Abstract
Advanced low-grade lymphomas are usually incurable with conventional-dose chemotherapy. It is uncertain whether cures are possible with high-dose therapy and bone marrow transplant from a human leukocyte antigen (HLA)-identical sibling. We sought to determine the outcome of HLA-identical sibling bone marrow transplants in advanced low-grade lymphoma in an observational study of 113 patients conducted at 50 centers participating in the International Bone Marrow Transplant Registry (IBMTR). The median patient age was 38 years (range, 15 to 61). Eighty percent had stage IV disease at the time of transplantation. The median number of prior chemotherapy regimens was two (range, 0 to 5). Thirty-eight percent had refractory disease and 29% a Karnofsky performance score (KPS) less than 80%. All patients underwent allogeneic bone marrow transplantation from a HLA-identical sibling donor. The conditioning regimen included total-body irradiation (TBI) in 82% of patients; cyclosporine was used for graft-versus-host disease prophylaxis in 74%. Survival, disease-free survival, recurrence rate, treatment-related mortality, and causes of death were determined. Three-year probabilities of recurrence, survival, and disease-free survival were 16% (95% confidence interval [CI], 9% to 27%), 49% (95% CI, 39% to 60%), and 49% (95% CI, 39% to 59%), respectively. Higher survival was associated with pretransplant KPS ≥90%, chemotherapy-sensitive disease, use of a TBI-containing conditioning regimen, and age less than 40 years. We conclude that high-dose therapy followed by transplantation from a HLA-identical sibling leads to prolonged survival in some patients with advanced low-grade lymphoma. Most mortality is treatment-related, and recurrences are rare.
© 1998 by The American Society of Hematology.
ADVANCED-GRADE LYMPHOMAS are relatively indolent, but are incurable with conventional treatments.1-4 The median survival duration from diagnosis is 7 to 9 years. High-dose therapy and a blood cell or bone marrow autotransplant reportedly results in sustained remission in some patients.5-9 Recurrences are common and there is concern about posttransplant myelodysplastic syndromes.5,10-12Prolonged remissions have been reported in small numbers of patients treated with allogeneic bone marrow transplantation.13-19We studied 113 patients with advanced low-grade lymphoma who received a bone marrow transplant from a human leukocyte antigen (HLA)-identical sibling.
PATIENTS AND METHODS
Patients.
We reviewed all HLA-identical sibling transplants for low-grade lymphoma performed between 1984 and 1995 and reported to the International Bone Marrow Transplant Registry (IBMTR) by 50 centers worldwide. The study included 113 patients with a diagnosis of low-grade lymphoma at diagnosis and at the time of transplant. This included patients with diffuse well-differentiated lymphocytic lymphoma (Working Formulation group A), follicular small cleaved-cell lymphoma (Working Formulation group B), and follicular mixed-cell lymphoma (Working Formulation group C).20 Patients initially diagnosed with low-grade lymphoma, but whose disease transformed to intermediate-grade or high-grade lymphoma before transplantation, were excluded.
Pathology reports confirming the diagnosis of low-grade lymphoma were reviewed by Koen van Besien. Discrepancies in nomenclature among centers were resolved using the recent publication on the Revised European-American Lymphoma (REAL) classification.21
IBMTR.
The IBMTR is a voluntary working group of more than 300 transplant teams worldwide that contribute detailed data on their allogeneic bone marrow transplants to the Statistical Center at the Medical College of Wisconsin. Participants are required to report all consecutive transplants; compliance is monitored by on-site audits. Approximately two thirds of all active transplant centers report their data to the IBMTR. The IBMTR database includes 40% to 45% of all allogeneic transplant recipients since 1970. Patients are monitored longitudinally. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality.
Statistical methods.
Primary outcomes were survival, disease-free survival (survival without lymphoma posttransplant), recurrence, and treatment-related mortality (nonrelapse death). For treatment-related mortality, patients were considered treatment failures at the time of death from any cause in the first 28 days posttransplant or at time of death in continuous remission for those surviving more than 28 days posttransplant; patients with recurrent lymphoma were censored at the time of relapse and those alive in remission were censored at the last follow-up evaluation. For disease-free survival, patients were considered treatment failures at the time of relapse or death from any cause; patients alive in continuous remission were censored at the last follow-up evaluation. Patients who never achieved remission were analyzed as having recurrent lymphoma on day 28.
Other outcomes examined were acute graft-versus-host disease (GVHD), chronic GVHD, and survival. Acute GVHD was defined as moderate to severe (grade II to IV) disease using established criteria; patients surviving more than 21 days with evidence of engraftment were considered at risk.22 Chronic GVHD was determined by clinical criteria in patients surviving more than 90 days with evidence of engraftment.23
Probabilities of outcomes were calculated using the Kaplan-Meier product-limit estimate and expressed as probabilities with a 95% confidence interval (CI) computed using the arcsine-square root transformation. Patient-, disease-, and transplant-related variables were studied for associations with survival. Univariate comparisons used the log-rank test. Multivariate analyses used Cox proportional hazards regression with stepwise forward variable selection. As disease-free survival was nearly identical to survival, multivariate analyses were performed only for survival. The incidence of lymphoma relapse was too low to allow a multivariate analysis of this parameter.
RESULTS
Patient characteristics.
Patient-, disease-, and transplant-related characteristics are listed in Table 1. Fifty-eight percent of patients were male. The median age was 38 years (range, 15 to 61). Twenty-nine percent had a Karnofsky performance score (KPS) less than 90%.
Patient-, Disease-, and Transplant-Related Characteristics of 113 Recipients of HLA-Identical Sibling Bone Marrow Transplants for Low-Grade Non-Hodgkin’s Lymphoma Reported to the IBMTR by 50 Centers Worldwide
| Variable . | No. Assessable . | Patients . | |
|---|---|---|---|
| No. . | % . | ||
| Patient characteristics | |||
| Male gender | 113 | 66 | 58 |
| Age at transplant (yr) | 113 | ||
| Median | 38 | ||
| Range | 15-61 | ||
| <40 | 63 | 56 | |
| ≥40 | 50 | 44 | |
| KPS ≤ 80% | 113 | 33 | 29 |
| Disease characteristics at diagnosis | |||
| Histology | 113 | ||
| Small lymphocytic | 20 | 18 | |
| Follicular small cleaved | 52 | 46 | |
| Follicular mixed | 41 | 36 | |
| Disease stage | 113 | ||
| I | 2 | 2 | |
| II | 9 | 8 | |
| III | 10 | 9 | |
| IV | 92 | 81 | |
| Extranodal involvement | 110 | ||
| None | 19 | 17 | |
| Bone marrow | 66 | 60 | |
| Bone marrow + other-150 | 13 | 12 | |
| Other-150 | 12 | 11 | |
| Disease characteristics at transplant | |||
| Disease stage | 113 | ||
| Complete remission | 16 | 14 | |
| I | 2 | 2 | |
| II | 7 | 6 | |
| III | 7 | 6 | |
| IV | 80 | 71 | |
| Unknown | 1 | 1 | |
| Extranodal involvement | 110 | ||
| None | 30 | 27 | |
| Bone marrow | 64 | 58 | |
| Bone marrow + other-151 | 11 | 10 | |
| Other-151 | 5 | 5 | |
| Response to chemotherapy | 105 | ||
| Sensitive | 66 | 63 | |
| Resistant | 39 | 37 | |
| No. of prior chemotherapy regimens | 110 | ||
| Median | 2 | ||
| Range | 1-5 | ||
| Prior complete remission | 112 | 46 | 41 |
| Disease duration (mo) | 113 | ||
| Median | 24 | ||
| Range | 5-130 | ||
| Transplant characteristics | |||
| Year of transplant | 113 | ||
| 1984-1987 | 3 | 3 | |
| 1988-1989 | 15 | 13 | |
| 1990-1991 | 23 | 20 | |
| 1992-1993 | 35 | 31 | |
| 1994-1995 | 37 | 33 | |
| Donor-recipient sex match | 113 | ||
| Male-male | 35 | 31 | |
| Male-female | 29 | 26 | |
| Female-male | 30 | 27 | |
| Female-female | 19 | 16 | |
| Donor-recipient CMV status | 109 | ||
| +/+ | 36 | 33 | |
| −/+ | 19 | 17 | |
| Donor-recipient CMV status (Cont’d) | |||
| +/− | 21 | 19 | |
| −/− | 33 | 30 | |
| Conditioning regimen | 113 | ||
| TBI + Cy | 32 | 28 | |
| TBI + Cy + VP16 ± other | 43 | 38 | |
| TBI + Cy + other (not VP16) | 11 | 10 | |
| TBI ± other | 7 | 6 | |
| LFR + Cy + Bu | 1 | 1 | |
| Cy + BCNU + VP16 | 1 | 1 | |
| Cy + Bu | 15 | 13 | |
| Other | 3 | 3 | |
| GVHD prophylaxis | 113 | ||
| MTX + CsA ± other | 68 | 60 | |
| MTX ± other | 2 | 2 | |
| CsA ± other | 17 | 15 | |
| T-cell depletion ± other | 25 | 22 | |
| FK506 + corticosteroids | 1 | 1 | |
| Variable . | No. Assessable . | Patients . | |
|---|---|---|---|
| No. . | % . | ||
| Patient characteristics | |||
| Male gender | 113 | 66 | 58 |
| Age at transplant (yr) | 113 | ||
| Median | 38 | ||
| Range | 15-61 | ||
| <40 | 63 | 56 | |
| ≥40 | 50 | 44 | |
| KPS ≤ 80% | 113 | 33 | 29 |
| Disease characteristics at diagnosis | |||
| Histology | 113 | ||
| Small lymphocytic | 20 | 18 | |
| Follicular small cleaved | 52 | 46 | |
| Follicular mixed | 41 | 36 | |
| Disease stage | 113 | ||
| I | 2 | 2 | |
| II | 9 | 8 | |
| III | 10 | 9 | |
| IV | 92 | 81 | |
| Extranodal involvement | 110 | ||
| None | 19 | 17 | |
| Bone marrow | 66 | 60 | |
| Bone marrow + other-150 | 13 | 12 | |
| Other-150 | 12 | 11 | |
| Disease characteristics at transplant | |||
| Disease stage | 113 | ||
| Complete remission | 16 | 14 | |
| I | 2 | 2 | |
| II | 7 | 6 | |
| III | 7 | 6 | |
| IV | 80 | 71 | |
| Unknown | 1 | 1 | |
| Extranodal involvement | 110 | ||
| None | 30 | 27 | |
| Bone marrow | 64 | 58 | |
| Bone marrow + other-151 | 11 | 10 | |
| Other-151 | 5 | 5 | |
| Response to chemotherapy | 105 | ||
| Sensitive | 66 | 63 | |
| Resistant | 39 | 37 | |
| No. of prior chemotherapy regimens | 110 | ||
| Median | 2 | ||
| Range | 1-5 | ||
| Prior complete remission | 112 | 46 | 41 |
| Disease duration (mo) | 113 | ||
| Median | 24 | ||
| Range | 5-130 | ||
| Transplant characteristics | |||
| Year of transplant | 113 | ||
| 1984-1987 | 3 | 3 | |
| 1988-1989 | 15 | 13 | |
| 1990-1991 | 23 | 20 | |
| 1992-1993 | 35 | 31 | |
| 1994-1995 | 37 | 33 | |
| Donor-recipient sex match | 113 | ||
| Male-male | 35 | 31 | |
| Male-female | 29 | 26 | |
| Female-male | 30 | 27 | |
| Female-female | 19 | 16 | |
| Donor-recipient CMV status | 109 | ||
| +/+ | 36 | 33 | |
| −/+ | 19 | 17 | |
| Donor-recipient CMV status (Cont’d) | |||
| +/− | 21 | 19 | |
| −/− | 33 | 30 | |
| Conditioning regimen | 113 | ||
| TBI + Cy | 32 | 28 | |
| TBI + Cy + VP16 ± other | 43 | 38 | |
| TBI + Cy + other (not VP16) | 11 | 10 | |
| TBI ± other | 7 | 6 | |
| LFR + Cy + Bu | 1 | 1 | |
| Cy + BCNU + VP16 | 1 | 1 | |
| Cy + Bu | 15 | 13 | |
| Other | 3 | 3 | |
| GVHD prophylaxis | 113 | ||
| MTX + CsA ± other | 68 | 60 | |
| MTX ± other | 2 | 2 | |
| CsA ± other | 17 | 15 | |
| T-cell depletion ± other | 25 | 22 | |
| FK506 + corticosteroids | 1 | 1 | |
Abbreviations: KPS, Karnovsky performance score; CMV, cytomegalovirus; TBI, total-body irradiation; Cy, cyclophosphamide; VP16, etoposide; LFR, limited-field radiation; Bu, busulfan; BCNU, nitrosurea; MTX, methotrexate; CsA, cyclosporine.
Other = pleura, liver, bone, skin, lung, kidney, epidural space.
Other = pleura, liver, kidney, bone, lung, brain.
Eighteen percent of patients had small lymphocytic lymphoma, 46% had follicular small cleaved-cell lymphoma, and 36% had follicular mixed-cell lymphoma. Eighty-one percent were diagnosed with stage IV disease, most commonly due to bone marrow involvement. Only 14% were in complete remission at transplant. Seventy-one percent had stage IV disease at transplant, despite a median of two prior chemotherapy regimens. Diverse chemotherapy regimens were used pretransplant; 37% of patients were felt to have chemotherapy-resistant lymphoma (ie, they had achieved less than a partial remission to the last chemotherapy regimen administered before transplant).
Eighty-four percent of the transplants were performed after 1990. The median interval from diagnosis to transplant was 24 months (range, 5 to 130). The pretransplant conditioning regimen included total-body irradiation (TBI) in 82% of cases. Among the 20 patients (18%) not receiving TBI for conditioning regimens, only three had received prior radiation. Twenty-two percent of patients received T-cell–depleted transplants.
Outcomes.
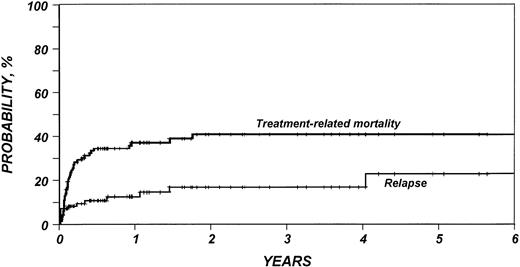
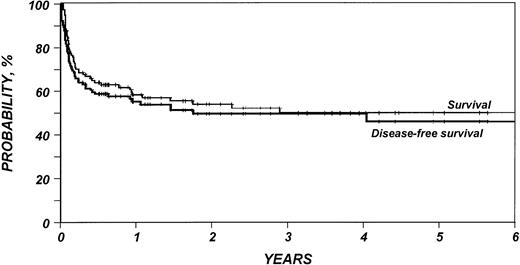
Outcomes are summarized in Table 2. The median follow-up duration of surviving patients was 25 months (range, 4 to 95). Three-year probabilities of recurrence and treatment-related mortality were 16% (95% CI, 9% to 27%) and 40% (95% CI, 30% to 50%), respectively (Fig1). Three-year probabilities of survival and disease-free survival were both 49% (95% CI, 39% to 59%) (Fig 2). Among 33 patients monitored for more than 2 years after transplantation, only one relapse was documented.
Probabilities (±95% CI) of Transplant Outcomes (at 3 years unless otherwise stated)
| . | 3-Year Probability (%) . | No. Assessable . |
|---|---|---|
| Graft failure | 1 (0-5) | 101 |
| Relapse* | 16 (9-27) | 93 |
| Treatment-related mortality | 40 (30-50) | 113 |
| Acute GVHD (at 100 days)† | 27 (19-37) | 99 |
| Chronic GVHD‡ | 66 (53-77) | 67 |
| Survival | 49 (39-60) | 113 |
| Disease-free survival | 49 (39-59) | 113 |
| . | 3-Year Probability (%) . | No. Assessable . |
|---|---|---|
| Graft failure | 1 (0-5) | 101 |
| Relapse* | 16 (9-27) | 93 |
| Treatment-related mortality | 40 (30-50) | 113 |
| Acute GVHD (at 100 days)† | 27 (19-37) | 99 |
| Chronic GVHD‡ | 66 (53-77) | 67 |
| Survival | 49 (39-60) | 113 |
| Disease-free survival | 49 (39-59) | 113 |
*Among patients surviving ≥28 days posttransplant.
Among patients surviving ≥21 days with evidence of engraftment.
Among patients surviving ≥90 days with evidence of engraftment.
Probability of relapse and treatment-related mortality after HLA-identical sibling bone marrow transplant for low-grade non-Hodgkin’s lymphoma.
Probability of relapse and treatment-related mortality after HLA-identical sibling bone marrow transplant for low-grade non-Hodgkin’s lymphoma.
Probability of survival and disease-free survival after HLA-identical sibling bone marrow transplant for low-grade non-Hodgkin’s lymphoma.
Probability of survival and disease-free survival after HLA-identical sibling bone marrow transplant for low-grade non-Hodgkin’s lymphoma.
In multivariate analysis, KPS, chemotherapy-resistance, conditioning regimen, and age significantly predicted survival (Table3).
Factors Significantly Associated With Survival in Multivariate Analysis of 113 Recipients of HLA-Identical Sibling Bone Marrow Transplants for Low-Grade Non-Hodgkin’s Lymphoma Reported to the IBMTR by 50 Centers Worldwide
| Covariate . | No. . | RR of Death . | 95% CI . | P Value* . |
|---|---|---|---|---|
| KPS pretransplant | ||||
| <90% | 33 | 1.00 | — | |
| ≥90% | 80 | 0.42 | (0.23-0.77) | .005 |
| Conditioning regimen | ||||
| Chemotherapy alone | 20 | 1.00 | — | |
| TBI + chemotherapy | 93 | 0.47 | (0.24-0.93) | .03 |
| Sensitivity to chemotherapy | ||||
| Resistant | 66 | 1.00 | — | |
| Sensitive | 39 | 0.50 | (0.27-0.91) | .02* |
| Unknown | 8 | 1.02 | (0.35-2.99) | .97* |
| Age at transplant (yr) | ||||
| <40 | 63 | 1.00 | — | |
| ≥40 | 50 | 1.85 | (1.05-3.24) | .03* |
| Covariate . | No. . | RR of Death . | 95% CI . | P Value* . |
|---|---|---|---|---|
| KPS pretransplant | ||||
| <90% | 33 | 1.00 | — | |
| ≥90% | 80 | 0.42 | (0.23-0.77) | .005 |
| Conditioning regimen | ||||
| Chemotherapy alone | 20 | 1.00 | — | |
| TBI + chemotherapy | 93 | 0.47 | (0.24-0.93) | .03 |
| Sensitivity to chemotherapy | ||||
| Resistant | 66 | 1.00 | — | |
| Sensitive | 39 | 0.50 | (0.27-0.91) | .02* |
| Unknown | 8 | 1.02 | (0.35-2.99) | .97* |
| Age at transplant (yr) | ||||
| <40 | 63 | 1.00 | — | |
| ≥40 | 50 | 1.85 | (1.05-3.24) | .03* |
Abbreviation: RR, relative risk.
P value for pairwise comparison of specific category with the reference (baseline) group.
Fifty-one patients died; causes of death are summarized in Table4. Pulmonary complications were most common, including interstitial pneumonitis (n = 7), acute respiratory distress syndrome (n = 5), and pulmonary hemorrhage (n = 1). Two patients died of acute GVHD and three of chronic GVHD.
Causes of Death of 51 Recipients of HLA-Identical Sibling Bone Marrow Transplants for Low-Grade Non-Hodgkin’s Lymphoma
| Cause of Death . | Patients . | |
|---|---|---|
| No. . | % . | |
| Persistant or recurrent lymphoma | 11 | 22 |
| Interstitial pneumonia | 10 | 20 |
| VOD | 7 | 14 |
| GVHD (chronic or acute) | 6 | 12 |
| Adult respiratory distress syndrome | 5 | 10 |
| Sepsis | 4 | 8 |
| Organ failure (not VOD) | 2 | 4 |
| Hemorrhage | 2 | 4 |
| Unknown | 2 | 4 |
| Pulmonary embolism | 1 | 2 |
| Stroke | 1 | 2 |
| Cause of Death . | Patients . | |
|---|---|---|
| No. . | % . | |
| Persistant or recurrent lymphoma | 11 | 22 |
| Interstitial pneumonia | 10 | 20 |
| VOD | 7 | 14 |
| GVHD (chronic or acute) | 6 | 12 |
| Adult respiratory distress syndrome | 5 | 10 |
| Sepsis | 4 | 8 |
| Organ failure (not VOD) | 2 | 4 |
| Hemorrhage | 2 | 4 |
| Unknown | 2 | 4 |
| Pulmonary embolism | 1 | 2 |
| Stroke | 1 | 2 |
Abbreviation: VOD, venoocclusive disease.
DISCUSSION
This report evaluates the outcome of HLA-identical sibling bone marrow transplants for advanced low-grade lymphomas among centers reporting consecutive patients to the IBMTR. Not surprisingly, the data indicate that transplants are mainly offered to younger patients with advanced disease. Most patients in this study received extensive prior therapy. Many had chemotherapy-resistant disease and low performance scores. Most were not candidates for autotransplants because of extensive bone marrow involvement. Characteristics of these patients and their outcomes are consistent with those reported in three smaller single-institution series.13,18 19 Only 22 of the patients in this study were included in those series. Given the unfavorable characteristics of this population, the observed lymphoma-free and overall survival rates of 49% and the recurrence rate of only 16% are encouraging.
Interestingly, there was only one recurrence among 33 patients monitored for more than 2 years. This seems lower than has been reported for autotransplants and is consistent with other recent reports.24,25 The low recurrence rate, if true, may be due to graft-versus-lymphoma effects as suggested by some,26-28or, alternatively, to lack of tumor contamination of the allogeneic graft.29 30 Our results should be interpreted cautiously. Although we tried to obtain current information on all patients, follow-up methods and accuracy of restaging varies considerably among reporting centers; failure to detect early recurrences may at least partially explain the results.
Multivariate analyses identified poor KPS and chemotherapy-resistance as adverse prognostic factors. Better patient selection and earlier transplants could improve outcome. Use of TBI for pretransplant conditioning was also associated with better survival. Radiation is effective in low-grade lymphoma and is frequently used in autotransplant conditioning regimens. However, in a recent analysis of autotransplants for low-grade lymphoma, there was a trend (P = .09 in multivariate analysis) for poorer survival among patients receiving TBI.31 Only 18% of the patients in this series received non-TBI regimens and it is possible that such patients differed for unknown but important (latent) covariates.
The 3-year probability of treatment-related mortality was 40% (95% CI, 30% to 50%). Most treatment-related deaths were from pulmonary complications, similar to observations in allogeneic transplants for Hodgkin’s disease.32 This high incidence of pulmonary complications may be related to the use of busulfan or TBI. Chronic GVHD, though common, was a rare cause of death.
In conclusion, this analysis establishes the potential of allogeneic transplantation to achieve survival in patients with advanced low-grade lymphoma. Our data provide a rationale for prospective studies of allogeneic transplants earlier in the course of the disease.
ACKNOWLEDGMENT
The authors thank the coordinators and physicians of the participating teams who provided us with additional data on these patients.
APPENDIX
Supported by Public Health Service Grant No. P01-CA-40053 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute, of the US Department of Health and Human Services; and grants from Alpha Therapeutic Corporation; Amgen, Inc; Anonymous; Astra Pharmaceutical; Baxter Healthcare Corp; Bayer Corp; Biogen; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Co; Frank G. Brotz Family Foundation; CellPro, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Glaxo Wellcome Co; Hoechst Marion Roussel, Inc; ICN Pharmaceuticals; Immunex Corp; Janssen Pharmaceutica; Kettering Family Foundation; Kirin Brewery Co; Robert J. Kleberg, Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Eli Lilly Co Foundation; Nada and Herbert P. Mahler Charities; MDS Nordian; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Ortho Biotech Corp; John Oster Family Foundation; Elsa U. Pardee Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; Pharmacia and Upjohn; Principal Mutual Life Insurance Co; RGK Foundation; Rockwell-Automation Allen-Bradley Co; Schering-Plough International; Walter Schroeder Foundation; Searle; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Charities; and Wyeth-Ayerst Laboratories.
See Appendix for support data.
Address reprint requests to Mary M. Horowitz, MD, MS, IBMTR/ABMTR Statistical Center, Medical College of Wisconsin, PO Box 26509, 8701 Watertown Plank Rd, Milwaukee, WI 53226.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal