Abstract
BB-10010 is a variant of the human form of macrophage inflammatory protein-1 (MIP-1), which has been shown in mice to block the entry of hematopoietic stem cells into S-phase and to increase their self-renewal capacity during recovery from cytotoxic damage. Its use may constitute a novel approach for protecting the quality of the stem cell population and its capacity to regenerate after periods of cytotoxic treatment. Thirty patients with locally advanced or metastatic breast cancer were entered into the first randomized, parallel group controlled phase II study. This was designed to evaluate the potential myeloprotective effects of a 7-day regimen of BB-10010 administered to patients receiving six cycles of 5-fluorouracil (5-FU), adriamycin, and cyclophosphamide (FAC) chemotherapy. Patients were randomized, 10 receiving 100 μg/kg BB-10010, 11 receiving 30 μg/kg BB-10010, and nine control patients receiving no BB-10010. BB-10010 was well-tolerated in all patients with no severe adverse events related to the drug. Episodes of febrile neutropenia complicated only 4% of the treatment cycles and there was no difference in incidence between the treated and nontreated groups. Studies to assess the generation of progenitor cells in long-term bone marrow cultures were performed immediately preceding chemotherapy and at the end of six dosing cycles in 18 patients. Circulating neutrophils, platelets, CD 34+ cells, and granulocyte/macrophage colony-forming cell (GM-CFC) levels were determined at serial time points in cycles 1, 3, and 6. The results showed similar hemoglobin and platelet kinetics in all three groups. On completion of the six treatment cycles, the average pretreatment neutrophil levels were reduced from 5.3 to 1.7 × 109/L in the control patients and from 4.3 to 1.9 and 4.5 to 2.5 × 109/L in the 30/100 μg/kg BB-10010 groups, respectively. Relative to their pretreatment values, 50% of the patients receiving BB-10010 completed the treatment with neutrophil values significantly higher than any of the controls (P = .02). Mobilization of GM-CFC was enhanced by BB-10010 with an additional fivefold increase over that generated by chemotherapy alone, giving a maximal 25-fold increase over pretreatment values. Bone marrow progenitor assays before and after this standard regimen of chemotherapy indicated little long-term cumulative impairment to recovery from chemotherapy. Despite the limited cumulative damage to the bone marrow, which may have minimized the protective value of BB-10010 during this regimen of chemotherapy, better recovery of neutrophils in the later treatment cycles with BB-10010 was indicated in a number of patients.
© 1998 by The American Society of Hematology.
DOXORUBICIN-BASED chemotherapy represents the mainstay of treatment for patients with breast cancer, both in the adjuvant and advanced setting. Dose escalation results in a higher response rate, but it is also associated with increased hematopoietic toxicity and dose-limiting mucosal toxicity.1,2 In the adjuvant setting, repeated cycles of doxorubicin-based treatment have been shown to produce cumulative and long-lasting damage in the bone marrow (BM) progenitor cell populations.3 The effects of short-term hematopoietic damage resulting from chemotherapy can be overcome to some extent by the concurrent use of granulocyte colony-stimulating factor (G-CSF) to encourage regeneration of neutrophils.2,4 However, this approach is accompanied by progressive thrombocytopenia and probable cumulative BM damage as reflected by a reduction in the quality of mobilized progenitor cells over successive cycles of treatment.5 6
Currently, there is considerable interest in chemotherapy dose intensification as a means of improving tumor response rates and perhaps survival.7-9 The introduction of G-CSF and other hematopoietic growth factors has facilitated this movement, but the small gain (typically less than twofold) has been compounded by increased hematopoietic toxicity with progressive thrombocytopenia,5,10,11 probably as a consequence of incremental damage to the BM stem cell pool.6,12 The ability to maintain stem cells in a quiescent state during periods of chemotherapy treatment in animals has been shown to attenuate the cytotoxic effects on hematopoeisis13-20 and epithelium,21 the former reflected in an accelerated BM and peripheral blood cell recovery.
Macrophage inflammatory protein-1α (MIP-1α), a C-C chemokine22 has been recognized as a hematopoietic stem cell proliferation inhibitor that enhances stem cell recovery, postcytotoxic treatment, not only as a consequence of cytoprotection, but also via secondary effects on stem cell self-renewal.23BB-10010 is an active, nonaggregating variant of human MIP-1α.24 In mice, it reduced the degree of accumulated hematopoietic damage after repeated sublethal irradiations25 and, in a further model, enhanced leukocyte recovery and progenitor cell mobilization after cyclophosphamide.26
In phase-I clinical studies, BB-10010 was extremely well-tolerated with no maximum tolerated dose defined at up to 300 μg/kg administered subcutaneously and up to 100 μ/kg intravenously.27 Only a few mild local injection site reactions were observed despite its earlier classification as a proinflammatory chemokine22 and a single subcutaneous (SC) injection resulted in sustained plasma levels over a 24-hour period.
We now report the effects of BB-10010 on the maintenance of hematopoiesis in humans, using BM and peripheral blood parameters, following a repeated daily dosing schedule, administered with every cycle of 5-fluorouracil, adriamycin, and cyclophosphamide (FAC) chemotherapy in patients with advanced breast cancer.
MATERIALS AND METHODS
Between August, 1995 and March 1996, 30 patients with advanced breast cancer were recruited from three centers: 20 at the Christie Hospital, Manchester, 9 at Guy’s Hospital, London, and 1 at the Institute of Cancer Studies, Birmingham. The protocol was approved by the South Manchester Ethical Committee and written informed consent was obtained from all patients. Patient inclusion criteria included the following: females aged 18 years or older with advanced breast cancer and Eastern Cooperative Oncology Group (ECOG) performance status < 2. White cell count (WCC) ≥ 3 × 109/L, platelets ≥ 100 × 109/L, and hemoglobin (Hb) ≥ 11 g/dL. They had normal renal function tests, normal bilirubin, and transaminases less than two times the upper limit of normal. Exclusion criteria included previous chemotherapy, except standard adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy at least 6 months previously and previous radiotherapy to more than one third of the axial skeleton.
Randomization.
Patients were randomized to receive either 0, 30, or 100 μg BB-10010/kg SC daily for 7 days (days −1, 0, 1, 2, 3, 4, and 5) administered at the start of each of the six cycles of FAC chemotherapy (day 0), which were given at 21-day intervals. Control patients received chemotherapy only. Subjects were balanced by the method of minimization according to whether they had received previous CMF adjuvant chemotherapy or not, plus the extent of any metastases in their hematopoietic system. These were categorized as follows: (1) locally advanced disease with a negative bone scan; (2) evidence of metastatic disease in less than three marrow containing areas of skeleton; and (3) evidence of metastatic disease in all three of the principal marrow containing areas of the skeleton (ribs/pelvis/spine).
Cytotoxic chemotherapy.
All patients received intravenous FAC chemotherapy (5-FU 600 mg/m2 day 0, doxorubicin 50 mg/m2, and cyclophosphamide 600 mg/m2) on day 0 and subsequently at 21-day intervals. In each cycle, the chemotherapy was administered 24 hours after the start of the BB-10010 dosing. All subjects received a 7-day course of prophylactic ciprofloxacin and fluconazole if their neutrophil count dropped below 0.5 × 109/L at any time during the study. If neutrophils or platelets had not recovered to ≥1 × 109/L or ≥100 × 109/L, respectively, chemotherapy was to be delayed by up to 2 weeks. Dose reductions of 25% were to be made in succeeding cycles in event of either (1) an episode of neutropenic sepsis (temperature ≥ 38°C and neutrophils < 0.5 × 109/L); (2) any nonhematopoietic National Cancer Institute (NCI) toxicity of grade 3 or 4; or (3) failure of the neutrophil count to recover following a 1-week delay.
BB-10010.
BB-10010 was supplied by British Biotech Pharmaceuticals Ltd as a sterile solution at 2 mg/mL concentration in phosphate-buffered saline (PBS). Ampules of drug were stored at −20°C then thawed within 7 days before administration and stored at 4°C. The correct volume of BB-10010 was drawn into a 25-gauge 1 inch sterile needle from either the 10 mg/mL ampule (for the 100 μg/kg dose level) or the 2 mg/mL ampule (for the 30 μg/kg dose level). The BB-10010 was administered as a daily (7 days) SC injection, the first dose being given 24 hours before each cycle of FAC chemotherapy.
Peripheral blood assays.
Full blood counts (FBC) were taken at screening and on days 7, 10, 14, 16, and 20 of cycles 1, 3, and 6 plus day 20 of cycles 2, 4, and 5. Circulating progenitor cells were assayed on days 14, 16, and 20 of cycles 1, 3, and 6, in 11 of the 20 patients attending the Manchester center, using a clonogenic assay for GM-CFC. CD34+ cells were assessed by flow cytometry.
GM-CFC assay.
At the time points specified above, mononuclear cells (MNC) from peripheral blood were separated on Ficoll Hypaque (density 1.077 g/mL; Life Technologies, Paisley, UK) and suspended in Iscove’s medium supplemented with 4 × 10−3 mol/L glutamine, 10−7 mol/L sodium selenite, 2.5 × 10−4 mol/L α thioglycerol, 30% pretested fetal calf serum (FCS), 1% deionized bovine serum albumin (BSA) (Sigma Chemical Co, Poole, UK), and 2 U of recombinant erythropoietin (Epo) (Boehringer Mannheim, Lewis, UK) per mL of culture. Medium conditioned by the cell line 5637 was added at 10% by volume as source of growth factors. Cells were cultured in 1.35% methylcellulose in 24-well standard tissue culture plates (Falcon; Fred Baker Supplies, Runcorn, Cheshire, UK) in triplicate, at a final concentration of 105 mononuclear cells/mL/well. The cells were incubated at 37°C in a humidified atmosphere of 5% C02 and 5% O2 in nitrogen. Colonies were counted and classified after 14 days growth as granulocyte-macrophage colonies, as previously described.28
Determination of CD34+ cells.
The number of CD34+ cells in the peripheral blood was determined on days 14, 16, and 20 of cycles 1, 3, and 6. Fifty-microliter aliquots of the samples were incubated with a mouse anti-CD34 fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MoAb) (anti-human progenitor cell antigen-2; Becton Dickinson, San Jose, CA) for 30 minutes at 4°C. Red blood cells were lysed by addition of 2 mL ammonium chloride solution (Ortho-mune lysing Reagent; Ortho Diagnostic Systems, Inc, Raritan, NJ). Cells were washed twice in PBS, stored in a fixative of 1% formaldehyde solution, and analyzed on a fluorescence-activated cell sorter (FACS). A nonspecific isotype matched FITC-conjugated MoAb was used as a negative control.29
BM assays.
A total of 2 mL of BM was aspirated under aseptic conditions from the right posterior iliac crest of the Manchester patients before starting treatment and at day 28 after the start of the sixth cycle of FAC chemotherapy. Mononuclear cells were separated and assayed for GM-CFC and CD34+ cells as described above. The pretreatment marrow samples were also assessed for tumor infiltration, GM-CFC, and CD34+ cells. Long-term BM cultures (LTBMC) were established from both pretreatment and posttreatment samples.
LTBMC.
Between 5 × 105 and 2 × 107nucleated BM cells after separation from red blood cells using methylcellulose28 in 10 mL of LTBMC medium was added to 25 cm2 tissue culture flasks (Falcon). Each 10 mL of LTBMC medium consisted of 1 mL FCS, 1 mL horse serum, 7.9 mL of Iscove’s modified Dulbecco’s medium (IMDM) (350 mOsm/kg) and 0.1 mL of 5 × 10-5 mol/L hydrocortisone succinate (Sigma). After inoculation, the flasks were gassed with 5% carbon dioxide in air, then capped, and incubated at 33°C in the dark. Cultures were fed routinely on a weekly basis by replacing half the volume of supernatant with fresh cell-free medium. The cells in the harvested medium were assayed for GM-CFC. At 4 and 8 weeks of culture, some cultures were sacrificed for assay of GM-CFC in the adherent layer after trypsin digestion as described by Couthino et al.28
Statistical methods.
To assess differences between the treatment groups with respect to timing and depth of neutrophil nadir, stabilization of counts during the first 7 days of each cycle (corresponding with BB-10010 dosing) and also recovery in counts at day 20 across each of the three cycles, the absolute neutrophil counts were subjected to repeated measures analysis of variance (ANOVA) multivariate method using Wilk’s λ test.
The magnitude of GM-CFC and total nucleated cell generation in pre- and posttreatment LTBMCs were expressed by the area-under-the-curve (AUC) over 8 weeks. The Kruskal-Wallis test and analysis of covariance were used to compare the patient groups (control, BB-10010, 30 and 100 μg/kg) and differences between pre- and posttreatment values within the groups. Repeated measures of covariance were performed to identify differences in peripheral blood progenitor cell mobilization.
RESULTS
Patient characteristics are shown in Table1. Their mean ages (and range) were 45 (29 to 63), 49 (34 to 62), and 56 (41 to 69) for the control (no BB-10010) and the 30 and 100 μg/kg BB-10010 groups, respectively. The groups were well-matched for number of sites of disease and previous treatment.
Patient Characteristics
| BB-10010 dose (μg/kg) | 0 | 30 | 100 |
| No. | 9 | 11 | 10 |
| Age, mean (range) | 45 (29-63) | 49 (34-62) | 56 (41-69) |
| Mean performance status | 0.7 | 0.2 | 0.6 |
| Median performance status (range) | 0.5 (0-2) | 0 (0-1) | 1 (0-2) |
| Number of sites of secondary disease | |||
| 1 | 6 | 8 | 6 |
| 2 | 1 | 2 | 2 |
| 3 | 2 | 0 | 1 |
| >3 | 0 | 1 | 1 |
| No. of patients with secondary disease in bone* | 5 | 4 | 4 |
| No. of patients with definable BM involvement-151 | 2 | 0 | 0 |
| No. of patients completing 6 cycles-152 | 7 | 10 | 10 |
| Previous adjuvant CMF chemotherapy | 3 | 3 | 2 |
| Previous endocrine therapy | 5 | 5 | 7 |
| BB-10010 dose (μg/kg) | 0 | 30 | 100 |
| No. | 9 | 11 | 10 |
| Age, mean (range) | 45 (29-63) | 49 (34-62) | 56 (41-69) |
| Mean performance status | 0.7 | 0.2 | 0.6 |
| Median performance status (range) | 0.5 (0-2) | 0 (0-1) | 1 (0-2) |
| Number of sites of secondary disease | |||
| 1 | 6 | 8 | 6 |
| 2 | 1 | 2 | 2 |
| 3 | 2 | 0 | 1 |
| >3 | 0 | 1 | 1 |
| No. of patients with secondary disease in bone* | 5 | 4 | 4 |
| No. of patients with definable BM involvement-151 | 2 | 0 | 0 |
| No. of patients completing 6 cycles-152 | 7 | 10 | 10 |
| Previous adjuvant CMF chemotherapy | 3 | 3 | 2 |
| Previous endocrine therapy | 5 | 5 | 7 |
*On bone scan.
On trephine biopsy (out of 20).
Patients included in the analysis.
All patients tolerated the FAC chemotherapy treatment extremely well with a low incidence of neutropenic sepsis (4% of cycles, duration 3 to 6 days) in all groups. Three control patients were withdrawn, two due to excessive disease progression and one withdrawn (and excluded from Table 1) for surgery after a good response to chemotherapy. One patient receiving 30 mg/kg BB-10010 was also withdrawn due to excessive disease progression. Of the patients with neutropenic sepsis, 1 was from the control group, 2 received 30 μg/kg BB-10010, 1 required a single 1-week dose delay, and 1 required a 2-week delay; 2 patients received 100 μg/kg BB-10010; 1 required a 1-week delay and the other required a 2-week dose delay. Two of the four BB-10010 patients requiring dose delays showed extremely poor neutrophil recovery even in the first cycle of chemotherapy (see below). However, these delays have been ignored in the following analyses because they were minimal (2% of the total treatment cycles) and distributed at a low level among all three patient groups. Nonhematopoietic toxicity was typically mild with no episodes of oral mucositis ≥2 NCI grade. Nausea was well-controlled with standard antiemetics. BB-10010 administration was well-tolerated and was not associated with any serious adverse events. Three patients (one at 30 μg/kg and two at 100 μg/kg BB-10010) suffered a mild self-limiting cutaneous reaction characterized by erythema and swelling at the injection site. No patient developed any systemic inflammatory response or anaphylaxis.
Pharmacokinetics of BB-10010.
Plasma concentrations of BB-10010 in blood samples taken on days −1, 0, 2, and 6 of the first cycle of FAC treatment were assayed, under contract, at Huntingdon Life Sciences (Huntingdon, UK), by hMIP-1α enzyme-linked immunosorbent assay (ELISA) (R & D Systems, hMIP-1α Quantikine Kit; Abingdon, UK). This ELISA measures hMIP-1α and BB-10010 equally well and, as in the Phase I trial,27no endogenous MIP-1α was detected in the controls: all were below the detection limit of 93.6 pg/mL. At 30 μg/kg, 33 assays over all time points gave an average concentration of 258 ± 146 pg/mL including two, which were below the detection limit. At 100 μg/kg, 41 assays gave an average concentration of 1,604 ± 2,050 pg/mL, including one assay below the limit of detection. The large standard deviation was the effect of two individual measurements that gave readings of 3.4 and 8 times the average for that group. The reasons for this variability are not clear, but the overall excess levels of circulating BB-10010 over the whole injection schedule indicate its continuous availability, and both dose levels used were therefore considered adequate for effective dosing in the BM.27
Peripheral blood.
All patients, irrespective of their treatment group, retained good levels of Hb and platelets throughout the period of treatment, both parameters remaining well within their normal range and variations (Table 2).
Peripheral Blood Status at Pretreatment and at the End of Treatment Cycles 1, 3, and 6
| BB-10010 (μg/kg) . | Hb (g/dL) . | Platelets (×10−9)/L . | Neutrophils (×10−9)/L . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment . | C1 . | C3 . | C6 . | Pretreatment . | C1 . | C3 . | C6 . | Pretreatment . | C1 . | C3 . | C6 . | |
| 0 (n = 7) | 13.4 ± 0.4 | 12.7 ± 0.5 | 12.1 ± 0.4 | 11.4 ± 0.5 | 272 ± 23 | 339 ± 41 | 326 ± 31 | 308 ± 36 | 5.3 ± 0.6 | 3.8 ± 0.6 | 3.4 ± 0.4 | 1.7 ± 0.3 |
| 30 (n = 10) | 12.9 ± 0.4 | 11.9 ± 0.3 | 11.9 ± 0.3 | 11.7 ± 0.4 | 273 ± 23 | 371 ± 36 | 372 ± 34 | 319 ± 32 | 4.4 ± 0.4 | 2.9 ± 0.7 | 3.0 ± 0.8 | 1.9 ± 0.5 |
| 100 (n = 10) | 13.4 ± 0.4 | 12.3 ± 0.4 | 11.5 ± 0.4 | 11.7 ± 0.3 | 270 ± 12 | 332 ± 19 | 357 ± 26 | 318 ± 23 | 4.5 ± 0.3 | 3.3 ± 0.5 | 3.8 ± 0.6 | 2.5 ± 0.4 |
| BB-10010 (μg/kg) . | Hb (g/dL) . | Platelets (×10−9)/L . | Neutrophils (×10−9)/L . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment . | C1 . | C3 . | C6 . | Pretreatment . | C1 . | C3 . | C6 . | Pretreatment . | C1 . | C3 . | C6 . | |
| 0 (n = 7) | 13.4 ± 0.4 | 12.7 ± 0.5 | 12.1 ± 0.4 | 11.4 ± 0.5 | 272 ± 23 | 339 ± 41 | 326 ± 31 | 308 ± 36 | 5.3 ± 0.6 | 3.8 ± 0.6 | 3.4 ± 0.4 | 1.7 ± 0.3 |
| 30 (n = 10) | 12.9 ± 0.4 | 11.9 ± 0.3 | 11.9 ± 0.3 | 11.7 ± 0.4 | 273 ± 23 | 371 ± 36 | 372 ± 34 | 319 ± 32 | 4.4 ± 0.4 | 2.9 ± 0.7 | 3.0 ± 0.8 | 1.9 ± 0.5 |
| 100 (n = 10) | 13.4 ± 0.4 | 12.3 ± 0.4 | 11.5 ± 0.4 | 11.7 ± 0.3 | 270 ± 12 | 332 ± 19 | 357 ± 26 | 318 ± 23 | 4.5 ± 0.3 | 3.3 ± 0.5 | 3.8 ± 0.6 | 2.5 ± 0.4 |
Data given for day 20 in cycles (C) 1, 3, and 6 of FAC therapy (mean values for each patient group ± SE).
Neutrophils.
The mean and standard deviations of the neutrophil counts, pretreatment and at the ends of cycles 1, 3, and 6, are shown in Table 2 and for the complete picture, as a percentage of their pretreatment levels in Fig 1. Pretreatment values were 20% lower in the BB-10010 groups than in the control group. However, the difference was statistically not significant. Each cycle of chemotherapy caused a dramatic reduction of the neutrophil counts with nadirs, generally lasting from days 10 to 16 of each cycle, of 0.4 to 1.3 × 109/L followed by recovery by day 20. Table 3 charts the recovery pattern relative to the starting point in each cycle. From this (and Fig 1), it can be seen that after an initial fall to about 71% of pretreatment values in all groups at the end of cycle 1, the second cycles showed no further net damage; 97% recovery in the controls and 91% and 100% recovery in the two BB-10010-treated groups. Thereafter, the control group showed a progressive decline (92%, 76%, 65%) in its recoveries over cycles 3 to 6, resulting in an overall reduction to 32% at the end of six cycles of FAC chemotherapy. By contrast, little additional damage occurred in the two treatment groups with 113%, 79%, and 82% recoveries in cycles 3 to 6 and ending at 50% of pretreatment neutrophil levels. It should be noted, however, that of the control group, no patient exceeded 43% of her pretreatment neutrophil count at day 20 of cycle 6. By contrast, of the 10 patients receiving 30 μg/kg BB-10010, four repeatedly failed to attain 2 × 109neutrophils/L (40% pretreatment value) even in the first treatment cycle and a further two also failed to surpass the maximal level (43% of their pretreatment value) seen in the control cohort at the end of six cycles. The remaining four in this group completed the trial with higher neutrophil counts than the best of the controls. Similarly, in the 100 μg/kg BB-10010 treated group, 6 of 10 patients completed the trial with higher neutrophil counts than the best of the controls. Overall, therefore, 50% of the BB-10010–treated patients performed better than the control patients and a variance ratio test on the standard deviations of these two groups showed them to be different (P = .02).
Mean neutrophil counts over cycles 1, 3, and 6 of FAC chemotherapy, presented as a percentage of each patient’s pretreatment neutrophil count. Arrows on the abscissa indicate day 0 of the treatment-cycle (C). (•), control (no BB-10010); (○), 30 μg/kg BB-10010; (▪), 100 μg/kg BB-10010.
Mean neutrophil counts over cycles 1, 3, and 6 of FAC chemotherapy, presented as a percentage of each patient’s pretreatment neutrophil count. Arrows on the abscissa indicate day 0 of the treatment-cycle (C). (•), control (no BB-10010); (○), 30 μg/kg BB-10010; (▪), 100 μg/kg BB-10010.
Percent Recovery of Neutrophil Count in Successive Cycles
| BB-10010 (μg/kg) . | Cycle No. . | |||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4/5 . | 6 . | 0-6 . | |
| 0 | 72 | 97 | 92 | 76 | 65 | 32 |
| 30 | 67 | 100 | 107 | 81 | 76 | 44 |
| 100 | 73 | 91 | 127 | 76 | 86 | 56 |
| BB-10010 (μg/kg) . | Cycle No. . | |||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4/5 . | 6 . | 0-6 . | |
| 0 | 72 | 97 | 92 | 76 | 65 | 32 |
| 30 | 67 | 100 | 107 | 81 | 76 | 44 |
| 100 | 73 | 91 | 127 | 76 | 86 | 56 |
Mean value for each treatment group. Presented as a percentage of the value at the start of each given cycle of FAC chemotherapy. 4/5 indicates the fall between the end of cycle 3 and the start of cycle 6.
Owing to the large standard deviations (interpatient variations), however, few of these changes between cycles or between treatments attained statistical significance. Repeated measures of ANOVA using multivariate method was used to study the patterns of absolute neutrophil counts over time and to relate them to the dose of BB-10010. Of the 30 patients, three were omitted from the analysis, as they did not complete the full six cycles of treatment. For the remaining 27 patients, the nadir days were averaged leaving 81 sets of measurements over the three cycles (27 in cycle 1, 28 in cycle 3, and 26 in cycle 6). Although counts in the control group recovered from the chemotherapy-induced nadirs by day 20 to levels that were statistically equivalent to days 0 and 7 in each cycle, repeated measures analysis used to study the change across cycles gave clear evidence of a difference between cycles (Wilk’s λ: P < .001). The contrast between recoveries in cycles 1 and 3 was not significant (P = .79), but that between cycles 1, 3, and 6 was highly significant (P < .001). However, the difference between means of the control and BB-10010 groups at the end of cycle 6, based on the raw data counts, was statistically not significant (Wilk’s λ: P = .2).
Progenitor and CD34+ cells.
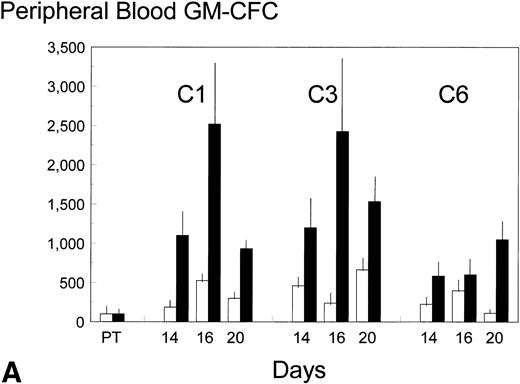
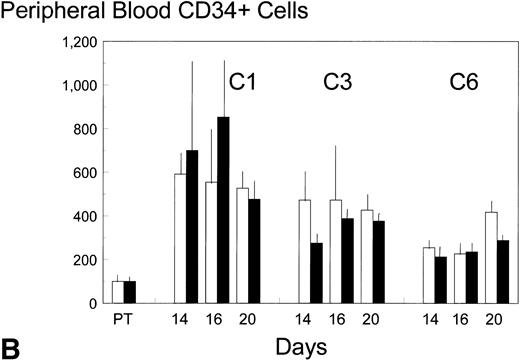
Pretreatment, peripheral blood in the control patients contained 8 ± 3 (standard error [SE]) GM-CFC per mL and 1,100 ± 900 (SE) CD34+ cells per mL. Each cycle of FAC treatment alone induced a modest mobilization of both cell types, over days 16 to 20, to maximum levels of 53 and 6,500 per mL, respectively (approximately up to sixfold). Additional BB-10010 treatment increased GM-CFC mobilization up to a maximum of 151 per mL, and maximal levels of about 25 times the pretreatment level. A full description of the levels of mobilization is shown in Fig 2A and B. Although mobilization in the sixth cycle was less striking, BB-10010 still increased GM-CFC levels above those with FAC therapy alone (Fig 2A). CD34+ cells were also mobilized in all cycles of treatment (again up to about sixfold normal), but BB-10010 had little further impact (Fig 2B). However, the considerable interpatient variability in the progenitor and CD34+ cell assays meant there were no statistically significant differences (P > .05) between the groups at any individual time point. Nevertheless, GM-CFC were mobilized to a greater extent in all of the nine sets of assays (Fig 2A) and the average increase (4.7 ± 1.1-fold) may be seen as significant (P = .01).
Mobilization kinetics of hematopoietic progenitor cells through cycles C1, C3, and C6 of FAC chemotherapy. Data are presented as a percentage of the pretreatment (PT) levels of GM-CFC (A) and of CD34+ (B), (±SE bars). Control (□) n = 4; BB-10010 (▪) n = 7 at 30 plus 5 at 100 μg/kg.
Mobilization kinetics of hematopoietic progenitor cells through cycles C1, C3, and C6 of FAC chemotherapy. Data are presented as a percentage of the pretreatment (PT) levels of GM-CFC (A) and of CD34+ (B), (±SE bars). Control (□) n = 4; BB-10010 (▪) n = 7 at 30 plus 5 at 100 μg/kg.
BM.
BM was sampled before the first treatment cycle and after the completion of the last cycle. Both GM-CFC and CD34+ cells were well maintained with recoveries after completion of the full treatment schedules being close to their pretreatment levels (Table 4).
CD34+ and GM-CFC Content in Fresh BM Harvested Before and After Administration of 6 Cycles of FAC Chemotherapy
| BB10010 (μg/kg) . | GM-CFC . | CD34+ . | ||
|---|---|---|---|---|
| Pretreatment . | Posttreatment . | Pretreatment . | Posttreatment . | |
| 0 (n = 4) | 25 ± 10 | 18 ± 4 | 0.7 ± 0.2 | 2.7 ± 0.83-150 |
| 30 (n = 7) | 36 ± 16 | 21 ± 9 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| 100 (n = 5) | 23 ± 21 | 26 ± 13 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| BB10010 (μg/kg) . | GM-CFC . | CD34+ . | ||
|---|---|---|---|---|
| Pretreatment . | Posttreatment . | Pretreatment . | Posttreatment . | |
| 0 (n = 4) | 25 ± 10 | 18 ± 4 | 0.7 ± 0.2 | 2.7 ± 0.83-150 |
| 30 (n = 7) | 36 ± 16 | 21 ± 9 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| 100 (n = 5) | 23 ± 21 | 26 ± 13 | 0.8 ± 0.2 | 0.8 ± 0.2 |
Data are shown as mean ± SE. Values represent absolute CD34+ cells (×10−5) per 107methylcellulose separated BM cells. GM-CFC data are colony counts per 105 BMMNC.
P (v pretreatment level) = 0.06; not significant.
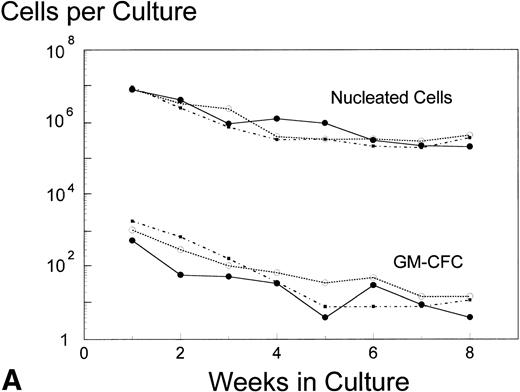
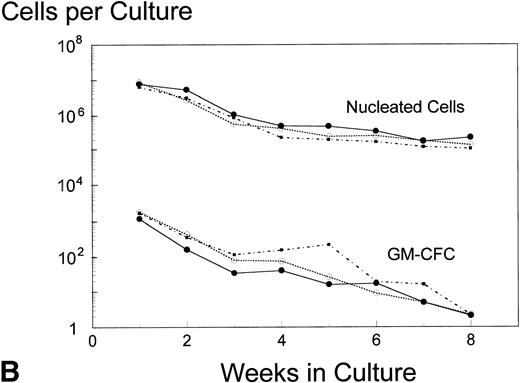
Analysis of AUC to assess the numbers of either nucleated cells or GM-CFC in the supernatant of LTBMC by the pre- and posttreatment marrows did not show any significant difference in the BB-10010–treated patients compared with controls (Fig 3). However, in cultures sacrificed at 4 weeks (though not at 8 weeks), posttreatment control patients had about 10 times lower numbers of GM-CFC per culture adherent layer compared with pretreatment (Table 5). Both groups of BB-10010–treated patients, however, retained their pretreatment levels (P = .04).
Hematopoiesis over 8 weeks (GM-CFC and nucleated cells) in LTBMCs established from patients before (A) and after completion of six cycles of FAC chemotherapy (B). Symbol key: •, control group (n = 5); ○, BB-10010, 30 μg/kg (n = 7); ▪, BB-10010, 100 μg/kg (n = 6). Values represent the mean ± SE. Differences in the AUC between weeks 1 and 8 of culture for each patient group, pretreatment and posttreatment, groups were not statistically significant (P > .05).
Hematopoiesis over 8 weeks (GM-CFC and nucleated cells) in LTBMCs established from patients before (A) and after completion of six cycles of FAC chemotherapy (B). Symbol key: •, control group (n = 5); ○, BB-10010, 30 μg/kg (n = 7); ▪, BB-10010, 100 μg/kg (n = 6). Values represent the mean ± SE. Differences in the AUC between weeks 1 and 8 of culture for each patient group, pretreatment and posttreatment, groups were not statistically significant (P > .05).
GM-CFC in the Adherent Layers of 4- and 8-Week Old LTBMC Established Before and After Administration of 6 Cycles of FAC Chemotherapy
| BB10010 (μg/kg) . | Pretreatment . | Posttreatment . | ||
|---|---|---|---|---|
| Week 4 . | Week 8 . | Week 4 . | Week 8 . | |
| 0 (n = 4) | 57 ± 49 | 13 ± 6 | 5 ± 4 | 23 ± 17 |
| 30 (n = 7) | 44 ± 17 | 40 ± 10 | 33 ± 21 | 6 ± 3 |
| 100 (n = 5) | 27 ± 13 | 11 ± 7 | 54 ± 33 | 17 ± 17 |
| BB10010 (μg/kg) . | Pretreatment . | Posttreatment . | ||
|---|---|---|---|---|
| Week 4 . | Week 8 . | Week 4 . | Week 8 . | |
| 0 (n = 4) | 57 ± 49 | 13 ± 6 | 5 ± 4 | 23 ± 17 |
| 30 (n = 7) | 44 ± 17 | 40 ± 10 | 33 ± 21 | 6 ± 3 |
| 100 (n = 5) | 27 ± 13 | 11 ± 7 | 54 ± 33 | 17 ± 17 |
Numbers per culture are shown. The data are presented as mean ± SE.
DISCUSSION
This study reports on the potential myeloprotective effects of MIP-1α when administered during all cycles of treatment in a multicyclic program of chemotherapy in a clinical trial. MIP-1α, in a formulation recognized as BB-10010,24 was administered at two dose levels in combination with standard dose FAC chemotherapy. Its 7-day injection schedule (starting 1 day before FAC therapy) was based on preclinical models that had demonstrated superior efficacy of MIP-1α as a protracted administration.25,26 In this trial, BB-10010 was administered at dose levels, which had been shown in preliminary pharmacokinetic studies and a phase I healthy human volunteer toxicity study, to be safe and to produce measurable plasma levels of MIP-1α.26 Its myelotoxic protective capacity was assessed in terms of neutrophil recovery rate in each 21-day cycle of FAC chemotherapy, mobilization of hematopoietic progenitor cells into the peripheral blood, and the quality of progenitor cells in the BM before and after six cycles of FAC treatment. The parallel group-controlled design of this study, with BB-10010 administered in every treatment cycle, allowed direct comparison of a protection arm versus control (chemotherapy only). All patients tolerated the chemotherapy well and only 5% of the treatment cycles were subject to delays due to suboptimal recovery (primary center). There was no mucosal toxicity and in keeping with our previous phase I studies, BB-10010 itself was not associated with any significant toxicity, although three patients developed mild recurring injection site reactions characterized by erythema and weal formation.27 A recently published Phase II study also found BB-10010 to be safe and with no mucosal toxicity.
After chemotherapy, neutrophils in the blood underwent the anticipated changes of decline to a very significant nadir and, with sequential cycles of treatment, a reducing capacity to recover to pretreatment levels by day 20 (Fig 1, Table 3). At no point were the results in the BB-10010 treatment arms significantly different from those in the control arm, however there was a high degree of interpatient variation in all arms of this relatively small study. Furthermore, there were also two patients in each of the two BB-10010 treatment arms who were barely making the neutrophil recovery criterion between chemotherapy cycles. The reasons for their failure are unclear, but seem unrelated to their health status at the start of the study. All four satisfactorily met the inclusion criteria and each had only one site of secondary disease (Table 1). Two had breast/soft tissue involvement, one had bone, and one had liver metastases. Therefore, they were not excluded from any of these analyses.
Because by chance, the average pretreatment neutrophil counts in both the BB-10010 treatment arms were nearly 20% lower than in the control arm, we have also presented the results as a percentage of the pretreatment value, calculated as an average from each individual patient. Other than the nadir days (days 10 to 16 of each cycle), the neutrophil counts in the BB-10010–treated patients were generally higher (Fig 1). Fall to the nadir appeared to be delayed (day 7) and 20-day neutrophil recoveries, particularly in the final treatment cycle, were enhanced. There was even an indication of a BB-10010 dose-related response in the final treatment cycle. Because no obvious protection was afforded by BB-10010 in the first treatment cycle, the progressive protection over cycles 2 to 6 becomes more evident: the 63% subsequently accrued failure to recover fully in the controls was reduced to only 23% by the treatment with BB-10010. At this stage, despite being in a poorer group, 50% of the patients treated with BB-10010 had neutrophil counts that were higher than those seen in any of the controls (Fig 4). A recently published phase II trial, limited to one cycle of cytotoxic treatment only,30 was also unable to record any enhanced neutrophil recovery due to BB-10010. The pattern of recovery now seen in the later cycles, however, is in full accord with those seen in preclinical studies with mice, where the full protective effects of BB-10010 were seen only after several cycles of sublethal irradiation25or bis-chloro-nitroso-urea treatment (unpublished data, E.M.). Furthermore, it cannot be ascribed to any demargination phenomenon, as phase I studies showed only a modest dose-related monocytophilia due to BB-10010 and no effect on levels of circulating neutrophils.27 This latter finding was also consistent with the observation that human neutrophils are 10-fold less responsive to BB-10010 than monocytes.31
Histogram showing the distribution of neutrophil counts (as percent of pretreatment value for each patient) at the end of the study (cycle 6, day 20), comparing 20 patients receiving BB-10010 with seven who did not. (□), Control; (▪), BB-10010 treatments.
Histogram showing the distribution of neutrophil counts (as percent of pretreatment value for each patient) at the end of the study (cycle 6, day 20), comparing 20 patients receiving BB-10010 with seven who did not. (□), Control; (▪), BB-10010 treatments.
Because MIP-1α was originally studied as a hematopoietic stem cell protection factor, most of the in vivo, preclinical work in animals relates to BM responses. In those published studies, which included mature cells, there was some acceleration of neutrophil production as a result.16,26 In studies using radiation and noncycle-specific cytotoxic drugs, long-term BM damage, damage that was ameliorated by administration of BB-10010, accumulated in murine hematopoietic spleen colony-forming units.25,26 Such effects were not seen, at least in the GM-CFC and CD34+cell populations, in this study. Marrow harvested both before and after completion of the six cycles of treatment contained a normal complement of progenitor cells (Table 4). Furthermore, the marrows performed equally well in generating these progenitor cells in long-term BM cultures (Fig 3). BB-10010 did not influence these observations except in maintaining some additional capacity for progenitor cell mobilization into the peripheral blood. This was in agreement with experimental findings in mice26,32 and consistent with the preliminary findings of a phase I trial,33 which also indicated about a fivefold increase relative to chemotherapy alone. The extended time scale of release in these patients is, however, different from that in mice, where BB-10010 induced short-term mobilization. The reasons for this difference are not known. It is recognized, however, that chemotherapy extends the mobilization process34 and it is possible that the damage caused by therapy is also permissive for an extended mobilizing effect of BB-10010. Perhaps surprisingly, the additional mobilization was not reflected by CD34+ cells. This population, however, is comprised of a much wider range of hematopoietic cells and its larger complement may well have concealed changes in the smaller GM-CFC component it contained. The only deficiency in the quality of the BM after FAC treatment alone appeared in its reduced capacity to establish the production of GM-CFC in the adherent layers of long-term cultures within 4 weeks (Table 5). The posttreatment kinetics of culture development were normal in marrow from the BB-10010–treated patients, whereas the controls were slower to establish their pretreatment performance. The different kinetics in posttreatment BM harvests, together with the higher numbers of mobilized progenitors seen in the BB-10010–treated patients (Fig 2), may indicate somewhat more active marrows in post-BB–10010-treated patients. The significant shortfall in the sixth cycle control neutrophil recovery may well be a direct consequence of a less active marrow in the control patients. The posttreatment marrows were harvested 1 week after the completion of cycle 6 and the point at which the final neutrophil counts were recorded. It is possible that this delay would have allowed further recovery in the control neutrophils to the levels recorded in the BB-10010–treated patients 1 week earlier. Unfortunately, the appropriate kinetic studies on the BM, necessary to resolve these questions, were not included in the trial design. Nevertheless, it is clear that in these groups of patients, the intensity of the FAC treatment regimen was insufficient to cause significant long-term manifestation of accumulated myelotoxicty. Compared with the preclinical animal experiments where cumulative toxicity resulted in a reduction in BM spleen colony-forming cells of about 65%, this was clearly insufficient to allow significant manifestation of the potential of BB-10010 for myeloprotection.
In conclusion, therefore, it is clear that BB-10010 is well-tolerated by the patients and, although basically considered to be an inhibitor of hematopoiesis, does not cause additional myelosuppression. The standard level of FAC therapy used was also well-tolerated and BB-10010 did not cause any significant changes in neutropenic sepsis. Although there was no statistically significant benefit derived from BB-10010 in this study, there was some evidence to suggest dose-dependent improvements in neutrophil recovery at the end of cycles 3 and 6. The ability to maintain acceptable levels of recovery in the face of additional accumulating myelotoxicity due to high-dose cytotoxic treatment or to an extended sequence of therapy cycles would allow the potential for greater damage to the tumor(s) under treatment. On day 20 of cycle 6, the last data point in this study, we note that 50% of the BB-10010–treated patients completed the trial with neutrophil counts higher than those in the control group. BB-10010 did, therefore, indicate a measure of myeloprotection against repeated cycles of FAC chemotherapy. This suggested that additional cycles of treatment may have demonstrated more fully the ability to maintain acceptable levels of recovery in the face of additional accumulating myelotoxicity. Further studies of additional cycles of therapy or using more intensive regimens of cytotoxicity, both designed to increase the degree of accumulated myelotoxicity, will be necessary to establish whether this apparent protection is real and to determine the efficiency and therapeutic benefit of BB-10010.
Supported by the Cancer Research Campaign of Great Britain and British Biotech Pharmaceuticals Ltd for whom Dr Ros Puttick coordinated the trial and the Biometrics Department gave valuable advice. M.C. and E.M. were supported by grants from The Leukaemia Research Fund (London, UK), and J.D. is supported by a grant from the European Society for Molecular Oncology (Brussels, Belgium).
Address reprint requests to Prof Anthony Howell, MD, CRC Department of Medical Oncology, Christie Hospital NHS Trust, Manchester, M20 4BX, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal