Abstract
Wiskott-Aldrich syndrome (WAS) and X-linked thrombocytopenia (XLT) are caused by mutations of the WAS protein (WASP) gene. All hematopoietic stem cell-derived lineages, including platelets, express WASP. Platelets from WAS patients are smaller than their normal counterparts and defects in platelet aggregation and actin polymerization have been reported. To determine if WASP is important for normal platelet function, we examined its role in signal transduction. We found that collagen but not thrombopoietin or thrombin induces a rapid and robust increase in tyrosine phosphorylation of platelet-associated WASP. Collagen-induced tyrosine phosphorylation of WASP was inhibited by cytochalasin D and wortmannin, respectively, suggesting that actin polymerization and phosphatidylinositol 3-kinase (PI3-kinase) play a role in the induction of tyrosine phosphorylation of WASP. Binding of glutathion S-transferase (GST)-Grb2 to WASP was seen in the lysate of resting platelets. The binding was reduced when lysates from collagen-stimulated platelets were incubated with GST-Grb2, suggesting that tyrosine phosphorylation of WASP may directly or indirectly modulate the adapter function of WASP. Although thrombin- and thrombopoietin-induced increase in tyrosine phosphorylation of WASP is negligible or marginal, WASP from thrombin-activated platelets became incorporated into the Triton X-100–insoluble 10,000gsedimentable residue in an aggregation-dependent manner, suggesting that it may have a regulatory role in platelet cytoskeletal processes during aggregation. Lastly, we found that WASP is cleaved in response to activation of calpain, a protease that may have a role in postaggregation signaling processes. Our data suggest that collagen specifically induces an increase in tyrosine phosphorylation of WASP and that WASP is involved in signaling during thrombin-induced aggregation by its redistribution to the cytoskeleton and its cleavage during aggregation.
© 1998 by The American Society of Hematology.
MUTATIONS IN THE classic Wiskott-Aldrich syndrome (WAS) protein (WASP) gene may result in WAS or in a milder phenotype, X-linked thrombocytopenia (XLT).1-4Whereas the immune defect in XLT is either mild or absent, thrombocytopenia and platelets of small size are common features for both phenotypes, suggesting that WASP may have a unique and essential role in platelet production and the regulation of platelet function in the circulation.5-8 WASP is a 64-kD protein expressed exclusively in hematopoietic cells.9,10 Because WASP does not have a known catalytic domain, it has been postulated that it may serve as an adapter protein for other signaling and cytoskeletal molecules. This possibility has been further supported by the reports that WASP binds in vitro to several tyrosine kinases (c-Src, Fyn, Tec family kinases, and cERG), Grb2, p47nck, and phospholipase Cγ1. Fyn and p47nck have also been shown to associate with WASP in vivo. The interaction of WASP with these molecules is thought to be mediated through the binding of the PXXY motif of WASP to Src homology (SH) 3 domains of the signaling molecules.10-16 Many adapter molecules, such as Shc, Grb2, p47nck, and the 85-kD subunit for phosphatidylinositol 3-kinase (PI3-kinase), are known to become tyrosine phosphorylated and thus create the binding sites for SH2 or protein tyrosine binding (PTB) domains of other signaling molecules, contributing to the efficient assembly of a large signaling complex.17-19 Although WASP has five tyrosine residues, no inducible tyrosine phosphorylation of WASP has been documented so far. For example, it was reported that, in HL60 cells, various types of stimulation, including treatment with interleukin-4 (IL-4) or cross-linking of Fcγ receptors, failed to induce tyrosine phosphorylation of WASP.16 Similarly, the cross-linking of B-cell receptors (BCRs) did not seem to induce a significant increase in tyrosine phosphorylation of WASP,12although a role for WASP in BCR signaling has been suggested and WASP has been reported to be associated with BTK, a B-cell–specific tyrosine kinase expressed in B cells. Interestingly, recent studies have suggested that collagen may activate platelets through signal transduction pathways, analogous to BCR signaling in B cells.20-23 Thus, glycoprotein VI (gpVI), one of the receptors for collagen on platelets, has been found to be associated with the γ subunit of Fcγ receptor.20,21 The cross-linking of the γ subunit of the Fcγ receptor may trigger activation of src family kinases and the syk tyrosine kinase, leading to activation of phospholipase Cγ2, which may, in turn, mediate hydrolysis of polyphosphoinositides. The essential role of the syk kinase and the γ subunit of Fcγ receptor in collagen-induced platelet activation were confirmed by studies of platelets from syk tyrosine kinase-null or Fcγ receptor-null mice generated by gene targeting. Platelets, which are deficient in both of the two molecules, failed to respond to collagen.23
Given the similarity between BCR signaling in B cells and collagen-induced signalings in platelets, we postulated that normal platelets, which express WASP and BTK (unpublished observation), represent a model system to investigate the role of WASP in signal transduction.
To accomplish this, we examined whether WASP becomes uniquely tyrosine phosphorylated after treatment with collagen. We found that collagen-treated but not thrombin- or thrombopoietin-treated platelets substantially increase tyrosine phosphorylation of WASP. In contrast, thrombin induces redistribution of WASP into the Triton X-100–insoluble residue in an aggregation-dependent fashion. Finally, similar to many proteins incorporated into the Triton X-100–insoluble residue after platelet aggregation, such as integrin β3, c-Src, talin, and actin-binding protein, we found that WASP was an endogenous substrate for calpain, suggesting a role for WASP in postaggregation events.
MATERIALS AND METHODS
Materials.
Prostaglandin E1 (PGE1), cytochalasin D, wortmannin, calcium ionophore A23187, Arg-Gly-Asp-Ser (RGDS) peptide, dibucaine, dimethyl sulfoxide (DMSO), aspirin, apyrase (type VIII), N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), sodium dodecyl sulfate (SDS), 2-mercaptoethanol, sodium orthovanadate, chicken egg albumin, protein A-Sepharose, Triton X-100, and Tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma (St Louis, MO). Polyvinylidene difluoride (PVDF) membranes (pore size, 0.45 mm) were obtained from Millipore Corp (Bedford, MA). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) molecular standards and enhanced chemiluminesence (ECL) reagents, including secondary antibodies, were purchased from Amersham (Arlington Heights, IL). Calpeptin was obtained from Calbiochem (La Jolla, CA). The antiphosphotyrosine murine monoclonal antibody (4G10) and the anti-WASP rabbit polyclonal antibody (503) were described previously.10 24-26 Purified human thrombin was generously provided by Green Cross Co Ltd (Osaka, Japan). Agarose conjugated-glutathion S-transferase (GST), -GST-Grb2, and -GST-Grb2-SH2 domain were purchased from Santa Cruz (Santa Cruz, CA). Recombinant thrombopoietin was a generous gift from Kirin Brewery Co Ltd (Maebashi, Japan). Horse tendon collagen (type I) was purchased from Hormon Chemie (Munich, Germany).
Platelet preparation.
Human blood from healthy volunteers was drawn by venipuncture into 1/10 vol of 3.8% (wt/vol) trisodium citrate and gently mixed. Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 200g for 20 minutes. PRP was aspirated and incubated with aspirin (2 mmol/L) for 30 minutes at room temperature. After the addition of PGE1 (1 μmol/L) from a stock solution in absolute ethanol (1 mmol/L), the PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL of a modified HEPES-Tyrode buffer (129 mmol/L NaCl, 8.9 mmol/L NaHCO3, 0.8 mmol/L KH2PO4, 0.8 mmol/L MgCl2, 5.6 mmol/L dextrose, and 10 mmol/L HEPES, pH 7.4) also containing apyrase (2 U/mL) and washed twice. Platelets were resuspended at a concentration of 3 × 108 cells/mL in the same buffer containing apyrase (2 U/mL) at 37°C. RGDS peptide (200 μmol/L) was added whenever it was necessary to inhibit platelet aggregation.
Gel electrophoresis and Western blotting to detect tyrosine phosphorylated proteins or WASP.
Platelet stimulation was terminated by the addition of an equal volume of 2× concentrated Laemmli’s sample buffer (10% glycerol, 1% SDS, 5% 2-mercaptoethanol, 50 mmol/L Tris-HCl [pH 6.8], and 0.002% bromophenol blue), 10 mmol/L EGTA, and 1 mmol/L sodium orthovanadate. After boiling at 95°C for 5 minutes, one-dimensional SDS-electrophoresis was performed on 10% or 7.5% to 15% polyacrylamide gels.24-26 Separated proteins were electrophoretically transferred from the gel onto PVDF membranes in a buffer containing Tris (25 mmol/L), glycine (192 mmol/L), and 20% methanol at 0.2 amps for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (Tris-buffered saline [TBS]: 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.6 with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 1.0 μg/mL for antibody 4G10 and 1:1,000 dilution for antibody 503 in TBST. The primary antibody was removed and the blots were washed four times in TBST and incubated with horse radish peroxidase-conjugated second antibody diluted 1:3,000 in TBST. Blots were then washed four times in TBST. Antibody reactions were detected with chemiluminescence according to the manufacturer’s instructions.
Immunoprecipitation.
Platelet stimulation was terminated by the addition of an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 [vol/wt], pH 7.4). After 20 minutes on ice, the lysates were centrifuged at 10,000g (at 4°C) for 20 minutes. The supernatant was removed, incubated with protein A-Sepharose (40 μL of 50% slurry) for 1 hour, and centrifuged to obtain the precleared supernatant. The anti-WASP polyclonal antibody 503 was then added and the mixtures were incubated for 2 to 3 hours on ice (concentration, 2 μL/mL supernatant). Protein A-Sepharose (40 μL of 50% slurry/mL supernatant) was added and incubated for several hours. The immune complexes were washed with 1 mL of cold washing buffer (the same as the lysis buffer except the concentration of Triton X-100 [vol/wt] was 1%) three times and then resuspended in Laemmli’s sample buffer.
Isolation of platelet cytoskeleton.
The Triton X-100–insoluble cytoskeleton was isolated as described,25 26 with the following modification. An equal amount of lysis buffer was added to platelet suspensions to solubilize platelets. After 5 minutes on ice, the lysates were centrifuged at 10,000g. The resulting pellet was washed twice in washing buffer. For one-dimensional SDS electrophoresis, the Triton X-100–insoluble residue was solubilized in SDS sample buffer. The supernatant was diluted with an equal volume of 2× concentrated SDS sample buffer.
RESULTS
Collagen-induced tyrosine phophorylation of WASP.
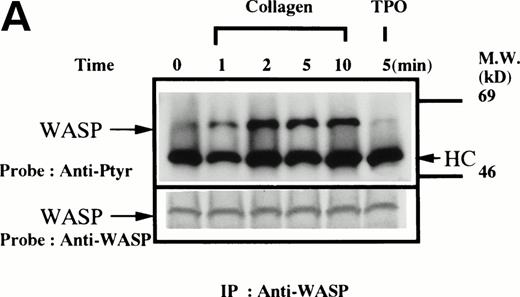
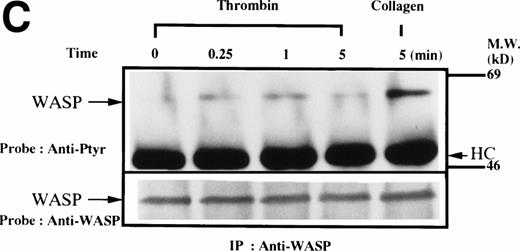
Platelets suspended in the presence of 200 μmol/L RGDS peptide were treated with either collagen (50 μg/mL), thrombin (1 U/mL), or thrombopoietin (100 ng/mL). After incubation for various times, the platelets were lysed and the lysates were incubated with anti-WASP antiserum 503 and protein A Sepharose. The same amount of a 64-kD protein recognized by the anti-WASP antiserum was immunoprecipitated from all cellular lysates in each set of experimemts (Fig 1A through D, lower panels). After treatment with collagen, WASP was increasingly tyrosine phosphorylated over the low basal level (Fig 1A, upper panel). Tyrosine phosphorylation reached a plateau within 2 minutes. In contrast, thrombin and thrombopoietin induced a marginal increase in tyrosine phosphorylation of WASP in platelets (Fig 1B and C), but the degree of phosphorylation by either of the reagents was much smaller than that induced by collagen. When platelet suspensions were stirred in the absence of RGDS peptide, thrombin-induced increase in tyrosine phosphorylation of WASP was still minimal (Fig 1D).
(A through D, upper panels) Tyrosine phosphorylation of WASP in platelets stimulated by collagen (50 μg/mL), thrombopoietin (TPO; 100 ng/mL), or thrombin (1 U/mL) at the time intervals indicated. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to collagen, TPO, or thrombin. To prevent platelet aggregation, platelets were treated with RGDS (200 μmol/L), except in lanes 1 through 3 in (D). For experiments shown in (D), we used lysis buffer containing 0.2% SDS and sonicated the lysates. WASP was immunoprecipitated with the polyclonal anti-WASP antiserum (503). Immune complexes were resuspended in SDS sample buffer and divided into two. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. One immunoblot was probed with antiphosphotyrosine antibodies and bands were visualized by chemiluminescence (upper panels, A through D). The other blot was probed for WASP (lower panels, A through D).
(A through D, upper panels) Tyrosine phosphorylation of WASP in platelets stimulated by collagen (50 μg/mL), thrombopoietin (TPO; 100 ng/mL), or thrombin (1 U/mL) at the time intervals indicated. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to collagen, TPO, or thrombin. To prevent platelet aggregation, platelets were treated with RGDS (200 μmol/L), except in lanes 1 through 3 in (D). For experiments shown in (D), we used lysis buffer containing 0.2% SDS and sonicated the lysates. WASP was immunoprecipitated with the polyclonal anti-WASP antiserum (503). Immune complexes were resuspended in SDS sample buffer and divided into two. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. One immunoblot was probed with antiphosphotyrosine antibodies and bands were visualized by chemiluminescence (upper panels, A through D). The other blot was probed for WASP (lower panels, A through D).
Collagen-induced tyrosine phosphorylation was inhibited by cytochaslsin D and wortmannin.
Because of recent reports suggesting a role of WASP in the initiation of actin polymerization in platelets27 as well as in T cells28 and because of evidence that PI3-kinase is also involved in the regulation of cytoskeletal reorganization of platelets,29 30 we examined the effects of cytochalasin D (an inhibitor of actin polymerization) and wortmannin (a PI3-kinase inhibitor) on WASP tyrosine phosphorylation. Platelets were treated with 0.1% DMSO (the vehicle for cytochalasin D and wortmannin) for 30 minutes, cytochalasin D (10 μmol/L) for 10 minutes, or wortmannin (50 nmol/L) for 30 minutes. Cytochalasin D or wortmannin but not DMSO inhibited the collagen-induced increase in tyrosine phosphorylation of WASP (Fig 2).
Collagen-induced tyrosine phosphorylation of WASP is inhibited by cytochalasin D or wortmannin. Platelet suspensions were incubated in the presence of RGDS peptide (200 μmol/L) for 5 minutes. After treatment with DMSO (0.1%) for 30 minutes, cytochalasin D (10 μmol/L) for 10 minutes, or wortmannin (50 nmol/L) for 30 minutes, collagen (50 μg/mL) was added to the suspension for 5 minutes as indicated (lanes 2 through 4). Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before (resting, lane 1) and after the addition of collagen (lanes 2 through 4). Tyrosine phosphorylation of WASP was detected as described in Fig1.
Collagen-induced tyrosine phosphorylation of WASP is inhibited by cytochalasin D or wortmannin. Platelet suspensions were incubated in the presence of RGDS peptide (200 μmol/L) for 5 minutes. After treatment with DMSO (0.1%) for 30 minutes, cytochalasin D (10 μmol/L) for 10 minutes, or wortmannin (50 nmol/L) for 30 minutes, collagen (50 μg/mL) was added to the suspension for 5 minutes as indicated (lanes 2 through 4). Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before (resting, lane 1) and after the addition of collagen (lanes 2 through 4). Tyrosine phosphorylation of WASP was detected as described in Fig1.
WASP associates with the cytoskeleton after platelet aggregation.
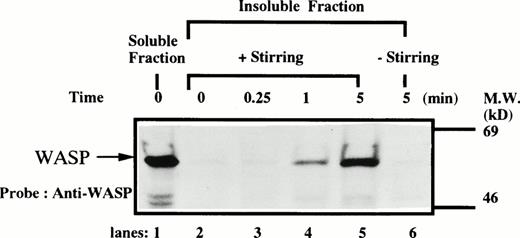
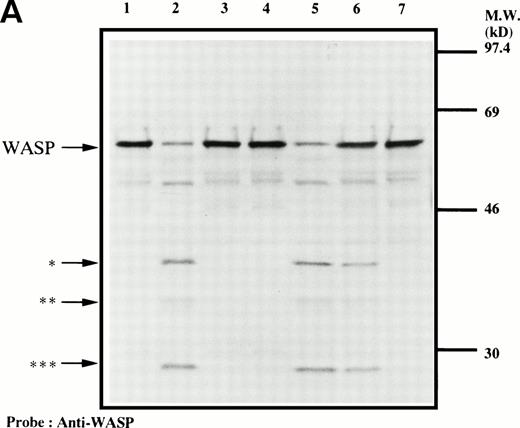
We next examined the possibility that WASP plays a role in signaling after platelet aggregation. Because it has been shown that many signaling molecules, including c-Src, become incorporated into the integrin-rich cytoskeleton after platelet aggregation,25,26,31-33 we tested the possibility that WASP may be incorporated into the cytoskeleton. In resting platelets, WASP was found predominantly in Triton X-100–soluble fraction (Fig 3). However, after stimulation of platelets by adding thrombin and stirring to induce platelet aggregation, WASP became associated with the Triton X-100–insoluble residue. This association of WASP with the cytoskeleton was inhibited by omitting stirring to minimize platelet aggregation (lane 6, Fig 3), as has been reported for c-Src, Vav, Crkl, Grb2, c-Cbl, and αIIbβ3.25,26,31-33 Because activation of calpain is known to accompany platelet aggregation,33-37 we examined whether WASP is a substrate for calpain and, if so, is cleaved during platelet aggregation. Activation of μ-calpain is known to be accompanied by cleavage of the enzyme, although it is unclear whether cleavage is necessary for activation.33,34 When platelets were treated with calcium ionophore A23187 or dibucaine, both known to induce calpain activation in platelets in the presence of extracellular calcium, we observed a significant loss of immunoreactive WASP and the generation of bands (Fig 4A, indicated by the arrows) of lower molecular weights reactive with anti-WASP (lane 2 and 5, Fig 4A). Inhibition of calpain activation by adding EGTA or calpeptin resulted in an inhibition of cleavage of WASP (lanes 3, 4, and 7, Fig 4A). Activation of calpain by dibucaine does not require extracellular calcium, whereas A23187 does. As expected, WASP cleavage induced by dibucaine was not inhibited by EGTA (lane 6, Fig 4A), but A23187-induced cleavage was (lane 3, Fig 4A). Thus, only under conditions in which calpain is activated was WASP cleaved. These data suggest that WASP may be a substrate for calpain, although we cannot rule out the possibility that activation of calpain may result in activation of other proteases that are responsible for the cleavage of WASP. When platelets were activated with thrombin and simultaneously stirred to induce platelet aggregation, a significant loss of WASP immunoreactivity was also observed (Fig 4B), resulting in the appearance of the same set of cleaved bands as observed in Fig 4A (compare lanes 1 and 2, Fig 4B). Omitting stirring to minimize aggregation resulted in an inhibition of the loss of immunoreactive WASP (lane 3, Fig 4B). Because activation of calpain could occur during lysis of platelets, these experiments were performed by the addition of EGTA and EDTA immediately before lysis of platelets to prevent artificial cleavage.38
Association of WASP with the Triton X-100–insoluble residue. Platelets were lysed with Triton X-100-EGTA buffer before or after stimulation with thrombin (1 U/mL), with or without stirring. Lysates were separated by high speed centrifugation into soluble and insoluble residues. Proteins from each fraction were separated by 10% SDS-PAGE and immunoblotted with anti-WASP antiserum 503. Lane 1, Triton X-100–soluble residue of resting cells (7.5 × 106cells). Lane 2, Triton X-100–insoluble residue of resting cells (3.0 × 107 cells). Lanes 3 through 5, Triton X-100–insoluble residue from 3.0 × 107 cells, prepared 15 seconds, 1 minute, and 5 minutes after exposure to thrombin (1 U/mL) with stirring, respectively. Lane 6, Triton X-100–insoluble residue of cells (3.0 × 107 cells) stimulated for 5 minutes with thrombin but without stirring.
Association of WASP with the Triton X-100–insoluble residue. Platelets were lysed with Triton X-100-EGTA buffer before or after stimulation with thrombin (1 U/mL), with or without stirring. Lysates were separated by high speed centrifugation into soluble and insoluble residues. Proteins from each fraction were separated by 10% SDS-PAGE and immunoblotted with anti-WASP antiserum 503. Lane 1, Triton X-100–soluble residue of resting cells (7.5 × 106cells). Lane 2, Triton X-100–insoluble residue of resting cells (3.0 × 107 cells). Lanes 3 through 5, Triton X-100–insoluble residue from 3.0 × 107 cells, prepared 15 seconds, 1 minute, and 5 minutes after exposure to thrombin (1 U/mL) with stirring, respectively. Lane 6, Triton X-100–insoluble residue of cells (3.0 × 107 cells) stimulated for 5 minutes with thrombin but without stirring.
Cleavage of WASP. (A) Platelets were incubated with either calpeptin (20 μmol/L) or DMSO (vehicle of calpeptin; final concentration, 0.1%) for 5 minutes. Platelets were then treated with calcium ionophore A23187 (1 μmol/L for 5 minutes) or dibucaine (1 mmol/L for 15 minutes) in the presence of 1 mmol/L CaCl2 or 5 mmol/L EGTA. Whole platelet lysates (1.5 × 107 cells/lane) were analyzed by 10% SDS-PAGE. Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes. WASP was detected by immunoblotting with polyclonal antibody 503. The top arrow indicates the relative position of the intact 64-kD subunit of WASP. The lower arrows indicate the positions of apparently cleaved products of WASP. Lane 1, control, DMSO + CaCl2; lane 2, A23187 + CaCl2; lane 3, A23187 + EGTA; lane 4, A23187 + CaCl2 + calpeptin ; lane 5, dibucaine + CaCl2; lane 6, dibucaine + EGTA; lane 7, dibucaine + CaCl2 + calpeptin. (B) Cleavage of WASP during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After the addition of EGTA (5 mmol/L) and EDTA (5 mmol/L), platelets were lysed by boiling in SDS sample buffer. WASP was detected by immunoblotting as described in Fig 1. Lane 1, resting platelets; lane 2, thrombin stimulation of platelets for 30 minutes with stirring; lane 3, thrombin stimulation for 30 minutes without stirring. The arrows indicate the same cleaved fragments of WASP as in (A).
Cleavage of WASP. (A) Platelets were incubated with either calpeptin (20 μmol/L) or DMSO (vehicle of calpeptin; final concentration, 0.1%) for 5 minutes. Platelets were then treated with calcium ionophore A23187 (1 μmol/L for 5 minutes) or dibucaine (1 mmol/L for 15 minutes) in the presence of 1 mmol/L CaCl2 or 5 mmol/L EGTA. Whole platelet lysates (1.5 × 107 cells/lane) were analyzed by 10% SDS-PAGE. Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes. WASP was detected by immunoblotting with polyclonal antibody 503. The top arrow indicates the relative position of the intact 64-kD subunit of WASP. The lower arrows indicate the positions of apparently cleaved products of WASP. Lane 1, control, DMSO + CaCl2; lane 2, A23187 + CaCl2; lane 3, A23187 + EGTA; lane 4, A23187 + CaCl2 + calpeptin ; lane 5, dibucaine + CaCl2; lane 6, dibucaine + EGTA; lane 7, dibucaine + CaCl2 + calpeptin. (B) Cleavage of WASP during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After the addition of EGTA (5 mmol/L) and EDTA (5 mmol/L), platelets were lysed by boiling in SDS sample buffer. WASP was detected by immunoblotting as described in Fig 1. Lane 1, resting platelets; lane 2, thrombin stimulation of platelets for 30 minutes with stirring; lane 3, thrombin stimulation for 30 minutes without stirring. The arrows indicate the same cleaved fragments of WASP as in (A).
Binding of GST-Grb2 but not GST to WASP.
Because WASP is thought to function as an adapter protein in lymphocytes, we next asked whether WASP in platelets may also bind to other proteins and, if so, whether collagen-induced stimulation may affect the adapter function of WASP. Because it is impossible to examine all SH2/SH3 proteins expressed in platelets for binding to WASP,25,26,31-33 we focused our attention on Grb2, which is known to be constitutively associated with WASP in lymphocytes.10 12 Platelet lysates obtained before and 5 minutes after collagen stimulation were equally divided for immunoprecipitation with anti-WASP antiserum or for GST-fusion protein-binding studies. WASP was immunoprecipitated by WASP antiserum 503 but not by the preimmune serum from both lysates equally well (data not shown), confirming that the lysates have the same amount of WASP. As expected, tyrosine phosphorylation of the 64-kD protein in platelets was dramatically increased after collagen stimulation (data not shown). The 64-kD tyrosine phosphorylated protein was not precipitated by preimmune serum. GST-Grb2 but not GST on agarose precipitated WASP from the same lysates (Fig 5). The amount of WASP precipitated by GST-Grb2 on agarose was significantly reduced when lysates from collagen-stimulated platelets were used (Fig 5, lane 2).
Binding of GST-Grb2 but not GST to WASP. Platelets were stimulated in nonaggregating conditions and lysed as described for Fig1. Lysates before (−) and 5 minutes after (+) collagen stimulation were equally divided into four aliquots. The two samples from each of the lysates were incubated either with agarose-bound GST-Grb2 (10 μg, lanes 1 and 2) or GST (10 μg, lanes 3 and 4) on ice for 3 hours. After three washes in phosphate-buffered saline (150 mmol/L, pH 7.4) containing orthovanadate (200 μmol/L), the precipitates were incubated in SDS sample buffer. The SDS samples were subjected to Western blotting to detect WASP by polyclonal anti-WASP antiserum 503.
Binding of GST-Grb2 but not GST to WASP. Platelets were stimulated in nonaggregating conditions and lysed as described for Fig1. Lysates before (−) and 5 minutes after (+) collagen stimulation were equally divided into four aliquots. The two samples from each of the lysates were incubated either with agarose-bound GST-Grb2 (10 μg, lanes 1 and 2) or GST (10 μg, lanes 3 and 4) on ice for 3 hours. After three washes in phosphate-buffered saline (150 mmol/L, pH 7.4) containing orthovanadate (200 μmol/L), the precipitates were incubated in SDS sample buffer. The SDS samples were subjected to Western blotting to detect WASP by polyclonal anti-WASP antiserum 503.
DISCUSSION
Although platelet abnormalities are a hallmark of WAS, the role of WASP in platelet release and function is not understood.5-8,27,28 Because of reports that, in other cells, WASP may serve as an adapter protein for other molecules involved in signaling or cytoskeletal reorganization, we explored signal transduction in human platelets and found that collagen, a natural agonist for platelets, is a strong inducer of tyrosine phosphorylation of WASP. Because WASP is believed to be an adapter-like molecule and protein tyrosine phosphorylation is essential for collagen-induced platelet activation,20-23 it is tempting to speculate that the induction of tyrosine phosphorylation of WASP is generating the potential binding site(s) for proteins containing SH2 and/or PTB domains, thus contributing to collagen-induced signaling in platelets. The robust induction of tyrosine phosphorylation of WASP seems to be a unique response to collagen-induced stimulation, because exposure to thrombin or thrombopoietin resulted in only a small increase in tyrosine phosphorylation of WASP. The observation that tyrosine phosphorylation of WASP during thrombin-induced platelet aggregation was also marginal may be misleading, being caused by the artificial dephosphorylation during the lysis of platelet aggregates, as has been reported by Law et al39 for integrin β3. Thus, it is possible that the increase in tyrosine phosphorylation of WASP has been underestimated by the current study. The observation that both cytochalasin D and wortmannin inhibited collagen-induced tyrosine phosphorylation of WASP suggests that the kinase(s) responsible for WASP phosphorylation may require both actin polymerization and activation of PI-3 kinase. Candidates for such kinases in platelets include Fak, RAFTK, and Tec. However, these kinases are not exclusively expressed in platelets.31,32,40 41 Thus, platelets may have unique mechanisms that enable these kinases to phosphorylate WASP or they may express unique and unknown platelet-specific WASP kinase(s).
WASP may also play a role during platelet aggregation, as evidenced by the redistribution of WASP to the cytoskeleton after thrombin-stimulated platelet aggregation. This is similar to what has been described for Cdc42, c-Src, Fyn, c-Cbl, Vav, Crkl, and αIIbβ3 integrin.25,26,31-33 The carboxyl terminal portion of WASP contains regions that show homology to several actin-binding proteins, such as verprolin and cofilin, which may allow binding of WASP to filamentous actin.1,2 This is also consistent with the proposed role for WASP in the regulation of actin polymerization through interaction with Cdc42 and/or WIP.42-44 On the other hand, we prefer to interpret our data to suggest that tyrosine phosphorylation of WASP acts downstream of actin polymerization. Thus, WASP may not simply regulate actin polymerization in platelets, but the function of WASP, in turn, could be modulated by polymerization of actin. WASP was shown to be associated with Grb2 in studies of other hematopoietic cells.9-16 Because the experiments reported here have demonstrated that platelet WASP is associated in vitro with Grb2, WASP and Grb2 may recruit more signaling molecules into the cytoskeleton. This hypothesis is also supported by our previous finding that Grb2 redistributes to the platelet cytoskeleton in an aggregation-dependent fashion.45 Such redistribution could be essential for platelet aggregation, because WAS platelets show defective aggregation in response not just to collagen, but also to other agonists such as thrombin or ADP.8 46Binding of Grb2 to WASP appreared to be reduced after stimulation of platelets by collagen.
In other cellular elements studied, WASP was not significantly tyrosine phosphorylated after stimulation of the cells, although WASP was found to be constitutively associated with Grb2.10-16 Because induction of tyrosine phosphorylation seems to be unique to collagen-stimulated platelets, it is attractive to suggest that tyrosine phosphorylation of WASP may directly or indirectly reduce avidity or accessibility of Grb2 to bind WASP. However, because we have not determined the stoichiometry of tyrosine phosphorylation of WASP in collagen-stimulated platelets, we cannot rule our the possibility that Grb2 binding to WASP is reduced by a mechanism that is unrelated to WASP tyrosine phosphorylation.
The finding that WASP is an endogenous substrate for calpain and that it is cleaved during aggregation is intriguing in view of the recent report that the integrin β3 subunit is also cleaved during platelet aggregation.36 It appears possible that WASP, β3, and calpain colocalize during platelet aggregation and that WASP and β3 are cleaved by the same protease. The hypothesis is in aggreement with the findings by Remold-O’Donnell et al47 suggesting that the protein defective in WAS is related to the regulation of calpain and that inappropriate activation of calpain in platelets and its release may be responsible for the consistent loss of CD43 and other glycoproteins on the surface of T lymphocytes. They further argue that, as a consequence, lymphocytes, especially T lymphocytes, lose functionally important cell surface molecules and surface-associated receptors, contributing to the immunodeficiency observed in WAS patients.47 Although the degree of cleavage of WASP in aggregated platelets seems to be small, this is generally true for most substrates for calpain in platelets.33 For example, most of the talin or actin binding protein that is cleaved in calcium ionophore- or dibucaine-treated platelets in the presence of extracellular ionic calcium shows cleavage that is less than 10% of total. It has been suggested that this small degree of cleavage may play a significant role in the shedding of procoagulant microparticles from platelets or in the relaxation of fibrin-platelet clots.33 48
Although we have shown that tyrosine phosphorylation of WASP may modulate its binding to Grb2 in vitro, it is imperative to search for the physiological ligands for WASP associated phosphotyrosines. Furthermore, it is also essential to examine the effects of tyrosine phosphorylation of WASP on the binding of other SH3-containing proteins, including PSTPIP, a recently characterized ligand for WASP.49 The unique and robust tyrosine phosphorylation of WASP observed in collagen-stimulated platelets makes them an attractive system to further analyze the physiologic functions of WASP. This becomes even more attractive in view of the fact that platelets contain many SH2/SH3 proteins that are potential candidates for WASP ligands.25,26,31-33 45 Finally, our data suggest that WASP may also play a role in aggregation events, based on our observation that WASP redistributes to the cytoskeleton during platelet aggregation and that it is an endogenous substrate for calpain.
Supported in part by grants from The Ministry of Education, Science, Technology and Sport of Japan (Y.I. and A.O.), The Ryoichi Naito Foundation for Medical Research (A.O.), Research Grants for Life Sciences and Medicine, Keio University Medical Science Fund (A.O.), the National Institutes of Health (HD17427; H.D.O.), and the DeJoria Wiskott-Aldrich Research Fund (H.D.O.).
Address reprint requests to Hans D. Ochs, MD, Professor, Department of Pediatrics, RD-20, 1959 NE Pacific St, Box 356320, University of Washington Medical School, Seattle, WA 98195.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal