Abstract
The possibility of primitive hematopoietic cell ex vivo expansion is of interest for both gene therapy and transplantation applications. The engraftment of autologous rhesus peripheral blood (PB) progenitors expanded 10 to 14 days were tracked in vivo using genetic marking. Stem cell factor (SCF)/granulocyte colony-stimulating factor (G-CSF)–mobilized and CD34-enriched PB cells were divided into two equal aliquots and transduced with one of two retroviral vectors carrying the neomycin-resistance gene (neo) for 4 days in the presence of interleukin-3 (IL-3), IL-6, and SCF in the first 5 animals, IL-3/IL-6/SCF/Flt-3 ligand (FLT) in 2 subsequent animals, or IL-3/IL-6/SCF/FLT plus an autologous stromal monolayer (STR) in the final 2. At the end of transduction period, one aliquot (nonexpanded) from each animal was frozen, whereas the other was expanded under the same conditions but without vector for a total of 14 days before freezing. After total body irradiation, both the nonexpanded and expanded transduced cells were reinfused. Despite 5- to 13-fold higher cell and colony-forming unit (CFU) doses from the expanded fraction of marked cells, there was greater short- and long-term marking from the nonexpanded cells in all animals. In animals receiving cells transduced and expanded in the presence of IL-3/IL-6/SCF/FLT, engraftment by the marked expanded cells was further diminished. This discrepancy was even more pronounced in the animals who received cells transduced and expanded in the presence of FLT and autologous stroma, with no marking detectable from the expanded cells. Despite lack of evidence for expansion of engrafting cells, we found that the addition of FLT and especially STR during the initial brief transduction period increased engraftment with marked cells into a clinically relevant range. Levels of marked progeny cells originating from the nonexpanded aliqouts were significantly higher than that seen in previous 4 animals receiving cells transduced in the presence of IL-3/IL-6/SCF, with levels of 10% to 20% confirmed by Southern blotting from the nonexpanded IL-3/IL-6/SCF/FLT/STR graft compared with 0.01% in the original IL-3/IL-6/SCF cohort. These results suggest that, although expansion of PB progenitors is feasible ex vivo, their contribution towards both short- and long-term engraftment is markedly impaired. However, a brief transduction in the presence of specific cytokines and stromal support allows engraftment with an encouraging number of retrovirally modified cells.
This is a US government work. There are no restrictions on its use.
PRIMITIVE HEMATOPOIETIC stem cells (HSCs) are particularly suitable as targets for genetic manipulation based on the ease of their collection and the potential for their manipulation ex vivo. The use of integrating vectors, such as retroviruses, permits the passage of transferred genetic material to all progeny cells, potentially allowing definitive treatment of a wide variety of congenital and acquired diseases, including hemoglobinopathies, immunodeficiencies, metabolic storage disorders, and cancer. However, early preclinical and clinical studies using retroviral vectors to transduce human and nonhuman primate hematopoietic progenitor and stem cells have resulted in levels of engraftment with genetically altered cells too low to hope for clinical benefit in most disorders.1
Ex vivo expansion of retrovirally transduced HSCs could theoretically increase the overall number of gene-corrected cells infused and therefore improve competition against endogenous nontransduced stem cells. More importantly, even if transduction of stem cells is inefficient, expansion of these cells after positive selection for a retrovirally encoded marker gene such as a cell surface protein might result in a safe transplantation dose of almost pure genetically modified cells. This approach has shown promise in vitro.2,3 In murine models, transplantation of positively selected cells has resulted in high-level engraftment with vector-containing cells, but ex vivo expansion postselection has not been explored.4,5 Given the requirement for cell division with current vector systems, strategies effective in expanding engrafting cells ex vivo might also improve transduction efficiency.6
The use of ex vivo-expanded hematopoietic cells to improve the safety and efficacy of autologous and allogeneic transplantation is another application that has generated intense interest and experimentation over the past 5 years.7 8 Ex vivo expanded or activated progenitor and stem cells might result in more rapid recovery after autologous transplantation, allow cells from a single short apheresis procedure or outpatient bone marrow (BM) harvest to support a transplantation procedure, increase applicability of cord blood transplantation to larger recipients, and allow more effective tumor cell purging if expanded cell populations could reliably provide long-term or even short-term engraftment.
In mice, ex vivo culture of marrow cells for 7 days in the presence of multiple hematopoietic growth factors accelerates hematopoietic recovery and increases radioprotection.9 Successful secondary transplantation of cells from the primary recipients suggests that the long-term proliferative potential of murine stem cells is not lost during ex vivo culture.10 However, when cultured cells are competed against fresh cells, a significant engraftment defect was observed.11,12 Many groups have reported ex vivo expansion of human BM or peripheral blood (PB) cells using combinations of hematopoietic growth factors or stromal support systems, with up to several log expansion of both total cell number and colony-forming units (CFU).13-15 Flow cytometric analysis at the end of the culture period has shown the majority of the amplification occurring via terminal differentiation. However, quantitation of more primitive stem cell surrogates such as long-term culture-initiating cells (LTCIC) suggest that these cells can be maintained or expanded under some conditions.16 17
There have been several human clinical trials designed to document the safety and feasibility of infusing expanded progenitor cells in the setting of high-dose chemotherapy. Rapid hematopoietic recovery has been reported after administration of PB CD34-enriched cells expanded for 10 days. Long-term repopulating ability could not be definitively assessed in this study, because fully myeloablative conditioning treatment was not used.18 More worrisome was the report that several patients did not engraft after receiving expanded PB cells in the setting of full myeloablation.19
To more clearly assess the engrafting ability of ex vivo-expanded hematopoietic cells in a model with relevance to human gene therapy and transplantation, we used retroviral gene marking to track the effect of ex vivo expansion on the engraftment of mobilized rhesus monkey PB cells. Our studies indicate that ex vivo expansion of transduced CD34-enriched PB cells in the presence of interleukin-3 (IL-3), IL-6, and stem cell factor (SCF), with or without flt 3 ligand (FLT) or autologous stromal cells (STR), does not increase short-term or long-term engraftment as compared with transduced but nonexpanded cells and diminishes or at best maintains long-term repopulating ability. Our data does not support any contribution to engraftment, even transiently, by committed progenitors such as colony-forming unit–granulocyte-macrophage (CFU-GM). However, a brief ex vivo culture in the presence of flt3 ligand and stromal cells in addition to IL-3, IL-6, and SCF resulted in transduction of repopulating cells at levels of 10% to 20%, a clinically relevant range.
MATERIALS AND METHODS
PB progenitor cell mobilization and harvesting.
Young rhesus macaques (Macaca mulatta) were housed and handled in accordance with the guidelines set forth by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHHS Publication No. NIH 85-23), and the protocol was approved by the Animal Care and Use Committee of the National Heart and Lung and Blood Institute. The animals received recombinant pegylated human SCF (200 μg/kg; Amgen, Thousand Oaks, CA) and recombinant human granulocyte colony-stimulating factor (G-CSF; 10 μg/kg; Amgen) subcutaneously daily for 5 days, followed by apheresis of 2.5 times the blood volume on day 6.20 The mononuclear fraction was purified by density gradient centrifugation over lymphocyte separation media (LSM; Organon Teknika, Durham, NC). Enrichment for primitive progenitor and stem cells was performed using the Ceprate LC CD34 immunoabsorption column as directed (Cellpro, Bothell, WA). The degree of progenitor enrichment was calculated from CFU-GM assays performed before and after column purification.
Ex vivo transduction and expansion.
The producer cell lines G1Na and LNL6 were grown to confluence in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Herndan, VA) supplemented with 10% fetal calf serum (FCS; GIBCO/BRL, Gaithersburg MD).21,22 The biologic titer of these supernatants assayed by transfer of G418-resistance to NIH 3T3 cells ranged from 1 to 5 × 106 cfu/mL. Undiluted vector supernatant was collected and passed through a 0.4-μm filter before transduction of target CD34-enriched cells. For each animal, CD34-enriched PB cells were divided into two equal fractions and each was transduced under identical conditions with one of the two vectors. Transductions were performed as previously described for a total of 96 hours at a starting concentration of 1 × 105 cells/mL with daily replacement of vector supernatant and cytokines.23 All transduction cultures were supplemented with 4 μg/mL protamine sulfate (Sigma, St Louis, MO), 20 ng/mL recombinant human IL-3 (Sandoz, East Hanover, NJ), 50 ng/mL recombinant human IL-6 (Sandoz), and 100 ng/mL recombinant human SCF (Amgen). For 2 animals, 100 ng/mL recombinant human FLT (Research Diagnostics, Flanders, NJ) was also used in the transduction and expansion cultures. An additional 2 animals had their cells cocultured on a preformed irradiated autologous marrow stromal layer (STR) along with all four cytokines. This stromal layer was produced by culturing mononuclear cells from a 10-mL BM aspirate for 2 days in DMEM plus 10% FCS and then removing nonadherent cells by vigorous washing with phosphate-buffered saline (PBS) and continuing to culture the adherent cells until a confluent monolayer formed (∼14 days of culture). Before adding CD34-enriched PB cells, the stromal flask was irradiated with 1,500 cGy.
At the conclusion of the 96-hour transduction period, all the cells transduced with one vector (the nonexpanded fraction) from each animal were harvested by vigorous washing with PBS, followed by trypsinization to remove any adherent cells, and cryopreserved in DMEM, 50% autologous serum, and 10% dimethyl sulfoxide (DMSO; Sigma) using a controlled rate freezer. The other fraction (expanded) from each animal was replated in fresh DMEM, 10% FCS, and cytokines and returned to culture in the same flask, without further exposure to viral supernatant, under the identical culture conditions used during the transduction. Media and cytokines were replenished at day 7 of the culture, and the total volume of the culture was doubled at that time. On day 10 or 14, the expanded cells were harvested and cryopreserved as described above.
Autologous transplantation.
The animals received 650 cGy total body γ-irradiation daily for 2 days. The following day, both the nonexpanded and expanded fractions were thawed in a 37°C water bath and infused. Recombinant human G-CSF (Amgen) was administered at a dose of 5 μg/kg/d as a continuous infusion until the white blood cell count (WBC) reached 6,000/μL. Standard supportive care was administered posttransplantation.24
Analysis of transduction and expansion.
Total cell number and colony-forming units (CFU-GM, CFU-G, CFU-M, and burst-forming unit-erythroid [BFU-E]) were determined for the mononuclear cells precolumn immunoabsorption, for the CD34-enriched cells postcolumn, at the end of the 96-hour transduction period for both fractions, at the end of the expansion period for the expanded fraction, and postthaw for both nonexpanded and expanded fractions. CFU assays were performed using methylcellulose media (StemCell Technologies, Vancouver, British Columbia, Canada) supplemented with 5 U/mL recombinant human erythropoietin (Epo; Amgen), 10 ng/mL IL-3 (Sandoz), 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz), and 100 ng/mL SCF (Amgen). At day 14, colonies containing greater than 50 cells were counted, and 24 individual colonies from each fraction were plucked into 50 μL of distilled water, digested with 20 mg/mL proteinase K at 55°C for 1 hour followed by 99°C for 10 minutes, and assessed for vectorneo sequences by polymerase chain reaction (PCR) as described below. Simultaneous PCR for β-actin sequences was performed on each plucked colony and the percentage of transduction was calculated by dividing the number of CFU positive for the neo gene by the number of CFU positive for β-actin, as previously described.21 23
PB and BM samples were obtained at the time of recovery of the neutrophil count to greater than 1,000/μL, and at time intervals of 1 month, 3 months, 6 months, 9 months, and 1 year posttransplantation. PB and BM mononuclear cells were purified by density centrifugation over LSM. The sedimented pellet containing granulocytes was layered over LSM a second time, and the granulocyte pellet was shown to be greater than 95% pure on cytospin analysis. DNA was extracted as directed using the QIAamp Blood Kit (Qiagen, Santa Clarita, CA). For isolation of T and B cells, PB cells were stained with anti-CD2-fluorescein isothiocyanate (FITC)/CD19-phycoerythrin (PE) (Immunotech, Marseille, France) and sorted using a Coulter Epics Elite instrument (Coulter, Hialeah, FL). Sorted populations had purities of greater than 95%.
PCR analyses for neo and β-actin sequences were performed using primers and conditions as previously described.23Negative controls consisted of normal rhesus PB samples extracted concurrently with each set of test samples and a reagent control. A positive control dilution series was prepared by making serial dilutions of DNA from the producer cell line G1Na (containing 2 integrated copies per cell) into normal rhesus PB DNA. Additionally, DNA from the LNL6 producer line was mixed with DNA from the G1Na producer cell line in ratios of 0/100, 20/80, 50/50, 80/20, and 100/0 and diluted into control rhesus BM DNA to an combined percentage of 2%. All neo and β-actin PCR reactions were optimized to yield linear amplifications in the range of the intensity of the in vivo samples. The neo PCR products were run on a 25-cm 8% polyacrylamide gel (National Diagnostics, Atlanta, GA) to allow separation of the 16-bp difference between the G1Na and LNL6 radiolabeled amplification products. Phosphorimager technology was used to measure the intensity of each band (Molecular Dynamics, Sunnyvale, CA). Neo band intensity was normalized for amplifiable DNA content based on the β-actin signal, and the overall contribution of each vector to in vivo marking was calculated by plotting the signal intensities of each band to those of a standard curve generated from the positive control dilutions.
For Southern blotting, 10 μg of genomic DNA was cut with KpnI (Boehringer Mannheim, Indianapolis, IN) for 8 hours at 37°C and electrophoresed on a 0.8% agarose gel. The DNA was transferred to a nylon membrane and subsequently hybridized with a radiolabeled 611-bpneo probe and analyzed by autoradiography.
Statistical analysis.
The paired two-tailed Student’s t-test, ANOVA, and regression analysis were performed using Microsoft Excel 5.0 (Microsoft, Seattle, WA).
RESULTS
Experimental design.
Figure 1 summarizes the experimental design used to test the competitive repopulating ability of genetically marked nonexpanded CD34-enriched PB cells compared with genetically marked expanded cells. SCF/G-CSF–mobilized PB cells from each rhesus monkey were CD34-enriched and then divided into two equal fractions. Each fraction was transduced with one of two retroviral vectors (G1Na or LNL6) containing the bacterial neomycin resistance phosphotransferase gene (neo). At the completion of the 96-hour transduction period, one fraction was frozen and the other was cultured under the same culture conditions but without vector supernatant for a total of 10 to 14 days. The vector (LNL6 v G1Na) used to transduce the nonexpanded versus the expanded fraction was alternated in sequential monkeys to control for possible differences in each vector’s gene transfer efficiency into primitive hematopoietic cells despite equivalent in vitro biologic titers as assessed on 3T3 cells and CFU-GM. Table 1 summarizes the transduction/expansion culture conditions used for each animal.
Experimental design. Rhesus macaques underwent mobilization with G-CSF/SCF followed by apheresis of 2.5 times their blood volume. The apheresis product was enriched for primitive progenitors by CD34 selection and split into two equal fractions for a 96-hour transduction with either of two retroviral vectors: G1 (G1Na) or LN (LNL6). One aliquot was frozen at the end of transduction, whereas the other was returned to the original culture conditions without further exposure to retrovirus for a total of 10 to 14 days before freezing. Culture condition A: IL-3, IL-6, SCF; culture condition B: IL-3, IL-6, SCF, Flt-3 ligand; culture condition C: IL-3, IL-6, SCF, Flt-3 ligand, and an autologous stromal monolayer. After 650 cGy TBI on 2 consecutive days, both the transduced (nonexpanded) and the transduced and expanded aliquots were thawed and simultaneously reinfused.
Experimental design. Rhesus macaques underwent mobilization with G-CSF/SCF followed by apheresis of 2.5 times their blood volume. The apheresis product was enriched for primitive progenitors by CD34 selection and split into two equal fractions for a 96-hour transduction with either of two retroviral vectors: G1 (G1Na) or LN (LNL6). One aliquot was frozen at the end of transduction, whereas the other was returned to the original culture conditions without further exposure to retrovirus for a total of 10 to 14 days before freezing. Culture condition A: IL-3, IL-6, SCF; culture condition B: IL-3, IL-6, SCF, Flt-3 ligand; culture condition C: IL-3, IL-6, SCF, Flt-3 ligand, and an autologous stromal monolayer. After 650 cGy TBI on 2 consecutive days, both the transduced (nonexpanded) and the transduced and expanded aliquots were thawed and simultaneously reinfused.
Ex Vivo Culture, Infusion, and Engraftment Data on All Animals
| Animal . | Culture Conditions* . | Total Cell (no./kg × 106) . | CFU (no./kg × 105) . | Day ANC >500/μL¶ . | ||||
|---|---|---|---|---|---|---|---|---|
| Postcolumn-151 . | End Transduction-152 . | End Expansion-153 . | Postcolumn-151 . | End Transduction-152 . | End Expansion-153 . | |||
| RQ813 | IL-3/IL-6/SCF:10 | 6.4 | 11.0 | 114 | 3.6 | 2.0 | 7.4 | 5 |
| RQ923 | IL-3/IL-6/SCF:10 | 5.2 | 10.4 | 104 | 3.9 | 2.5 | 8.3 | 5 |
| RQ1271 | IL-3/IL-6/SCF:14 | 7.1 | 8.0 | 186 | 3.9 | 3.0 | 63.7 | 6 |
| RQ1094 | IL-3/IL-6/SCF:14 | 4.0 | 11.0 | 130 | 3.3 | 2.6 | 27.9 | 6 |
| L161 | IL-3/IL-6/SCF/FLT:14 | 5.8 | 11.7 | 131 | 3.3 | 4.1 | 29.5 | 7 |
| J039 | IL-3/IL-6/SCF/FLT:14 | 1.3 | 3.1 | 41.1 | 0.9 | 1.5 | 4.5 | 17 |
| RC402 | IL-3/IL-6/SCF/FLT/STR:14 | 5.4 | 12.8 | 76.1 | 4.1 | 3.9 | 11.5 | 7 |
| 94E048 | IL-3/IL-6/SCF/FLT/STR:14 | 4.6 | 5.3 | 27.0 | 1.4 | 0.7 | 1.9 | 19 |
| Animal . | Culture Conditions* . | Total Cell (no./kg × 106) . | CFU (no./kg × 105) . | Day ANC >500/μL¶ . | ||||
|---|---|---|---|---|---|---|---|---|
| Postcolumn-151 . | End Transduction-152 . | End Expansion-153 . | Postcolumn-151 . | End Transduction-152 . | End Expansion-153 . | |||
| RQ813 | IL-3/IL-6/SCF:10 | 6.4 | 11.0 | 114 | 3.6 | 2.0 | 7.4 | 5 |
| RQ923 | IL-3/IL-6/SCF:10 | 5.2 | 10.4 | 104 | 3.9 | 2.5 | 8.3 | 5 |
| RQ1271 | IL-3/IL-6/SCF:14 | 7.1 | 8.0 | 186 | 3.9 | 3.0 | 63.7 | 6 |
| RQ1094 | IL-3/IL-6/SCF:14 | 4.0 | 11.0 | 130 | 3.3 | 2.6 | 27.9 | 6 |
| L161 | IL-3/IL-6/SCF/FLT:14 | 5.8 | 11.7 | 131 | 3.3 | 4.1 | 29.5 | 7 |
| J039 | IL-3/IL-6/SCF/FLT:14 | 1.3 | 3.1 | 41.1 | 0.9 | 1.5 | 4.5 | 17 |
| RC402 | IL-3/IL-6/SCF/FLT/STR:14 | 5.4 | 12.8 | 76.1 | 4.1 | 3.9 | 11.5 | 7 |
| 94E048 | IL-3/IL-6/SCF/FLT/STR:14 | 4.6 | 5.3 | 27.0 | 1.4 | 0.7 | 1.9 | 19 |
*IL-3 (20 ng/mL), IL-6 (50 ng/mL), SCF (100 ng/mL), FLT (100 ng/mL), and STR coculture on autologous marrow stromal cell layer. For each animal, these culture conditions were used for both the transduction and expansion periods. The total length of culture for the expanded fraction is shown in days.
The number of total cells or CFU present after CD34 column immunoabsorption, expressed per kilogram of recipient animal.
The number of total cells or CFU present in the nonexpanded fraction at the end of the 96-hour transduction period (note only 50% of the starting postcolumn CD34-enriched cells were used in this fraction) expressed per kilogram of recipient.
The number of total cells or CFU present in the expanded fraction at the end of the 10- to 14-day expansion period (note only 50% of the starting postcolumn CD34-enriched cells were used in this fraction) expressed per kilogram of recipient.
¶Day 0 defined as the day of reinfusion.
Total cell and CFU expansion and transduction.
CD34 column immunoabsorption of the mobilized PB mononuclear cells resulted in a 204- ± 64.5-fold enrichment of CFU. As shown in Fig 2, total cell numbers increased 3.85- ± 1.2-fold during the 96-hour transduction period and then a total of 42.3-± 18-fold during the expansion period. There was no statistically significant difference (P = .09, ANOVA) in the total cellular expansion between the three transduction/culture conditions, although there was a trend towards less expansion in the stromal cocultures (IL-3/IL-6/SCF, 47.4-fold; IL-3/IL-6/SCF/FLT, 54.4-fold; and IL-3/IL-6/SCF/FLT/STR, 19.8-fold).
Total cell numbers during transduction and expansion periods. For each animal, the total cell number present in the cultures at the following time points are shown: starting CD34 enriched fraction (Starting), the end of transduction for the nonexpanded fraction (End Transduction-NE), the end of transduction for subsequently expanded fraction (End Transduction-Exp), and the end of expansion (End Expansion). The culture conditions are shown below: IL-3, interleukin 3; IL-6, interleukin-6; SCF, stem cell factor; FLT, flt3 ligand; STR, autologous stromal monolayer.
Total cell numbers during transduction and expansion periods. For each animal, the total cell number present in the cultures at the following time points are shown: starting CD34 enriched fraction (Starting), the end of transduction for the nonexpanded fraction (End Transduction-NE), the end of transduction for subsequently expanded fraction (End Transduction-Exp), and the end of expansion (End Expansion). The culture conditions are shown below: IL-3, interleukin 3; IL-6, interleukin-6; SCF, stem cell factor; FLT, flt3 ligand; STR, autologous stromal monolayer.
The expansion of committed progenitors (CFU-GM, CFU-G, CFU-M, and BFU-E) is shown in Fig 3. CFU increased 1.8- ± 0.8-fold during the 96-hour transduction and a total of 11.8- ± 10.4-fold in the expanded fraction. There was no statistically significant difference (P = .55, ANOVA) in the total CFU expansion between the three culture conditions (IL-3/IL-6/SCF, 14.5-fold; IL-3/IL-6/SCF/FLT, 14.3-fold; and IL-3/IL-6/SCF/FLT/STR, 4.5-fold). The number of CFU in both nonexpanded and expanded fractions after thawing were not significantly different from prefreeze CFU content, and viability was greater than 95% (data not shown).
CFU during transduction and expansion periods. For each animal, the total CFU (CFU-GM, CFU-M, CFU-G, and BFU-E) present in the cultures at the following time points are shown: starting CD34 enriched fraction (Starting), the end of transduction for the nonexpanded fraction (End Transduction-NE), the end of transduction for subsequently expanded fraction (End Transduction-Exp), and the end of expansion (End Expansion). The culture conditions are shown below: IL-3, interleukin-3; IL-6, interleukin-6; SCF, stem cell factor; FLT, flt3 ligand; STR, autologous stromal monolayer.
CFU during transduction and expansion periods. For each animal, the total CFU (CFU-GM, CFU-M, CFU-G, and BFU-E) present in the cultures at the following time points are shown: starting CD34 enriched fraction (Starting), the end of transduction for the nonexpanded fraction (End Transduction-NE), the end of transduction for subsequently expanded fraction (End Transduction-Exp), and the end of expansion (End Expansion). The culture conditions are shown below: IL-3, interleukin-3; IL-6, interleukin-6; SCF, stem cell factor; FLT, flt3 ligand; STR, autologous stromal monolayer.
Figure 4 shows the percentage ofneo-marked CFU-GM present in the two fractions from each animal at the end of the 4-day transduction period, as well as in the expanded fraction at the end of the 10- to 14-day expansion period. Both aliquots (nonexpanded and to be expanded) were transduced with equivalent efficiencies as assessed by the percentage ofneo-marked CFU-GM at the end of the transduction period (51.0% ± 23.5% v 48.4% ± 29.2%, P= .73, two-tailed t-test). In the first 4 animals, transductions and expansions were performed in the presence of IL-3, IL-6, and SCF, and the mean transduction efficiency with either vector was 39.2% ± 22.2%. In the 2 animals whose cells were transduced and expanded with the addition of FLT, the mean transduction efficiency was 47.9% ± 28.7%. In the final 2 animals whose cells were transduced and expanded with the addition of both FLT and STR, the mean transduction efficiency was 72.4% ± 18.4%. There was a trend towards a higher in vitro transduction efficiency for cultures including FLT (P = .099, ANOVA). Importantly, the percentage ofneo-positive CFU from the end of the expansion period was in every case almost identical to the percentage of neo-positive CFU present in the same fraction aliquot before expansion (48.4% ± 29.2% end transduction v 45.3% ± 26.3% on the same fractions at the end of expansion; P = .64, paired two-tailedt-test), indicating no selective loss of successfully transduced CFU during the expansion period, and similar levels of transduction of cells fated to become CFU during the expansion period.
Marking efficieny of CFU in nonexpanded and expanded fractions. In vitro transduction efficiency of CFU was assessed at the end of transduction for both fractions and the end of expansion for the expanded fraction. The efficiency was calculated by from the percentage of individual plucked CFU positive for neo sequences by PCR. For each animal, the percentage of CFU positive for the neogene is shown at the end of transduction for the nonexpanded fraction (End Transduction-NE), at the end of transduction for the fraction subsequently expanded (End Transduction-Exp), as well as colonies plated at the end of the expansion period for the expanded fraction (End Expansion).
Marking efficieny of CFU in nonexpanded and expanded fractions. In vitro transduction efficiency of CFU was assessed at the end of transduction for both fractions and the end of expansion for the expanded fraction. The efficiency was calculated by from the percentage of individual plucked CFU positive for neo sequences by PCR. For each animal, the percentage of CFU positive for the neogene is shown at the end of transduction for the nonexpanded fraction (End Transduction-NE), at the end of transduction for the fraction subsequently expanded (End Transduction-Exp), as well as colonies plated at the end of the expansion period for the expanded fraction (End Expansion).
Engraftment kinetics.
Engraftment was prompt in the majority of the animals, with a median of 7 days (range, 5 to 19 days) to reach a neutrophil count of 500/μL (Table 1). The time to engraftment correlated well with the starting CFU dose per kilogram placed into culture from each animal (r = .941, linear regression analysis), but not with the end expansion CFU dose (r = .440). Engraftment was more rapid than in historical animals transplanted by our program using CD34-enriched BM grafts (neutrophil recovery days 18 to 24) or combined mobilized PB and primed BM CD34-enriched grafts (days 10 to 14).25,26 However, the seemingly more rapid engraftment did not appear to be due to the use of ex vivo expanded cells, because more recently a series of animals receiving equivalent doses of transduced but not expanded SCF/G-CSF–mobilized PB cells have also engrafted as early as days 5 to 7 (Y. Hanazono and J. Tisdale, data not shown). The very rapid engraftment is most likely due to progressively better apheresis and CD34-enrichment techniques, resulting in higher total primitive cell dosages, and agrees with murine data relating engraftment time to primitive cell dose.27
In vivo detection of transduced cells.
The use of the two vectors differing by 16 bp to transduce the nonexpanded versus expanded fractions of cells from each animal allowed simple quantitation of the relative contribution of each fraction to in vivo marking (example shown in Fig 5). Figure 6A and B summarizes the in vivo marking data from all 8 animals observed long-term, with levels of marked cells derived from the nonexpanded and expanded fractions shown and the ratio of marking in cells derived from the nonexpanded to expanded fractions calculated for each time point. In the first cohort of 4 animals, transductions and expansions were performed in the presence of IL-3/IL-6/SCF (Fig 6A). Low-level long-term marking was detected, with only 1/10,000 cells or 0.01% containing the vector, assuming a single copy of vector per marked cell. In almost every cell type analyzed for each time point, the ratio of marked cells derived from the nonexpanded to the expanded fraction was greater than 1, reflecting a greater contribution towards in vivo marking in progeny cells derived from the nonexpanded marked fraction. The level of marked cells originating from the expanded aliquots did not reflect the in vitro transduction efficiency of CFU, despite the 10-fold or greater numbers of total cells, total CFU, and marked CFU infused from the expanded aliquots. On the day of engraftment, the level of marked cells originating from the expanded aliquot was no higher than the level of marked cells originating from the nonexpanded aliquot, suggesting that marked CFU were not contributing to early hematopoietic reconstitution.
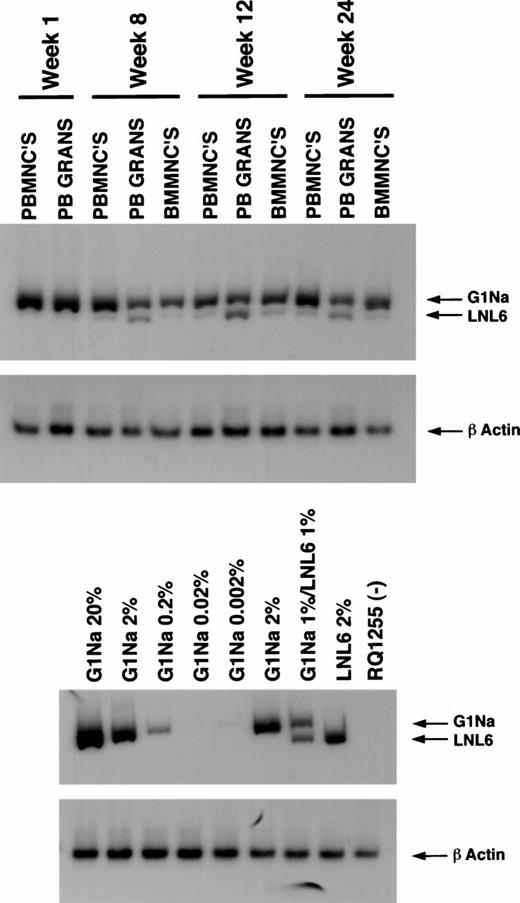
PCR analysis of posttransplantation samples on animal L161. L161 received cells that were transduced (G1Na) and transduced (LNL6) and expanded in the presence of IL-3, IL-6, SCF, and Flt-3 ligand. PB mononuclear cells (PBMNCs), granulocytes (PB GRANS), and BM mononuclear cells (BMMNCs) were analyzed by PCR for the neogene. The 16-bp difference between the vectors in the amplified neoregion allows simultaneous assessment of marking in progeny from the nonexpanded (G1Na) and the expanded (LNL6) infused fractions. Concurrent amplification of β-actin was performed on each sample to quantitate the amount of amplifiable template DNA. Serial dilutions and mixtures of DNA from the producer cell lines containing a known copy number of the neo gene into control rhesus PB DNA at the indicated percentages were used to quantitate the percentage of marked cells posttransplant. RQ1255 represents a sample simultaneously processed and extracted from a negative control animal.
PCR analysis of posttransplantation samples on animal L161. L161 received cells that were transduced (G1Na) and transduced (LNL6) and expanded in the presence of IL-3, IL-6, SCF, and Flt-3 ligand. PB mononuclear cells (PBMNCs), granulocytes (PB GRANS), and BM mononuclear cells (BMMNCs) were analyzed by PCR for the neogene. The 16-bp difference between the vectors in the amplified neoregion allows simultaneous assessment of marking in progeny from the nonexpanded (G1Na) and the expanded (LNL6) infused fractions. Concurrent amplification of β-actin was performed on each sample to quantitate the amount of amplifiable template DNA. Serial dilutions and mixtures of DNA from the producer cell lines containing a known copy number of the neo gene into control rhesus PB DNA at the indicated percentages were used to quantitate the percentage of marked cells posttransplant. RQ1255 represents a sample simultaneously processed and extracted from a negative control animal.
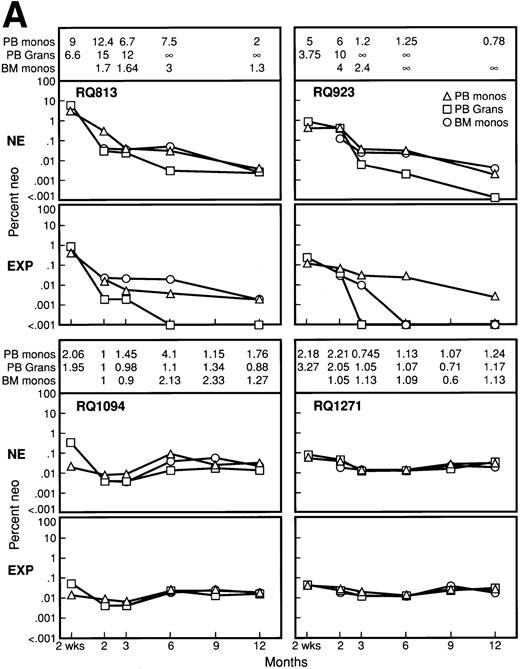
Percentage of marking calculated by semiquantitative PCR originating from both the nonexpanded and expanded population of marked cells posttransplant for all 8 animals. For each animal, the upper panel represents the percentage of marking originating from transduced but nonexpanded cells (NE), while the lower panel represents the percentage of marking from the expanded cells (EXP). Marking was assessed in PB mononuclear cells (PB monos), PB granulocytes (PB Grans), and BM mononuclear cells (BM monos) at 2 weeks, 2 months, 3 months, 6 months, 9 months, and 12 months. In each panel, the ratio of marking from the nonexpanded cells to the marking from the expanded cells is given for each fraction analyzed at each time point in the upper portion of each panel. A ratio greater than 1 signifies higher marking from the nonexpanded cells, and ∞ denotes no detectable marking from the expanded cells, at least a 3 log difference given the PCR sensitivity.
Percentage of marking calculated by semiquantitative PCR originating from both the nonexpanded and expanded population of marked cells posttransplant for all 8 animals. For each animal, the upper panel represents the percentage of marking originating from transduced but nonexpanded cells (NE), while the lower panel represents the percentage of marking from the expanded cells (EXP). Marking was assessed in PB mononuclear cells (PB monos), PB granulocytes (PB Grans), and BM mononuclear cells (BM monos) at 2 weeks, 2 months, 3 months, 6 months, 9 months, and 12 months. In each panel, the ratio of marking from the nonexpanded cells to the marking from the expanded cells is given for each fraction analyzed at each time point in the upper portion of each panel. A ratio greater than 1 signifies higher marking from the nonexpanded cells, and ∞ denotes no detectable marking from the expanded cells, at least a 3 log difference given the PCR sensitivity.
In an attempt to improve engraftment by expanded cells, FLT with or without an autologous stromal monolayer (STR) was included during both transduction and expansion, based on multiple reports of the unique properties of FLT in stimulating division of very primitive hematopoietic cells.28-31 In the 2 animals receiving cells marked and expanded in IL-3/IL-6/SCF/FLT, the relative defect in engraftment of marked cells originating from the expanded fraction was even greater (Figs 5 and 6B). This was partly due to improved marking from the nonexpanded fraction compared with nonexpanded cells transduced without FLT (average levels of 1% compared with 0.01%), with no improvement in long-term marking from the expanded cells. In 1 animal (J039), no marking from the expanded fraction was detectable despite 83.3% marked CFU infused. In the 2 animals receiving cells transduced and expanded in the presence of FLT and STR in addition to IL-3/IL-6 and SCF (RC402 and 94E048), no marking from the expanded cell population could be detected (sensitivity of the PCR assay 0.001%). However, marking from the nonexpanded fraction was further improved, with initial levels of greater than 10% estimated by PCR (Figs 5 and6B).
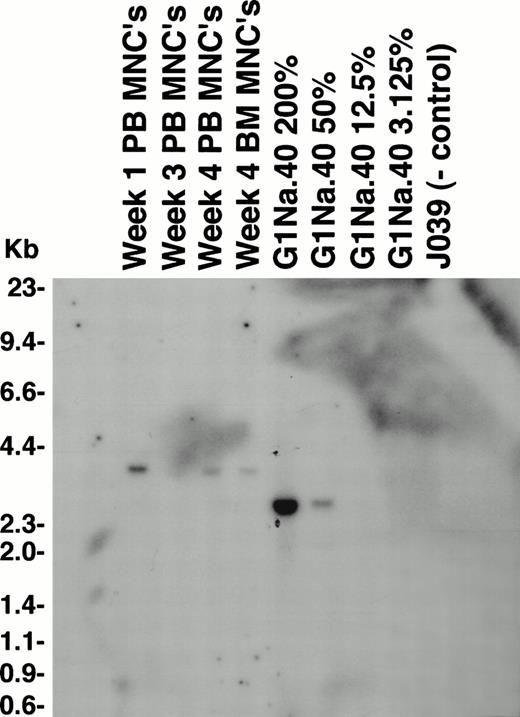
Southern blotting confirmed the PCR-quantitated marking levels in animal RC402, with posttransplantation mononuclear cells and granulocyte samples having levels of 12.5% to 50% marked cells, all originating from the nonexpanded cell fraction (LNL6), and no detectable marking from the expanded cell fraction (G1Na) (Fig 7). Digestion of the same DNA samples with an enzyme that cuts once within the proviral DNA for insertion site analysis resulted in no detectable bands, suggesting that the marking did not originate from a single transduced clone and was at least oligoclonal (data not shown). Sorted populations of PB B cells, T cells, and granulocytes collected just before death from radiation enteritis 3 months posttransplantation showed equivalent marking in each cell fraction of approximately 5%. Southern blotting of DNA from postmortem tissues showed bands corresponding to the nonexpanded graft in the spleen, axillary lymph node, and BM at levels of 3% to 12%. Five of 16 CFU (31.3%) from a postmortem BM sample were positive for the neo gene by PCR.
Southern blot analysis of genetic marking in animal RC402. Ten micrograms of genomic DNA was digested with Kpn I, which cuts within the viral LTRs. In this animal, the nonexpanded fraction was transduced with LNL6 and the expanded fraction with G1Na in the presence of IL-3, IL-6, SCF, Flt-3 ligand, and an autologous stromal monolayer. PB mononuclear cells (PB MNCs) and BM mononuclear cells (BM MNCs) were analyzed, along with positive control dilutions of G1Na producer cell line DNA mixed with normal rhesus PB DNA at the percentages shown. The band present in these samples (3.0 kb) match the predicted size for LNL6 (3.0 kb).
Southern blot analysis of genetic marking in animal RC402. Ten micrograms of genomic DNA was digested with Kpn I, which cuts within the viral LTRs. In this animal, the nonexpanded fraction was transduced with LNL6 and the expanded fraction with G1Na in the presence of IL-3, IL-6, SCF, Flt-3 ligand, and an autologous stromal monolayer. PB mononuclear cells (PB MNCs) and BM mononuclear cells (BM MNCs) were analyzed, along with positive control dilutions of G1Na producer cell line DNA mixed with normal rhesus PB DNA at the percentages shown. The band present in these samples (3.0 kb) match the predicted size for LNL6 (3.0 kb).
DISCUSSION
Ex vivo expansion of hematopoietic progenitor cells remains an area of active interest in the field of stem cell transplantation and HSC gene transfer, yet the long-term fate of ex vivo-expanded cells remains unresolved.7,8 Using a relevant large animal model and genetic marking, we were able to track the fate of the progeny of ex vivo-expanded hematopoietic cells transduced and expanded under a variety of conditions.32 The experimental design allowed simultaneous tracking of cells derived from both nonexpanded and expanded marked cells within the same animal after complete myeloablation, serving as an in vivo competitive repopulation assay and allowing assessment of the relative engrafting ability of marked cells under nonexpanded and expanded conditions. In vitro measures of expansion yielded results similar to those reported by others, with significant expansion of both total cell number and CFU.7There was no selective loss of marked CFU during the expansion period, and animals received a log or greater marked expanded as compared with nonexpanded cells. Despite this difference, in vivo marking did not reflect the relative number of marked nonexpanded versus expanded CFU infused at any time posttransplantation, even on the day of engraftment. There was no evidence of expansion of marked long-term repopulating cells; at best, the cells were maintained and, in fact, there was a significant defect in long-term marked engraftment originating from the expanded cell fraction compared with the nonexpanded fraction under some culture conditions.
Time to engraftment, although rapid, correlated with the number of primitive cells present in the original CD34-enriched collection and not with that from prolonged ex vivo expansion. PCR analysis of granulocytes, even on the day of recovery, showed an equal or greater signal originating from the nonexpanded graft at levels not reflecting the high percentage of marked CFU infused, directly confirming data from the murine model that lineage-committed CFU do not contribute to engraftment and that numbers of ex vivo-cultured CFU do not predict engraftment.27,33 In human transplantation, the dose of unmanipulated CD34+ cells or the number of CFU-GM may predict time to engraftment and other important clinical outcomes, but it may be ill advised to assume that these surrogate stem cell assays are meaningful after ex vivo manipulation.34-36 We did not quantitate LTCIC or attempt FACS analysis of primitive cell phenotypes, but culture conditions similar to those shown to expand the numbers of LTCIC or CD34+/CD38− cells in culture did not in our primate model translate into improved in vivo engrafting ability.17 31
One animal initially entered on this study did not mobilize well and had a very low number of CD34-enriched cells available for transduction and expansion in the presence of IL-3/IL-6/SCF. Despite a 21-fold increase in total cell number and 26-fold increase in CFU over the expansion period, bringing these values into a safe range for rapid engraftment using fresh cells (1.6 × 106 CFU/kg), only a transient increase in neutrophil count was seen without any evidence of platelet recovery, and the animal had to be killed at day 38 for failure to engraft. A similar phenomena has recently been reported in a clinical trial in which ex vivo-expanded cells were infused without unmanipulated cells in a small number of patients (4) after myeloablative conditioning; lack of sustained hematopoietic recovery prompted infusion of the unmanipulated cryopreserved back-up cells.19 Problems with excessive cell loss due to clumping in 1 patient, heavy pretreatment in a second (including a prior autograft), and fungal contamination of the culture in the third left only 1 patient evaluable; therefore, this study serves as a caution but not definitive proof that ex vivo expansion is detrimental to engraftment of hematopoietic progenitors. Taken together with our results, this clinical trial underscores the need for more predictive and rapid assays of human hematopoiesis and the need for carefully designed large animal studies before large-scale human clinical applications of HSC ex vivo expansion technology.
There are several caveats to the interpretation of our data. First, we are only able to follow cells long-term that have been successfully transduced, which in the first cohort of animals, especially, represented a very small fraction of those cells contributing to hematopoiesis. There may be specific characteristics of transducible primitive cells that preclude ex vivo expansion. For instance, these cells must have cycled early during the culture period to be transduced, and perhaps cycling at this time resulted in phenotypic changes precluding homing or other necessary steps in engraftment, even if the cells avoided apoptosis and terminal differentiation. One group has suggested that the engraftment defect seen in a murine model using ex vivo-cultured cells results from an inability of cycling cells to engraft.11,37 There is also evidence that only certain subsets of murine repopulating cells express receptors for amphotropic retroviral vectors, and extrapolation of this data might suggest that cells expressing the receptor can not be expanded ex vivo.38 Thus, our only definitive conclusion can be that retovirally marked engrafting cells were not expanded ex vivo. This has important implications for gene therapy protocols, but not necessarily for other applications in clinical transplantation.
Second, we have evaluated only G-CSF/SCF–mobilized, CD34-enriched PB cell grafts. Although these results may also apply to other cell sources such as BM or cord blood, this should be tested. Some encouraging in vitro and xenograft data have suggested that cord blood stem cells may have unique expansion capacities.39-41 The ability to expand cord blood-engrafting cells would also have the most immediate clinical application, because the success and widespread use of allogeneic cord blood transplantation in adult recipients is currently limited by low cell doses and slow engraftment.42,43 Third, only three culture conditions were tested. Other cytokine combinations, stromal support systems, or even the use of bioreactor technology might have improved expansion of engrafting cells.15,44 Other culture components aimed at preventing apoptosis or terminal differentiation, such as anti–transforming growth factor-β antibodies, or new cytokines with activity on primitive cells, such as thrombopoietin, should also be tested.45-47 In one study, expansion of LTCIC was achieved only when relatively high doses of hematopoietic growth factors were used, yet these high doses were not required to expand total cell number or CFU.17 A relatively prolonged ex vivo culture period was used (10 to 14 days). Shorter culture periods may produce more modest increases in cell number or CFU, but allow retention or even expansion of true engrafting cells. Data from the NOD/SCID xenograft model suggests that 4 days of ex vivo expansion allows good engraftment of human cells, but longer periods are very detrimental.48
Although ex vivo expansion in our study was detrimental to marking in vivo for all three culture conditions studied, marking from the nonexpanded but transduced fraction was markedly improved by manipulations that were designed to enhance expansion. In the second cohort of animals receiving cells transduced in the presence of FLT, stable long-term marking of 1% was seen, compared with a large number of animals in this and other studies with levels of only 0.01% long-term when transductions were performed in the presence of IL-3/IL-6/SCF alone. With the addition of FLT and autologous stroma, clinically relevant levels of greater than 10% in vivo gene transfer were obtained. These results are encouraging for the eventual successful application of hematopoietic stem cell gene transfer and need to be confirmed and studied in more detail in a larger cohort of nonhuman primates and ultimately in humans.
ACKNOWLEDGMENT
The authors thank Malcolm Brenner, Arthur Nienhuis, and Neal Young for helpful discussions; Martha Kirby for FACS; Barrington Thompson and Earl West for their assistance in caring for the animals; and Amgen for providing cytokines.
Address reprint requests to J.F. Tisdale, MD, Bldg 10/Room 7C208, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892-1652; e-mail: tisdalej@gwgate.nhlbi.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions in its use.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal