Abstract
It has been shown that interferons (IFNs) exert their signals through receptor-associated Janus kinases (JAKs) and signal transducers and activators of transcription (STATs). However, molecular mechanism of regulation of IFN signaling has not been fully understood. We have reported novel cytokine-inducible SH2 protein (CIS) and JAK binding protein (JAB) family genes that can potentially modulate cytokine signaling. Here we report that JAB is strongly induced by IFN-γ but not by IFN-β in mouse myeloid leukemia M1 cells and NIH-3T3 fibroblasts. NIH-3T3 cells ectopically expressing JAB but not CIS3 lost responsiveness to the antiviral effect of IFN-β and IFN-γ. M1 leukemic cells stably expressing JAB were also resistant to IFN-γ and IFN-β–induced growth arrest. In both NIH-3T3 and M1 transformants expressing JAB, IFN-γ did not induce tyrosine phosphorylation and DNA binding activity of STAT1. Moreover, IFN-γ–induced activation of JAK1 and JAK2 and IFN-β–induced JAK1 and Tyk2 activation were inhibited in NIH-3T3 JAB transformants. These results suggest that JAB inhibits IFN signaling by blocking JAK activity. We also found that IFN-resistant clones derived from LoVo cells and Daudi cells expressed high levels of JAB without stimulation. In IFN-resistant Daudi cells, IFN-induced STAT1 and JAK phosphorylation was partially reduced. Therefore, overexpression of JAB could be, at least in part, a mechanism of IFN resistance.
© 1998 by The American Society of Hematology.
A DIRECT SIGNAL transduction pathway from the interferon (IFN) receptor to the nucleus has been discovered.1,2 A novel subfamily of protein tyrosine kinases, known as Janus kinases (JAKs), was found to play an important role in IFN receptor signaling. JAKs associate with IFN receptors and are stimulated when IFNs bind to their cognate receptors. In particular, IFN-α/β activates JAK1 and Tyk2, whereas IFN-γ activates JAK1 and JAK2.1,2 The activated JAKs in turn convert latent cytoplasmic transcription factors, known as signal transducer and activator of transcription (STATs), into active forms by tyrosine phosphorylation. The tyrosine-phosphorylated STATs form homodimers or heterodimers and translocate into the nucleus, where they bind to their specific target sequences. STAT1 and STAT2 are phosphorylated in response to IFN-α/β and heterotrimerize together with a 48-kD protein, then bind to the IFN-α–specific DNA element, IFN-stimulated response element (ISRE). STAT1 is activated by IFN-γ, homodimerized, and then binds to a different DNA element, IFN-γ–activated site (GAS). Many cytokines including interleukins and colony-stimulating factors use similar JAK-STAT pathways.3
IFNs, especially IFN-α, have been used for treatment of hairy cell leukemia, melanoma, and chronic myelogenous leukemia (CML). However, patients in the late chronic phase, accelerated phase, or blastic phase of CML are usually resistant to the antiproliferative effects of IFNs.4 Efforts to understand the molecular basis of the cellular resistance to IFN have been made, using somatic cell mutants resistant to IFNs. Stark et al identified genes necessary for IFN signaling and have shown that defects of any signaling molecule, including receptors, JAKs, and STATs, could be mechanisms of IFN unresponsiveness and resistance.1,2 In fact, marked reduction in the level of STAT1 was found in IFN-resistant melanoma patients.5 Such IFN resistance should be a recessive phenotype. However, the molecular basis of the dominant phenotype of IFN resistance is not clear.6
Negative regulation of JAK-STAT signaling has only just begun to be studied. Selective degradation or dephosphorylation of receptors and STATs has been reported.7-10 Naturally occurring dominant negative variants of STAT5 and a specific STAT3 inhibitor, PIAS3, have also been found.11-13 Deregulation of the negative feedback of the JAK-STAT pathway could be involved in IFN resistance. We cloned a cytokine-inducible SH2 protein, CIS.14 CIS gene is a direct target of STAT5, and its product tightly binds to the tyrosine-phosphorylated interleukin-3 (IL-3) receptor and erythropoietin (EPO) receptor. It partially suppresses STAT5 activation, probably through masking of STAT5 docking sites on the receptor.15 We recently cloned another CIS family member, JAB, which directly binds to the JAK2 tyrosine kinase domain and inhibits tyrosine kinase activity.16 Overexpression of JAB resulted in the suppression of all cytokine signaling using JAKs. We also found five additional CIS family members (CIS2-CIS6), and the original CIS has been referred to as CIS1.17 CIS3 binds to JAK2-JH1 tyrosine kinase domain like JAB; however, the interaction between JH1 and CIS3 seems to be weaker than that between JH1 and JAB.17 Forced expression of CIS3 and JAB at physiological levels inhibited IL-6 or leukemia inhibitory factor (LIF)-mediated growth arrest of M1 leukemia cells. Two other groups have reported on related genes. One referred to JAB, CIS2, and CIS3 as SOCS-1, SOCS-2, and SOCS-3,18 respectively, and the other as SSI-1, SSI-2, and SSI-3, respectively.19 20 Because the CIS family genes (CISs) seem to be involved in regulation of cytokine signaling, we examined their role in IFN signaling. We found that forced expression of JAB but not CIS1, CIS2, and CIS3 in M1 leukemia cells or NIH-3T3 cells conferred resistance to the action of IFNs. Because elevated expression of JAB was found in two independent IFN-resistant cell lines, JAB could potentially be involved in IFN resistance or reduced response to IFNs.
MATERIALS AND METHODS
Cells and transformants.
M1 myelogenous leukemia cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% horse serum. M1 transformants were obtained by electroporation with pcDNA3 carrying Myc-tagged full-length CISs and JAB and maintained in the presence of 0.5 mg/mL G418 as described previously.17 NIH-3T3 cells were cultured in DMEM containing 10% calf serum (CS). NIH-3T3 transformants expressing CIS1 were obtained by electroporation with pEFneo-CIS1 (without epitope tag). Other transformants were obtained by infection of retrovirus vector pLXSN carrying Myc-tagged CIS2, CIS3, or JAB.16 NIH-3T3 transformants were selected with 0.7 mg/mL G418, and two to three positive clones for each transfection were maintained in DMEM containing 0.5 mg/mL G418. Isolation and characterization of IFN-α–resistant Daudi clone (DaudiR) will be described elsewhere. Briefly, it was obtained by long-term exposure of parental cells to IFN-α (Sumitomo Pharmaceutical Co, Osaka, Japan) and cloning with colony formation. The resistant clone was 10,000 times more resistant to IFN-α than parental cells. Daudi and its IFN-α–resistant clone were cultured in RPMI containing 10% fetal calf serum (FCS). LoVo and its IFN-resistant clones were maintained in Ham’s F12 containing 10% FCS.6 IFN-resistant phenotype of Daudi-and LoVo-derived clones remained in the absence of IFNs for more than 1 year.
IFN-induced growth arrest of M1 cells.
Two to four independent clones were tested for IFN-β– (a gift from TORAY Research Lab, Kamakura, Japan) or IFN-γ– (Hayashibara Biochemical Lab, Okayama, Japan) induced growth arrest. Briefly, 0.5 × 104 parental M1 cells and transformants expressing Myc-tagged CISs or JAB were cultured in medium containing 10% horse serum supplemented with or without the indicated amount of IFNs for 6 days, then the viable cells were determined by Trypan blue exclusion and counted in a hemocytometer.
IFN-induced activation of JAKs and STAT1.
Immunoblotting with antiphosphotyrosine, anti-STAT1 (C20; Santa Cruz, Santa Cruz, CA), or anti–tyrosine-phosphorylated STAT1 (New England BioLabs, Beverly, MA) was performed as described15-17 after cells were stimulated with either saline or 1,000 IU/mL IFNs for 15 minutes to 18 hours at 37°C. Electrophoretic mobility shift assay (EMSA) was performed as described.21 Immunoprecipitation with anti-JAK1 and JAK2 (UBI) from 2 × 106 cells and immunoblotting with antiphosphotyrosine were performed as described.15
Northern hybridization.
Cells were stimulated with 1,000 IU/mL IFN-β or IFN-γ for indicated periods. For Northern blotting, total RNA (5 μg) was separated on 1.0% agarose gels containing 2.4% formaldehyde, then transferred to positively charged nylon membranes. After fixation under calibrated ultraviolet irradiation, the membranes were hybridized with DIG-labeled riboprobes and visualized using alkaline-phosphatase–labeled anti-DIG antibody according to the manufacturer’s instructions (Boehringer, Mannheim, Germany). Probe cDNAs for CIS3, JAB, IRF-1, FcγRI, and G3PDH have been described previously.17 22
Antiviral assay.
NIH3T3 cells (5,000 cells/well) were plated in 96-well microplates. After IFN-β and IFN-γ treatments for 24 hours, the medium was changed to that containing vesicular stomatitis virus (VSV) at 8,000 plaque-forming units (PFU) per well. At 72 hours postinfection, the cytopathic effects were estimated by measuring the number of living cells using the methylthiotetrazole (MTT) assay as described.23 The cytopathic effects were expressed as the percentage of MTT reduction activity of IFN-treated cells to that of untreated, uninfected cells.
RESULTS
Induction of JAB expression by IFN-γ.
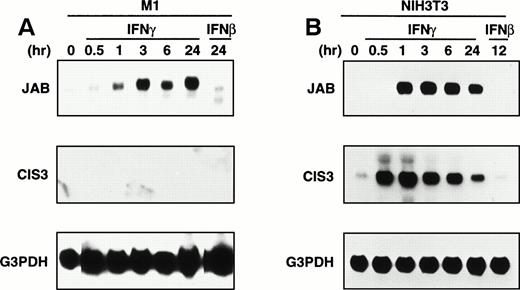
Because CIS family members have shown to be a cytokine-inducible gene, we examined induction of CIS1, 2, 3, and JAB by IFNs using Northern hybridization. CIS1 and CIS2 were not induced in M1 leukemic cells and NIH-3T3 fibroblast cells by either IFN-β or IFN-γ (data not shown). IFN-β did not induce CIS3 and JAB in either cell line (Fig 1A and B). IFN-γ induced JAB but not CIS3 in M1 cells, whereas CIS3 and JAB were induced by IFN-γ in NIH-3T3 cells. Expression of JAB was detected as long as IFN-γ was present for at least 24 hours after stimulation in both M1 and NIH-3T3 cells, whereas induction of CIS3 in NIH-3T3 cells was rapid and transient. We examined several cell lines and cytokines including IL-6, LIF, and granulocyte-macrophage colony-stimulating factor (GM-CSF) and found that JAB was most frequently and strongly induced by IFN-γ (data not shown). Although LIF and IL-6 have shown to induce JAB expression in M1 cells,18 19 JAB induction by IFN-γ was much higher (Fig 2). Thus, JAB seemed to be an IFN-γ–inducible gene.
Expression of endogenous JAB or CIS3 with IFNs. M1 (A) and NIH3T3 (B) cells were stimulated with IFNs (1,000 IU/mL) for the indicated time, and the total RNA was subjected to Northern blotting with probe for JAB, CIS3, or control G3PDH.
Expression of endogenous JAB or CIS3 with IFNs. M1 (A) and NIH3T3 (B) cells were stimulated with IFNs (1,000 IU/mL) for the indicated time, and the total RNA was subjected to Northern blotting with probe for JAB, CIS3, or control G3PDH.
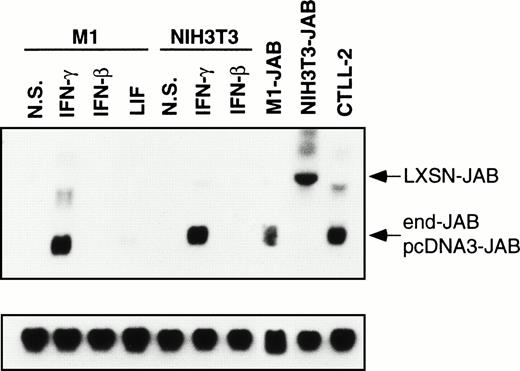
Expression of JAB in M1 and 3T3 transformants. Parental M1 or NIH-3T3 cells were stimulated without (N.S.) or with 1,000 IU/mL IFN-γ, 1,000 IU/mL IFN-β, or 10 ng/mL LIF for 12 hours, and then the total RNA was isolated. Total RNA was also isolated from untreated M1 transformant expressing JAB (M1-JAB), NIH-3T3 transformant (NIH3T3-JAB), and CTLL2 cells. The RNAs were subjected to Northern blotting with probe for JAB and control G3PDH. The arrowheads indicate JAB message derived from transfected cDNA in pLXSN (LXSN-JAB) in NIH3T3-JAB transformant, that in pcDNA3 (pcDNA3-JAB) in M1-JAB transformant, and endogenous JAB (end-JAB).
Expression of JAB in M1 and 3T3 transformants. Parental M1 or NIH-3T3 cells were stimulated without (N.S.) or with 1,000 IU/mL IFN-γ, 1,000 IU/mL IFN-β, or 10 ng/mL LIF for 12 hours, and then the total RNA was isolated. Total RNA was also isolated from untreated M1 transformant expressing JAB (M1-JAB), NIH-3T3 transformant (NIH3T3-JAB), and CTLL2 cells. The RNAs were subjected to Northern blotting with probe for JAB and control G3PDH. The arrowheads indicate JAB message derived from transfected cDNA in pLXSN (LXSN-JAB) in NIH3T3-JAB transformant, that in pcDNA3 (pcDNA3-JAB) in M1-JAB transformant, and endogenous JAB (end-JAB).
Forced expression of JAB conferred resistance to IFNs on M1 and NIH-3T3 cells.
We examined the effect of forced expression of JAB and CISs on the biological action of IFNs in M1 leukemia cells and NIH-3T3 fibroblastic cells. In M1 transformants, the expression level of transfected JAB was comparable to or less than that of endogenous JAB in IFN-γ–treated parental M1 or NIH-3T3 cells (Fig 2). The expression level of JAB in NIH-3T3 transformants was as high as that in IFN-γ–treated parental cells or in CTLL2 cells (Fig 2). Immunoblotting with anti-Myc antibody revealed that the expression levels of Myc-CIS1, CIS2, CIS3, and JAB were similar as described.17 In NIH-3T3 transformants, the expression level of each CIS was about fivefold higher than that of M1 cells as judged by immunoblotting (data not shown).
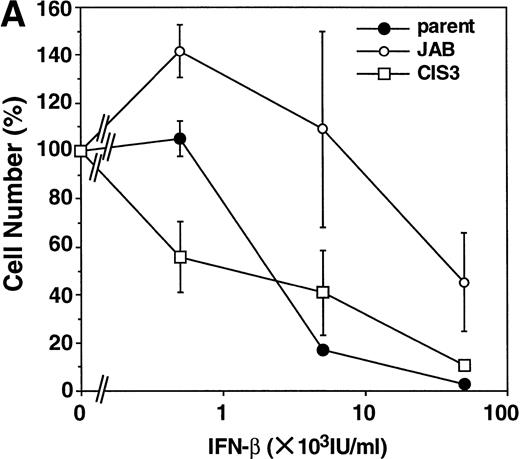
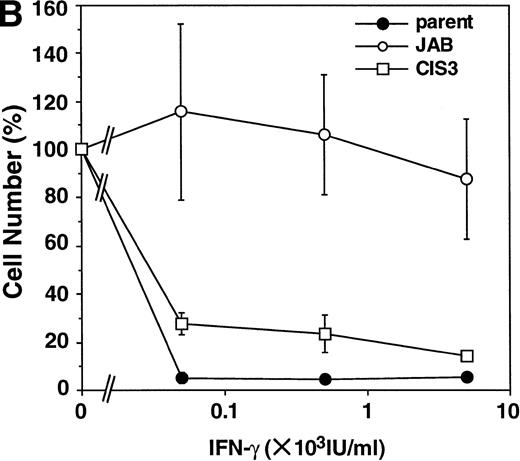
First, we examined the antiproliferative effect of IFNs in M1 cells, because our NIH-3T3 exhibited only weak growth arrest in response to IFNs. As shown in Fig 3B, IFN-γ induced growth arrest of parental M1 cells and CIS3 transformants, whereas JAB transformants were highly resistant to the antiproliferative effect of IFN-γ. Although IFN-β required higher concentrations for growth inhibition than IFN-γ, the growth of parental M1 cells and CIS3 transformants was also suppressed by IFN-β (Fig 3A). JAB transformants were 10 to 30 times more resistant to IFN-β than parental M1 cells and CIS3 transformants.
Effect of forced expression of JAB and CIS3 on IFN-induced growth inhibition of M1 cells. Parental M1 cells and CIS3 or JAB transformants (5,000 cells/well) were plated in 24-well plates and cultured for 6 days in the presence of the indicated amount of IFN-β (A) or IFN-γ (B). Then the viable cells were counted by Trypan blue exclusion. The viability of the cells is expressed as the percentage of the number of IFN-treated cells to that of untreated cells. The numbers of untreated cells (100%) are 2.4 × 105 (parental M1 cells), 2.4 × 105 (JAB transformant), and 2.1 × 105 (CIS3 transformant).
Effect of forced expression of JAB and CIS3 on IFN-induced growth inhibition of M1 cells. Parental M1 cells and CIS3 or JAB transformants (5,000 cells/well) were plated in 24-well plates and cultured for 6 days in the presence of the indicated amount of IFN-β (A) or IFN-γ (B). Then the viable cells were counted by Trypan blue exclusion. The viability of the cells is expressed as the percentage of the number of IFN-treated cells to that of untreated cells. The numbers of untreated cells (100%) are 2.4 × 105 (parental M1 cells), 2.4 × 105 (JAB transformant), and 2.1 × 105 (CIS3 transformant).
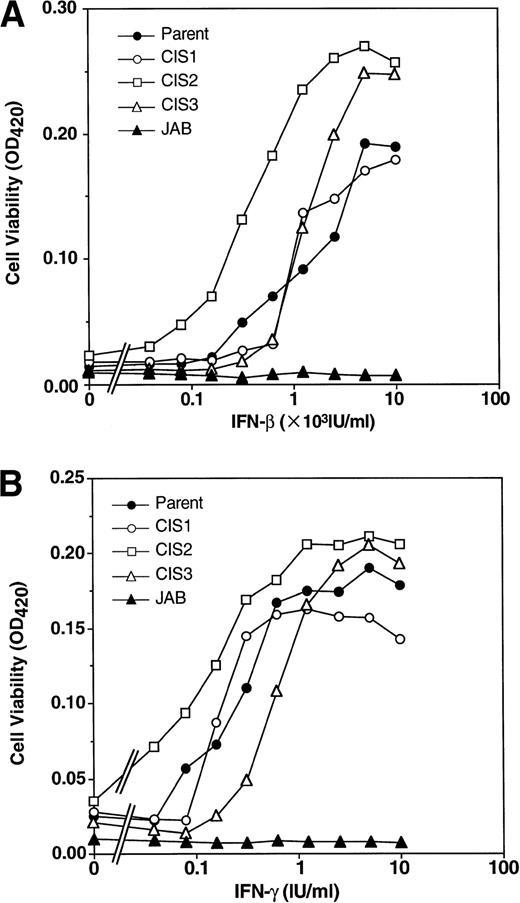
Next, we examined the IFN-induced antiviral effect using NIH-3T3 transformants. Transformants expressing CIS1, CIS2, and CIS3 were as sensitive as parental NIH-3T3 cells to both IFN-β and IFN-γ, whereas JAB transformants were almost completely insensitive to the IFN-induced antiviral effect (Fig 4). Thus, forced overexpression of JAB conferred IFN resistance on both hematopoietic and fibroblastic cells.
Effect of CIS family members and JAB on the antiviral effect of IFNs. Parental NIH3T3 cells and transformants were infected with VSV for 3 days after incubation with the indicated amount of IFN-β (A) or IFN-γ (B) for 24 hours. The viable cells were measured by MTT assay. The optical density (OD) value at 420 nm that represents viable cell number is shown.
Effect of CIS family members and JAB on the antiviral effect of IFNs. Parental NIH3T3 cells and transformants were infected with VSV for 3 days after incubation with the indicated amount of IFN-β (A) or IFN-γ (B) for 24 hours. The viable cells were measured by MTT assay. The optical density (OD) value at 420 nm that represents viable cell number is shown.
Inhibition of STAT1 activation by forced expression of JAB.
To elucidate the mechanism of inhibition of IFN-γ–mediated growth arrest and the antiviral effect by JAB, we first examined the IFN-γ–dependent gene expression (Fig 5A and B). IFN-induced growth arrest of M1 cells has been shown to be accompanied by induction of interferon regulatory factor (IRF)-1. FcγRI has been used as a marker of IFN-γ action. Promoter regions of IRF-1 and FcγRI contain GAS sequences and could be primary targets of STAT1 and STAT3. IFN-γ induced the expression of IRF-1 in both parental M1 and NIH-3T3 cells within 1 hour, and a high level of expression was maintained thereafter. Similar induction of IRF-1 was observed in CIS3 transformants. In contrast, IRF-1 expression was only marginally elevated in M1 and NIH-3T3 transformants expressing JAB. FcγRI expression was gradually induced from 1 hour after stimulation in parental M1 cells and CIS3 transformants, whereas it was not induced within 6 hours in JAB transformants. We could not detect FcγRI expression in NIH-3T3 cells. These data suggest that JAB but not CIS3 can efficiently inhibit IFN-γ–induced STAT1 activation.
Effect of JAB and CIS3 on IFN-γ inducible genes. The cells (A; M1-derived cells, B; NIH3T3-derived cells) were stimulated with 1,000 IU/mL IFN-γ for the indicated periods (h) and analyzed with Northern hybridization using IRF1, FcγRI, and control G3PDH probes. FcγRI was not detected in parental NIH3T3 cells (not shown).
Effect of JAB and CIS3 on IFN-γ inducible genes. The cells (A; M1-derived cells, B; NIH3T3-derived cells) were stimulated with 1,000 IU/mL IFN-γ for the indicated periods (h) and analyzed with Northern hybridization using IRF1, FcγRI, and control G3PDH probes. FcγRI was not detected in parental NIH3T3 cells (not shown).
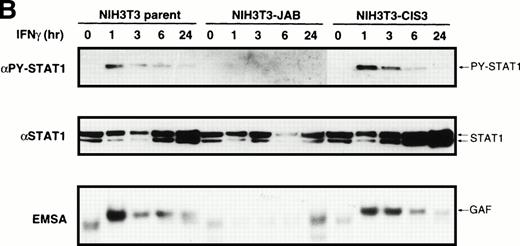
Next, we examined IFN-γ–induced STAT1 activation directly. Parental M1 and NIH-3T3 cells or their transformants expressing CIS3 or JAB were stimulated with or without IFN-γ, then cell extracts were blotted with antibody specific to tyrosine-phosphorylated STAT1 (Fig 6; αPY-STAT1) or to STAT1 (Fig 6; αSTAT1). STAT1 was tyrosine phosphorylated in response to IFN-γ in parental M1 cells and transformants expressing CIS3 for over 24 hours. On the other hand, the tyrosine phosphorylation of STAT1 in NIH-3T3 cells was transient. In both M1 and NIH-3T3 JAB transformants, IFN-γ–induced tyrosine phosphorylation of STAT1 was largely reduced. Interestingly, STAT1 content was markedly increased after stimulation with IFN-γ in parental M1 cells, NIH-3T3 cells, and their CIS3 transformants, suggesting a positive feedback regulation of STAT1 synthesis. No such increase in STAT1 content in response to IFN-γ was evident in JAB transformants. The DNA binding activity of STATs was assessed by EMSA using the IRF-1 GAS probe21 (Fig 6; EMSA). STAT1/DNA binding complexes appeared after stimulation with IFN-γ for 60 minutes in parental cells and transformants expressing CIS3. DNA binding activity of STAT1 in M1 cells lasted over 24 hours, whereas that in NIH-3T3 cells was transient, which is consistent with the time course of STAT1 phosphorylation. IFN-γ–induced DNA binding activity of STAT1 was not detected in either M1 or NIH-3T3 transformants expressing JAB (Fig 6; EMSA).
Inhibition of IFN-γ–induced STAT1 activation by JAB. Cells (A; M1-derived cells, B; NIH3T3-derived cells) were stimulated with or without IFN-γ (1,000 IU/mL) for the indicated periods and lysed. The postnuclear supernatant was subjected to immunoblotting with anti–tyrosine-phosphorylated STAT1 (PY-STAT1) or anti-STAT1 (STAT1) and to EMSA with IRF-1 probe (EMSA).
Inhibition of IFN-γ–induced STAT1 activation by JAB. Cells (A; M1-derived cells, B; NIH3T3-derived cells) were stimulated with or without IFN-γ (1,000 IU/mL) for the indicated periods and lysed. The postnuclear supernatant was subjected to immunoblotting with anti–tyrosine-phosphorylated STAT1 (PY-STAT1) or anti-STAT1 (STAT1) and to EMSA with IRF-1 probe (EMSA).
Impaired JAK activation in JAB transformants in response to IFN-γ and IFN-β.
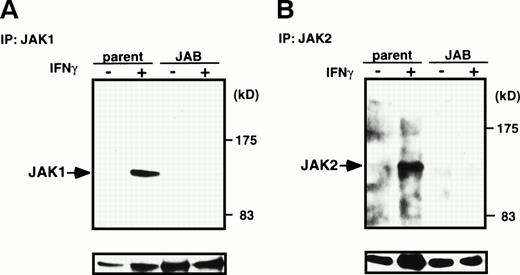
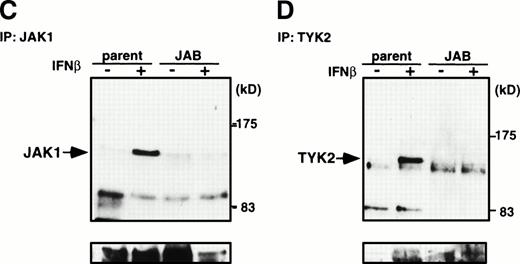
To elucidate further molecular basis of the inhibition of STAT1 activation by JAB, we examined IFN-γ–induced activation of JAK1 and JAK2 and IFN-β–induced activation of JAK1 and Tyk2 in NIH-3T3 cells. Tyrosine phosphorylation of JAKs in response to IFNs in M1 cells was not very evident, probably because of very low content of JAKs or receptors. After stimulation of parental NIH-3T3 cells or JAB transformants with IFN-γ for 15 minutes, cells were lysed and then immunoprecipitated with anti-JAK1 or JAK2 antibody and probed with antiphosphotyrosine antibody (Fig 7A and B). Tyrosine phosphorylation of JAK1 and JAK2 was largely impaired in JAB transformants. Similarly, tyrosine phosphorylation of JAK1 and Tyk2 in response to IFN-β was largely reduced in JAB transformants (Fig 7C and D). As shown previously,16 fibroblast growth factor (FGF) induced tyrosine phosphorylation and activation of the FGF receptor normally, suggesting that the inhibition of tyrosine kinase activity in JAB transformants was specific to JAKs. Thus, the IFN unresponsiveness of JAB transformants can be explained by inhibition of activation of JAKs by JAB.
Inhibition of IFN-β– and IFN-γ–induced JAK activation by JAB. NIH3T3 parental cells (parent) and JAB transformants (JAB) were stimulated with 1,000 IU/mL IFN-γ (A and B) or IFN-β (C and D) for 15 minutes and lysed. The postnuclear supernatant was immunoprecipitated with anti-JAK1 (A and C) anti-JAK2 (B), or anti-Tyk2 (D). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels), then the membrane was stripped and reprobed with anti-JAK1 (A and C), anti-JAK2 (B), or anti-Tyk2 (C) antibodies (lower panels).
Inhibition of IFN-β– and IFN-γ–induced JAK activation by JAB. NIH3T3 parental cells (parent) and JAB transformants (JAB) were stimulated with 1,000 IU/mL IFN-γ (A and B) or IFN-β (C and D) for 15 minutes and lysed. The postnuclear supernatant was immunoprecipitated with anti-JAK1 (A and C) anti-JAK2 (B), or anti-Tyk2 (D). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels), then the membrane was stripped and reprobed with anti-JAK1 (A and C), anti-JAK2 (B), or anti-Tyk2 (C) antibodies (lower panels).
Elevated expression of JAB in IFN-resistant cell lines.
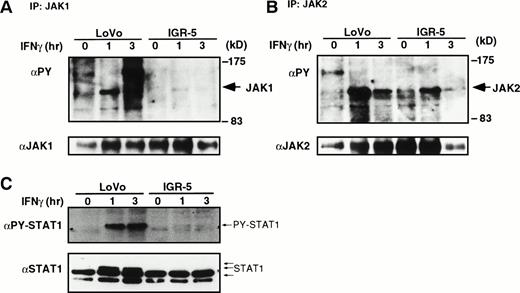
To extend the potential involvement of JAB expression in the IFN-resistant phenotype, we examined JAB expression in IFN-resistant somatic cell mutants derived from a human Burkitt’s lymphoma cell line, Daudi, and a colon adenocarcinoma cell line, LoVo. Daudi cells are highly sensitive to IFN-α–induced growth arrest, although IFN-γ exhibits no effect. An IFN-α–resistant clone was obtained by colony formation in the presence of IFN-α. Two IFN-γ–resistant mutants, IGR-5 and IGR-53, with dominant phenotypes were obtained from LoVo cells previously.6 IGR-5 is highly resistant to IFN-γ and weakly to IFN-α, and IGR-53 is highly resistant to both. IGR-53 cells lack functional IFN-γ receptors, whereas IGR-5 possesses IFN-γ receptors. The molecular mechanism of IFN resistance of IGR-5 has not been clarified. We examined JAB and CIS3 expression in these mutants. As shown in Fig 8, IFN-α–resistant Daudi cells and IGR-5, but not IGR-53, exhibited elevated expression of JAB. The level of JAB in DaudiRcells was comparable to that in CTLL2 cells (data not shown). IFN-α–induced tyrosine phosphorylation of JAK1 and Tyk2 as well as that of STAT1 were reduced to about 50% in IFN-α–resistant Daudi cells compared with parental Daudi cells (Fig 9). Remarkable reduction in tyrosine phosphorylation of JAK1, JAK2, and STAT1 was observed in response to IFN-γ in IGR-5 cells (Fig 10). Therefore, overexpression of JAB could be, at least in part, a mechanism of IFN resistance in vitro.
Expression of JAB and CIS3 mRNA in IFN-resistant clones from Daudi and LoVo cells. Total RNAs from exponentially growing parental LoVo cells (LoVo), IFN-γ–resistant IGR-5 cells [IGR-5(γ)], IFN-– and IFN-γ–resistant IGR-53 cells [IGR-53(,γ)], parental Daudi cells (Daudi), and IFN-–resistant Daudi cells [DaudiR()] were extracted and analyzed with Northern hybridization using JAB, CIS3, and G3PDH probes.
Expression of JAB and CIS3 mRNA in IFN-resistant clones from Daudi and LoVo cells. Total RNAs from exponentially growing parental LoVo cells (LoVo), IFN-γ–resistant IGR-5 cells [IGR-5(γ)], IFN-– and IFN-γ–resistant IGR-53 cells [IGR-53(,γ)], parental Daudi cells (Daudi), and IFN-–resistant Daudi cells [DaudiR()] were extracted and analyzed with Northern hybridization using JAB, CIS3, and G3PDH probes.
IFN-–induced tyrosine phosphorylation of JAK1, Tyk2, and STAT1 in Daudi and its IFN-resistant clone. Parental Daudi cells (Daudi) or IFN-resistant cells (DaudiR) were stimulated with IFN- (1,000 IU/mL) for 15 minutes and lysed. The postnuclear supernatants were immunoprecipitated with anti-JAK1 (A) or anti-Tyk2 (B). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels), then the membrane was stripped and reprobed with anti-JAK1 (A) or anti-Tyk2 (B) antibodies (lower panels). (C) Total cell extracts were also blotted with anti-phospho STAT1 (PY-STAT1) and anti-STAT1 (STAT1).
IFN-–induced tyrosine phosphorylation of JAK1, Tyk2, and STAT1 in Daudi and its IFN-resistant clone. Parental Daudi cells (Daudi) or IFN-resistant cells (DaudiR) were stimulated with IFN- (1,000 IU/mL) for 15 minutes and lysed. The postnuclear supernatants were immunoprecipitated with anti-JAK1 (A) or anti-Tyk2 (B). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels), then the membrane was stripped and reprobed with anti-JAK1 (A) or anti-Tyk2 (B) antibodies (lower panels). (C) Total cell extracts were also blotted with anti-phospho STAT1 (PY-STAT1) and anti-STAT1 (STAT1).
IFN-γ–induced tyrosine phosphorylation of JAK1, JAK2, and STAT1 in LoVo and IGR-5. Parental LoVo cells (LoVo) or IFN-resistant cells (IGR-5) were stimulated with IFN-γ (1,000 IU/mL) for indicated periods (h) and lysed. The postnuclear supernatants were immunoprecipitated with anti-JAK1 (A) or anti-JAK2 (B). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels, PY), then the membrane was stripped and reprobed with anti-JAK1 (A) or anti-JAK2 (B) antibodies (lower panels). (C) Total cell extracts were also blotted with anti-phospho STAT1 (PY-STAT1) and anti-STAT1 (STAT1).
IFN-γ–induced tyrosine phosphorylation of JAK1, JAK2, and STAT1 in LoVo and IGR-5. Parental LoVo cells (LoVo) or IFN-resistant cells (IGR-5) were stimulated with IFN-γ (1,000 IU/mL) for indicated periods (h) and lysed. The postnuclear supernatants were immunoprecipitated with anti-JAK1 (A) or anti-JAK2 (B). The immunoprecipitates were blotted with antiphosphotyrosine antibody (upper panels, PY), then the membrane was stripped and reprobed with anti-JAK1 (A) or anti-JAK2 (B) antibodies (lower panels). (C) Total cell extracts were also blotted with anti-phospho STAT1 (PY-STAT1) and anti-STAT1 (STAT1).
DISCUSSION
In this study, we found that JAB is an IFN-γ–inducible gene and that forced expression of JAB conferred resistance not only to IFN-γ but also to IFN-β in murine cell lines. The molecular mechanism of the inhibition of IFN signaling by JAB is most likely to be the inhibition of activation of JAKs, thereby blocking STATs activation and target gene expression. Several lines of evidence suggest a direct inhibition of tyrosine kinase activity by binding of JAB to JAKs. As shown previously, coexpression of JAB efficiently inhibits tyrosine phosphorylation of JAK as well as cellular proteins in 293 cells.16 LIF- or IL-6–induced tyrosine phosphorylation of STAT3 and gp130 was largely impaired in M1 cells expressing JAB.18-20 However, in M1 cells it has been very difficult to show tyrosine phosphorylation of JAKs in response to LIF or IFNs. In the present study, we showed that in NIH-3T3 cells expressing JAB that do not respond to IFNs, IFN-induced tyrosine phosphorylation of JAK1, JAK2, and Tyk2 was strongly inhibited. These inhibitions probably lead further inhibition of STAT1 activation, IRF-1 expression, and induction of an antiviral state. Recently, we showed inhibition of JAK2 tyrosine kinase activity in vitro using purified recombinant JAK2 and JAB (Yasukawa et al, unpublished data, February 1998). Thus, JAB is probably an intrinsic inhibitor of JAKs.
Starr et al reported that in mouse bone marrow cells, SOCS-1/JAB is highly induced by GM-CSF, IL-13, and IFN-γ.18We also observed that JAB was induced by GM-CSF in UT7 myeloid leukemia cells and by EPO in F36E erythroleukemia cells.17 However, as far as we ascertained, IFN-γ is the most potent inducer of JAB in a wide variety of cell lines. The promoter region of JAB contains GAS motif,24 suggesting the involvement of STAT1 in the induction of JAB by IFN-γ. Previously, JAB/SOCS1 was reported to be induced by IL-6 in mouse liver and M1 cells and to inhibit IL-6 signaling in M1 cells, suggesting that JAB is a negative feedback regulator of IL6/STAT3. However, our experiments suggest that IL-6 or LIF induces expression of JAB in M1 cells much less efficiently than IFN-γ (Fig 2 and data not shown). We found that IFN-γ induces JAB expression in M1 cells at a level probably high enough to inhibit not only IL-6 but also IFN-γ signaling (see Fig 2). Thus, JAB could be a negative feedback regulator of IFN-γ/STAT1 and also could be involved in IFN-γ–induced downregulation of other cytokine responsiveness. We also found that JAB expression is very high in CTLL2 cells without IFN-γ (see Fig 2). As expected, CTLL2 did not respond to IFN-γ and IFN-β for growth inhibition (Sakamoto et al, unpublished data, March 1998). It also has been shown that exogenously expressed EPO receptor and granulocyte colony-stimulating factor (G-CSF) receptor are not functional in this cell line.25,26 Because the molecular basis of such a selective inhibition of particular cytokine receptor signaling has not been elucidated, JAB could be a good candidate for the factor that is involved in this cytokine unresponsiveness in CTLL2. Although EPO does not induce tyrosine phosphorylation of JAK2 in CTLL2 cells expressing exogenous EPO receptor,27 it is not clear why CTLL2 still can respond to IL-2. JAB may have a lower affinity to JAK3 than to JAK1 and JAK2, thereby allowing activation of IL-2 signaling but not EPO or G-CSF signaling.
If JAB is a negative feedback regulator, why cannot JAB induced by IFN-γ inhibit further IFN-γ signaling to produce an antiviral effect? The signaling events induced by early JAK activation until the appearance of JAB (usually these inductions take a few hours) may be essential and sufficient for further irreversible changes inside cells. Indeed, tyrosine phosphorylation of STAT1 in response to IFN-γ is usually transient even in the continuous presence of IFN-γ (see Fig6B). JAK activity is also transient in most cases. JAB mRNA is detected at 1 to 3 hours and remains after 24 hours of stimulation (Fig 1). Thus, JAB expression could be a mechanism of downmodulation of IFN-γ–induced JAK/STAT1 activity. However, in M1 cells, activation of STAT1 induced by IFN-γ lasted for more than 24 hours. Similarly, prolonged activation and increased levels of STAT3 were observed in M1 cells after stimulation with IL-6.28 The molecular mechanism of such prolonged activation of STATs in M1 cells has not been elucidated but may be partly due to the increase of the levels of STAT1 (Fig 6) and STAT3.28 Similar IFN-γ “priming” effect for IFN-α–induced STAT1 and STAT2 tyrosine phosphorylation has been reported.29 However, the efficiency of STAT1 phosphorylation compared with the level of STAT1 content apparently decreased in M1 cells after continuous IFN-γ exposure, suggesting a downregulation of JAK activation even in M1 cells (Fig 6).
Our present study suggests disregulated overexpression of JAB can confer IFN resistance on cells. JAB could be a mechanism of IFN resistance with dominant phenotype because resistance appeared on constitutive expression. Indeed, two independently isolated IFN-resistant clones expressed elevated levels of JAB. IFN resistance with recessive phenotype has been well described. Mutations in the receptors, JAKs, and STATs have been found in IFN-resistant mutants.1,2 Recently, marked reduction of STAT1 was consistently observed in IFN-resistant melanoma cells from patients.5 Exogenous expression of STAT1 improved responsiveness to IFN-β in an IFN-resistant cell line.5These are examples of IFN resistance with recessive phenotype. On the other hand, IFN resistance with dominant phenotype has not been well documented. As far as we know, IGR-5 and IGR-53 are the only examples of dominant resistant phenotypes.6 However, Raiph et al reported that resistance of melanoma cell lines to IFNs correlates with reduction of IFN-induced tyrosine phosphorylation of cellular proteins.30 Thus, as shown here, reduced activation of JAKs by JAB overexpression could be, at least in part, a mechanism of IFN resistance with dominant phenotype. At present, we do not know what kind of mutations can elevate JAB expression. However, constitutive activation of STATs by oncogenic tyrosine kinases such as Bcr-Abl can potentially be involved in high JAB expression. Although partial inhibition of JAK/STAT pathway by JAB may not be able to explain 10,000 times resistance to IFN-α of DaudiR cells, overexpression of JAB could contribute to some feature of IFN resistance obtained from sensitive cell lines by in vitro selection. Probably, DaudiR cells have multiple mutations that confer strong resistance to IFN-α–induced apoptosis and growth arrest. Our study strongly encourages the retrospective search of JAB expression in cells from patients, which may elucidate the involvement of JAB in actual IFN resistance in cancer or viral diseases.
IFN-γ is known as an inhibitory cytokine and is known to suppress many cytokine actions or cellular responses. For example, IL-4–induced IgE synthesis and germline ε transcription was suppressed in the presence of IFN-γ in B cells.31 JAB could be a part of the mechanism of the antagonistic effect of IFN-γ on other cytokines. JAB is also most abundantly expressed in thymus,18 which suggests a regulatory role for JAB in cytokine actions in T cells. IFN-γ has been shown to play an important role in immune responses, including the development of Th1 cells.32 IFN-γ–treated Th1 cells become resistant to growth inhibition by IFNs, which has been explained by loss of the IFN-γ receptor β chain.33 However, such loss of the receptor may need long-term incubation of cells with IFN-γ. Induction of JAB may partly explain early downregulation of IFN-γ responsiveness in Th1 cells. The physiological role of JAB on IFN-γ activity may be defined by the creation of mice lacking the JAB gene.
ACKNOWLEDGMENT
We thank Ms H. Ohgusu for excellent technical assistance.
Supported in part by grants from the Ministry of Science, Education and Culture of Japan, Ryoichi Naito Medical Foundation, Suzuken Memorial Foundation, TORAY Research Foundation, and Uehara Memorial Foundation.
Address reprint requests to Akihiko Yoshimura, Institute of Life Science, Kurume University, Aikawamachi 2432-3, Kurume 839-0861, Japan; e-mail: yosimura@lsi.kurume-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 8. Expression of JAB and CIS3 mRNA in IFN-resistant clones from Daudi and LoVo cells. Total RNAs from exponentially growing parental LoVo cells (LoVo), IFN-γ–resistant IGR-5 cells [IGR-5(γ)], IFN-– and IFN-γ–resistant IGR-53 cells [IGR-53(,γ)], parental Daudi cells (Daudi), and IFN-–resistant Daudi cells [DaudiR()] were extracted and analyzed with Northern hybridization using JAB, CIS3, and G3PDH probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1668/4/m_blod41709008w.jpeg?Expires=1767720841&Signature=PXQn~emfRLLJkIpfNsUkdUGX5gLxG8PJXZbuEDMsuZOBiCQ32D1uwwGQ0hhFjzeM5pOleEiMp0sJV7S2ywvuRkTFWcEk0Brve4fqbq~GnMjdn~YsVGU1lWD7CaHmSYlcCkiWTvrY6BSyDegdSHx9gDbEgOI-HVzA-pMCzvrgsJt7kYxrNyLuUYmSpxw2NELz8NTP4MPafbNJn6njNCzX0OAxVn17tsylwRKAPwqlE6ifGE9YTfftIsu2PGBJdhopS~L4AWR6rGB0jVV-wync586GwXHyDlnnGHlf53xU2xUDwiHCDJYtuyOh6WVOrpnZhD6pnffj2DhMCiyC6Zma0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal