Abstract
Thrombopoietin (TPO) is a hematopoietic growth factor that stimulates megakaryocytopoiesis and platelet production in vivo and promotes the development of identifiable megakaryocytes in vitro. We have developed a murine monoclonal antibody, BAH-1, raised against human megakaryocytic cells, which specifically recognizes the c-Mpl receptor and shows agonist activity by stimulating megakaryocytopoiesis in vitro. BAH-1 antibody specifically binds to platelets and to recombinant c-Mpl with high affinity. Similar to TPO, BAH-1 alone supported the formation of colony-forming unit-megakaryocyte (CFU-MK) colonies. The combination of BAH-1 plus interleukin-3 or of BAH-1 plus human TPO significantly increased the number of human CFU-MK colonies. In addition, BAH-1 monoclonal antibody stimulated the proliferation and maturation of primary bone marrow megakaryocytes in a dynamic heterogeneous liquid culture system. Individual large megakaryocytes as well as small megakaryocytic cells were observed in cultures of CD34+ CD41+cells in the presence of BAH-1 antibodies. Similar to TPO, BAH-1 antibody induced a significant response of murine immature megakaryocytes as observed by an increase in the detectable numbers of acetylcholinesterase-positive megakaryocytes. No effects of BAH-1 antibody were observed on colony-forming unit–granulocyte-macrophage, burst-forming unit-erythroid, or colony-forming unit-erythroid colonies. In vivo studies showed that BAH-1, alone or in combination with TPO, expands the numbers of megakaryocytic progenitor cells in myelosuppressed mice. This antibody should prove useful in understanding the structure-function aspects of the c-Mpl receptor as well as in evaluating the effects of the sustained activation of this receptor in preclinical models of severe thrombocytopenia.
© 1998 by The American Society of Hematology.
THROMBOPOIETIN (TPO) is the primary regulator of physiological platelet production. TPO has dramatic effects on both the proliferation and differentiation of megakaryocytes in vitro and in vivo and is the most potent thrombopoietic agent described to date.1-13 At therapeutic doses, TPO causes as much as a 10-fold increase in circulating platelet levels.12 The central role of TPO in megakaryocytopoiesis and thrombopoiesis is further shown by the severe thrombocytopenic phenotype of mice rendered null for the expression of either TPO or its receptor c-Mpl.14-16 The similarity in the phenotype of the TPO and c-Mpl knockout mice shows that the system is nonredundant and that there is one receptor for TPO and one ligand for c-Mpl. Because of this, it is presumed that binding of TPO to c-Mpl is solely responsible for its activation.
The cell surface receptor (c-Mpl) for TPO is a member of the hematopoietic growth factor receptor superfamily.17Extracellular domains of members of this family are typically composed of multiple β-sandwich modules related to the fibronectin type-III immunoglobulin fold, with a characteristic ligand-binding domain formed from two adjacent β-sandwich structures.18 The mechanism by which TPO activates c-Mpl appears to be similar to that of other hematopoietic growth factors (such as erythropoietin [EPO], growth hormone [GH], prolactin [PRL], and granulocyte colony-stimulating factor [G-CSF]), that are triggered by ligand-induced receptor homodimerization.19-21 Recently, two families of small peptides that bind to the c-Mpl and compete with the binding of the natural ligand TPO were identified from recombinant peptide libraries.22 The peptide dimer stimulated the in vitro proliferation and maturation of megakaryocytes from human bone marrow cells.22
An antibody having agonist activity that stimulates c-Mpl might serve as an attractive therapeutic option in situations in which a prolonged half-life is needed and in which less frequent administration is desired. Furthermore, mapping the binding site on the c-Mpl receptor of an agonist monoclonal antibody (MoAb) would improve our understanding of the structure-function relationships of this receptor. To these ends, we report on the development of a murine MoAb, termed BAH-1, raised against human megakaryocytic cells that specifically recognizes the c-Mpl receptor, shows agonist activity by stimulating megakaryocytopoiesis in vitro, and also expands the numbers of megakaryocytic progenitor cells in myelosuppressed mice.
MATERIALS AND METHODS
Immunization and cell fusion.
BALB/C mice (Jackson Laboratories, Bar Harbor, ME) received repeated injections of 107 CMK cells emulsified in complete Freunds adjuvant according to a previously reported protocol.23-27A final booster of 2 × 105 human primary bone marrow megakaryocytes plus 106 CMK cells was injected 3 to 4 days before the animals were sacrificed.
Spleen cells from the immunized mice were fused with a mouse myeloma cell line (X653). These spleen cells (approximately 1 × 108) were fused with 2 × 107 myeloma cells by the addition of 1 mL of 40% polyethylene glycol (Sigma Chemical Corp, St Louis, MO). They were then diluted with 15 mL of Dulbecco’s modified Eagle’s medium (DMEM), centrifuged, and rediluted into a complete (10% fetal calf serum) selective medium containing hypoxanthine/aminopterin/thymidine at 2 × 106cells/mL. The cells were then distributed into 96 wells (100 μL) in hypoxanthine/aminopterin/thymidine medium.24 After 10 to 16 days, duplicate aliquots of the supernatants were assayed for cell enzyme-linked immunosorbent assay (ELISA) binding. Hybridoma cells from positive testing wells were then transferred to 24 wells containing 0.5 mL of hypoxanthine and thymidine medium. After the cells were grown and retested, they were cloned and recloned by limiting dilution into 96-well plates. For the antibody assays, the supernatants from the hybridoma cells were tested directly.
A screening procedure with a whole cell ELISA technique was used to detect antibodies that specifically recognized the human megakaryocytic cell lines CMK, DAMI, Mo7e, and CMS but not T cells, B cells, and monocyte-macrophages.28,29 After the identification of candidate antimegakaryocyte antibodies, screening for recognition of known megakaryocytic surface structures was done by cross-blocking studies with a panel of antibodies against the integrins GpIb and GpIIb/IIIa.28,29 One MoAb (BAH-1) that appeared to be specific for megakaryocytes and did not recognize GpIb or GpIIb/IIIa was further characterized. The BAH-1 MoAb was isotyped by using a MoAb isotyping kit (Innogenetics, Antwerp, Belgium), and found to be IgG1 kappa. BAH-1 hybridoma cells were injected intraperitoneally into BALB/C mice primed with pristine, and the antibody-containing ascites fluid was collected 2 to 3 weeks later and affinity-purified as described.28
Growth factors.
Recombinant human interleukin-3 (IL-3) or recombinant murine IL-3, human granulocyte-macrophage colony-stimulating factor (GM-CSF) and human IL-6 were obtained from R&D Systems (Minneapolis, MN). These cytokines were determined to be free of endotoxin contamination. Plateau doses of each factor were determined from dose-response curves for each assay. Recombinant human thrombopoietin (hTPO) and murine thrombopoietin (mTPO; both from Genentech Inc, South San Francisco, CA) were used at 100 ng/mL as determined from dose-response curves in the megakaryocyte progenitor assays (colony formation and liquid cultures). In some experiments, as indicated, we used various dilutions of TPO or IL-3 (10-100 ng/mL) to assess the synergistic effects of both cytokines under conditions of subconcentration or optimum concentration on the megakaryocytic lineage.
Marrow megakaryocytes.
Human bone marrow was obtained by aspiration from the iliac crests of normal donors who gave their informed consent in a protocol approved by the Deaconess Hospital Institutional Review Board. The marrow was aspirated into preservative-free heparin (Sigma) and separated by centrifugation through Ficoll-Hypaque (Pharmacia Biotech Inc, Piscataway, NJ) at 1,200g at room temperature for 30 minutes. After two washes with sterile phosphate-buffered saline (PBS), the cells were resuspended in Iscove’s modified Dulbecco’s medium with 20% fetal calf serum (FCS), penicillin/streptomycin, and L-glutamine; seeded onto T-75 tissue culture flasks (Corning, Corning, NY); and incubated at 37°C in 5% CO2. After 24 hours, the nonadherent cells were gently removed. Human marrow megakaryocytes were isolated by a method using immunomagnetic beads with antihuman GpIIb/IIIa MoAb as described previously.11 The cells that rosetted with the immunomagnetic beads were collected with a dynal magnetic particle concentrator (Dynabeads M-450; Dynal Inc, Great Neck, NY) and were washed three times with a megakaryocyte medium, which consisted of Ca2+ − Mg2+ free PBS containing 13.6 mmol/L-sodium citrate, 1 mmol/L theophylline, 1% bovine serum albumin (BSA), fraction V (Sigma), and 11 mmol/L glucose, adjusted to pH 7.3 and an osmolarity of 290 mOSM/L. After purification, cells were labeled using a MoAb against von Willebrand’s factor (MoAb 4F9; AMAC Inc, Westbrook, ME), and more than 95% of the cells were stained. Twenty milliliters of bone marrow aspirate generally yielded about 1 × 105 megakaryocytes. Contaminating cells (1%-5%) were essentially monocytes and macrophages. Cells were cultured in RPMI-1640 supplemented with 2% platelet poor plasma (PPP)11 at 37°C in a 5% CO2 fully humidified atmosphere for 24 hours. Monocytes and macrophages were identified by morphology after May-Grunwald-Giemsa staining and by positive antibody staining using a MoAb directed against CD14 (monocytes), CD15 (granulocytes), CD16 (IgG Fc receptor-natural killer cells, granulocytes, and macrophages; AMAC Inc). These analyses indicated that the maximum potential degree of contamination of bone marrow megakaryocytes after 24 hours was about 5% to 10%.
Isolation of CD34+ cells by the immunomagnetic bead technique.
CD34+ cells were isolated as described.12 Cells were first incubated at 4°C for 30 minutes with the CD34+ MoAb at a concentration of 10 mg/mL, and then with paramagnetic beads coupled with goat antibody to mouse IgG (Dynabeads M-450; Dynal) with a bead-to-target cell ratio of 5:1. CD34+ cells were isolated by magnetic separation and detached from the beads by chymopapain treatment (Sigma; 130 U/mL for 10 minutes), which allows for the collection of CD34+ cells free of beads.
Megakaryocyte progenitor assay.
Low-density bone marrow cells were cultured in a semisolid medium by using the plasma clot technique.11 The medium consisted of RPMI-1640, 1% deionized BSA, 20 mg/mL asparagine, 28 mg/mL CaCl2, 10% PPP, and 2.5 × 105nonadherent bone marrow cells in the absence or presence of various dilutions of the MoAb BAH-1. Ten percent citrated bovine plasma (GIBCO, Gaithersburg, MD) was added as the last product. The PPP and citrated bovine plasma used in these cultures were assayed and determined to be devoid of any detectable IL-6 or endogenous transforming growth factor-β (TGF-β) by specific immunoenzymatic assays for IL-6 and TGF-β, respectively (R & D Systems). Cultures were incubated for 12 days at 37°C in duplicate. Quantitation of colonies was performed by an ABC labeling kit (Vector Laboratories, Burlingame, CA) by using anti-GpIIIa antibodies. Each dish was entirely scanned under a microscope at day 12 of culture, and each cluster of three or more megakaryocytes was scored as a colony. For the limiting dilution experiments, CD34+ CD41+ cells were directly sorted into 96-well tissue culture plates and the numbers of megakaryocytes were identified by a GpIIb/IIIa cell-based ELISA.11
Human megakaryocytic cell lines.
The megakaryoblastic cell lines CMK,30,31 DAMI, and Mo7e were provided by Dr T. Sato (Chiba University, Chiba, Japan), Dr S. Greenberg (Brigham and Women’s Hospital, Boston, MA) and Dr J. Hoxie (University of Pennsylvania, Philadelphia, PA), respectively. Each cell line was cultured as previously described.32 Jurkat T cells were obtained from the American Type Tissue Collection and maintained in liquid culture according to the specifications in the catalog.
Murine megakaryocyte assay.
To assess megakaryocytic differentiating activity, a single megakaryocyte growth assay was used.31 Single cell populations from bone marrow were prepared from the femurs of normal C57/BL6 mice. This preparation was performed by flushing the bones with DMEM containing 10% FCS. Immature megakaryocyte populations were obtained in 1.07 to 1.085 g/cm−3 fractions, from a suspension of single bone marrow cells separated in a Percoll gradient. The fractionated cells were cultured in 10% FCS in DMEM for 5 days at 37°C in a 10% CO2 humidified incubator. This procedure was performed in the presence of titrated doses of growth factors. Cultures were dried and stained for acetylcholinesterase. Growth and maturation of immature megakaryocytes were quantitated by assessing the number of single large megakaryocytes detected by light microscopy.
Mice treatments and assays.
For the myelosuppression experiments, 6- to 9-week-old female BALB/C mice received a single intraperitoneal injection of 1.2 mg carboplatin and 350 cGy whole-body 137Cs irradiation (Gammacell 40 irradiator; Atomic Energy of Canada Radiochemical Co, Kanata, Canada) on day 0. The following day, the mice were begun on daily intraperitoneal injections of vehicle (20 mmol/L Tris, pH 8.1/0.9% NaCl/0.25% rabbit serum albumin), recombinant murine TPO (40 kU/mouse/d) in vehicle, purified BAH-1 antibody (5 mg/mouse), or both TPO (40 kU) and BAH-1 antibody (5 mg/mouse).
Animals were sacrificed 13 to 14 days after the initiation of treatment, which was 2 to 3 days before the onset of platelet recovery in the TPO-treated mice.
After sacrifice by cervical dislocation, the femurs of each study mouse were harvested and single-cell suspensions were prepared by using standard techniques.33,34 From 0.5 to 2 × 105 cells/mL were plated for megakaryocytic colony formation (CFU-MK) using 20 ng/mL murine IL-3 plus 7 ng/mL murine TPO in agar as previously described.8 As these cultures contained optimal levels of IL-3, granulocyte-macrophage colonies (CFU-GM) were also enumerated. Assays for erythroid bursts (BFU-E) were performed using recombinant human EPO as previously described.8 Late erythroid progenitors (CFU-E) were assayed in a plasma clot in the presence of 0.5 U/mL EPO.35 Each assay was performed in duplicate.
Flow cytometric analysis of surface protein expression.
To detect the potential surface binding proteins that bind BAH-1, we used flow cytometric analysis (FACS staining). Cells were washed with sterile PBS, and 1 × 106 cells were resuspended in 0.1 mL of PBS. Cells were incubated with 10 mL of the BAH-1 MoAb or GpIIIa antibodies, mouse IgG as a control (Immunotech Inc, Westbrook, ME) or PBS at 4°C for 20 minutes. Fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG or goat antirabbit IgG was added at a final dilution of 1:500 and followed by incubation for 20 minutes at 4°C. Cells were washed twice and resuspended in 0.5 mL of 1% (vol/vol) paraformaldehyde in PBS. Cells were then analyzed by flow cytometry.
Immunoprecipitation and Western immunoblotting.
CMK cells (2 × 106/mL) were serum-starved for 4 to 5 hours in RPMI-1640 medium. Cells were centrifuged, then resuspended at 107 cells/mL in the RPMI-1640. Cells (20 × 106/precipitation) were placed on ice and lysed by the addition of 1/3 volumes of 3× lysis buffer (40 mmol/L Tris-HCl, pH 7.4/2 mmol/L MgCl2/2 mmol/L CaCl2/20% glycerol/2% NP-40/2 mmol/L Na3VO4/20 mg/mL leupeptin/20 mg/mL aprotinin/4 mmol/L phenylmethyl sulfonyl fluoride [PMSF]). Lysates were centrifuged at 10,000g for 15 minutes. The MoAbs BAH-1 or Mpl-R (Genzyme, Cambridge, MA) were added to the supernatant at 5 mg/precipitation. Tubes were incubated by rocking at 5°C for 3 hours, and then 40 mL of 1:1 Protein G-Sepharose (Pierce, Rockford, IL) were added. After 1 and a half hours, lysates were washed three times with 1× lysis buffer. Sodium dodecyl sulfate (SDS) sample buffer was added to the washed beads and samples were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 7.5% acrylamide). SDS polyacrylamide gels were electrophoretically transferred onto nitrocellulose membranes (BioRad, Hercules, CA). The membranes were blocked with 4% BSA in PBS/0.1% Tween 20 (PBST) and then incubated with the BAH-1 MoAb (0.2 mg/mL) for 1 and a half hours or with Mpl-R MoAb (0.2 mg/mL). Membranes were then washed three times in PBST and incubated for 45 minutes in horseradish peroxidase-linked secondary antibody (Amersham Corp, Arlington Heights, IL) diluted in PBST. Transfers were washed three times in PBST and developed by the enhanced chemiluminescence (ECL) method (Amersham).
Binding of BAH-1 to platelets and c-Mpl.
Platelet rich plasma was prepared by centrifugation of citrated whole blood at 400g for 5 minutes. Binding studies were conducted within 3 hours of collection. 125I-BAH-1 was prepared by indirect iodination and yielded a specific activity of 15 to 50 mCi/mg protein. 125I-BAH-1 (100 pmol/L) was incubated with 4 × 107 platelets in plasma at 37°C for 30 minutes with varying concentrations of unlabeled BAH-1 in triplicate. The reaction mixture was overlayed on 1 mL of Hank’s balanced salt solution containing 0.5% BSA and 20% sucrose, then microcentrifuged at 13,500 rpm for 5 minutes. The supernatants were aspirated, tube bottoms containing the cell pellets were cut off, and platelet-associated radioactivity was determined.
The binding of BAH-1 to recombinant c-Mpl was determined by using a solid phase assay as described.36 Ninety-six–well immunoplates were coated with 50 mL of rabbit antihuman IgG Fc (Jackson Labs; 2 mg/mL in carbonate buffer, pH 9.6) overnight at 4°C. After blocking for 1 hour with PBS containing 0.5% BSA, the plates were incubated with conditioned media from the Mpl-IgG transfected 293 cells. Plates were subsequently washed three times with PBS containing 0.5% BSA and 0.05% Tween 20 (assay buffer) and 125I-BAH-1 (100 pmol/L) was added with varying concentrations of unlabeled BAH-1. After 1 hour, the plates were washed five times with assay buffer and bound 125I-BAH-1 was eluted with 4% SDS in 0.1 mol/L acetic acid and then quantitated in a gamma counter.
Statistical analysis.
The results were expressed as the mean ± standard error of the mean (SEM) of data obtained from three or more experiments performed in triplicate. Statistical significance was determined using the Student’s t-test.
RESULTS
Characterization of the MoAb BAH-1.
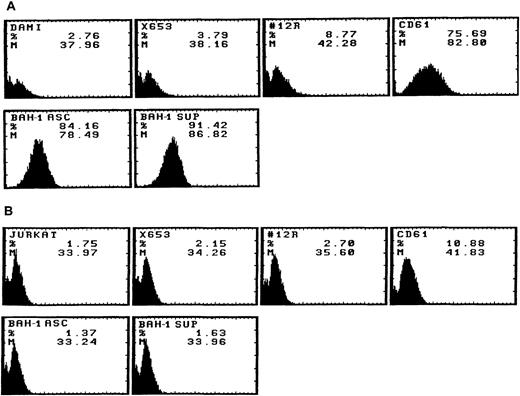
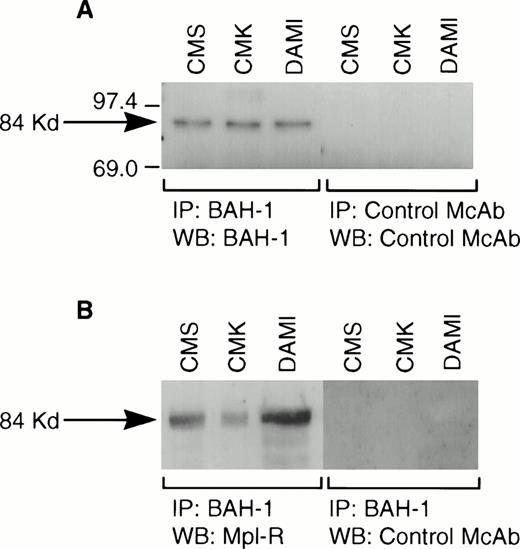
We derived one murine MoAb (BAH-1), which appeared to specifically interact with the human megakaryocytic cell line DAMI. Immunofluorescence staining showed that more than 90% of the DAMI cells stained positive with BAH-1 MoAb. Similar results were obtained with other megakaryoblastic cell lines, such as CMK, CMS, and Mo7e (data not shown). No staining was observed when Jurkat T cells were used (Fig 1). In addition, this antibody did not recognize the major megakaryocytic glycoproteins Ib or IIb/IIIa (data not shown). Immunoprecipitation and Western blot analysis showed that this MoAb reacted specifically with the c-Mpl protein (Fig 2). BAH-1 immunoprecipitated an 84-kD protein from CMK cell lysates that cross-reacted with a known c-Mpl antibody (Mpl-R).
Flow cytometric analysis of BAH-1 surface protein expression in a DAMI megakaryocytic cell line. Antigen expression was evaluated by FACS staining using BAH-1 and Mpl-R MoAbs (commercially available from Genzyme, MA), CD61 or control MoAb on DAMI (A) and Jurkat T cells (B). X653, supernatant from the mouse myeloma cell line used in cell fusion; 12R, a control nonrelevant MoAb (1:1,000); CD61, a positive control for megakaryocytic cells; BAH-1 Asc, ascites of BAH-1 (1:1,000 dilution); BAH-1 Sup, BAH-1 supernatant (1:1,000 dilution).
Flow cytometric analysis of BAH-1 surface protein expression in a DAMI megakaryocytic cell line. Antigen expression was evaluated by FACS staining using BAH-1 and Mpl-R MoAbs (commercially available from Genzyme, MA), CD61 or control MoAb on DAMI (A) and Jurkat T cells (B). X653, supernatant from the mouse myeloma cell line used in cell fusion; 12R, a control nonrelevant MoAb (1:1,000); CD61, a positive control for megakaryocytic cells; BAH-1 Asc, ascites of BAH-1 (1:1,000 dilution); BAH-1 Sup, BAH-1 supernatant (1:1,000 dilution).
Immunoprecipitation of Mpl-R protein. CMK cell extracts were immunoprecipitated with BAH-1 antibodies and subjected to SDS-PAGE followed by Western blotting using control MoAb or BAH-1 antibodies (A) or Mpl-R antibodies (B) as described in the Materials and Methods section. Arrow indicates the position of the c-Mpl. The reactive proteins were detected by using the ECL system (Amersham).
Immunoprecipitation of Mpl-R protein. CMK cell extracts were immunoprecipitated with BAH-1 antibodies and subjected to SDS-PAGE followed by Western blotting using control MoAb or BAH-1 antibodies (A) or Mpl-R antibodies (B) as described in the Materials and Methods section. Arrow indicates the position of the c-Mpl. The reactive proteins were detected by using the ECL system (Amersham).
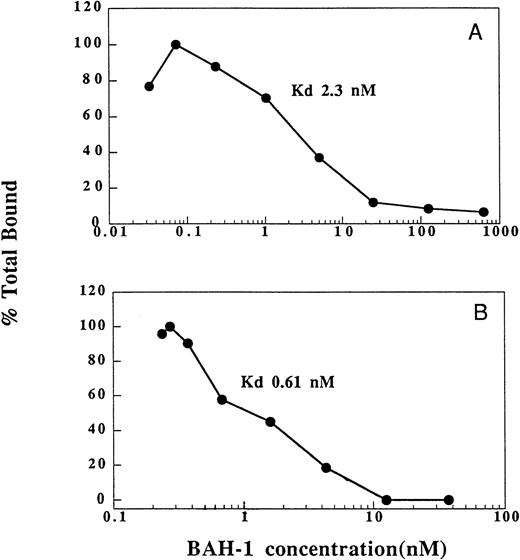
We also determined the ability of BAH-1 to bind to platelets and recombinant c-Mpl (Fig 3). In equilibrium binding studies, a kd of 2.3 nmol/L was determined for BAH-1 binding to platelets. This compares to a kd of 200 pmol/L for TPO.36 By using a recombinant truncated version of c-Mpl, which consists of the extracellular domain of c-Mpl fused to an Fc domain of IgG,36 we found that BAH-1 also bound specifically to purified c-Mpl with a kd of 0.61 nmol/L. These results show that BAH-1 specifically binds c-Mpl with high affinity. Interestingly, competition experiments showed that BAH-1 did not inhibit TPO binding to c-Mpl, suggesting that TPO and BAH-1 bind to different sites on the c-Mpl receptor (data not shown).
Binding of BAH-1 to human platelets and recombinant c-Mpl. Equilibrium binding studies for 125I-BAH-1 were performed on platelets (A) and c-Mpl-IgG fusion protein (B) as described in the Materials and Methods. The kd was determined as described.40
Binding of BAH-1 to human platelets and recombinant c-Mpl. Equilibrium binding studies for 125I-BAH-1 were performed on platelets (A) and c-Mpl-IgG fusion protein (B) as described in the Materials and Methods. The kd was determined as described.40
Functional characterization of BAH-1 MoAb.
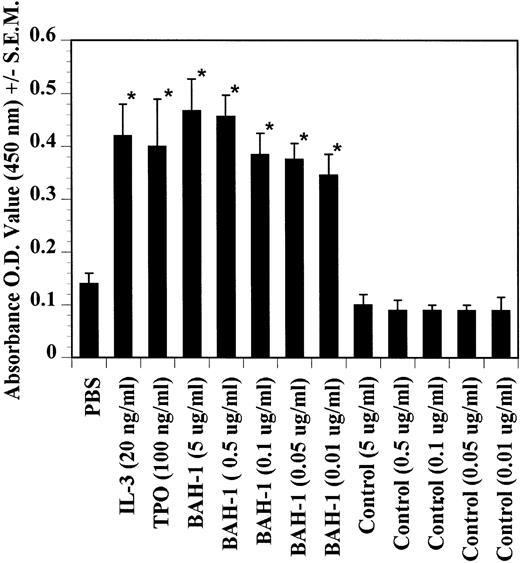
The effects of BAH-1 MoAb on megakaryocytopoiesis were evaluated in liquid suspension and fibrin clot megakaryocytopoiesis assays. For the liquid suspension assay, purified CD34+ cells (5 × 104/mL) were cultured in serum-free medium in the presence of various concentrations of BAH-1 for 10 days. As shown in Fig 4, BAH-1 stimulated the production of megakaryocytes in these cultures. A plateau was reached at 100 ng/mL of BAH-1, a level of stimulation that is similar to that caused by 100 ng/mL of TPO (Fig 4). Similarly, in the fibrin clot system BAH-1 also stimulated the production of CFU-MK progenitors (Fig 5A and B). In combination with IL-3, BAH-1 acted additively. No effects were observed on CFU-GM, BFU-E, or CFU-E colonies when BAH-1 antibody was used in various concentrations.
Effects of TPO, IL-3, and BAH-1 MoAb on megakaryocyte differentiation of CD34+ cells. CD34+ cells (1 × 104/mL) were cultured in 500 mL of serum-free culture medium. After 10 days, the increase in megakaryocytes was determined by ELISA using anti-GpIIb/IIIa antibodies. Results represent the mean optical density (OD) ± SEM of three independent experiments. *Statistically significant compared with PBS (P < .05).
Effects of TPO, IL-3, and BAH-1 MoAb on megakaryocyte differentiation of CD34+ cells. CD34+ cells (1 × 104/mL) were cultured in 500 mL of serum-free culture medium. After 10 days, the increase in megakaryocytes was determined by ELISA using anti-GpIIb/IIIa antibodies. Results represent the mean optical density (OD) ± SEM of three independent experiments. *Statistically significant compared with PBS (P < .05).
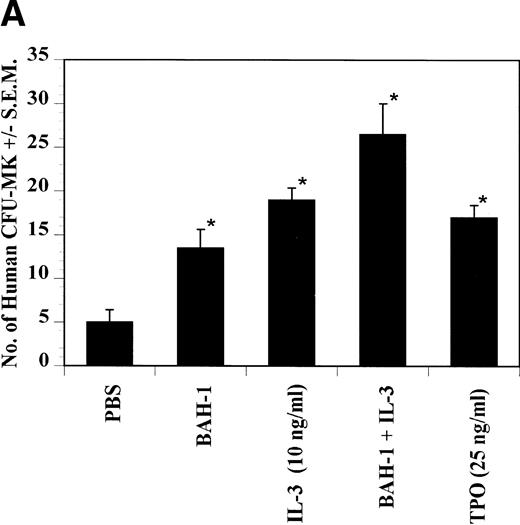
Effect of BAH-1 MoAb on human CFU-MK colonies. (A) CD34+ bone marrow cells were plated at 5 × 103/mL in the fibrin clot system (see the Materials and Methods). Results are expressed as the means ± SEM of megakaryocyte colonies. Experiments were performed in triplicate in four assays. *Statistically significant compared with PBS (P < .05). (B) Photographs of CFU-MK colonies in the presence of BAH-1 (B-I) or TPO (B-II).
Effect of BAH-1 MoAb on human CFU-MK colonies. (A) CD34+ bone marrow cells were plated at 5 × 103/mL in the fibrin clot system (see the Materials and Methods). Results are expressed as the means ± SEM of megakaryocyte colonies. Experiments were performed in triplicate in four assays. *Statistically significant compared with PBS (P < .05). (B) Photographs of CFU-MK colonies in the presence of BAH-1 (B-I) or TPO (B-II).
We also determined the ability of BAH-1 to directly stimulate the proliferation of early megakaryocyte progenitors. In limiting dilution experiments, CD34+ CD41+ cells were plated at a concentration of 1 to 50 cells (100 mL volume) in the presence of TPO, IL-3, or BAH-1 alone or in combination. After 5 days in culture, overall expansion was determined and megakaryocytes quantitated by staining with an anti-IIb/IIIa antibody. As shown in Fig 6, the effect of BAH-1 was similar to that of TPO. The percentage of positive megakaryocytes in each well was determined to be approximately 40% to 70%. Individual large megakaryocytes as well as small megakaryocytic cells were observed.
Effect of BAH-1 on CD34+CD41+ cells. CD34+ CD41+cells were cultured under serum-free conditions in the presence of rhTPO, IL-3 (100 ng/mL), the optimal plateau concentration as determined for IL-3 with this assay, or various concentrations of BAH-1. On day 5, megakaryocytes in culture were quantitated visually by using an inverted microscope. *Statistically significant compared with PBS (P < .05).
Effect of BAH-1 on CD34+CD41+ cells. CD34+ CD41+cells were cultured under serum-free conditions in the presence of rhTPO, IL-3 (100 ng/mL), the optimal plateau concentration as determined for IL-3 with this assay, or various concentrations of BAH-1. On day 5, megakaryocytes in culture were quantitated visually by using an inverted microscope. *Statistically significant compared with PBS (P < .05).
Effect of BAH-1 on murine megakaryocytopoiesis.
Dot blot analysis indicated that BAH-1 also cross-reacts with murine Mpl (data not shown). Because of this, we determined whether BAH-1 was also an agonist for murine megakaryocytopoiesis. In a liquid suspension assay,31 BAH-1 stimulated the proliferation of immature megakaryocytes (Fig 7). However, BAH-1 failed to stimulate murine CFU-MK colony formation (Fig 8) although it did appear to synergize with TPO or IL-3 in stimulating murine CFU-MK formation (Fig 8). These results suggest that BAH-1 does not induce complete differentiation of murine megakaryocytes.
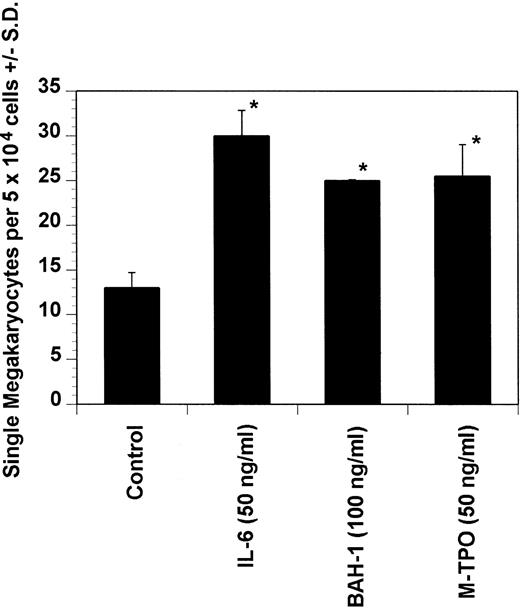
Effect of BAH-1 MoAb on murine immature megakaryocytes by using a single megakaryocyte growth assay. Single megakaryocytes were scored as the number of acetylcholinesterase-positive cells per fractionated 5 × 104 bone marrow cell cultures. Results are the means ± SEM from triplicate cultures from three experiments. *Results were significantly different (P < .01) from FCS control.
Effect of BAH-1 MoAb on murine immature megakaryocytes by using a single megakaryocyte growth assay. Single megakaryocytes were scored as the number of acetylcholinesterase-positive cells per fractionated 5 × 104 bone marrow cell cultures. Results are the means ± SEM from triplicate cultures from three experiments. *Results were significantly different (P < .01) from FCS control.
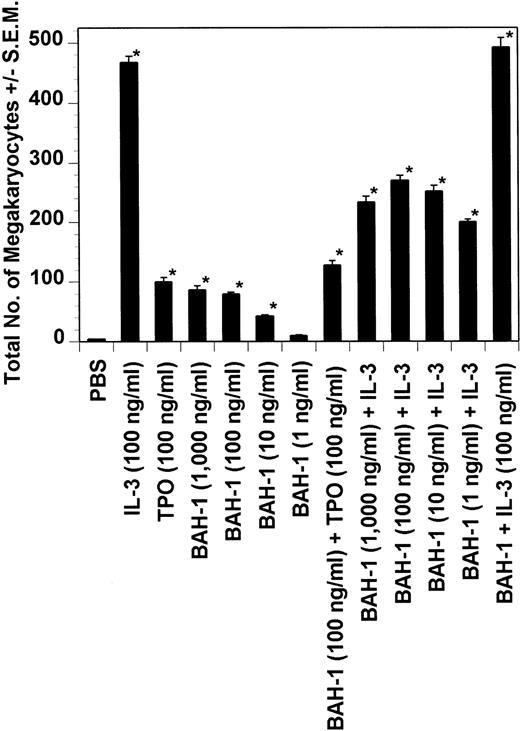
Effect of BAH-1 MoAb on murine CFU-MK colonies. CFU-MK megakaryocytes were scored as the number of colonies with three cells or more per 105 unfractionated cells. BAH-1 (1 μg/mL) was used in various dilutions as indicated. Results are the means ± SEM from triplicate cultures from three experiments. *Results were significantly different (P < .01) from FCS control.
Effect of BAH-1 MoAb on murine CFU-MK colonies. CFU-MK megakaryocytes were scored as the number of colonies with three cells or more per 105 unfractionated cells. BAH-1 (1 μg/mL) was used in various dilutions as indicated. Results are the means ± SEM from triplicate cultures from three experiments. *Results were significantly different (P < .01) from FCS control.
Effect of BAH-1 on hematopoiesis in myelosuppressed mice.
The effects of BAH-1 alone or in combination with TPO on hematopoietic progenitor cell numbers during the recovery phase after myelosuppressive therapy were evaluated in myelosuppressed mice. Only a modest increase in the numbers of CFU-MK colonies was observed in the marrow of BAH-1–treated mice (Table 1), whereas an increase in the numbers of CFU-MK colonies was observed in the BAH-1 plus TPO-treated mice (Table 1). No effects of BAH-1 were observed on the CFU-GM, BFU-E, or CFU-E colonies (Table 1). The modest effect of BAH-1 in this model is likely because of the relatively modest effect of BAH-1 on murine megakaryocytopoiesis.
Marrow Hematopoietic Progenitor Cell Levels After the Administration of BAH-1, TPO, Control MoAb, or TPO plus BAH-1 to Myelosuppressed Mice
| Treatment . | CFU-MK (×10−3) . | BFU-E (×10−3) Experiment 1 . | CFU-GM (×10−3) Experiment 1 . | ||
|---|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
| Control | 0 | 0 | 0 | 2.0 ± 1.0 | 9.0 ± 1.0 |
| TPO | 10.5 ± 0.5-150 | 3.0 ± 1.0-150 | 7.5 ± 1.0-150 | 7.0 ± 1.0-150 | 50.5 ± 9.5-150 |
| BAH-1 | 1.0 ± 0 | 2.0 ± 1.0 | 2.0 ± 1.0 | 2.5 ± 1.0 | 12.0 ± 1.0 |
| BAH-1 + TPO | 12.0 ± 1.0-150 | 4.5 ± 0.5-150 | 12.5 ± 1.0-150 | 7.5 ± 0.5-150 | ND |
| Treatment . | CFU-MK (×10−3) . | BFU-E (×10−3) Experiment 1 . | CFU-GM (×10−3) Experiment 1 . | ||
|---|---|---|---|---|---|
| Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
| Control | 0 | 0 | 0 | 2.0 ± 1.0 | 9.0 ± 1.0 |
| TPO | 10.5 ± 0.5-150 | 3.0 ± 1.0-150 | 7.5 ± 1.0-150 | 7.0 ± 1.0-150 | 50.5 ± 9.5-150 |
| BAH-1 | 1.0 ± 0 | 2.0 ± 1.0 | 2.0 ± 1.0 | 2.5 ± 1.0 | 12.0 ± 1.0 |
| BAH-1 + TPO | 12.0 ± 1.0-150 | 4.5 ± 0.5-150 | 12.5 ± 1.0-150 | 7.5 ± 0.5-150 | ND |
The results for femoral progenitors in each experiment represent the mean ± SEM of three animals in Experiments 1 and 2, whereas Experiment 3 included five animals in each treatment group.
Abbreviation: ND, not determined.
P < .005 compared with control.
DISCUSSION
We have generated a murine MoAb, BAH-1, against human megakaryocytic cells that specifically recognizes the TPO receptor, c-Mpl. BAH-1 specifically bound to platelets and recombinant c-Mpl with high affinity and had agonist activity in various assays of in vitro human and murine megakaryocytopoiesis, including the generation of CFU-MK megakaryocyte progenitors and the production of mature GpIIb/IIIa-expressing megakaryocytes in liquid cultures of heterogeneous bone marrow cells. Although BAH-1 was able to trigger cell proliferation and differentiation of human megakaryocytic precursors and immature murine megakaryocytes, by itself it failed to stimulate murine CFU-MK colony formation (Fig 7). However, it did synergize with IL-3 and TPO to stimulate murine CFU-MK formation. From these results, we conclude that BAH-1 is an agonist antibody specific for c-Mpl and is capable of stimulating human megakaryocyte growth and maturation.
TPO has an effect on stem cells as well as erythroid progenitors.15,34 However, BAH-1 alone had no effects on BFU-E and CFU-E colonies under the conditions tested, as described.15 The lack of potency of BAH-1 on the erythroid lineage could be caused by a cross-species effect, because BAH-1 is a murine antibody being tested in a human system. Alternatively, a cooperative effect with EPO could be needed to see an effect of BAH-1 on this lineage.
Homodimerization of the c-Mpl receptor by TPO on the cell surface is believed to be the key event in TPO-induced signal transduction.17 In support of this, TPO has recently been shown to contain two receptor binding sites that function to dimerize c-Mpl.37 Similarly, because of its bivalency, BAH-1 likely acts as an agonist by inducing homodimerization of c-Mpl. Indeed, monovalent Fab fragments of BAH-1 antibody, which cannot form receptor dimers, did not stimulate megakaryocytopoiesis (data not shown). These results suggest that the mechanism through which BAH-1 stimulates megakaryocytopoiesis is by “self-antagonism,” which has been described for agonist antibodies for GH, PRL, and EPO receptors.35,38 39
BAH-1 offers several opportunities for improving our understanding of the biology of megakaryocytopoiesis as well as its potential therapeutic applications. More detailed mapping of the binding site of this MoAb on c-Mpl may help define the sequences and confirmation of the native receptor, which are necessary and sufficient for activation and subsequent signal transduction. Studies of different c-Mpl constructs are in progress to achieve this aim. It is interesting to note that BAH-1 does not antagonize TPO binding to c-Mpl, suggesting that they have different binding sites.
Currently, it is not known if BAH-1 stimulates platelet production in vivo. In a murine myelosuppressive model, BAH-1 only modestly affected megakaryocytopoiesis. This may be caused by species specificity because BAH-1 was much more effective in stimulating human versus murine megakaryocytopoiesis in vitro.
Although the most immediate clinical trials are of recombinant human TPO, there may be certain opportunities for the use of a humanized agonist MoAb. Humanizing murine antibodies have successfully yielded a number of products currently in clinical trials that have minimal antigenicity while sustaining affinity and functional potency in the recognition of human antigens. Because of the prolonged half-life of antibodies, it might be feasible to administer an agonist antibody to the c-Mpl receptor on an intermittent basis, thereby sustaining the stimulation of megakaryocytopoiesis in patients with a compromised production of cells of this lineage.
ACKNOWLEDGMENT
We thank Dr Jerome E. Groopman for his helpful and valuable discussions. We are grateful to Janet Delahanty for editing and preparation of the figures for this manuscript, as well as Evelyn Gould for her help with the figures. We also thank Tee Trac for her typing assistance.
Supported in part by Genentech Inc and National Institutes of Health Grant No. RO1 51456.
Address reprint requests to Hava Avraham, PhD, Division of Experimental Medicine, Harvard Institutes of Medicine—BIDMC, 4 Blackfan Circle, 3rd Floor, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal