Abstract
In polycythemia vera (PV) erythroid colonies that grow in vitro in the absence of exogenous erythropoietin (EPO) arise from the abnormal clone that is responsible for overproduction of red blood cells. Although the mechanism of autonomous formation of burst-forming units-erythroid (BFU-E) is not fully understood, a spontaneous release of growth regulatory molecules by PV cells and/or by accessory cells is likely to be involved. Because of its cytokine synthesis inhibiting action, interleukin-10 (IL-10) could be a potentially useful molecule to modulate abnormal erythropoiesis in PV. We studied the effect of recombinant human IL-10 on the EPO-independent growth of erythroid bursts derived from peripheral blood mononuclear cells (PBMNCs) of patients with PV. IL-10 showed a profound, dose-dependent, and specific inhibitory effect on autonomous BFU-E formation. Ten nanograms per milliliter of IL-10 significantly suppressed spontaneous growth of erythroid colonies in methylcellulose in five of five PV patients tested with a mean inhibition by 81% (range, 72-94). To elucidate the possible mechanism of the inhibitory action of IL-10 we further studied the effect of anticytokine antibodies on autonomous BFU-E growth and the ability of exogenous cytokines to restore IL-10–induced suppression of erythroid colony growth. Among a panel of growth regulatory factors tested (granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-3, granulocyte colony-stimulating factor, stem cell factor, and insulin-like growth factor-1) GM-CSF was the only molecule for which both an inhibition of spontaneous BFU-E formation by its respective antibody as well as a significant restimulation of erythroid colonies in IL-10-treated cultures by exogenous addition was found. Moreover, inhibition of GM-CSF production by IL-10 was shown in PV PBMNCs at the mRNA level. Our data indicate that autonomous BFU-E growth in PV can be profoundly inhibited by IL-10 and that this inhibitory effect seems to be at least in part secondary to suppression of endogenous GM-CSF production.
© 1998 by The American Society of Hematology.
POLYCYTHEMIA VERA (PV) is a clonal disease of the multipotential progenitor cell characterized by an increased red blood cell mass and varying numbers of granulocytes and platelets in the peripheral blood (PB). Whereas erythroid progenitors from normal individuals require addition of exogenous erythropoietin (EPO) to form erythroid colonies in vitro, a distinct population of erythroid progenitors from patients with PV can form hemoglobinized colonies in the absence of added EPO.1 Because such spontaneous burst forming unit-erythroid (BFU-E) formation from PV cells can still be observed under serum-free culture conditions2 or in the presence of anti-EPO and anti-EPO-receptor antibodies,2 3 minute amounts of EPO in the culture system can be excluded as a possible cause of endogenous colony formation. Growth of BFU-E without addition of exogenous growth factors increases the possibility that PV cells secrete their own growth factors and/or are stimulated by growth factors released from accessory cells that might subsequently lead to hyperproliferation of erythroid cells in vitro and possibly in vivo.
Interleukin-10 (IL-10) is a 35-kD protein, originally identified by virtue of its ability to inhibit cytokine synthesis in T helper 1 clones.4,5 It is primarily produced by mononuclear cells (MNCs)6 and possesses a wide range of activities on a number of cell types including B cells,7 T cells,8 natural killer cells,9 mast cells,10 neutrophils,11eosinophils,12 and monocytes.13 The main feature of this cytokine is a suppressive effect on cytokine expression. Thus, IL-10 inhibits production of numerous cytokines in lipopolysaccharide or interferon-γ-activated monocytes, such as IL-1a, IL-1b, IL-6, IL-8, tumor necrosis factor-α, granulocyte- macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF).13 14
Because of its cytokine synthesis-inhibiting action, IL-10 could be a potentially useful molecule to modulate hematopoiesis in conditions in which the autocrine and/or paracrine secretion of cytokines plays a significant role. In fact, we have shown that IL-10 markedly suppresses the spontaneous formation of granulocyte-macrophage colonies (CFU-GM) from normal PBMNCs in semisolid cultures.15 More importantly, we showed a profound inhibitory effect of IL-10 on the massively increased autonomous CFU-GM growth in patients with chronic myelomonocytic leukemia (CMML), suggesting that IL-10 was a potential therapeutic agent in this disease.16 Here we investigated the effect of IL-10 on the spontaneous growth of erythroid colonies in patients with PV. We found that IL-10 profoundly inhibits EPO-independent BFU-E growth from such patients, at least in part through suppression of spontaneous release of GM-CSF.
MATERIALS AND METHODS
Patients.
Five patients clinically diagnosed as having PV with the help of the PV Study Group guidelines17 were used in this study. Clinical and laboratory data of these patients are shown in Table 1. All patients were being managed by phlebotomy at the time of study and none of them had been previously treated with cytostatic drugs or radioactive phosphorus.
Clinical and Laboratory Data in Patients With Polycythemia Vera at Time of Study
| Patient . | Age/Sex . | Duration (mo) . | RBC (×1012/L) . | Hct . | WBC (×109/L) . | Platelets (×103/μL) . | Spleen . | Therapy . |
|---|---|---|---|---|---|---|---|---|
| HR | 82/M | 5 | 6.88 | 42.3 | 17.4 | 594 | Enlarged | Phlebotomy |
| KJ | 75/M | 216 | 6.15 | 42.1 | 16.1 | 340 | Enlarged | Phlebotomy |
| KA | 31/M | 1 | 5.63 | 43.5 | 9.9 | 496 | Normal | Phlebotomy |
| JH | 70/F | 1 | 6.59 | 46.1 | 6.1 | 365 | Normal | Phlebotomy |
| SA | 54/M | 9 | 7.65 | 45.9 | 39.4 | 380 | Enlarged | Phlebotomy |
| Patient . | Age/Sex . | Duration (mo) . | RBC (×1012/L) . | Hct . | WBC (×109/L) . | Platelets (×103/μL) . | Spleen . | Therapy . |
|---|---|---|---|---|---|---|---|---|
| HR | 82/M | 5 | 6.88 | 42.3 | 17.4 | 594 | Enlarged | Phlebotomy |
| KJ | 75/M | 216 | 6.15 | 42.1 | 16.1 | 340 | Enlarged | Phlebotomy |
| KA | 31/M | 1 | 5.63 | 43.5 | 9.9 | 496 | Normal | Phlebotomy |
| JH | 70/F | 1 | 6.59 | 46.1 | 6.1 | 365 | Normal | Phlebotomy |
| SA | 54/M | 9 | 7.65 | 45.9 | 39.4 | 380 | Enlarged | Phlebotomy |
Abbreviations: RBC, red blood cells; Hct, hematocrit; WBC, white blood cells.
Preparation of cells.
After informed consent, PB was collected into sterile tubes containing EDTA. MNCs were isolated from PB of patients by Ficoll-Hypaque density gradient centrifugation (density 1.077 g/mL, 400g for 40 minutes). The low-density cells were collected from the interface between density solution and plasma, washed twice, and resuspended in Iscove‘s modified Dulbecco‘s medium (GIBCO, Paisley, Scotland).
Reagents.
Recombinant human IL-10 (rhIL-10; specific activity 1-2 × 106 U/mg) was kindly provided by Schering-Plough Corp (Kenilworth, NJ) and rhGM-CSF and rhIL-3 by Sandoz (Basel, Switzerland). RhG-CSF was purchased from British Biotechnology (Oxan, UK) and rhEPO from Boehringer Mannheim (Vienna, Austria). Recombinant human stem cell factor (rhSCF) and recombinant human insulin-like growth factor-1 (rhIGF-1) were obtained from Pharma Biotechnologie Hannover (Hannover, Germany). Antibodies directed against GM-CSF, IL-3, G-CSF, and SCF were purchased from Genzyme (Cambridge, MA), anti-IGF-1 from Serotec Ltd (Oxford, UK), and anti-IL-10 from R&D Systems Europe Ltd (Abington, UK).
Colony assay.
PBMNCs were cultured in 0.9% methylcellulose, 30% fetal calf serum (FCS; INLIFE, Wiener Neudorf, Austria), 10% bovine serum albumin (Behring, Marburg, Germany), α-thioglycerol (10-4 mol/L) and Iscove‘s modified Dulbecco‘s medium with or without the addition of cytokines or anticytokine antibodies. Cultures were plated in triplicate at 75 to 120 × 103 MNC/mL. In some experiments a neutralizing antibody against IL-10 was preincubated with IL-10 for 2 hours at room temperature. Neutralizing antibodies against GM-CSF, G-CSF, IL-3, SCF, or IGF-1 were used as recommended by the manufacturer. Plates were incubated at 37°C, 5% CO2, and full humidity. After a culture period of 14 days, cultures were examined under an inverted microscope. Aggregates with at least 50 hemoglobinized cells, easily recognizable by their red color, were counted as BFU-E–derived erythroid bursts. For cultivation of BFU-E from normal individuals, 2 U/mL EPO was added to culture dishes.
Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of GM-CSF transcripts.
A total of 2 × 107 PV PBMNCs were cultured in suspension both with and without IL-10 (10 ng/mL) for 48 hours. After incubation, cells were washed twice in diethylpyrocarbonate-treated water and 107 cell aliquots were lysed by addition of 1.6 mL RNAzol B (Biotecx, Houston, TX). Total RNA was extracted as described.18 The integrity of RNA was controlled by electrophoresis through formaldehyde agarose gels. High quality RNA was quantitated by measuring absorbance at 260 nm and 1 g total RNA was subjected to cDNA synthesis as recently described.19
For semiquantitative analysis of GM-CSF mRNA, an RT-PCR technique that allows measurements of relative transcript levels was applied.20,21 The oligonucleotide primer sequences for amplification of GM-CSF were 5′-CTGCTGCTGAGATGAATGAAACAG-3′ and 5′-TGGACTGGCTCCCAGCAGTCAAAG-3′, which bracketed a GM-CSF fragment of 286 bp.22 PCR amplification of ABL (Abelson) transcripts was used as a reference to assess variation of total RNA or cDNA between samples. The primer sequences for amplification of ABL were as follows: 5′-CAGCGGCCAGTAGCATCTGACTTTG-3′ and 5′-CCATTTTTGGTTTGGGCATCACACCATTCC-3′ resulting in the production of a PCR fragment of 228 bp.19 The linear ranges of PCR amplifications of GM-CSF and ABL were established as a function of cycle number and cDNA concentration as described.15,20 21Reaction conditions included 3 L cDNA, 20 pmol of each primer, 1.5 mmol/L MgCL2, 200 mol/L of each dNTP, 2.5 U Ampli Taq DNA Polymerase (Perkin Elmer Cetus, Norwalk, CT), and (32P) dCTP (150,000 cpm) in a 50-L reaction volume. The thermal cycling conditions were denaturation at 94°C (1 minute), annealing at 60°C (1 minute), and extension at 72°C (2 minutes), preceded by an initial denaturation step at 94°C for 5 minutes, and followed by a terminal extension of 10 minutes at 72°C. The number of PCR cycles for amplification of GM-CSF and ABL transcripts was 32 and 25 cycles, respectively. Reaction products were subjected to 6% polyacrylamide gels (Novex, San Diego, CA), and dried gels were exposed to Kodak XAR-5 films (Eastman Kodak, Rochester, NY) at −70°C for 12 hours.
For quantification of PCR products incorporated (32P) dCTP was measured on autoradiograms by using the Bio Rad 670 Imaging Densitometer and the system‘s volume integration program (Bio Rad Gel DOC 1000 system, Molecular Analyst/PC software; BioRad, Richmond, CA). Potential differences of total cellular RNA/cDNA in PCR analyses were corrected by dividing GM-CSF values by the ABL value (mean value of six PCR analyses). The relative level of GM-CSF transcripts was measured in patient samples in three PCR analyses in duplicate using freshly synthesized cDNA.
Statistical analysis.
The t-test was used to determine the significance of differences. A P value of <.05 was considered statistically significant.
RESULTS
Inhibitory effect of IL-10 on autonomous BFU-E growth in patients with PV.
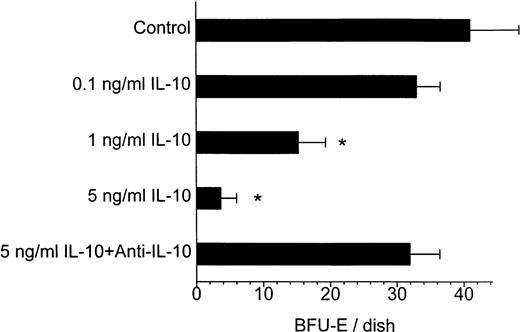
As it has been originally shown by Prchal and Axelrad,1erythroid colonies can grow in methylcellulose cultures containing PBMNCs from patients with PV in the absence of exogenous EPO. Treatment of PV cell cultures with IL-10 resulted in a profound and dose-dependent inhibition of autonomous BFU-E growth (Fig 1). The inhibitory effect of IL-10 became apparent at a concentration of 1 ng/mL and was even more pronounced at higher concentrations. This effect was specific, because a neutralizing anti-IL-10 antibody was able to prevent IL-10-induced suppression of BFU-E growth (Fig 1). The effect of 10 ng/mL IL-10 on autonomous BFU-E growth was investigated in PBMNCs from 5 patients with PV (Table 2). As shown in Table 2, autonomous formation of erythroid colonies greatly varied among different patients but was significantly inhibited by IL-10 in all of them. On average, 10 ng/mL of IL-10-inhibited EPO independent BFU-E growth from PV PBMNCs by 81% (range, 71% to 94%).
Dose-dependent inhibitory effect of IL-10 on autonomous BFU-E growth from PV cells and its abrogation by an anti-IL-10 antibody in patient HR. A total of 120 × 103 PBMNC/mL were cultured in methylcellulose with medium alone, with increasing concentrations of IL-10, or with IL-10 plus a neutralizing antibody against IL-10. Colony growth was assessed after 14 days. Results represent the mean values ± standard deviation (SD) from triplicates. * Significant change from control with P value at least <.05.
Dose-dependent inhibitory effect of IL-10 on autonomous BFU-E growth from PV cells and its abrogation by an anti-IL-10 antibody in patient HR. A total of 120 × 103 PBMNC/mL were cultured in methylcellulose with medium alone, with increasing concentrations of IL-10, or with IL-10 plus a neutralizing antibody against IL-10. Colony growth was assessed after 14 days. Results represent the mean values ± standard deviation (SD) from triplicates. * Significant change from control with P value at least <.05.
Inhibitory Effect of IL-10 on Autonomous BFU-E Growth in PV Patients
| Patient . | MNC/Dish × 103 . | BFU-E ± SD/Dish . | Percent of Inhibition . | P . | |
|---|---|---|---|---|---|
| Control . | +IL-10 . | ||||
| HR | 100 | 32.0 ± 3.5 | 9.3 ± 1.2 | 71 | <.001 |
| KJ | 100 | 43.0 ± 1.7 | 10.3 ± 1.5 | 76 | <.001 |
| KA | 100 | 5.0 ± 1.0 | 0.3 ± 0.6 | 94 | <.005 |
| JP | 116 | 15.8 ± 4.2 | 3.0 ± 0.0 | 81 | <.05 |
| SA | 75 | 107.3 ± 7.6 | 19.3 ± 2.5 | 82 | <.001 |
| Patient . | MNC/Dish × 103 . | BFU-E ± SD/Dish . | Percent of Inhibition . | P . | |
|---|---|---|---|---|---|
| Control . | +IL-10 . | ||||
| HR | 100 | 32.0 ± 3.5 | 9.3 ± 1.2 | 71 | <.001 |
| KJ | 100 | 43.0 ± 1.7 | 10.3 ± 1.5 | 76 | <.001 |
| KA | 100 | 5.0 ± 1.0 | 0.3 ± 0.6 | 94 | <.005 |
| JP | 116 | 15.8 ± 4.2 | 3.0 ± 0.0 | 81 | <.05 |
| SA | 75 | 107.3 ± 7.6 | 19.3 ± 2.5 | 82 | <.001 |
PBMNCs from PV patients were cultured as described in the Materials and Methods with or without 10 ng/mL IL-10. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates.
Effect of anticytokine antibodies on autonomous BFU-E growth from PV cells.
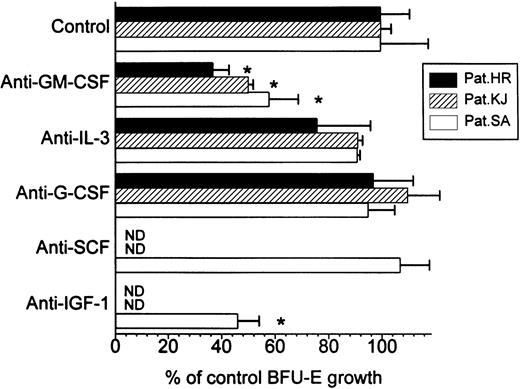
To elucidate the possible mechanism of the inhibitory action of IL-10, we first tried to identify the factor responsible for autonomous BFU-E growth in PV by adding neutralizing antibodies against GM-CSF, IL-3, G-CSF, SCF, and IGF-1 to cell cultures from three patients with marked colony growth (Fig 2). Not unexpectedly the IGF-1 antibody significantly inhibited autonomous BFU-E growth in our FCS containing culture system (patient SA), because IGF-1 has been reported as the major EPO-like activity in FCS.23 Among the other anticytokine antibodies tested only the anti-GM-CSF antibody significantly decreased spontaneous growth of erythroid colonies in all three patients tested, with a mean inhibition by 52% (range, 42% to 63%). This clearly suggested that GM-CSF and IGF-1 were involved in the spontaneous formation of erythroid colonies from PV PBMNCs in this culture system.
Effect of anticytokine antibodies on autonomous BFU-E growth from PV cells in three patients. PBMNCs were cultured in methylcellulose with medium alone or with antibodies against GM-CSF, IL-3, G-CSF, SCF, or IGF-1, respectively. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates. * Significant change from control with P value at least <.05.
Effect of anticytokine antibodies on autonomous BFU-E growth from PV cells in three patients. PBMNCs were cultured in methylcellulose with medium alone or with antibodies against GM-CSF, IL-3, G-CSF, SCF, or IGF-1, respectively. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates. * Significant change from control with P value at least <.05.
Effect of exogenous growth factors on IL-10-induced suppression of autonomous BFU-E growth from PV cells.
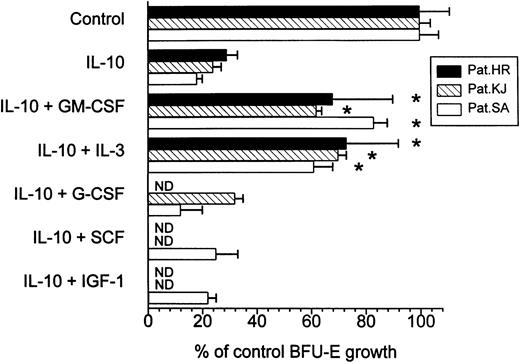
The fact that IL-10 has been shown to inhibit cytokine synthesis in different cell types of the mononuclear cell fraction4 13led us to hypothesize that inhibition of autonomous BFU-E growth by IL-10 was secondary to IL-10-induced suppression of endogenous release of growth regulatory molecules. If this was the case one would expect that exogenous addition of particular cytokines could at least in part reverse growth inhibition by IL-10. In contrast, restoration of colony growth by exogenous growth factors would not be observed if IL-10 had a direct cytotoxic effect on PV cells. In fact, exogenous GM-CSF and IL-3 significantly restimulated erythroid colony formation in IL-10 treated cultures (Fig 3). All other cytokines were not effective in correcting IL-10-induced growth inhibition. Also IGF-1 alone at concentrations up to 100 ng/mL was not able to restore autonomous BFU-E growth in IL-10 suppressed cultures, nor did it significantly potentiate the effects of GM-CSF when added in combination (Table 3). This observation and the suppressive effect of the anti-IGF-1 antibody in the culture system suggest that IGF-1 is important for EPO-independent erythroid colony formation in PV patients but is not involved in growth inhibition by IL-10.
Effect of exogenous growth factors on IL-10-induced suppression of BFU-E growth from PV cells. PBMNCs were cultured in methylcellulose with 10 ng/mL IL-10 in the presence or absence of exogenous GM-CSF (100 U/mL), IL-3 (10 U/mL), G-CSF (100 U/mL), SCF (10 ng/mL), and IGF-1 (10 ng/mL), respectively. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates. * Significant change from IL-10 treated cultures with P value at least <.05.
Effect of exogenous growth factors on IL-10-induced suppression of BFU-E growth from PV cells. PBMNCs were cultured in methylcellulose with 10 ng/mL IL-10 in the presence or absence of exogenous GM-CSF (100 U/mL), IL-3 (10 U/mL), G-CSF (100 U/mL), SCF (10 ng/mL), and IGF-1 (10 ng/mL), respectively. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates. * Significant change from IL-10 treated cultures with P value at least <.05.
Effect of IGF-1 Alone or in Combination With GM-CSF on IL-10–Induced Suppression of Autonomous BFU-E Growth From PV Cells
| Culture Conditions . | BFU-E ± SD/Dish . |
|---|---|
| Control (no cytokines) | 96.0 ± 8.2 |
| + 10 ng/mL IL-10 | 12.7 ± 3.1 |
| + 10 ng/mL IL-10 + 1 ng/mL IGF-1 | 10.3 ± 0.6 |
| + 10 ng/mL IL-10 + 10 ng/mL IGF-1 | 14.0 ± 2.7 |
| + 10 ng/mL IL-10 + 100 ng/mL IGF-1 | 13.7 ± 1.5 |
| + 10 ng/mL IL-10 + 100 U/mL GM-CSF | 83.7 ± 4.2 |
| + 10 ng/mL IL-10 + 100 U/mL GM-CSF + 10 ng/mL IGF-1 | 92.7 ± 24.6 |
| Culture Conditions . | BFU-E ± SD/Dish . |
|---|---|
| Control (no cytokines) | 96.0 ± 8.2 |
| + 10 ng/mL IL-10 | 12.7 ± 3.1 |
| + 10 ng/mL IL-10 + 1 ng/mL IGF-1 | 10.3 ± 0.6 |
| + 10 ng/mL IL-10 + 10 ng/mL IGF-1 | 14.0 ± 2.7 |
| + 10 ng/mL IL-10 + 100 ng/mL IGF-1 | 13.7 ± 1.5 |
| + 10 ng/mL IL-10 + 100 U/mL GM-CSF | 83.7 ± 4.2 |
| + 10 ng/mL IL-10 + 100 U/mL GM-CSF + 10 ng/mL IGF-1 | 92.7 ± 24.6 |
A total of 105 PBMNC/dish from patient SA were cultured in methylcellulose in the presence or absence of exogenous cytokines as indicated in the Table. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates.
Inhibitory effect of IL-10 on GM-CSF expression in PV PBMNCs.
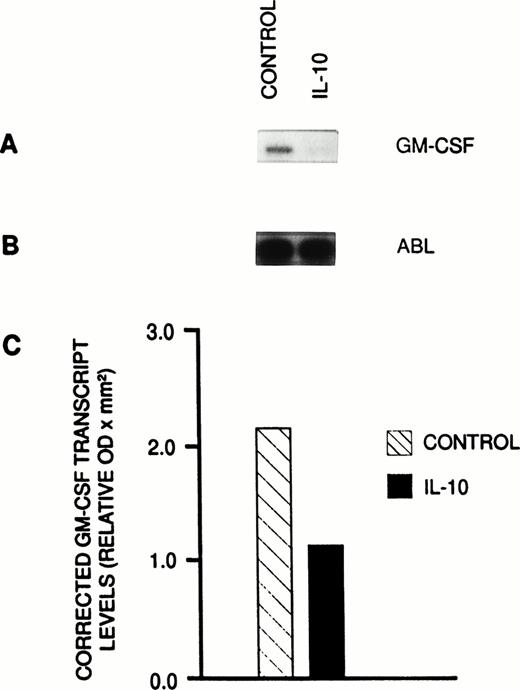
The demonstration of both the antiproliferative action of the anti-GM-CSF antibody on the autonomous BFU-E growth and the restoration of IL-10-induced growth suppression by exogenous GM-CSF strongly suggested that the growth inhibitory effect of IL- 10 in PV was at least in part caused by suppression of spontaneous GM-CSF production. Therefore, the effect of IL-10 on GM-CSF expression in unseparated PBMNCs from a patient with PV (HR) was studied by a semiquantitative PCR technique. We have previously shown that the PCR technique used by us allows at least semiquantitative measurements of GM-CSF transcript levels by establishing linear ranges of amplifications of GM-CSF as a function of cycle number and cDNA concentrations.15 After culturing PV cells in the presence of IL-10 (10 ng/mL) for 48 hours, GM-CSF transcript levels were substantially lower than that of cells kept in suspension without IL-10 (Fig 4A). In comparison, ABL transcripts, which served as a control, remained unchanged during the time of culture (Fig 4B). Figure 4C shows corrected GM-CSF mRNA levels in PV PBMNCs at 48 hours. Comparison of corrected GM-CSF transcript levels between PBMNCs cultured with and PBMNCs cultured without IL-10 showed a mean decrease by 47%.
Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiogram showing incorporated radioactivity of amplification products obtained from PV PBMNCs cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiogram showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected GM-CSF transcript levels in cultured PV cells. Each sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of 32P incorporated into PCR product was determined by densitometric scanning of the autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA. The results are shown as mean values.
Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiogram showing incorporated radioactivity of amplification products obtained from PV PBMNCs cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiogram showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected GM-CSF transcript levels in cultured PV cells. Each sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of 32P incorporated into PCR product was determined by densitometric scanning of the autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA. The results are shown as mean values.
Effect of IL-10 on EPO-dependent BFU-E growth in normal individuals.
To investigate the effect of IL-10 on the EPO-dependent in vitro growth of normal erythroid progenitors, PBMNCs from five healthy volunteers were cultured in the presence of EPO with or without 10 ng/mL IL-10 (Table 4). IL-10-induced suppression of EPO-dependent erythroid colony growth was seen in all five individuals, but this inhibition was less pronounced than in PV patients. The mean inhibition of normal EPO-dependent BFU-E growth by 10 ng/mL IL- 10 was 45% ± 12% (standard deviation), which was significantly less than the inhibition of EPO-independent erythroid colony growth in PV patients ( 81% ± 12%, P < .001)
Effect of IL-10 on EPO-Dependent BFU-E Growth in Normal Individuals
| Patient No. . | BFU-E ± SD/Dish . | Percent of Inhibition . | |
|---|---|---|---|
| Control . | +IL-10 . | ||
| 1 | 13.0 ± 5.0 | 8.3 ± 2.1 | 36 |
| 2 | 33.0 ± 2.7 | 13.7 ± 10.1 | 58 |
| 3 | 36.0 ± 4.4 | 23.7 ± 5.0 | 34 |
| 4 | 30.3 ± 2.1 | 19.0 ± 3.0 | 37 |
| 5 | 53.7 ± 3.5 | 22.7 ± 2.5 | 58 |
| Patient No. . | BFU-E ± SD/Dish . | Percent of Inhibition . | |
|---|---|---|---|
| Control . | +IL-10 . | ||
| 1 | 13.0 ± 5.0 | 8.3 ± 2.1 | 36 |
| 2 | 33.0 ± 2.7 | 13.7 ± 10.1 | 58 |
| 3 | 36.0 ± 4.4 | 23.7 ± 5.0 | 34 |
| 4 | 30.3 ± 2.1 | 19.0 ± 3.0 | 37 |
| 5 | 53.7 ± 3.5 | 22.7 ± 2.5 | 58 |
A total of 105 PBMNC/dish from 5 healthy volunteers were cultured in the presence of 2 U/mL EPO as described in the Materials and Methods with or without 10 ng/mL IL-10. Colony growth was assessed after 14 days. Results represent mean values ± SD from triplicates.
DISCUSSION
Elegant studies by Adamson et al24 analyzing the isoenzyme pattern in different cell types of PV patients heterozygous for G6PD have shown that circulating red blood cells, granulocytes, and platelets expressed the same isoenzyme, suggesting that these cells were unicellullar in origin and derived from an abnormal multipotential progenitor cell. Later, Prchal et al25 could show that erythroid colonies from PV patients that formed in the absence of exogenous EPO contained the same G6PD isoenzyme type as that expressed by peripheral blood elements. Thus, the so-called endogenous colonies arose from the abnormal clone that was responsible for the overproduction of red blood cells in PV. When exogenous EPO was added, increasing numbers of erythroid colonies were formed containing cells that expressed the other G6PD isoenzymes, indicating the existence of both malignant and nonmalignant populations of hematopoietic progenitor cells in PV marrow. Here we show a profound inhibitory effect of IL-10 on endogenous erythroid colony formation in vitro in patients with PV. A significant inhibition of spontaneous erythroid colony formation by IL-10 was observed in all five PV patients investigated. The effect of IL-10 was dose-dependent and specific, because a neutralizing anti-IL-10 antibody was able to prevent IL-10-induced suppression of BFU-E growth.
The mechanism of spontaneous erythroid colony formation in vitro and the factors that lead to in vivo expansion of clonal cells in patients with PV remain to be fully understood. Spontaneous erythroid colony formation in the presence of anti-EPO antibodies (3) and in serum-free culture conditions (2) exclude minute amounts of EPO in the culture system as a potential stimulus for endogenous colony formation. The marked reduction of spontaneous erythroid colony formation by cells from PV patients by depletion of monocytes and its restoration by readdition of adherent cells or adherent cell supernatant26,27 strongly suggests monocyte-derived molecules as stimulatory factors for erythroid colony formation in PV. Several cytokines including GM-CSF,28 IL-3,28SCF,29 and IGF-130 have been shown to enhance EPO independent BFU-E growth if added to semisolid cultures containing PV MNCs. Moreover a hypersensitivity of PV BFU-E to each of these growth factors compared with normal erythroid progenitors has been shown,28-30 which may be a principal factor in the profound marrow hyperplasia and increased blood counts of patients with PV. Recently, a defect in phosphatase activity in PV cells has been proposed as the molecular basis for the enhanced response of PV BFU-E to growth regulatory molecules. Thus, increased basal and induced tyrosine phosphorylation of the IGF-1 receptor β subunit in circulating MNCs in such patients has been reported.31Moreover, a diminished enhancement of erythroid colony formation in the presence of orthovanadate,32 an inhibitor of protein tyrosine phosphatases, in PV cells has been shown.
There is substantial evidence that the inhibitory effect of IL-10 on autonomous BFU-E growth in PV is indirect. Spontaneous formation of erythroid colonies from PV cells has been shown to be decreased after removal of adherent cells.26,27 In unseparated PV PBMNCs, we found, in agreement with observation by others,33 that autonomous BFU-E growth was markedly reduced by addition of an anti-GM-CSF antibody, suggesting spontaneous release of GM-CSF as an important mechanism of EPO-independent erythroid colony formation. Not unexpectedly, an anti-IGF-1 antibody also inhibited autonomous BFU-E growth in our culture system because IGF- 1 has been shown to be the major EPO-like activity in FCS.23 The antiproliferative action of anti-GM-CSF antibody on EPO-independent BFU-E formation in PV and the fact that IL-10 has been shown to inhibit cytokine synthesis, including GM-CSF in human monocytes,13 led us to hypothesize that inhibition of BFU-E growth by IL-10 was mainly caused by IL-10-induced suppression of endogenous GM-CSF release. Our observation that exogenous GM-CSF was able to, at least in part, reverse growth inhibition by IL-10 strongly supports this concept. In fact, analysis of the effect of IL-10 on GM-CSF expression in MNCs from a patient with PV clearly showed that the IL-10-induced inhibition of erythroid colony growth correlated with a substantial decrease in GM-CSF transcripts in PV PBMNCs. Exogenous IL-3 also restimulated autonomous BFU-E formation in IL-10-treated PV cell cultures, but an anti-IL-3 antibody had no antiproliferative effect on spontaneous growth of erythroid progenitors. These data suggest that in addition to GM-CSF-responsive PV BFU-E, there seems to exist an IL-3-responsive population, which however, cannot be sufficiently stimulated by the endogenously released growth factors in unstimulated cultures.
The inhibitory effect of IL-10 on hematopoietic colony formation is not specific for PV BFU-E. However the growth suppression of IL-10 on EPO-independent erythroid colony growth in PV seems to be more pronounced than that on EPO-dependent normal BFU-E. In contrast to a mean growth inhibition by 81% on autonomous BFU-E formation from PV PBMNCs, IL-10 decreased the number of EPO-stimulated BFU-E by approximately 45% in PBMNC cultures from normal individuals. Moreover, the inhibitory action of IL-10 is not restricted to the erythroid lineage, because we have also shown IL-10-induced inhibition of spontaneous formation of myeloid colonies in normal individuals15 and patients with CMML16 through suppression of endogenous GM-CSF release. Thus, the inhibition of GM-CSF synthesis by IL-10 makes it useful in indirectly modulating hematopoiesis in a variety of conditions.
Despite its well-defined effects in vitro, the in vivo role of GM-CSF in normal and abnormal hematopoiesis is much less clear. Mice deficient in GM-CSF (GM-/-) through homologous recombination in embryonal stem cells show no alterations in hematopoiesis34suggesting substantial overlap of function with other colony-stimulating factors. On the other hand, animals transplanted with cells containing the GM-CSF cDNA developed a fatal syndrome resembling myeloproliferation, which however, was non-neoplastic in nature.35 In PV patients it is not known if GM-CSF, as in the in vitro system, plays a significant role in the expansion of clonal cells. If this is the case, therapy with IL-10, a cytokine that has been well tolerated in first clinical trials,36 37could be considered as a strategy to suppress the excessive production of red blood cells in PV.
Supported by the “Medizinisch-Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien.”
Address reprint requests to Klaus Geissler, MD, 1st Medical Department, Division of Hematology, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal