Abstract
Until recently, the identification of cellular factors that govern the developmental program of human stem cells has been difficult due to the absence of repopulation assays that detect human stem cells. The transplantation of human bone marrow (BM) or cord blood (CB) into non-obese diabetic (NOD)/severe-combined immunodeficient (SCID) mice has enabled identification of primitive human cells capable of multilineage repopulation of NOD/SCID mice (termed the SCID–repopulating cell [SRC]). Here, we examined the effect of long-term in vivo treatment with various combinations of human cytokines on the developmental program of SRC. Detailed flow cytometric analysis of engrafted mice indicated that the vast majority of the human graft of untreated mice was comprised of B lymphocytes at various stages of development as well as myeloid and primitive cells; T cells were not reproducibly detected. Many studies, including murine in vitro and in vivo data and human in vitro experiments, have suggested that flt3 ligand (FL) and/or Interleukin-7 (IL-7) promotes T- and B-cell development. Unexpectedly, we found that treatment of engrafted mice with the FL/IL-7 combination did not induce human T- or B-cell development, but instead markedly reduced B-cell development with a concomitant shift in the lineage distribution towards the myeloid lineage. Effects on lineage distribution were similar in engrafted mice transplanted with highly purified cells indicating that the action of the cytokines was not via cotransplanted mature cells from CB or BM cells. These data show that the lineage development of the human graft in NOD/SCID mice can be modulated by administration of human cytokines providing a valuable tool to evaluate the in vivo action of human cytokines on human repopulating cells.
© 1998 by The American Society of Hematology.
CLINICAL APPLICATION OF hematopoietic cytokine treatment has increased significantly both in mobilizing peripheral blood progenitor cells before transplantation and promoting the generation of mature granulocytic, erythroid, and megakaryocytic cells after high-dose chemotherapy. However, evaluation of the effects of cytokines on early human hematopoietic cells has been hampered by the lack of an in vivo repopulation system that enables detection of human stem cells. Typically, preclinical studies on the effects of cytokines involve extrapolation from in vivo studies using other mammals and in vitro studies with human cells.
We have identified a novel primitive cell, termed the severe-combined immunodeficient (SCID) mouse-repopulating cell (SRC), which initiates the human graft after transplantation of human bone marrow (BM) or umbilical cord blood (CB) by intravenous injection into SCID or non-obese diabetic SCID (NOD/SCID) mice.1-5 Cell purification and gene-marking studies indicate that the SRC is more primitive than most clonogenic progenitors and long-term culture-initiating cells (LTC-IC) and the SRC are exclusively found in the CD34+CD38− cell fraction from human CB and BM.4,6 Flow cytometric analysis and progenitor assays from the BM of engrafted mice transplanted with highly purified cells show that multiple hematopoietic lineages including myeloid, erythroid, and multilineage progenitors as well as more mature myeloid cells and B lymphocytes are present, whereas no mature T cells were detected.6-8 These xenotransplant systems provide the foundation required for an in vivo system for the preclinical evaluation of cytokines.
It was necessary in our original studies, using beige/nude/xid (BNX) or SCID recipients, to treat mice with human cytokines including stem-cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-3 (IL-3) to achieve high-level human cell engraftment.1 However, detailed flow cytometric studies were not performed on large numbers of these mice. The increased immunodeficiency and more supportive BM microenvironment of NOD/SCID mice permit a high level of engraftment without the need for continuous cytokine administration (Gan et al, personal communication). Thus, the NOD/SCID system is ideal to determine if in vivo cytokine administration can modulate the lineage development from SRC irrespective of affecting the total level of human cell engraftment.
We were particularly interested in the effects of cytokines predicted to act on primitive cells and for combinations that may promote T-cell development to develop models for HIV and T-cell progenitor assays. Many studies on murine stem cells, both in vitro9-11 and in vivo,12,13 and on in vitro analyses of human cells14-17 have suggested that SCF18 and flt3 ligand (FL) act on primitive hematopoietic cells. The tyrosine-kinase receptors for both of these ligands are expressed on early cells including CD34+ human cells.18-20Although these two factors alone have little effect on the proliferation of primitive cells, both are potent synergistic factors able to induce marked proliferation of both myeloid, erythroid, and lymphoid lineages.18,21 With respect to lymphocyte development, murine studies have shown that the combination of FL and IL-7 induced marked in vitro proliferation of CD43+B220low B cells and fetal thymocytes.9,22 Moreover, FL and IL-7 promoted stromal-independent expansion and differentiation of human fetal pro-B cells in vitro.23 IL-7 alone is an important factor in the development of both T and B cells,24,25 although some reports have shown that IL-7 together with other myeloid factors can support myelopoiesis.26-28
Here, we report that long-term intraperitoneal administration of some combinations of SCF, FL, IL-3, IL-7, GM-CSF to NOD/SCID mice transplanted with human hematopoietic cells can modulate the lineage distribution of the human graft in vivo. Treatment of mice with FL and IL-7, alone or in combination, did not result in increased numbers of T cells or B cells, rather there was a marked reduction in the number of CD19+B cells with a concomitant increase in the proportion of myeloid cells. Because the results we observed were not predicted from some earlier in vitro and in vivo murine studies or in vitro human studies, this study shows that the in vivo NOD/SCID repopulation model can be used to evaluate the biological effect of cytokine treatment on the developmental capacity of the engrafting human cells within the complexity of an in vivo setting.
MATERIALS AND METHODS
Human cells.
One human BM sample was obtained from a harvest of a normal donor for allogeneic transplantation in accordance with procedures approved by the Human Experimentation Committee at the Ontario Cancer Institute (OCI), Toronto, Ontario, Canada. Mice transplanted with BM cells are indicated in Fig 1 by circles. Samples of CB were obtained from discarded placental and umbilical tissues. Both BM and CB samples were diluted (1 to 3) in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO, BRL, Gaithersburg, MD) and enriched for mononuclear cells by centrifugation on Ficoll-paque (Pharmacia, Baie d’Urfe, Quebec, Canada).
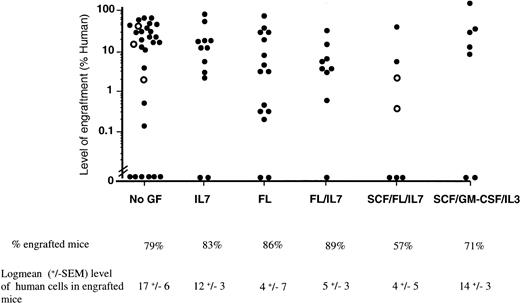
Effect of cytokine treatment on human cell engraftment in the BM of NOD/SCID mice. The proportion of CD45+ human cells present in the BM was determined by flow cytometry 6 to 8 weeks after the transplant of unseparated mononuclear CB or BM cells. Each dot represents the level of human cells detected in the BM of a single mouse. BM was used in one single experiment and indicated by open circles. Both the frequency of successful engraftment and the log10 mean level of human cells present in the group of engrafted mice are shown.
Effect of cytokine treatment on human cell engraftment in the BM of NOD/SCID mice. The proportion of CD45+ human cells present in the BM was determined by flow cytometry 6 to 8 weeks after the transplant of unseparated mononuclear CB or BM cells. Each dot represents the level of human cells detected in the BM of a single mouse. BM was used in one single experiment and indicated by open circles. Both the frequency of successful engraftment and the log10 mean level of human cells present in the group of engrafted mice are shown.
Purification of cell populations.
As previously described,6 mononuclear cells were stained with a mixture of lineage-specific antibodies provided by the manufacturer (Stem Cell Technologies, Vancouver, Canada), followed by addition of secondary antibody conjugated to metal colloid. Cells were then eluted through a magnetized column to enrich for cells not expressing lineage markers (Lin−). These cell fractions were then stained with anti-hu CD34-fluorescein isothiocyanate (FITC) and anti-hu CD38-phycoerythrin (PE), and analyzed and sorted on a FACStar Plus (Becton Dickinson, San Jose, CA) equipped with 5 W argon and 30 mW helium neon lasers. Fluorescence of FITC and PE excited at 482 nm (0.38 W) and 633 nm (30 mW), respectively, as well as known forward- and side-scatter properties of normal live human hematopoietic cells were used to establish sorting gates. Data acquisition and analysis were performed using LYSIS II software (Becton Dickinson). The purity of CD34+Lin− cells after FACS was 98% to 99% and the purity of CD34+/CD38− cells ranged from 96% to 98%.
Transplantation of human cells into NOD/SCID mice.
Eight-week-old NOD/LtSz-scid/scid (NOD/SCID) mice were bred from breeding pairs originally obtained from L. Shultz (Jackson Laboratory, Bar Harbor, ME), and maintained in the defined-flora animal facility located at the OCI. All animals were handled under sterile conditions and maintained under microisolators. Fifteen −20 × 106 unseparated CB or BM cells, 1800 to 6000 CD34+/CD38− cells, or 16 × 104−80×104CD34+Lin− cells were transplanted by tail-vein injection into sublethally irradiated mice (375 cGy using a137Cs irradiator) according to our standard protocol as previously described.1,3 6 Human cytokines were injected every second day intraperitoneally: huSCF, 10 μg, huIL-3 and huGM-CSF, 6 μg (Amgen, Thousand Oaks, CA); FL, 10 μg and huIL7, 10 μg (Immunex, Seattle, WA). Mice were killed 6 to 8 weeks after transplantation, and the BM from the femora, tibiae, humeri, and iliac crests of each mouse was flushed into IMDM containing 10% fetal calf serum (FCS).
Analysis of human cell engraftment in transplanted mice.
Genomic DNA was isolated from the BM and spleens of transplanted mice by standard extraction protocols.1EcoRI-digested DNA was separated by agarose-gel electrophoresis, transferred onto a positively charged nylon membrane, and probed with a labeled human chromosome 17-specific α-satellite probe (p17H8). The level of human cell engraftment was determined by comparing the characteristic 2.7 kb band with those of human:mouse DNA mixtures as controls (limit of detection 0.05% human DNA). The presence of human progenitors in the BM of transplanted mice was determined by plating BM cells in methylcellulose cultures under conditions that are selective for the growth of human cells.
Flow cytometric analysis of murine BM.
To prepare cells for flow cytometry, contaminating red cells were lysed with a 6% ammonium chloride solution and the remaining cells were washed in phosphate-buffered saline (PBS) containing 5% FCS. Approximately 106 cells were resuspended in 1 mL of PBS+5% FCS containing 5% human serum (to block Fc receptors) for 30 minutes at 4°C, washed, then incubated with monoclonal antibodies at a concentration of 5 μg/mL for 30 minutes at 4°C. CD45 was conjugated to PerCP; CD34, CD14, CD20, sIgM, CD3, CD4, and CD15 were conjugated to FITC; and CD38, CD33, CD19, CD8, CD7, and CD13 were conjugated to PE. Anti-CD45, -CD34, and -CD38 antibodies were purchased from Becton Dickinson, the antibody against surface IgM (sIgM) was from Cedar Lane (Hornby, Ontario, Canada), whereas all other antibodies were obtained from Coulter (Burlington, Ontario, Canada). Cells were then washed three times in PBS + 5% FCS and analyzed on a FACScan (Becton Dickinson). For each mouse analyzed, an aliquot of cells was also stained with mouse IgG conjugated to FITC, PE, and PerCP as an isotype control. BM cells from an untransplanted NOD/SCID were stained in parallel as an additional negative control. Fluorescence levels excluding greater than 98% of the cells in these negative controls were considered to be positive and specific for human staining.
Clonogenic progenitor assays.
Human clonogenic progenitors present in the BM of engrafted mice were detected by plating mononuclear cells in methylcellulose cultures as described under conditions selective for the growth of human progenitors.4
Statistics.
Comparison of treatment groups was performed using the Student’st test or the Mann-Whitney Rank Sum test, when appropriate.P values below .05 were considered significant. The analyses were done using the SigmaStat statistical software (SigmaStat for Windows, Jandel Corp, San Rafael, CA).
RESULTS
Effect of cytokine treatment on human cell engraftment.
Groups of sublethally irradiated mice were transplanted with mononuclear cells from CB or BM and treated with the following cytokine combinations: FL, IL-7, FL/IL-7, SCF/FL/IL-7, SCF/FL/IL-3, or SCF/GM-CSF/IL-3 every second day for 6 to 8 weeks. The murine bone marrow was examined for the presence of human cells by staining with a human-specific CD45 antibody. The results obtained by flow cytometry were confirmed by Southern blotting using a human-specific α-satellite probe (data not shown). As shown in Fig 1, the frequency of successful engraftment, obtained from analysis of 79 mice from 10 different donors, was similar among the different treatment groups. If only engrafted mice are analyzed, the mean level of engraftment amongst the different treatment groups ranged from 4% to 17%, with the lowest levels of human cells found in the groups treated with FL/IL-7 and FL/IL-7/SCF. Analysis of 27 mice (from six donors) that were engrafted with purified CD34+Lin− or CD34+CD38− cells showed similar frequency and levels of engraftment (data not shown).
Mobilization and/or induction of hematopoiesis in extramedullary sites such as the spleen has been observed in mice treated with cytokines, especially by IL-7.29-31 To determine if the distribution of human cells present in engrafted NOD/SCID mice could be changed due to cytokine treatment, we analyzed both the BM and spleen from 20 mice transplanted with three different donor CB samples that were untreated or treated with FL and FL/IL-7. The overall level of human cell engraftment of the BM of this untreated group was somewhat higher than the average shown in Fig 1. There was a lower level of human cells present in the spleens from these mice. Similar to the overall data shown in Fig 1, cytokine treatment of this group of mice resulted in a lower level of human cells in the BM (Fig 2). Concomitantly, the human cell engraftment of the spleen was also reduced with cytokine treatment. The correlation of human cell engraftment in the spleen and BM indicates that the reduction of human cells in the BM, from the FL- and FL/IL-7-treated mice, was not accompanied by a redistribution of human cells to the spleen.
The distribution of human cells in the BM and spleen after cytokine treatment. Three different CB samples were injected into 20 NOD/SCID mice and treated with the indicated cytokines. The amount of human cells present in the BM and the spleen was evaluated by DNA analysis 6- to 8-weeks post-transplant. Log10 mean levels of human cells are compared. The number of mice in each treatment group is shown in brackets.
The distribution of human cells in the BM and spleen after cytokine treatment. Three different CB samples were injected into 20 NOD/SCID mice and treated with the indicated cytokines. The amount of human cells present in the BM and the spleen was evaluated by DNA analysis 6- to 8-weeks post-transplant. Log10 mean levels of human cells are compared. The number of mice in each treatment group is shown in brackets.
Effect of cytokine treatment on human myeloid clonogenic progenitor cells.
To determine if cytokine treatment could affect the number of progenitors present in treated and untreated mice, progenitor assays were performed on the BM of engrafted NOD/SCID mice. Total number of colony-forming cells (CFC) ± standard error of mean (SEM)/2 × 105 cells in each group were: no treatment (n = 19), 149 ± 16; IL-7 (n = 10), 124 ± 25; FL (n = 11), 66 ± 30; FL/IL-7 (n = 7), 60 ± 8; SCF/FL/IL-7 (n = 4), 57 ± 25. Thus, the CFC content of the different treatment groups correlated with the level of human cell engraftment of the BM.
Effect of cytokine treatment on the lineage composition of the graft.
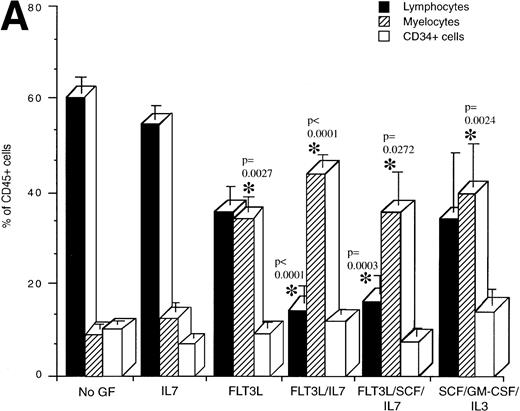
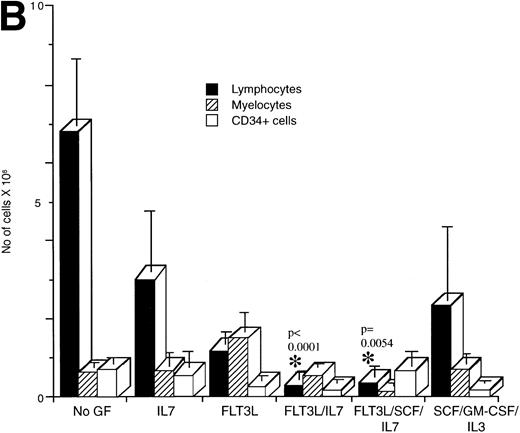
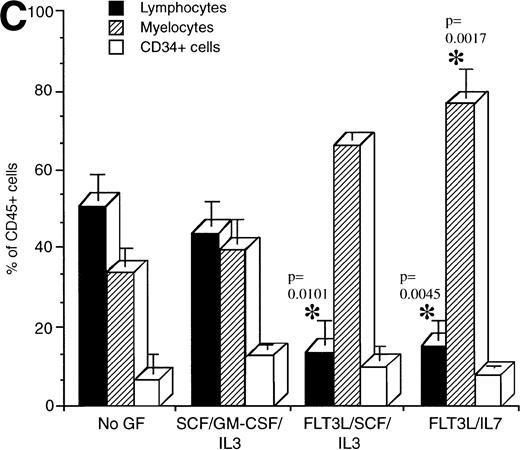
Flow cytometric analysis was performed on human cells present in engrafted mice from the various treatment groups to determine if cytokine treatment affected the development of specific lineages (Fig 3). The proportion of lymphocytes, myeloid cells, and CD34+ cells present in mice transplanted with mononuclear CB cells and treated with the indicated cytokines was determined (Fig 3A). Lymphocytes made up the majority of the human cells in engrafted NOD/SCID mice that were untreated or treated with IL-7 (Fig 3A). By contrast, mice treated with the combinations FL/IL-7 and FL/IL-7/SCF had significantly decreased proportions of lymphocytes (average of 15%, compared with 60% in the untreated group) with concomitant increases in the proportion of myeloid cells (average of 40%, compared with 9% in the untreated group). The mice treated with FL alone or SCF/GM-CSF/IL-3 had equal proportions of myeloid and lymphoid cells, the increase in the proportion of myeloid cells was significant for both treatment groups. The CD34+ cells remained similar amongst the treatment groups (∼10%). Calculation of the total number of lymphocytes, myeloid, and CD34+ cells present in these mice confirmed the reduction in the number of lymphocytes and shows that the absolute myeloid cell content was not increased in the FL/IL-7 treatment groups (Fig 3B). Similar trends were observed in the NOD/SCID mice transplanted with CD34+Lin− or CD34+CD38− cells indicating that the effect of the cytokine treatment on lymphocytes was not due to action on cotransplanted mature cells (Fig 3C).
Determination of the proportion of lymphocytes, myelocytes and primitive cells in engrafted mice treated with cytokines. The percentage of lymphoid, myeloid, and CD34+cells in the region of human CD45+ cells was measured by flow cytometry for each engrafted mouse in each treatment group. The lymphoid gate that contained >90% of CD19+ B lymphocytes and the myeloid gate that contained >90% CD33+ cells were established based on FSC and SSC characteristics.6 The histogram shows the mean ± SEM for each cell fraction. (A) Flow cytometric analysis of engrafted (>∼1% human cells) mice shown in Fig 1. In all cases, mice were transplanted with mononuclear CB cells except for the FL/SCF/IL-7 group that also contained two of four mice analyzed, which were transplanted with 1 BM sample. (B) Total number of cells present in the BM of the mice shown in A. This calculation was made by multiplying the percentage of cells with the total cell count obtained from four long bones. (C) Mice transplanted with purified CD34+Lin− or CD34+CD38− cells. Statistically significant differences with the untreated control mice are shown with the *.
Determination of the proportion of lymphocytes, myelocytes and primitive cells in engrafted mice treated with cytokines. The percentage of lymphoid, myeloid, and CD34+cells in the region of human CD45+ cells was measured by flow cytometry for each engrafted mouse in each treatment group. The lymphoid gate that contained >90% of CD19+ B lymphocytes and the myeloid gate that contained >90% CD33+ cells were established based on FSC and SSC characteristics.6 The histogram shows the mean ± SEM for each cell fraction. (A) Flow cytometric analysis of engrafted (>∼1% human cells) mice shown in Fig 1. In all cases, mice were transplanted with mononuclear CB cells except for the FL/SCF/IL-7 group that also contained two of four mice analyzed, which were transplanted with 1 BM sample. (B) Total number of cells present in the BM of the mice shown in A. This calculation was made by multiplying the percentage of cells with the total cell count obtained from four long bones. (C) Mice transplanted with purified CD34+Lin− or CD34+CD38− cells. Statistically significant differences with the untreated control mice are shown with the *.
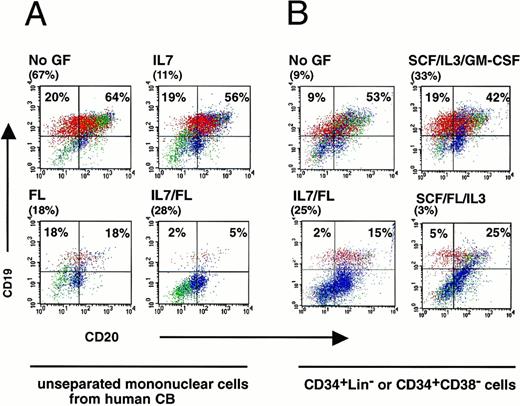
To examine the effect of cytokine treatment on the lymphocyte development, detailed flow cytometric analysis of engrafted mice was performed. A representative experiment of four mice transplanted with mononuclear cells from one CB sample is shown in Fig 4A and a detailed analysis on all mice is shown in Table 1A. Regardless of treatment, mice were engrafted at high levels (11% to 67% CD45+ cells). Without cytokine treatment, B cells made up 84% of the human cells present in the BM of the engrafted mouse as indicated by the presence of CD19+ cells. With the exception of the few mice with mature T cells (data not shown), normally more than 90% of cells in the lymphocyte gate were CD19-expressing B cells at different stages in B-cell development. Approximately, 5% to 10% of the CD19+ cells coexpressed CD34 indicating the presence of immature pro-B cells (data not shown), whereas, the detection of CD19+CD20+ cells shows that human pre-B cells were present (Fig 4A). A low proportion of these lymphocytes (8%) were mature and expressed sIgM (Table 1). Treatment with IL-7 had little effect on B-cell development, whereas the FL and FL/IL-7 groups had significantly lower levels (36% and 7%, respectively) of CD19+ and CD19+CD20+ cells in the human graft compared with untreated mice (84%) (Fig 4A). Similar effects of FL/IL-7 treatment on lymphocyte development were observed in mice transplanted with purified cells (Fig 4B). The similar reduction in B cells in mice transplanted with unseparated or purified cells indicates that the cytokine action on lineage development was not via cotransplanted nonrepopulating mature cells when whole CB or BM was used. The mice from all treatment groups in Fig 4 were well engrafted and there was no correlation between engraftment level and B-cell content showing that the effect of human cytokine treatment was specific to B-cell differentiation, rather than a general effect on human cell engraftment.
Flow cytometric analysis of the human B-cell population present in engrafted NOD/SCID mice treated with various cytokines. (A) Four NOD/SCID mice were transplanted with one CB donor and the BM was analyzed 6-weeks later for the presence of CD19+and/or CD20+ human B lymphocytes. These analyses were performed on gated CD45+ human cells. The level of human cell engraftment is shown in brackets for each mouse. In all cases the mice were treated with the indicated cocktail of cytokines every other day for the entire experiment. Lymphocytes (red) were distinguished from blasts (green) and myeloid cells (blue) according to their size and morphology using forward and side-scatter characteristics. (B) Mice were transplanted with CD34+CD38− cells for the no GF, SCF/IL-3/GM-CSF, FL/SCF/IL-3 treatment groups, or CD34+Lin− cells for the IL-7/FL treatment group.
Flow cytometric analysis of the human B-cell population present in engrafted NOD/SCID mice treated with various cytokines. (A) Four NOD/SCID mice were transplanted with one CB donor and the BM was analyzed 6-weeks later for the presence of CD19+and/or CD20+ human B lymphocytes. These analyses were performed on gated CD45+ human cells. The level of human cell engraftment is shown in brackets for each mouse. In all cases the mice were treated with the indicated cocktail of cytokines every other day for the entire experiment. Lymphocytes (red) were distinguished from blasts (green) and myeloid cells (blue) according to their size and morphology using forward and side-scatter characteristics. (B) Mice were transplanted with CD34+CD38− cells for the no GF, SCF/IL-3/GM-CSF, FL/SCF/IL-3 treatment groups, or CD34+Lin− cells for the IL-7/FL treatment group.
Phenotype of Human CD45+ Cells in the Bone Marrow of Mice Injected With Unsorted Cord Blood
| Treatment (no. of mice) . | Proportion of CD45+ Human Cells (range) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes . | Myelocytes . | CD19 . | CD20 . | IgM . | CD33 . | CD13 . | CD14 . | CD15 . | CD34 . | |
| NoGF | 60 | 9 | 77 | 47 | 8 | 15 | 12 | 6 | 6 | 10 |
| (23) | (35-84) | (2-18) | (48-96) | (30-68) | (5 mice, pooled) | (5 mice, pooled) | (5 mice, pooled) | (1-12) | (1-14) | (2-21) |
| SCF/GM-CSF/IL-3 | 34 | 32 | 53 | 37 | 13 | 33 | 27 | 16 | 14 | 15 |
| (5) | (7-81) | (9-48) | (43-74) | (26-51) | (8-19) | (8-44) | (8-39) | (0.2-24) | (4-20) | (2-21) |
| IL-7 | 55 | 12 | 73 | 50 | — | — | — | 6 | — | 7 |
| (10) | (38-68) | (5-25) | (62-89) | (26-68) | (3-10) | (3-16) | ||||
| FL | 36 | 34 | 47 | 29 | 4 | 48 | 37 | 14 | 17 | 8 |
| (8) | (20-50) | (15-45) | (28-71) | (18-53) | (2-25) | (5-13) | ||||
| FL/IL-7 | 14 | 44 | 28 | 21 | — | — | — | 29 | — | 12 |
| (8) | (3-29) | (36-60) | (5-44) | (4-31) | (18-36) | (6-16) | ||||
| FL/SCF/IL-7 | 16 | 36 | 23 | — | — | — | — | 12 | 10 | 8 |
| (4) | (6-25) | (14-49) | (18-28) | (14-17) | (9-15) | (5-12) | ||||
| Treatment (no. of mice) . | Proportion of CD45+ Human Cells (range) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes . | Myelocytes . | CD19 . | CD20 . | IgM . | CD33 . | CD13 . | CD14 . | CD15 . | CD34 . | |
| NoGF | 60 | 9 | 77 | 47 | 8 | 15 | 12 | 6 | 6 | 10 |
| (23) | (35-84) | (2-18) | (48-96) | (30-68) | (5 mice, pooled) | (5 mice, pooled) | (5 mice, pooled) | (1-12) | (1-14) | (2-21) |
| SCF/GM-CSF/IL-3 | 34 | 32 | 53 | 37 | 13 | 33 | 27 | 16 | 14 | 15 |
| (5) | (7-81) | (9-48) | (43-74) | (26-51) | (8-19) | (8-44) | (8-39) | (0.2-24) | (4-20) | (2-21) |
| IL-7 | 55 | 12 | 73 | 50 | — | — | — | 6 | — | 7 |
| (10) | (38-68) | (5-25) | (62-89) | (26-68) | (3-10) | (3-16) | ||||
| FL | 36 | 34 | 47 | 29 | 4 | 48 | 37 | 14 | 17 | 8 |
| (8) | (20-50) | (15-45) | (28-71) | (18-53) | (2-25) | (5-13) | ||||
| FL/IL-7 | 14 | 44 | 28 | 21 | — | — | — | 29 | — | 12 |
| (8) | (3-29) | (36-60) | (5-44) | (4-31) | (18-36) | (6-16) | ||||
| FL/SCF/IL-7 | 16 | 36 | 23 | — | — | — | — | 12 | 10 | 8 |
| (4) | (6-25) | (14-49) | (18-28) | (14-17) | (9-15) | (5-12) | ||||
The proportion of CD45+ human cells coexpressing the indicated cell-surface phenotype is given as the average (and range) from the indicated number of mice. The lymphocyte and myelocyte percentages were calculated as described in Fig 3.
Phenotype of Human CD45+ Cells in the Bone Marrow of Mice Injected With CD34+/Lin− or CD34+/CD38−Cells
| Treatment (no. of mice) . | Proportion of CD45+ Human Cells (range) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes . | Myelocytes . | CD19 . | CD20 . | IgM . | CD33 . | CD13 . | CD14 . | CD15 . | CD34 . | |
| CD34+/CD38− | ||||||||||
| NoGF | 45 | 34 | 53 | 31 | 9 | 44 | 30 | 7 | 10 | 13 |
| (2) | (32-57) | (26-42) | (46-60) | (29-33) | (7-10) | (31-56) | (19-41) | (6-8) | (7-12) | (4-21) |
| SCF/GM-CSF/IL-3 | 44 | 39 | 53 | 32 | 8 | 42 | 30 | 8 | 11 | 13 |
| (5) | (25-58) | (27-63) | (34-62) | (17-40) | (6-10) | (28-68) | (13-58) | (4-10) | (6-16) | (8-15) |
| FL/SCF/IL-3 | 14 | 66 | 17 | 11 | 4 | 72 | 50 | 14 | 13 | 10 |
| (3) | (4-23) | (63-68) | (12-23) | (8-16) | (0-7) | (63-90) | (31-73) | (10-17) | (8-17) | (5-17) |
| CD34+/Lin− | ||||||||||
| NoGF | 63 | 26 | 69 | 60 | 30 | 26 | 15 | 8 | 7 | 2 |
| (1) | ||||||||||
| FL/IL-7 | 15 | 77 | 10 | 7 | 4 | 84 | 51 | 21 | 27 | 8 |
| (2) | (4-26) | (63-93) | (4-15) | (1-12) | (0-8) | (77-90) | (41-60) | (18-24) | (21-32) | (6-9) |
| Treatment (no. of mice) . | Proportion of CD45+ Human Cells (range) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes . | Myelocytes . | CD19 . | CD20 . | IgM . | CD33 . | CD13 . | CD14 . | CD15 . | CD34 . | |
| CD34+/CD38− | ||||||||||
| NoGF | 45 | 34 | 53 | 31 | 9 | 44 | 30 | 7 | 10 | 13 |
| (2) | (32-57) | (26-42) | (46-60) | (29-33) | (7-10) | (31-56) | (19-41) | (6-8) | (7-12) | (4-21) |
| SCF/GM-CSF/IL-3 | 44 | 39 | 53 | 32 | 8 | 42 | 30 | 8 | 11 | 13 |
| (5) | (25-58) | (27-63) | (34-62) | (17-40) | (6-10) | (28-68) | (13-58) | (4-10) | (6-16) | (8-15) |
| FL/SCF/IL-3 | 14 | 66 | 17 | 11 | 4 | 72 | 50 | 14 | 13 | 10 |
| (3) | (4-23) | (63-68) | (12-23) | (8-16) | (0-7) | (63-90) | (31-73) | (10-17) | (8-17) | (5-17) |
| CD34+/Lin− | ||||||||||
| NoGF | 63 | 26 | 69 | 60 | 30 | 26 | 15 | 8 | 7 | 2 |
| (1) | ||||||||||
| FL/IL-7 | 15 | 77 | 10 | 7 | 4 | 84 | 51 | 21 | 27 | 8 |
| (2) | (4-26) | (63-93) | (4-15) | (1-12) | (0-8) | (77-90) | (41-60) | (18-24) | (21-32) | (6-9) |
The proportion of CD45+ human cells coexpressing the indicated cell-surface phenotype is given as the average (and range) from the indicated number of mice. The lymphocyte and myelocyte percentages were calculated as described in Fig 3.
Mature T cells that express CD3 and express CD4 and/or CD8 were found sporadically in the BM of 7 out of 63 mice (11%) that were engrafted with human cells from unseparated CB or BM (data not shown). However, no T cells were detected in mice transplanted with purified CD34+/CD38− or CD34+Lin− cells suggesting that some of the detected T cells could be derived from committed T-cell precursors, rather than from SRC. The detection of T cells did not correlate to any particular treatment group. From these data we conclude that human T-cell differentiation in NOD/SCID mice could not be promoted by treatment with FL and IL-7.
Detailed analysis of myeloid cells from untreated mice showed that from 15% to 44% of the human cells expressed CD33 when transplanted with either mononuclear or purified cells, respectively (Table 1A and B). Significant proportions of CD14+ monocytes and CD15+ granulocytes were also detected showing various stages of myeloid cell development in engrafted mice. As the proportion of CD19+ cells was reduced in the treatment groups, the proportion of each of these myeloid lineages increased concomitantly by several-fold indicating that there was no differential increase in one specific myeloid lineage (Table 1).
To determine if primitive cells were affected by cytokine treatment, we measured the levels of CD34+ cells present in engrafted mice. As reported earlier,3 6 the proportion of CD34+ cells present in the BM of engrafted NOD/SCID mice is higher (10%) than in the original CB sample (0.5%). No significant differences were found in the numbers of human CD34+progenitors comparing the different treatment groups indicating that long-term cytokine treatment did not alter the already elevated levels (Table 1, Fig 3).
DISCUSSION
The data reported here indicate that long-term human cytokine treatment can modulate the lineage distribution of human hematopoietic cells present in NOD/SCID mice transplanted with human unseparated CB or purified CD34+Lin− fractions. B cells comprised the major population in engrafted NOD/SCID mice that were not treated with cytokines, confirming recent reports.7 8Treatment with our standard cocktail of SCF/GM-CSF/IL-3 resulted in mice in which the human graft was composed of equal proportions of myeloid cells and B cells. The most dramatic effect of cytokine treatment was obtained in mice treated with FL/IL-7 or FL/SCF/IL-7 in which the proportion of human B cells was reduced by fivefold in the BM, compared with untreated mice. Treatment with FL alone was intermediate, with equal proportions of myeloid and B cells present, indicating that FL and IL-7 were acting synergistically to effect B-cell development in engrafted NOD/SCID mice. The reduced quantity of B cells in the BM was not due to redistribution to the spleen because there was no proportional increase in human cells in the spleens of treated mice. Although one of our original goals was to enhance T-cell development, no cytokine combinations affected T-cell development in the NOD/SCID mice. Mature T cells were only detected in a small proportion of engrafted mice that were transplanted with unfractionated CB and cytokine treatment did not increase the frequency of mice that contained T cells. Because T cells were never detected in mice transplanted with purified CB cell fractions that were depleted of T cells, it is likely that the T cells occasionally detected in mice transplanted with unseparated CB are derived from the expansion of committed T cells that were cotransplanted with the primitive repopulating cells.
The marked reduction in human B-cell development and the absence of an effect on T-cell development after in vivo FL/IL-7 treatment of engrafted NOD/SCID mice was not generally predicted from prior in vitro murine experiments with these cytokines. For example, IL-7 treatment in liquid culture of cell fractions, enriched for primitive B- and T-cell progenitors, stimulated growth and differentiation resulting in the generation of mature T cells, T-cell progenitors, and B-cell progenitors.9,17,23-25 However, it is noteworthy that when primitive Sca+Lin− cells were used as the target, IL-7 promoted myeloid progenitor growth.26IL-7-gene–deleted mice have reduced numbers of B and T cells indicating the important role this cytokine plays in lymphoid development.32 Similarly, FL plays an important role in lymphoid development although it is more pleiotropic and can synergize with many factors to affect virtually all hematopoietic lineages. FL receptor gene deleted mice show some impairment in the commitment and/or differentiation of pluripotent stem cells and a major depletion of pro-B cells.12 When FL is combined with IL-7, in vitro culture studies showed that stimulation of the growth of murine CD43+B220loCD24−lymphoid cells occurs.22 However, the optimal effect was seen when stroma was added indicating that some other factors were also important in promoting B lymphopoiesis. The combination of FL/IL-7 also stimulated the clonal growth of single Sca1+Lin− cells resulting in B cells after 12 days.33 However, other studies using unfractionated murine BM show that FL/IL-7 can stimulate the in vitro proliferation of clonogenic murine myeloid cells.26,28 33Taken together, these murine data indicate that although the predominate action of FL and IL-7 is on stimulation of lymphoid development, this cytokine combination can also promote the proliferation of myeloid progenitors under different conditions. It appears that the specificity of a particular cytokine treatment may be influenced by the defined conditions of in vitro culture and the nature of the purified target cells (eg, pluripotential or lineage committed).
The effects of FL/IL-7 on the in vitro culture of human cells have not been studied as extensively as in murine systems, however, many similarities exist. FL treatment of CD34+ cells alone resulted in the long-term maintenance of clonogenic progenitors, whereas a potent synergistic effect on virtually all lineages was observed when other cytokines (eg, IL-3, IL-6, EPO, etc) were combined with FL.16 FL alone is also able to induce the proliferation of primitive CD34+CD38−cells and improve long-term maintenance of progenitors during liquid culture.34 Treatment of human fetal CD34+CD19+ pro-B cells in liquid culture with FL/IL-7 resulted in promotion of pro-B cell growth and differentiation into pre-B cells and mature sIgM+ cells. The addition of IL-3 plus coculture with stromal cells resulted in even greater B-cell production and higher levels of mature B cells,23suggesting that additional costimulatory molecules are important for maximal B-cell stimulation. Interestingly, the combination of SCF and IL-7 resulted in marked increases in myeloid progenitors, although in contrast to the murine system, no effects were observed when IL-7 was used alone. Thus, the combination of cytokines that are added together with IL-7 in the liquid culture can greatly affect the lineage specificity of growth stimulation. Overall, this in vitro data would not have predicted the reduction in B-cell development we observed in vivo.
Although no in vivo murine studies have been reported on the coadministration of FL and IL-7, the most directly comparable study to ours was the report from Brasel et al13 on short-term (10d) treatment of mice with human FL. The peripheral blood lymphocytes increased by threefold whereas the monocytes increased by 78-fold and the granulocytes by 10-fold. In the BM, the increases were more modest, B cells increased about twofold whereas monocytes and granulocytic cells increased by fivefold. Thus, the increased proportion of myeloid cells is consistent with our data, whereas the increased B-cell development was not. Because in vivo systems are inherently complex, it is difficult to identify a specific explanation for these contrasting results. There are several possibilities. (A) These data may reflect inherent differences in the cytokine requirements for B-cell commitment between murine and human hematopoietic cells. (B) The murine data are derived from steady-state mice, whereas our NOD/SCID system is a repopulation system and our mice received a longer duration of treatment. (C) There may be subtle differences in the response of primitive human repopulating cells to these cytokines compared with murine stem cells. Because engraftment in the NOD/SCID recipients is derived from the primitive human SRC, it is possible that the cytokines normally present in engrafted mice when combined with FL, direct myeloid development at the expense of B-cell development. (D) Finally, because these cytokines are not species-specific it is possible that they are impacting on the human cells indirectly via the murine hematopoietic or stromal cells. However, because the murine FL study showed increases in B cells under similar treatment conditions, one would need to postulate that the human B-cell commitment is differentially affected compared with murine B cells by the factors acting “indirectly.”
In conclusion, this study shows the importance of examining the effects of cytokine treatment on primitive human cells in an in vivo repopulation setting. Although in vivo systems, such as the NOD/SCID system, are inherently complex compared with defined in vitro cultures, they provide a means to ascertain the net biological effect of cytokine treatment, in the milieu of all the other cytokines and stromal factors that are present in vivo, on primitive human hematopoietic populations. This system may be a useful preclinical tool to evaluate the biological consequence of cytokine treatment on human hematopoiesis being proposed for clinical trials.
ACKNOWLEDGMENT
We thank S. Lyman at Immunex for gifts of FL and IL7, I. McNiece at Amgen for SCF, L. McWhirter for providing cord blood specimens, N. Jamal and H. Messner for providing bone marrow samples, and members of the laboratory for critically reviewing the manuscript.
Supported by grants to J.E.D. from the Medical Research Council of Canada (MRC), the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society, the Canadian Genetic Diseases Network of the National Centers of Excellence, an MRC Scientist award (J.E.D.), postdoctoral fellowships from the Deutsche Krebshilfe (U.K.), the NCIC (M.B.), and the Human Frontier Science Organization Program and the French Cancer Research Association (D.B.).
Address correspondence to John E. Dick, MD, Department of Genetics, Research Institute, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada, M5G 1X8; e-mail:dick@sickkids.on.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal