Abstract
Activation of the transcription factor NFκB in peripheral blood T cells from patients with renal cell carcinoma (RCC) is compromised. This impaired signaling function results from a failure of RelA and c-Rel to translocate to the nucleus though normal levels of Rel proteins are present in the cytoplasm. We demonstrate here in a subset of RCC patients that the defect in NFκB activation is attributable to the absence of phosphorylation and degradation of the inhibitor IκB. In patient T cells there was no stimulus dependent decrease in the cytoplasmic level of IκB. Coimmunoprecipitation studies showed that RelA was in complex with IκB and was not released after stimulation. Moreover, the phosphorylated form of IκB detected in normal T cells after activation is absent in patient T cells. Additional experiments showed that soluble products from RCCs (RCC-S) can reproduce the same phenotype in T cells from healthy individuals. Supernatant fluid from cultured explants of RCC, but not normal kidney, inhibited the stimulus dependent nuclear translocation of NFκB without altering the cytoplasmic levels of RelA, c-Rel, and NFκB1. Phosphorylation and degradation of IκB was also blocked by RCC-S. The mechanistic similarities between patient-derived T cells and normal T cells cultured with RCC-S suggest that the tumor-derived products may be the primary mediators of impaired T-cell function in this tumor system.
© 1998 by The American Society of Hematology.
EXPOSURE OF LYMPHOCYTES to antigen and certain cytokines results in the activation of the transcription factor, NFκB.1-3 NFκB consists of multiple proteins belonging to the Rel family that include p105/p50(NFκB1), p65(RelA), p100/p52(Lyt10, NFκB2), c-Rel, and RelB.4-13 Different family members can associate in various homodimeric and heterodimeric forms through a highly conserved N-terminal sequence referred to as the Rel homology region.3-8,14,15 NFκB members participate in the transcriptional control of a diverse set of genes whose products play a significant role in T-lymphocyte activation and the development of cellular immunity.2-4 The RelA/NFκB1 heterodimer has been most thoroughly studied and is known to have transactivating function, whereas the NFκB1 homodimer appears to function most commonly as a transcriptional suppressor.4,7,8,15,16 NFκB is sequestered in an inactive form in the cytoplasm of T cells through interaction with one or more inhibitory proteins collectively termed IκBs.4,17-19 IκBα, the best-characterized IκB, blocks both DNA binding activity and nuclear localization of RelA and c-Rel.4,17-19 IκBα appears to be a main regulator of NFκB, because the degradation of IκBα matches NFκB translocation to the nucleus.17-19 After stimulation with different inducers, NFκB activation follows the phosphorylation and subsequent degradation of IκBα.17-21 The phosphorylation of IκBα on serine residues 32 and 36 is thought to mark this inhibitory protein for ubiquitination and degradation by a proteasome pathway, allowing Rel dimers to translocate to the nucleus and initiate transcription.22 23
Although antitumor immunity is frequently associated with the activation of T cells, multiple studies suggest that T-cell immunity fails to develop adequately in cancer patients.24-30Defects in proliferation, lytic activity, and cytokine production have been noted in peripheral blood T cells; however, more pronounced alterations have been reported for T cells infiltrating the tumor.24-30 Impaired T-cell function is reported to involve alterations in stimulus-induced signaling responses and reports from multiple laboratories have demonstrated alterations in TCRζ chain expression and reduced tyrosine kinase activity.31-38 Our laboratory and others have reported that T cells isolated from tumor-bearing mice and from patients with renal cell carcinoma (RCC) showed impaired stimulus-dependent activation of κB-specific DNA binding activity.39-41 In tumor-bearing mice, the suppression of NFκB activation correlated with tumor progression.39 The mechanism(s) directly responsible for reduced NFκB activation in T cells from cancer patients remains obscure.
In the present report, we provide analysis of the mechanism responsible for impaired NFκB activation in a subset of RCC patients. In patients whose T cells failed to exhibit normal activation of NFκB, stimulus-dependent degradation of the inhibitor IκBα was also blocked. The absence of induced degradation of IκBα in patient T cells was associated with a failure of IκBα phosphorylation. A tumor-derived product may be directly responsible for the suppression of NFκB in patient T cells, because culture supernatant from explants of RCC (RCC-S) inhibited the activation of NFκB in T cells from healthy volunteers by blocking IκBα phosphorylation and degradation.
MATERIALS AND METHODS
Antibodies and other reagents.
Antibodies used in Western blotting for NFκB1 (p50), c-Rel, RelA (p65), and IκBα/MAD-3 were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antibodies to RelA, c-Rel, and NFκB1 were used at final concentration of 1.5 μg/mL, whereas rabbit antihuman IκBα/MAD-3 was used at 1.0 μg/mL. The secondary antibody was donkey antirabbit for c-Rel, RelA, NFκB1, and IκBα. Antibodies used in magnetic T-cell separation were bead-conjugated monoclonal antihuman CD14 (monocyte), CD56/CD16 (NK cell), and CD19 (B cell) antibodies (StemCell Technologies Inc, Vancouver, British Columbia, Canada). Phorbol myristate acetate (PMA; 20 ng/mL) and ionomycin (0.75 μg/mL) were obtained from Sigma (St Louis, MO), whereas tumor necrosis factor α (TNFα; 10 ng/mL) was purchased from R & D Systems Inc (Minneapolis, MN).
Patient population.
Peripheral blood lymphocytes (PBL) were obtained from 7 patients with confirmed diagnosis of RCC seen at the Cleveland Clinic Foundation (Cleveland, OH). Tumor tissue was obtained from an additional 16 RCC patients and was used to make tumor supernatants. Control lymphocytes were obtained from healthy volunteers that had been leukophoresed (n = 26). T cells from 10 normal donors were used as positive controls for patient T-cell studies and 16 were used for coculturing with tumor supernatant.
Isolation of normal and patient T cells.
PBL were isolated from healthy volunteers and RCC patients and T cells were purified as previously described.34,42 In brief, PBL were subjected to Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation and then depleted of macrophages, B cells, and NK cells by negative selection using magnetic separation with microbeads coated with antibodies to CD14, CD19, and CD16/CD56, as previously described42 (StemCell Technologies Inc). The T-cell isolation procedure yielded cells that were more than 95% positive for CD3 as determined by three-color immunocytometry. T cells (1 × 106/mL) were activated by culturing in the absence and presence of stimulus (PMA/ionomycin) for various lengths of time (0, 30, and 120 minutes). The medium used was RPMI 1640 supplemented with 5% fetal calf serum (FCS).
Preparation of tumor supernatants.
To generate tumor supernatant fluid, primary RCC were obtained from patients undergoing nephrectomy at the Cleveland Clinic Foundation. A 3 × 3 mm tumor explant was incubated overnight in RPMI 1640 medium. Thereafter, 1 g of 3 × 3 mm explant was placed into a T-75 flask with 15 mL of Dulbecco’s modified Eagle’s medium (DMEM) without additional supplements. After 3 to 4 days of culture at 37°C with 95% air and 5% CO2 the supernatant fluid was harvested, filtered, and stored at −70°C . As a control, nonneoplastic tissue from the same kidney was also incubated in vitro under the same conditions as the tumor explants. The presence of metabolically active cells in the explants was demonstrated by measuring dehydrogenase activity (Colorimetric assay kit; Boehringer Mannheim, Indianapolis, IN).64
To determine the effect tumor-derived products have on T-cell activation, cells were cultured in complete RPMI 1640 with and without RCC-S as well as explants from an uninvolved area of the same kidney (normal kidney cell supernatant [NKC-S]). The volume of supernatant fluid added to the T-cell cultures varied between 20% and 50% of the total volume depending on the experiment. The viability (trypan blue dye exclusion) of the T cells after exposure to RCC-S was greater than 80%.
Western blotting and immunoprecipitation.
Protein samples (10 to 20 μg) were mixed with an equal volume of 2× Laemmli sample buffer, boiled, and resolved by electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE).42 The proteins were transferred from the gel to nitrocellulose membranes using a semidry electroblotting apparatus (Bio-Rad, Hercules, CA). Membranes were blocked by incubating in 5% nonfat dry milk in TBST overnight to inhibit nonspecific protein binding. The membranes were sequentially incubated with specific antibody (2 hours for anti-IκBα, anti–c-Rel, anti-RelA, and anti-NFκB1) and then with horseradish peroxidase-conjugated donkey antirabbit IgG (Amersham, Chicago, IL; 1:2,000 dilution in TBST). Membranes were washed extensively with TBST after each of the incubation steps. Specific immune complexes were detected by enhanced chemiluminescence as specified by the manufacturer (Dupont, Boston, MA).
Immunoprecipitation of RelA was performed by adding 1 μg of rabbit antihuman RelA antibody to 150 μg of cytoplasmic extract protein for 2 hours at 4°C. Thirty microliters of protein G Sepharose-conjugated beads was added for 1 hour. Immunoprecipitates were washed 3 times with lysis buffer and proteins were eluted by boiling for 7 minutes in Laemmli sample buffer. Equivalent amounts of proteins were resolved on 10% SDS-PAGE and transferred to nitrocellulose membranes. To determine if IκBα coimmunoprecipitated with the Rel proteins, immunoblotting with anti-IκBα antibody was performed. As a specificity control, immunoprecipitation of cytoplasmic extract from normal T cells was performed using normal rabbit Ig (Sigma).
Densitometry scanning of immunoblots was performed. The developed X-OMAT AR film (Kodak, Rochester, NY) was placed on a white light box by Fotodym and its images were captured by a High Resolution CCD camera (Sierra Scientific, Sunnyvale, CA). Image 1.57 (National Institutes of Health, Bethesda, MD) was the program used to analyze the density of each band by graphically plotting the images and calculating the area under each peak.
Peptide blocking and phosphatase treatment.
To demonstrate immunochemical identity of bands reactive with polyclonal antisera, peptide competition experiments were performed. Aliquots of the anti-IκBα antibody were incubated with 0.5 or 2.0 μg/mL of IκBα peptide (Santa Cruz Biotechnology Inc) for 1 hour before incubation with membranes for Western blotting. For phosphatase treatment, T cells were stimulated with PMA/ionomycin for 30 minutes. Equal amounts of cytoplasmic extracts were prepared in the presence and absence of phosphatase inhibitors: 50 mmol/L NaF, 20 mmol/L β-glycerophosphate, 1.0 mmol/L sodium orthovanadate, and 10 mmol/L sodium molybdate. The extracts were then incubated at 37°C for 20 minutes either in phosphatase buffer alone or in buffer containing 24 U of calf intestine alkaline phosphatase (CIP) before analysis by immunoblotting with anti-IκBα antibody.
Electrophoretic mobility shift assay (EMSA).
Cytoplasmic and nuclear extracts were prepared according to Schreiber et al,43 with minor modifications. Briefly, T cells (107) were harvested and washed with cold phosphate-buffered saline (PBS) and then sedimented by centrifugation at 1,500 rpm for 5 minutes. The cell pellet was resuspended in 150 mL of buffer A (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, pH 8.0, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol [DTT], 100 μg/mL phenylmethylsulfonyl fluoride [PMSF], 1 μg/mL aprotinin, 2 μg/mL leupeptin, 100 μg/mL Pefabloc, and 100 μg/mL chymostatin) by gentle pipetting. The cells were incubated on ice for 15 minutes and then 10 μL of 10% Nonidet-P-40 solution (Sigma) was added, and cells were vigorously mixed for 10 seconds before centrifugation. The supernatant containing the cytoplasmic proteins was transferred to another tube and aliquoted. Pelleted nuclei were resuspended in 50 μL of buffer C (25% glycerol, 20 mmol/L HEPES, pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, pH 8.0, 1 mmol/L EGTA, 1 mmol/L DTT, 100 μg/mL PMSF, 1 μg/mL aprotinin, 2 μg/mL leupeptin, 100 μg/mL Pefabloc, and 100 μg/mL chymostatin). After mixing at 4°C for 20 minutes, the nuclei were centrifuged for 10 minutes at 13,000 rpm, and supernatants containing the nuclear proteins were stored at −70°C. Protein concentration was measured using the BCA method (Pierce Chemical Co, Rockford, IL), as specified by the manufacturer. EMSA was performed using extracts obtained at various times before and after stimulation with either TNFα (10 ng/mL) or PMA (20 ng/mL) plus ionomycin (0.75 μg/mL). Briefly, nuclear extracts (5 to 10 μg of protein) were incubated in a 25 μL total reaction volume containing 20 mmol/L HEPES (pH 7.9), 80 mmol/L NaCl, 0.1 mmol/L EDTA, 1 mmol/L DTT, 8% glycerol, and 2 μg of poly (dI-dC) (Pharmacia) for 15 minutes at 4°C. The reaction mixture was then incubated with radiolabeled oligonucleotide (2 × 105cpm) for 20 minutes at room temperature. Samples were analyzed by electrophoresis in a 6% nondenaturing polyacrylamide gel with 0.25× TBE buffer (22.3 mmol/L Tris, 22.2 mmol/L boric acid, 0.5 mmol/L EDTA). The gels were dried and analyzed by autoradiography.
For preparation of the probe, radiolabeled double-stranded oligonucleotides were prepared by annealing coding strand template to a complementary 10-base primer and filling in the overhang with the Klenow fragment of DNA polymerase I in the presence of (α32P)dCTP.
Oligonucleotide corresponding to κB element from IL-2Rα gene was prepared by using an Applied Biosystems (Foster City, CA) oligonucleotide synthesizer (model 381 A). The sequence of the oligonucleotide was 5′-CAACGGCAGGGGAATCTCCCTCTCCTT-3′. The underlined sequence represents the κB motif.
RESULTS
T cells from a subset of RCC patients show impaired activation of NFκB and no degradation of the inhibitor, IκBα.
Previously, we reported that T cells obtained from the peripheral blood of RCC patients displayed impaired activation of NFκB after stimulation through the TCR/CD3 complex.40 Indeed, impaired activation of NFκB was observed in circulating T cells from 17 of 25 RCC patients examined to date. The present study was undertaken to identify the molecular site of this defect. The inability to activate NFκB was not due to an alteration in levels or function of the TCR/CD3 complex, because peripheral blood T cells expressed normal levels of CD3ε and TCRζ as well as the associated kinases p56lck and p59fyn and at least some early signal transduction events linked to the TCR/CD3 complex were intact. Furthermore, NFκB is not activated in patient T cells stimulated with PMA/ionomycin, indicating that the defect lies downstream of the TCR complex and immediate receptor-coupled signaling events.
The inability to activate κB binding activity in patient T cells could result from diminished expression of the Rel proteins themselves. To evaluate this possibility cytosolic and nuclear extracts from normal and patient T cells were analyzed for Rel protein content both with and without stimulation by PMA/ionomycin. In normal T cells there is a stimulus-dependent movement of RelA, c-Rel, and NFκB1(p50) into the nucleus within 15 minutes (Fig 1). The nuclear translocation of the Rel proteins coincided with the appearance of κB-specific binding activity (data not shown). In contrast, PMA/ionomycin stimulation of patient-derived T cells did not result in the nuclear translocation of RelA, c-Rel, or NFkB1 at any of the times (0, 15, 30, and 120 minutes) examined. However, RelA, c-Rel, and NFκB1(p50) were present in normal amounts in the cytoplasm. The data shown are representative.
The nuclear translocation of Rel proteins is impaired in patient T cells. T cells isolated from normal donors and RCC patients were stimulated for the times indicated with PMA (20 ng/mL) plus ionomycin (0.75 μg/mL). Thereafter, cytoplasmic and nuclear extracts derived from T cells of a healthy donor and from 2 RCC patients (KG and BH) were subjected to immunoblotting using antibodies to c-Rel, RelA, and p50 (NFκB1). In normals, there was a time-dependent increase in the nuclear level of Rel proteins that coincided with κB binding activity (data not shown). In patient T cells, there was no nuclear localization of Rel proteins after stimulation, even though they were constitutively expressed in the cytoplasm. There was also no induction of κB-specific DNA binding activity as defined by EMSA in nuclear extract from patient T cells (data not shown). These findings were consistent for all 7 patients and 10 normals.
The nuclear translocation of Rel proteins is impaired in patient T cells. T cells isolated from normal donors and RCC patients were stimulated for the times indicated with PMA (20 ng/mL) plus ionomycin (0.75 μg/mL). Thereafter, cytoplasmic and nuclear extracts derived from T cells of a healthy donor and from 2 RCC patients (KG and BH) were subjected to immunoblotting using antibodies to c-Rel, RelA, and p50 (NFκB1). In normals, there was a time-dependent increase in the nuclear level of Rel proteins that coincided with κB binding activity (data not shown). In patient T cells, there was no nuclear localization of Rel proteins after stimulation, even though they were constitutively expressed in the cytoplasm. There was also no induction of κB-specific DNA binding activity as defined by EMSA in nuclear extract from patient T cells (data not shown). These findings were consistent for all 7 patients and 10 normals.
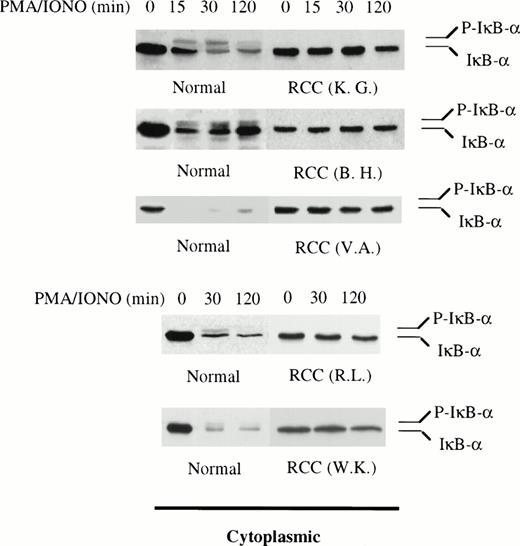
Because patient T cells contain normal amounts of Rel proteins, the defect apparently results from a blockade in the intracellular signaling pathway involved in normal activation of κB binding activity. We examined the behavior of the inhibitor IκBα in stimulated patient-derived T cells, because the phosphorylation and degradation of this protein represent critical events in the NFκB signaling process.17,19 As reported previously for normal T cells, stimulation with PMA/ionomycin causes degradation of IκBα within 15 minutes, coincident with the appearance of RelA, c-Rel, and NFκB1 in the nucleus4,17,21 22(Fig 2). Densitometric analysis of IκBα in T cells from normals (n = 5) demonstrated a mean decrease of 73.4% (±6.7% SEM) and 68.5% (±11.3% SEM) in cytoplasmic protein levels after 30 minutes and 2 hours of stimulation, respectively. In 10 of the 17 patients whose T cells exhibited defective κB binding activity, IκBα degradation occurred normally and could not be distinguished from normal cells (data not shown). In the remaining patients (7 of 17), the stimulus-dependent degradation of IκBα was greatly reduced or lost. IκBα levels only decreased by 10.1% (±4.9% SEM) after 30 minutes and 18.8% (±6.3% SEM) after 2 hours (Fig 2). Therefore, in these patients, the defect in NFκB activation coincided with markedly reduced degradation of IκBα and cytoplasmic retention of the Rel proteins. The remaining discussion will focus on this latter subset of patients (n = 7) in which IκBα degradation was lost.
Impaired IκB degradation in T cells derived from a subset of RCC patients. Western blotting for IκB was performed on cytoplasmic extract from patient T cells (n = 5). The levels of IκB in the remaining 2 patients (RH and LS) were determined by immunoprecipitating RelA and immunoblotting for IκB (data from RH presented in Fig 3). T cells from 5 different healthy donors served as positive controls. Densitometry analysis of IκB in T cells from normals showed a 73.4% (±6.7% SEM) and 68.5% (±11.3% SEM) decrease after 30 minutes and 2 hours of stimulation, respectively. In patient T cells, IκB levels decreased 10.1% (±4.9% SEM) after 30 minutes and 18.8% (±6.3% SEM) after 2 hours. The data presented here also showed that, within 15 to 30 minutes of stimulation, the phosphorylated form of IκB (P-IκB) was detected in normal T cells but not in patient T cells. In 1 normal, the phosphorylated form of IκB was not detected because of the rapid and total degradation of the inhibitor in 15 minutes.
Impaired IκB degradation in T cells derived from a subset of RCC patients. Western blotting for IκB was performed on cytoplasmic extract from patient T cells (n = 5). The levels of IκB in the remaining 2 patients (RH and LS) were determined by immunoprecipitating RelA and immunoblotting for IκB (data from RH presented in Fig 3). T cells from 5 different healthy donors served as positive controls. Densitometry analysis of IκB in T cells from normals showed a 73.4% (±6.7% SEM) and 68.5% (±11.3% SEM) decrease after 30 minutes and 2 hours of stimulation, respectively. In patient T cells, IκB levels decreased 10.1% (±4.9% SEM) after 30 minutes and 18.8% (±6.3% SEM) after 2 hours. The data presented here also showed that, within 15 to 30 minutes of stimulation, the phosphorylated form of IκB (P-IκB) was detected in normal T cells but not in patient T cells. In 1 normal, the phosphorylated form of IκB was not detected because of the rapid and total degradation of the inhibitor in 15 minutes.
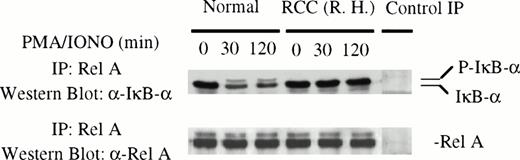
To verify that RelA was bound to IκBα in the cytoplasm of patient T cells and that this binding was not disrupted after stimulation with PMA/ionomycin, coimmunoprecipitation experiments were performed. T cells from normal and RCC patients were stimulated for various times (0, 30, and 120 minutes) before preparation of cytoplasmic extracts and immunoprecipitation with anti-RelA antibody. The immunoprecipitates were subjected to Western blot analysis with antibody to RelA or IκBα. As expected, IκBα coimmunoprecipitated with RelA in normal T cells and the level of IκBα bound to RelA decreased after stimulation (Fig 3). In unstimulated patient T cells, RelA was also bound to IκBα. However, stimulation with PMA/ionomycin did not alter the amount of the IκBα in complex with RelA. Similar results were obtained with T cells from 2 other patients (data not shown).
Rel A protein was in complex with IκB in the cytoplasm of patient T cells and was insensitive to stimulation. Cytoplasmic extracts from control and patient T cells were immunoprecipitated with anti-RelA antibody and the immunoprecipitates were subjected to immunoblotting using anti-IκB and anti-RelA antibodies, respectively. In control immunoprecipitations, normal rabbit IgG was added to cytoplasmic extract from a healthy individual. Similar results were obtained with 2 additional patients.
Rel A protein was in complex with IκB in the cytoplasm of patient T cells and was insensitive to stimulation. Cytoplasmic extracts from control and patient T cells were immunoprecipitated with anti-RelA antibody and the immunoprecipitates were subjected to immunoblotting using anti-IκB and anti-RelA antibodies, respectively. In control immunoprecipitations, normal rabbit IgG was added to cytoplasmic extract from a healthy individual. Similar results were obtained with 2 additional patients.
To determine if the impaired activation of NFκB was related to the stimulus, T cells were treated with TNFα, a potent inducer of NFκB activity with associated degradation of IκBα.4,17 19 In normal T cells, TNFα caused degradation of IκBα within 5 minutes that was paralleled by the nuclear appearance of Rel proteins. However, in patient T cells, TNFα did not stimulate either the degradation of IκBα or the nuclear translocation of RelA, c-Rel, or NFκB1 (Fig 4).
Suppression of TNF-induced nuclear localization of Rel proteins in RCC patients. T cells from a normal donor and an RCC patient (VA) were stimulated with 10 ng/mL of TNF for the times indicated. The nuclear and cytoplasmic extracts were then obtained and subjected to immunoblotting using antibodies to c-Rel, RelA, NFκB1 (p50), and IκB. Representative data are presented and similar results were obtained in 2 additional experiments.
Suppression of TNF-induced nuclear localization of Rel proteins in RCC patients. T cells from a normal donor and an RCC patient (VA) were stimulated with 10 ng/mL of TNF for the times indicated. The nuclear and cytoplasmic extracts were then obtained and subjected to immunoblotting using antibodies to c-Rel, RelA, NFκB1 (p50), and IκB. Representative data are presented and similar results were obtained in 2 additional experiments.
Phosphorylation of IκBα is impaired in T cells from RCC patients.
Phosphorylation of IκBα represents a critical event in stimulus-induced degradation of this inhibitor.4,17,21,44-49 The lack of IκBα degradation in T cells from a subset of RCC patients raises the question of whether phosphorylation of the inhibitor may be impaired in their T cells. Therefore, we examined phosphorylation of IκBα in normal and patient T cells. In resting T cells, IκBα is present in the cytoplasm as a hypophosphorylated 37-kD protein.4 17 After stimulation of normal T cells with PMA/ionomycin, a slower migrating band immunoreactive with anti-IκBα antibody is detected in the lysates along with the 37-kD form of IκBα (Figs 2 and5). The slower migrating band is IκBα, because its reactivity with anti-IκBα antibody can be blocked by IκBα peptide (Fig 5). To determine if the slower migrating IκBα is a phosphorylated form, cytoplasmic extracts from activated normal T cells were treated with alkaline phosphatase in the presence and absence of phosphatase inhibitor before analysis for IκBα. Pretreatment with alkaline phosphatase eliminated the more slowly migrating band and this effect was not observed in extracts that were also treated with a phosphatase inhibitor, indicating this represents a phosphorylated form of IκBα (Fig 5). Phosphorylated IκBα was not detected in patient T-cell populations exhibiting loss of stimulus-dependent IκBα degradation (Figs 2 and 3). These findings are consistent with the notion that, at least in T cells from this subset of RCC patients, IκBα is not phosphorylated after stimulation and this lack of phosphorylation may prevent degradation of IκBα and the nuclear translocation of NFκB.
There is stimulus-dependent phosphorylation of IκB in normal but not patient T cells. T cells isolated from a healthy donor were stimulated with PMA (20 ng/mL) and ionomycin (0.75 μg/mL) and subjected to cytoplasmic extract preparation. (A) Immunoblotting of the cytoplasmic extracts shows the presence of 2 immunoreactive bands within 30 minutes of stimulation. (B) The antibody anti-IκB was incubated with different concentrations of IκB peptide before incubation with membrane and immunoblotting. (C) The cytoplasmic extracts with and without phosphatase inhibitors were incubated with buffer containing 24 U of CIP (+) or buffer alone (−). Equal amounts of the cytoplasmic extract were analyzed by immunoblotting with anti-IκB antibody. The phosphorylated band of IκB is indicated by P-IκB. The upper band of IκB was not detectable in any of the cell lysates from the 7 RCC patients studied. These findings suggest that phosphorylation of IκB was inhibited in T cells from RCC patients.
There is stimulus-dependent phosphorylation of IκB in normal but not patient T cells. T cells isolated from a healthy donor were stimulated with PMA (20 ng/mL) and ionomycin (0.75 μg/mL) and subjected to cytoplasmic extract preparation. (A) Immunoblotting of the cytoplasmic extracts shows the presence of 2 immunoreactive bands within 30 minutes of stimulation. (B) The antibody anti-IκB was incubated with different concentrations of IκB peptide before incubation with membrane and immunoblotting. (C) The cytoplasmic extracts with and without phosphatase inhibitors were incubated with buffer containing 24 U of CIP (+) or buffer alone (−). Equal amounts of the cytoplasmic extract were analyzed by immunoblotting with anti-IκB antibody. The phosphorylated band of IκB is indicated by P-IκB. The upper band of IκB was not detectable in any of the cell lysates from the 7 RCC patients studied. These findings suggest that phosphorylation of IκB was inhibited in T cells from RCC patients.
Supernatant from RCC but not normal kidney explants can suppress the degradation of IκBα and activation of NFκB in normal T lymphocytes.
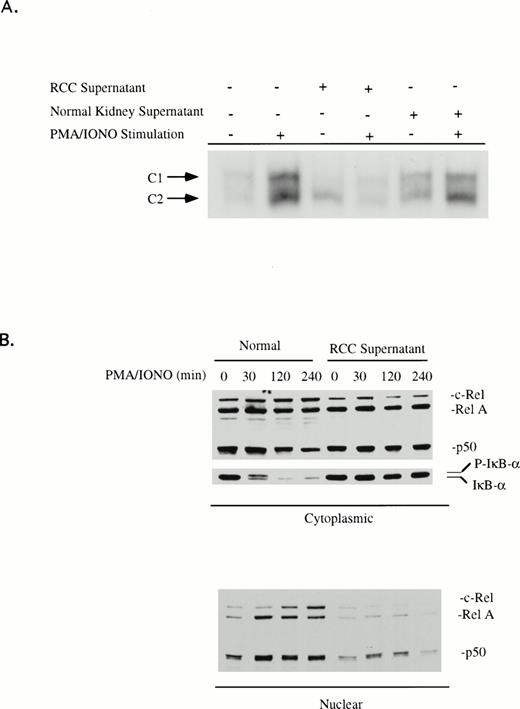
Although T cells from RCC patients are impaired in terms of NFκB activation, the origin of this defect is not known. We wished to determine if products from the tumor could alter activation of NFκB in normal T cells. Peripheral blood T cells from healthy volunteers were cultured in either medium or in RCC-S overnight and then stimulated with PMA/ionomycin before the preparation of nuclear extracts and analysis of κB binding activity by EMSA. In the presence of RCC-S, T cells demonstrated impaired activation of κB-specific binding activity. The major forms of NFκB were either not detectable or dramatically decreased after stimulation (Fig 6). Analysis of the Rel protein content of nuclear and cytoplasmic extracts confirmed that T cells cultured with RCC-S were unable to respond. Such treated cell populations contained normal levels of NFκB1, RelA, and c-Rel in the cytoplasm (Fig 6). Supernatants obtained from the cultures of 12 of 16 RCC tumors (75%) were able to suppress NFκB activation. This percentage was comparable to the percentage of patients that displayed this defect in their peripheral blood T cells (65%). This suppressive activity was tumor-derived, because culture supernatant from explant normal kidney tissue showed no effect on NFκB activation (Fig 6).
(A) RCC tumor supernatant suppresses κB-specific binding activity. Human peripheral blood T cells from healthy volunteers were cultured in medium, RCC-S, or NKC-S for 18 hours and then stimulated with PMA (20 ng/mL)/ionomycin (0.75 μg/mL) for 2 hours. Nuclear extracts were isolated and EMSA assays were performed with the oligonucleotide that corresponds to the κB sequence of the IL2R gene. (B) T cells from the healthy donor were cultured with medium or RCC-S for 18 hours and then stimulated with PMA (20 ng/mL)/ionomycin (0.75 μg/mL) for the times indicated. The nuclear and cytoplasmic extracts were prepared and then analyzed by immunoblotting for RelA, c-Rel, NFκB1 (p50), and IκB. Suppression of NFκB activation was observed in 12 of the 16 RCC-S. In addition, 6 of 11 RCC-S tested inhibited IκB phosphorylation and suppressed IκB degradation.
(A) RCC tumor supernatant suppresses κB-specific binding activity. Human peripheral blood T cells from healthy volunteers were cultured in medium, RCC-S, or NKC-S for 18 hours and then stimulated with PMA (20 ng/mL)/ionomycin (0.75 μg/mL) for 2 hours. Nuclear extracts were isolated and EMSA assays were performed with the oligonucleotide that corresponds to the κB sequence of the IL2R gene. (B) T cells from the healthy donor were cultured with medium or RCC-S for 18 hours and then stimulated with PMA (20 ng/mL)/ionomycin (0.75 μg/mL) for the times indicated. The nuclear and cytoplasmic extracts were prepared and then analyzed by immunoblotting for RelA, c-Rel, NFκB1 (p50), and IκB. Suppression of NFκB activation was observed in 12 of the 16 RCC-S. In addition, 6 of 11 RCC-S tested inhibited IκB phosphorylation and suppressed IκB degradation.
The ability of RCC-S to modulate the phosphorylation of IκBα was examined by Western blot analysis of cytoplasmic extracts from control and RCC-S–treated T cells before and after stimulation with PMA/ionomycin. Of 11 tumor supernatants examined, 6 inhibited the phosphorylation of IκBα and prevented degradation of IκBα after stimulation (representative data; Fig 6), whereas the remaining tumor supernatants produced inhibition of NFκB but did not inhibit degradation of IκBα. Again, this frequency is similar to the frequency with which this defect is seen in the RCC patient T cell. Thus, there appears to be at least 2 mechanisms by which RCC tumor products can inhibit the activation of NFκB.
DISCUSSION
NFκB controls expression of a number of genes during T-cell activation.20,50-54 In resting T lymphocytes, preformed NFκB is present in the cytoplasm in an inactive form bound to IκBs.50-54 The activation of NFκB involves the dissociation of RelA and c-Rel containing dimers from IκBα after phosphorylation and degradation of IκBα by the ubiquitin-proteasome pathway.17-23 Although phosphorylation of IκBα is not sufficient for dissociation, it is thought to target IκBα for degradation.22,23 Even though the signaling pathways leading to phosphorylation of IκBα are not well defined, several kinases have been implicated in the activation of NFκB, including PKA,55 PKCζ,56 Raf-1,57 and PKR.58 Recently, a large multisubunit complex termed IκBα kinase was shown to phosphorylate IκBα at serine 32 and 36; its activation appears to be regulated by mitogen-activated protein kinase/ERK kinase kinase 1.59 60 The findings reported here demonstrate that, in T cells from a subset of RCC patients, stimulus-dependent degradation of IκBα and the subsequent nuclear localization of κB binding activity is markedly suppressed. In this group of patients, the absence of IκBα degradation appears to be attributable to impaired phosphorylation of IκBα. Stimulation of patient T cells with either PMA/ionomycin or TNFα failed to induce the phosphorylated form of IκBα. It remains to be determined if RCC-derived T cells have altered expression or function of kinases implicated in IκBα phosphorylation.
There appears to be at least 2 mechanisms through which RCC tumors suppress the activation of κB binding activity. This is suggested by the identification of 2 distinct patterns of function observed in different RCC patients. We have observed 17 RCC patients in which the activation of NFκB in response to PMA/ionomycin was defective. In 7 patients from this group, this defect appears to result from a block in the signaling pathway before the phosphorylation of IκBα. In these samples, IκBα is not degraded and retains NFκB in the cytosol. In the remaining patients (n = 10), IκBα was degraded after stimulation without apparent nuclear localization of RelA or c-Rel. This outcome can result from interaction with other IκBs or from alterations in the signaling pathway downstream of IκB. Our recent findings show that, in T cells from 2 of 6 patients, IκBα degradation occurred in the absence of any change in IκBβ and IκBε levels, suggesting that in some cases Rel dimers are being retained in the cytoplasm by other inhibitors. In the remaining patients (n = 4), IκBβ and IκBε degraded along with IκBα. Under this circumstance, impaired κB binding activity may result from increased proteolysis of Rel proteins after their release from IκBs. Truncated forms of NFκB1 have been reported in T cells from tumor-bearing mice with defective κB binding activity.39However, in our study, we did not detect any altered forms of RelA, c-Rel, or NFκB1.
An important issue is whether a tumor product is responsible for the impaired activation of NFκB. The gradual loss of NFκB activation in T cells from mice with progressing tumors supports the view that the tumor can induce this alteration in signal transduction.39,41 The findings presented here show that soluble product(s) from RCC but not from uninvolved kidney tissue can suppress the activation of NFκB in normal peripheral blood T cells. That RCC culture supernatant can induce in normal T cells the same phenotype observed in patient T cells is consistent with the possibility that loss of NFκB activation in vivo may be due to a product of the renal cell tumor or associated cells. Similar mechanisms could be responsible for the suppression of other signal transduction pathways previously reported in T cells from cancer patients. Reduction in the levels of TCRζ and p56lck has been described in T cells isolated from patients with a number of different tumors and the most severe alterations are observed in tumor-infiltrating lymphocytes.31-38 Furthermore, recent work has suggested that tumor-associated macrophages may be responsible for the depressed expression of TCRζ and p56lck in tumor-bearing mice since H2O2 produced by these cells can downregulate TCRζ levels.61,62 TCRζ expression also can be inhibited through cell-cell contact when T cells are cocultured with human tumor cells.63 The tumor and/or associated stroma may produce a variety of immunosuppressive molecules that could inhibit the activation of NFκB. Although the biochemical nature of the suppressive product(s) in RCC supernatants is not known, it is currently under investigation.
T cells from animals bearing the mouse RENCA tumor show reduced activation of NFκB and this correlates with diminished production of interferon γ.39,41 We have found that culture supernatants derived from RCC that inhibited NFκB activation in the current study also suppressed the proliferation of normal T cells64 and the stimulus-dependent expression of genes known to be regulated by NFκB including IL-2Rα64 and IL-2 (Uzzo et al, unpublished data). It is also possible that poor NFκB activation may contribute to the lack of IL-2Rα and IL-2 gene expression in tumor-infiltrating lymphocytes.29 65 Downregulation of NFκB-dependent gene expression caused by suppression of κB DNA binding activity may represent a mechanism by which tumor cells can inhibit the development of antitumor immunity.
ACKNOWLEDGMENT
The authors thank Jan Kodish for assisting in the preparation of the manuscript.
Supported by US Public Health Services Grant No. CA56937.
Address reprint requests to James Finke, PhD, Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal