 μg of pActC, pAct-wTPO, or pAct-mTPO, respectively. By Northern blot analysis, TPO mRNA level in the transfected cells showed no definite difference between pAct-wTPO and pAct-mTPO. (B) TPO production definitely differed between pAct-wTPO and pAct-mTPO. TPO concentration in the conditioned media was analyzed by ELISA. TPO released by pAct-wTPO transfected L929 cells was arbitrarily assigned to a value of 1.0, and the results are shown as the mean ± SD. The analysis was performed in duplicate assays and the results were reproducible.
(A) Mutant-type TPO manifested the same level ofTPO mRNA as wild-type. L929 cells were transfected with 10 μg of pActC, pAct-wTPO, or pAct-mTPO, respectively. By Northern blot analysis, TPO mRNA level in the transfected cells showed no definite difference between pAct-wTPO and pAct-mTPO. (B) TPO production definitely differed between pAct-wTPO and pAct-mTPO. TPO concentration in the conditioned media was analyzed by ELISA. TPO released by pAct-wTPO transfected L929 cells was arbitrarily assigned to a value of 1.0, and the results are shown as the mean ± SD. The analysis was performed in duplicate assays and the results were reproducible.
Luciferase assay.
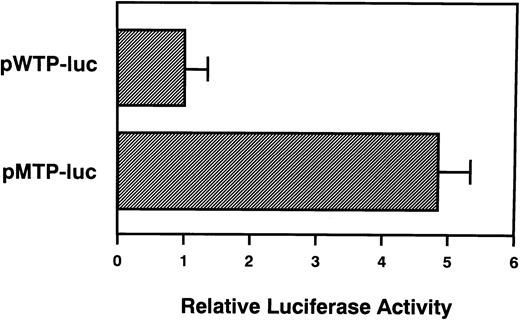
We next examined whether the restricted region of TPO gene could affect gene expression. TPO-luciferase fusion genes were constructed. The 5′-UTR and initial 30 bases of the coding region ofTPO cDNA were cloned upstream of the full-lengthluciferase gene. This fusion gene was then inserted downstream of the SV40 late promoter. The vector bearing wild-type TPOcDNA was named pWTP-luc, and the vector bearing the mutation was named pMTP-luc. After transfection of either pWTP-luc or pMTP-luc into L929 cells, luciferase activity was analyzed. As expected, pMTP-luc manifested almost 5 times higher luciferase activity than pWTP-luc (Fig4). This result suggested that the restricted region of the TPO gene may be critical to regulateTPO gene expression.
A restricted region within the TPO gene affects heterologous gene expression. L929 cells were transfected with 3 μg of pWTP-luc or pMTP-luc, respectively. Luciferase assay was performed as described in Materials and Methods. Luciferase activity of pWTP-luc was assigned to a value of 1.0, and the results are shown as the mean ± SD. The analysis was performed in duplicate assays and the results were reproducible.
A restricted region within the TPO gene affects heterologous gene expression. L929 cells were transfected with 3 μg of pWTP-luc or pMTP-luc, respectively. Luciferase assay was performed as described in Materials and Methods. Luciferase activity of pWTP-luc was assigned to a value of 1.0, and the results are shown as the mean ± SD. The analysis was performed in duplicate assays and the results were reproducible.
DISCUSSION
TPO is believed to be a critical cytokine to regulate platelet production, and platelet production depends mostly on the TPO–c-Mpl system. Either c-Mpl– or TPO-deficient mice exhibited a greater than 80% decrease in their platelet count,16-18 and TPO-transgenic mice displayed a marked elevation of platelet count.19 In this study, we observed that serum levels of TPO were markedly elevated only in the affected members of familial ET. These findings suggested that the elevation of TPO contributes to the pathogenesis of the disease. One-base deletion in the 5′-UTR of theTPO gene was identified only in the ET-affected family members. Furthermore, we showed that this mutation of the TPO gene directly increased the production of TPO. Collectively, these findings strongly suggest that the identified mutation could be the molecular cause of this case of familial ET.
The TPO gene was cloned in 1994 by several groups.5-7,10,11,13,14 It has structural homology to erythropoietin (EPO). Both the EPO receptor and TPO receptor, c-Mpl, belong to the cytokine superfamily.20,21 As with familial ET, a few cases of familial polycythemia have been reported. Patients show lineage-specific proliferation of erythrocytes, which is transmitted by autosomal-dominant inheritance.22,23Affected family members carry structural alterations of the EPO receptor gene, which result in increased sensitivity of the EPO receptor.24 25 In our case of familial ET, no mutations were detected in the coding sequence of the c-Mpl gene (data not shown). Thus, the mechanism causing familial ET in this particular case seems to be completely different from that of familial polycythemia.
Although TPO is believed to be an essential cytokine to maintain platelet count, the precise relationship between TPO production and platelet count under pathologic conditions has not been fully elucidated. TPO is produced mainly by liver and kidney, and the levels of TPO mRNA in these organs are found to be constant, regardless of the thrombocytopenic state. Consequently, a model has been proposed in which circulating TPO is wasted by c-Mpl expressed by platelets and megakaryocytes. Accordingly, elevation of serum TPO levels in thrombocytopenic state is due to the decreased expression of c-Mpl.26,27 Based on our findings, we suggest that the 5′-UTR of the TPO gene may be crucial to regulate TPO gene expression, probably through a posttranscriptional mechanism. As previously reported for several genes, such asbcl-228 or ferritin,29 the 5′-UTR contributes to the regulation of gene expression. In some genes,28 the upstream open reading frame attenuates the efficiency of translation. The TPO gene also harbors upstream open reading frames. For other genes,29 a regulatory molecule should exist that could exert its effect by interacting with 5′-UTR of mRNA. Considering the structure of 5′-UTR of TPOgene, we suppose that it may play a role in regulating TPO production by posttranscriptional mechanism. At this point, in vitro transcription-translation assay did not show any difference in translation efficiency between wild-type and mutant-type of TPOcDNA. Therefore, we speculate that additional factors regulating TPO production may be involved.
A recent study reported almost normal or slightly elevated serum concentrations of TPO in sporadic ET patients despite increased thrombopoiesis. This was thought to be a consequence of marked decrease of c-Mpl expression on pathological platelets.30 Thus, the question arises as to whether the molecular mechanism causing familial ET may be different from that of sporadic ET. Analysis of two cases of sporadic ET showed no mutations within the 5′-UTR of the TPOgene (data not shown). Furthermore, considering previous reports,12,30 the level of serum TPO in familial ET seems to be much higher than in sporadic cases. Collectively, in familial ET, elevated TPO production may constitute the primary event, and the proliferation of megakaryocyte lineage should be a secondary event. Thus, the myeloproliferative state may not be clonal, and the affected patients are not clinically distinguishable from sporadic cases of ET. In conclusion, familial ET may represent an independent entity within myeloproliferative disorders of the megakaryocyte lineage. Most recently, Wiestner et al31 reported that hereditary thrombocythemia accompanied by marked elevation of TPO production occurred as the consequence of exon skipping in the TPO gene. The exon containing the region of the mutation described here was skipped out by the mutation of splice donor site.31Collectively, these findings further support the notion that this particular region of the TPO gene may be important for the regulation of TPO expression.
ACKNOWLEDGMENT
The authors thank Drs T. Taniguchi, N. Tanaka (Tokyo University), H. Shibuya (Okazaki National Research Institute), and M. Kitagawa (Yale University) for invaluable advice; Drs T. Kato and H. Miyazaki (Kirin Brewery Co) for technical support to measure serum concentration of TPO; Drs M. Musashi (Hokkaido University), T. Fukuhara (Asahikawa City Hospital), N. Fujimoto, T. Miyagishima (Kushiro Rosai Hospital), and H. Naruse (Wakkanai Municipal Hospital) for blood sampling; and M. Yanome for secretarial assistance in preparing the manuscript.
Address reprint requests to Takeshi Kondo, MD, PhD, Third Department of Internal Medicine, Hokkaido University School of Medicine, Kita 15 Nishi 7, Kita-ku, Sapporo, Hokkaido 060-8038, Japan; e-mail:t-kondoh@med.hokudai.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal