Abstract
Anti-idiotype (Id) antibodies (Abs) have been shown to be effective in treatment of B-cell lymphoma in animal models and in clinical trials. The combination of interleukin-2 (IL-2) can augment the therapeutic effect of anti-Id Abs. To further improve the power of the combined therapy, a monoclonal anti-Id Ab, S5A8, specifically recognizing a murine B-cell lymphoma 38C13, was genetically modified to contain the IL-2 domain and thus use the unique targeting ability of Abs to direct IL-2 to the tumor site. Two forms of the anti-Id–IL-2 fusion proteins were constructed: one configuration consisting of mouse-human chimeric IgG (chS5A8–IL-2) and the other containing only the variable light (VL) and variable heavy (VH) Ab domains covalently connected by a peptide linker (scFvS5A8-IL-2). Both forms of the anti-Id–IL-2 fusion proteins retained IL-2 biological activities and were equivalent in potentiating tumor cell lysis in vitro. In contrast, the antigen-binding ability of scFvS5A8–IL-2 was 30- to 40-fold lower than that of the bivalent chS5A8–IL-2. Pharmacokinetic analysis showed that scFvS5A8–IL-2 was eliminated about 20 times faster than chS5A8–IL-2. Finally, it was shown that chS5A8–IL-2 was very proficient in inhibiting 38C13 tumor growth in vivo, more effectively than a combined therapy with anti-Id Abs and IL-2, whereas scFvS5A8–IL-2 did not show any therapeutic effect. These results demonstrate that the anti-Id–IL-2 fusion protein represents a potent reagent for treatment for B-cell lymphoma and that the intact IgG fusion protein is far more effective than its single-chain counterpart.
© 1998 by The American Society of Hematology.
THE ABILITY OF monoclonal antibody (MoAb) to specifically localize in tumor tissues in vivo offers an attractive therapeutic approach for cancer therapy. However, natural effector functions of Abs such as complement activation or Ab-dependent cellular cytotoxicity (ADCC), although effective in mediating tumor cell destruction in vitro, are generally not sufficient to completely suppress tumor cell growth in vivo.1 Treatment with MoAb alone has only achieved very limited success in the clinic.1 Interleukin-2 (IL-2) is a potent biological mediator of the immune system and has demonstrated the ability to augment ADCC2 and potentiate the antitumor activity of natural killer (NK) cells3-5 in vitro. Preclinical studies showed that application of IL-2, either independently or combined with infusion of lymphokine-activated killer cells, resulted in the induction of a long-lasting antitumor response, leading to the rejection of an otherwise lethal tumor.6-8 However, clinical experiences of IL-2 therapy were not as encouraging as the preclinical studies, only producing responses in some patients with melanoma and renal cell carcinoma.9,10 The poor clinical responses were in part due to failure to achieve long-lasting therapeutic concentrations in the tumor and by the severe systemic toxicity associated with high-dose IL-2 therapy.11 12
Under physiological conditions, cytokines usually exert their effector functions in a paracrine fashion. It is generally thought that specifically targeting IL-2 to the tumor microenvironment not only would achieve better antitumor activity, but also would help alleviate IL-2 toxicity. To achieve a local concentration of IL-2 at the tumor site, IL-2 has been applied either intratumorally13 or in a form of a minipellet that slowly releases IL-2 by diffusion and dissolution.14 Both methods resulted in consistent but limited inhibition of tumor growth. Another approach to generate high concentrations of specific cytokines at the tumor site involved the transduction of tumor cells with cytokine genes.15,16 The sustained release of cytokines by such cytokine-secreting tumor cells produced dramatic local inflammatory response without significant systemic toxicity and often resulted in the ultimate destruction of the transduced tumor cells. In some cases, systemic immunity was induced against wild-type parent tumors challenged at distant locations.17,18 Fusion proteins consisting of Ab and cytokine molecules have been constructed and found to maintain both cytokine activity and antigen specificity.19-21 It is conceivable that the unique targeting ability of such Ab-cytokine fusion proteins can specifically direct biologically active cytokines to the tumor site, achieving concentrations sufficient to stimulate immune destruction of tumor cells.
A murine B-cell lymphoma, 38C13, was used as an animal model to test this hypothesis. The idiotype (Id) of the surface Ig expressed by 38C13 tumor cells can serve as a unique tumor-specific antigen. It was demonstrated that Id-specific MoAb was effective in treatment of 38C13 tumors22 and that the therapeutic efficacy was strongly dependent on the Ab isotypes, with IgG2a being most effective and IgG2b least effective.23 Treatment with monoclonal anti-Id Abs was also reported to result in tumor regression in a number of human patients with B-cell lymphoma.24,25 In a preclinical study, it was demonstrated that a combined therapy consisting of anti-Id Abs and high-dose IL-2 resulted in a dramatic improvement in the antitumor activity compared with either agent used alone.26 In this report, we investigated whether the therapeutic efficacy of the anti-Id Ab and IL-2 combined therapy could be further enhanced if IL-2 were specifically directed to the tumor site. To this end, we made two forms of anti-Id–IL-2 fusion proteins: one configuration consisting of mouse-human chimeric IgG and the other containing a smaller single-chain (scFv) Ab comprising linked variable light (VL) and variable heavy (VH) Ab domains. The in vitro characterization and in vivo therapeutic efficacy of these two forms of anti-Id–IL-2 fusion proteins were compared.
MATERIALS AND METHODS
Mice.
Female C3H/HeN mice, 6 to 8 weeks old, were purchased from National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) and housed at the Laboratory Animal Facility, Institute of Biomedical Sciences, Academia Sinica (Taipei, Taiwan).
Cell lines.
38C13 murine B-cell lymphoma is a carcinogen (DMBA)-induced tumor originally produced in a T-cell–depleted C3H/eB mouse.27V1-1 is a subclone of an Id-negative variant V1 that was derived from the original 38C13 tumor by selection with passive Ab therapy in vivo.28 A1-2 is a hybridoma cell line that secretes 38C13 Id (IgM, κ).29 S5A8 is a hybridoma-secreting MoAb (IgG2b) specifically against 38C13 Id.29 HT-2 is a murine helper T-cell line dependent on IL-2 or IL-4 for growth. Cell lines were maintained in RPMI 1640, 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μmol/L 2-ME (RPMI-10) at 37°C, 5% CO2 in a humidified incubator. HT-2 cells were grown in the above-noted medium supplemented with 1% supernatant of a transfectoma cell line producing Id–IL-2 fusion protein.30 Sf21 insect cells were cultured at 27°C in TMN-FH medium (PharMingen, San Diego, CA) containing 10% heat-inactivated FCS.
Construction and production of chimeric IgG anti-Id–IL-2 fusion protein.
The VH and VL genes of S5A8 were obtained by reverse transcription and polymerase chain reaction (PCR) amplification using upstream primers containing an Nco I site overlapping the translation start codon ATG and downstream primers containingKpn I sites located in the J segments. The NcoI-Kpn I fragments were gel-purified and replaced the corresponding VH and VL genes in the heavy-chain (p3079) and light-chain (p3077) expression vectors as previously described.31 The resulting light-chain plasmid, p301, contained the VLS5A8 joined to the human κ constant region gene (Cκ), and the heavy-chain plasmid, p302, contained the VHS5A8 joined to the human γ1 constant region gene (Cγ1). To construct the anti-Id–IL-2 fusion protein, VHS5A8 was used to replace the VH gene in plasmid p316330 to produce plasmid p119 containing the human Cγ1 gene followed by two Gly codons and the mature murine IL-2 sequence. The heavy-chain vector (p302 or p119) was then cotransfected with the light-chain vector p301 into an Ig-nonproducing plasmacytoma cell line, P3X63Ag8.653, by electroporation as described.31Transfectomas were selected by resistance to G418 and the highest producers of each construct (chS5A8, chS5A8–IL-2) were selected for protein production. To make a chain-shuffle IL-2 fusion protein (TAY–IL-2), plasmid p119 containing VHS5A8 was cotransfected with a light-chain vector, p3112, containing the VL gene from a human B-cell lymphoma (RF) joined to the human Cκ gene.32 Transfectoma clones were expanded for large-scale production in RPMI 1640 containing 1% low IgG FCS and purified by protein A chromatography. Bound Abs were eluted from the column with buffer containing 0.1 mol/L glycine, 0.15 mol/L NaCl, pH 2.4, and brought to neutral pH with 0.5 mol/L sodium phosphate, pH 8. Purified proteins were dialyzed extensively versus phosphate-buffered saline (PBS) and sterilized by filtration. The concentrations of purified Abs were determined by a bicinchoninic acid-based protein assay (Pierce, Rockford, IL).
Construction and production of scFv anti-Id–IL-2 fusion protein.
A S5A8 scFv in VL-VH orientation was assembled by overlapping PCR, as previously described,33 with the 14 amino acid 212 linker,34 GSTSGSGKSSEGKG, covalently bridging the VL and VH domains. The S5A8 scFv PCR fragment was then digested with BamHI andEcoRI and gel-purified before ligation into the polycloning region of the baculovirus expression vector pAcGP67B (PharMingen), which contains the signal peptide of the glycoprotein gp67 of the baculovirus strain AcNPV to facilitate protein secretion. The fragment containing a mature mouse IL-2 sequence was generated by PCR from plasmid pmut-1 (ATCC 37553; American Type Culture Collection, Rockville, MD). The upstream primer contained two Gly codons 3′ to theEcoRI site, and the downstream primer contained a stop codon 5′ to the Pst I site. The PCR product was then digested withEcoRI and Pst I, gel-purified, and ligated to the 3′ end of the scFv in the expression vector to produce plasmid p221. To develop the recombinant baculovirus, Sf21 cells were cotransfected with plasmid p221 and linearized BaculoGold viral DNA (PharMingen) according to the manufacturer’s instructions. Five days after transfection, supernatants containing the recombinant viruses were harvested and used to infect a monolayer of Sf21 cells seeded at 9 × 105 per well in a 6-well tissue culture plate. After 1 hour of incubation at 27°C, supernatants were removed and cells were overlaid with 1.5 mL of 0.8% L.M.P. agarose (GIBCO BRL, Gaithersburg, MD). The putative recombinant viral plaques were isolated and tested for Ab production. Clones producing the highest levels of proteins were selected, expanded to produce large amounts of scFv anti-Id–IL-2 fusion protein, and purified by affinity chromatography prepared with 38C13 Id purified as previously described.35
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analyses.
SDS-PAGE and transfer of proteins to nitrocellulose by semidry electroblotting were performed as previously described.36Blots were probed with either 38C13 Id at 1 μg/mL or rat antimouse IL-2 MoAb (JES6-1A12; IgG2a; PharMingen) at 1:500. The bound 38C13 Id and anti–IL-2 Ab were then reacted with horseradish peroxidase-conjugated goat antimouse IgM (1:1,000; Cappel, Organon Teknika, Veedijk, Belgium) and goat antirat IgG (1:1,000; Cappel), respectively. Blots were developed by the enhanced chemiluminescence Western blot detection system (Amersham, Little Chalfont, UK).
Antigen binding assay.
The Id-binding ability of the various anti-Id Abs were determined by a competitive enzyme-linked immunosorbent assay (ELISA) binding assay.37 Wells of the microtiter plates were coated with 1 μg/mL of purified 38C13 Id and blocked with 10% bovine calf serum in PBS (BCS/PBS). The unlabeled anti-Id Abs at a concentration of 50 nmol/L were applied to the first row of microtiter plates and then titered by a factor of 1:3 with 10% BCS/PBS so that 5 logs in concentration could be analyzed. The final volume was 100 μL in each well. One hundred microliters of biotinylated S5A8 was immediately added to each well to reach a final concentration of 0.1 nmol/L and incubated overnight at 4°C. The bound biotinylated S5A8 was then detected with alkaline phosphatase-conjugated streptavidin (PharMingen), developed with p-nitrophenyl phosphate (Sigma, St Louis, MO) as the substrate, and absorbance at 405 nm was measured using an ELISA plate reader. The inhibition of biotinylated S5A8 binding by each anti-Id Abs was calculated relative to the biotin binding in the absence of competing Ab. The inhibition curves were plotted and the 50% inhibition value was calculated for each tested Ab.
Fluorescence-activated cell sorting (FACS) analysis.
38C13 or V1-1 cells (1 × 106) were reacted at 4°C with 10 μg/mL of S5A8, chS5A8, chS5A8–IL-2, or scFvS5A8–IL-2. An irrelevant mouse IgG2b MoAb was included as a control. The bound S5A8 and the control mouse Ab were detected with fluorescein isothiocyanate (FITC)-labeled goat antimouse IgG Ab (1:100; Sigma). The bound chS5A8 was detected with FITC-labeled goat antihuman κ Ab (1:100; Cappel). The bound chS5A8–IL-2 and scFvS5A8–IL-2 were detected using a biotin-conjugated rat antimouse IL-2 MoAb (1 μg/mL; PharMingen) followed by FITC-labeled avidin (1:500; Cappel). Cells were then fixed in 2% paraformaldehyde/PBS for analysis on a FACS (FACSstation; Becton Dickinson, Mountain View, CA).
Cytokine proliferation assays.
The biological activity of test samples was assayed by their ability to support the proliferation of a murine IL-2/IL-4–responsive T-cell line, HT-2. Samples were added in triplicate to 96-well plates in RPMI-10 with 5 × 103 HT-2 cells to a total volume of 0.1 mL and incubated for 16 to 24 hours at 37°C, 5% CO2 in a humidified incubator. In the assays that involved membrane-bound IL-2 activity, 2 × 106 irradiated (2,000 rad) 38C13 cells were incubated with 10 μg/mL of test samples at 4°C for 30 minutes, followed by treatment with 1% paraformaldehyde/PBS for 10 minutes at room temperature (RT). The Ab-coated cells were then harvested and added to the HT-2 culture. After 16 hours, 1 μCi3H-thymidine (Amersham) in 50 μL of growth medium was added to each well, cells were harvested 4 to 6 hours later using a FilterMate (Packard, Meriden, CT) automatic cell harvester, and incorporated radioactivity was determined by a TopCount microplate scintillation counter (Packard). Recombinant murine IL-2 (PharMingen) was included in the assay as a positive control. All results are presented as the mean cpm ± standard deviation (SD).
Cytotoxicity assays.
The 125IUdR release assay38 was used to detect the cell-mediated cytotoxicity. The effector cells were prepared by incubating murine splenocytes with anti-CD3 MoAb (1:100 dilution of culture supernatants of 145-2C11 hybridoma cells) for 2 days, followed by treatment with 30 to 50 U/mL of recombinant murine IL-2 in the absence of anti-CD3. 38C13 cells were labeled with 125IUdR and incubated for 20 hours with effector cells in the presence of various anti-Id Abs. Recombinant IL-2 at doses equivalent to the fusion protein or a combination of chS5A8 and recombinant IL-2 was also included for comparison. The results are expressed as total percentage of lysis and net percentage of lysis according to the following formula:
The spontaneous release (media alone) usually ranged from 5% to 15% of the total lysis. The standard error obtained with total lysis was usually between 0.5% and 5% of the mean. One lytic unit (LU) was defined as the number of effectors required to give 30% specific lysis for 5 × 103 target cells. LU per 106 cells was calculated by plotting a curve derived from specific lysis obtained from 3 to 4 effector-to-target (E/T) ratios.
In vivo clearance studies.
Female BALB/c mice were injected intravenously (IV) via the tail vein with 25 μg of the various anti-Id Abs. For S5A8, chS5A8, and chS5A8–IL-2, serum was collected by a microcapillary tube from the tail vein at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, and 72 hours after injection. Because of the short half-life of the scFv anti-Id–IL-2 fusion protein, blood samples were collected from the orbital plexus at 5, 10, 20, 30, 40, 50, 60, 120 and 240 minutes after injection. The specific Ab concentration in each sample was determined by ELISA for anti-Id determinant. Half-life values were determined based on the two-compartment open model, as previously described.39
Tumor challenge and Ab therapy.
C3H/HeN mice received 103 38C13 tumor cells subcutaneously (SC). Treatment was started 1, 3, or 7 days after tumor inoculation by IV injection of 10 μg of various Abs over a period of 5 days, once a day. Treatment with a combination of chS5A8 and recombinant IL-2 was included as a control. Tumors were measured every other day, and the tumor volume (in cubic millimeters) was approximated by using the ellipsoidal formula: length (mm) × width (mm) × height (mm) × 0.52 (derived from π/6).40 The mean volume and SD of each group were calculated. Animals were observed until the SC tumors measured more than 3,000 mm3 or until any mouse was observed to be suffering or appeared to be moribund. Animals under these conditions were euthanized humanely, according to institutional policy. Sacrifice dates were recorded, and the mean survival of each group was calculated. The statistical significance of differential findings between experimental groups of animals was determined by the Student’s t test. Findings were regarded as significant if two-tailed P values were ≤.05.
RESULTS
Construction and characterization of anti-Id–IL-2 fusion proteins.
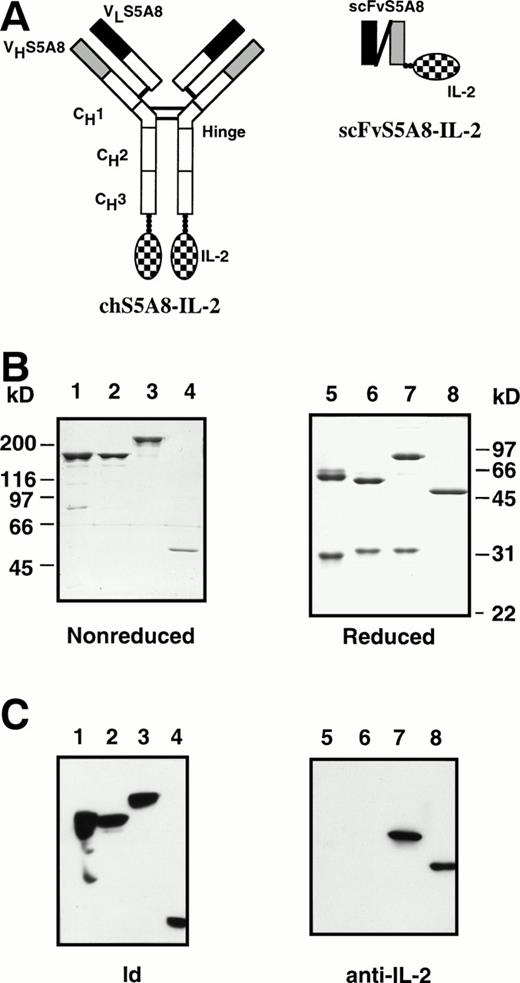
Anti-Id MoAbs have been shown to be effective in vivo in the treatment of 38C13 murine B-cell lymphoma.22,23 A combined therapy consisting of anti-Id MoAbs and IL-2 was reported to be more effective than treatment with anti-Id alone.26 However, high-dose IL-2 administered systematically usually causes severe toxicity11,12 and thus limits its clinical use. We reasoned that Ab-cytokine fusion proteins may offer an alternative approach to deliver large amounts of IL-2 to the tumor site in such a way as to minimize the toxicity. To this end, we made two forms of anti-Id–IL-2 fusion proteins: chS5A8–IL-2 consisting of a mouse-human chimeric IgG molecule with mouse IL-2 attached to each of the carboxy-terminal ends of the Ig CH3 domain, and scFvS5A8–IL-2 containing only the VL and VH domains covalently connected by a peptide linker (Fig 1A). We used human IgG1 isotype in this study, because it has been identified as the isotype of choice for building Abs with high avidity in complement fixation and ADCC.41,42 The chimeric chS5A8–IL-2 were produced from transfectoma and purified by affinity chromatography using protein A-Sepharose. The scFvS5A8–IL-2 fusion protein was produced in baculovirus-infected insect cells and purified by affinity chromatography prepared with 38C13 Id protein, as previously described.35 To serve as controls for chS5A8–IL-2, a mouse-human chimeric version of S5A8, designated chS5A8, and a chain-shuffle IgG IL-2 fusion protein, designated TAY–IL-2, were similarly constructed. The structure of TAY–IL-2 was identical to chS5A8–IL-2, except that its VL domain was derived from an unrelated Ig light chain and was expected to lose the 38C13 Id-binding activity.
Schematic diagram of the chimeric and scFv anti-Id–IL-2 fusion proteins. Solid and shaded areas represent light-chain and heavy-chain variable regions from an anti-Id mAb, S5A8. Open areas represent human γ1 and κ constant regions. Checkered regions represent the mature IL-2 sequence. (B) SDS-PAGE of S5A8 (lanes 1 and 5), chS5A8 (lanes 2 and 6), chS5A8–IL-2 (lanes 3 and 7), and scFvS5A8–IL-2 (lanes 4 and 8) under nonreducing (lanes 1 through 4) or reducing (lanes 5 through 8) conditions. The MW is determined by marker proteins. (C) Immunoblot analysis of the various anti-Id Abs. S5A8 (lanes 1 and 5), chS5A8 (lanes 2 and 6), chS5A8–IL-2 (lanes 3 and 7), and scFvS5A8–IL-2 (lanes 4 and 8) under nonreducing (lanes 1 through 4) or reducing (lanes 5 through 8) conditions were subjected to SDS-PAGE followed by electroblotting to nitrocellulose. Nitrocellulose strips were reacted with 38C13 Id (lanes 1 through 4) or rat antimouse IL-2 (lanes 5 through 8) and detected with horseradish peroxidase-conjugated second-step reagents.
Schematic diagram of the chimeric and scFv anti-Id–IL-2 fusion proteins. Solid and shaded areas represent light-chain and heavy-chain variable regions from an anti-Id mAb, S5A8. Open areas represent human γ1 and κ constant regions. Checkered regions represent the mature IL-2 sequence. (B) SDS-PAGE of S5A8 (lanes 1 and 5), chS5A8 (lanes 2 and 6), chS5A8–IL-2 (lanes 3 and 7), and scFvS5A8–IL-2 (lanes 4 and 8) under nonreducing (lanes 1 through 4) or reducing (lanes 5 through 8) conditions. The MW is determined by marker proteins. (C) Immunoblot analysis of the various anti-Id Abs. S5A8 (lanes 1 and 5), chS5A8 (lanes 2 and 6), chS5A8–IL-2 (lanes 3 and 7), and scFvS5A8–IL-2 (lanes 4 and 8) under nonreducing (lanes 1 through 4) or reducing (lanes 5 through 8) conditions were subjected to SDS-PAGE followed by electroblotting to nitrocellulose. Nitrocellulose strips were reacted with 38C13 Id (lanes 1 through 4) or rat antimouse IL-2 (lanes 5 through 8) and detected with horseradish peroxidase-conjugated second-step reagents.
The various anti-Id Abs were subjected to SDS-PAGE (Fig 1B). Under reducing conditions, the light and heavy chains of S5A8 and chS5A8 migrated at an apparent molecular weight (MW) of 29 and 31 kD and 57 and 55 kD, respectively (Fig 1B, lanes 5 and 6). Reduction of the chS5A8–IL-2 fusion protein gave a 31-kD light chain similar to that of chS5A8, but the heavy chain molecule migrated at an apparent MW of 76 kD, indicating that it contained an IL-2 tail (Fig 1B, lane 7). The heavy and light chains were properly assembled to give tetrameric proteins of MW 158 kD (S5A8 and chS5A8) and 212 kD (chS5A8–IL-2), as could be observed on a nonreducing gel (Fig 1B, lanes 1, 2, and 3). The scFvS5A8–IL-2 fusion protein was produced as a monomer with an apparent MW of 51 kD, as shown in the reducing and nonreducing gel (Fig1B, lanes 4 and 8). Immunoblot analysis showed that all S5A8-derived anti-Id Abs retained the 38C13 Id-binding activity (Fig 1C, lanes 1 through 4), but only the chS5A8–IL-2 and scFvS5A8–IL-2 fusion proteins were recognized by the specific anti–IL-2 Ab (Fig 1C, lanes 7 and 8).
Id-binding and IL-2 biological activities of anti-Id–IL-2 fusion proteins.
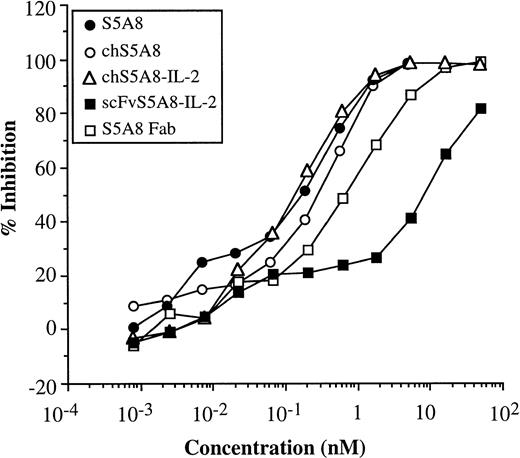
Competition assays were conducted to evaluate the immunoreactivity of purified anti-Id–IL-2 fusion proteins against 38C13 Id. Increasing concentrations of S5A8, chS5A8, and chS5A8–IL-2 and two monovalent Abs, scFvS5A8–IL-2 and S5A8 Fab fragment, were evaluated for their ability to inhibit binding of biotinylated S5A8 to 38C13 Id (Fig2). The relative Id-binding activities of S5A8, chS5A8, and chS5A8–IL-2 were comparable: maximum inhibition (100%) was achieved at 5 nmol/L, whereas 50% inhibition was at 0.2 to 0.3 nmol/L. The monovalent S5A8 Fab had about threefold lower binding activity to 38C13 Id with maximum and 50% inhibition achieved at 17 and 0.7 nmol/L, respectively. The scFvS5A8–IL-2 fusion protein had an even more significant reduction in the Id-binding activity: 100% inhibition was not achieved over the range of concentrations tested and its 50% inhibition was at 8.7 nmol/L. Thus, the relative binding ability of chS5A8–IL-2 was comparable to that of the parent S5A8 or the chimeric chS5A8 anti-Id Abs; however, the scFvS5A8–IL-2 fusion protein was about 30- to 40-fold lower in its Id-binding activity than the bivalent Abs.
Competition assays comparing the relative inhibition of binding of biotinylated S5A8 by unlabeled S5A8, chS5A8, and chS5A8–IL-2 Abs as well as two monovalent anti-Id Abs, scFvS5A8–IL-2 and S5A8 Fab fragment. Microtiter plates were coated with purified 38C13 Id. Serial dilution of each unlabeled competitor anti-Id Abs were added to each well together with a fixed amount of biotinylated S5A8 and incubated overnight at 4°C. The bound biotinylated S5A8 were then quantitated. The percentage of inhibition values represent the mean of triplicate trials. SE values were within 10% of the mean.
Competition assays comparing the relative inhibition of binding of biotinylated S5A8 by unlabeled S5A8, chS5A8, and chS5A8–IL-2 Abs as well as two monovalent anti-Id Abs, scFvS5A8–IL-2 and S5A8 Fab fragment. Microtiter plates were coated with purified 38C13 Id. Serial dilution of each unlabeled competitor anti-Id Abs were added to each well together with a fixed amount of biotinylated S5A8 and incubated overnight at 4°C. The bound biotinylated S5A8 were then quantitated. The percentage of inhibition values represent the mean of triplicate trials. SE values were within 10% of the mean.
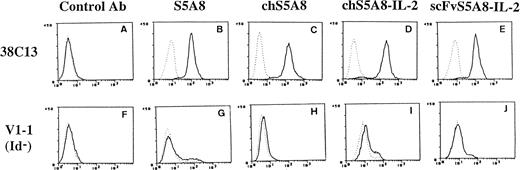
The ability of anti-Id–IL-2 fusion proteins to bind to tumor cells was examined by reacting the fusion proteins with 38C13 tumor or with V1-1, an Id-negative variant.28 Figure3 shows the result of flow cytometry analysis demonstrating that chS5A8–IL-2 and scFvS5A8–IL-2 fusion proteins recognized 38C13 tumor cells equally well as the parent S5A8 or the chimeric chS5A8 Abs (Fig 3B through E). However, none of the various anti-Id Abs reacted with the V1-1 variant (Fig 3G through J), indicating that the recognition of 38C13 cells was Id specific. Experiments using an Ig-negative variant of 38C13 have shown similar results (data not shown). Thus, both forms of the anti-Id–IL-2 fusion proteins were specific for the 38C13 Id and were capable of binding to the tumor cells. We also showed that the chain-shuffle TAY–IL-2 fusion protein, containing the S5A8 VH and a nonspecific VL, did not bind to 38C13 tumor cells (data not shown), indicating that both the heavy and light chains of S5A8 made a contribution to the Id-binding activity.
FACS analysis of anti-Id–IL-2 fusion proteins. A control MoAb (A and F), S5A8 (B and G), chS5A8 (C and H), chS5A8–IL-2 (D and I), or scFvS5A8–IL-2 (E and J) was reacted with 38C13 cells (A through E) or V1-1, an Id-negative variant of 38C13 (F through J). The bound anti-Id Abs were detected with second-step FITC-conjugated Abs or with second-step biotin-labeled Abs followed by FITC-labeled avidin, as detailed in the text. Dashed lines represent fluorescence from cells without addition of the tested anti-Id Abs.
FACS analysis of anti-Id–IL-2 fusion proteins. A control MoAb (A and F), S5A8 (B and G), chS5A8 (C and H), chS5A8–IL-2 (D and I), or scFvS5A8–IL-2 (E and J) was reacted with 38C13 cells (A through E) or V1-1, an Id-negative variant of 38C13 (F through J). The bound anti-Id Abs were detected with second-step FITC-conjugated Abs or with second-step biotin-labeled Abs followed by FITC-labeled avidin, as detailed in the text. Dashed lines represent fluorescence from cells without addition of the tested anti-Id Abs.
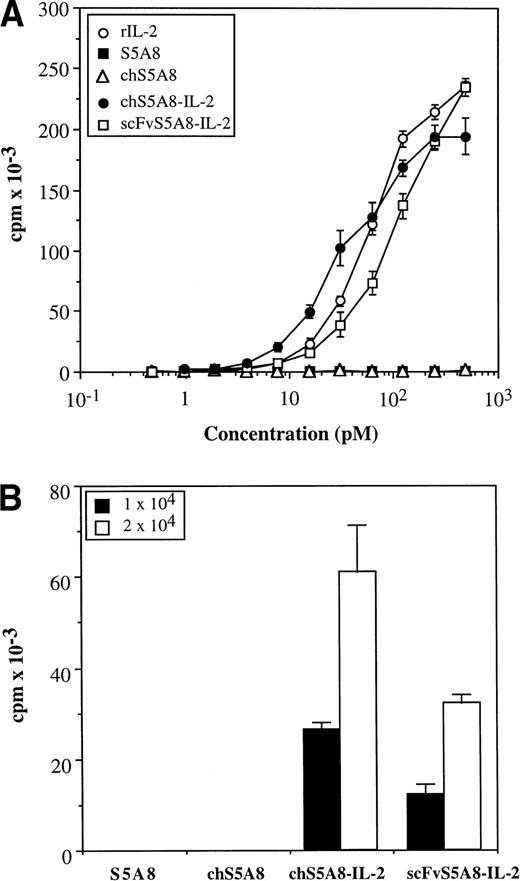
To determine IL-2 activity of the fusion proteins, purified proteins were analyzed for their ability to support the proliferation of an IL-2–dependent T-cell line, HT-2. The free anti-Id Abs, S5A8 and chS5A8, were completely negative in this assay. In contrast, both anti-Id–IL-2 fusion proteins clearly demonstrated the ability to stimulate the growth of HT-2 cells in a dose-dependent manner (Fig4A). On a molar basis, chS5A8–IL-2 and scFvS5A8–IL-2 had approximately 200% and 50%, respectively, of the activity required to produce 50% maximum proliferation of HT-2 cells compared with the recombinant IL-2 standard. The chain-shuffle TAY–IL-2 fusion protein, although having lost Id-binding ability, exhibited IL-2 activities similar to the Id-specific chS5A8–IL-2 fusion protein (data not shown).
IL-2 bioactivity of anti-Id–IL-2 fusion proteins. (A) HT-2 cells were incubated with various concentrations of S5A8, chS5A8, chS5A8–IL-2, scFv5A8–IL-2 or recombinant mouse IL-2. (B) Irradiated 38C13 cells were incubated with 10 μg/mL of various anti-Id Abs and fixed with paraformaldehyde. HT-2 cells were then incubated with 1 × 104 or 2 × 104 Ab-coated cells. Proliferation was measured by 3H-thymidine uptake 16 to 24 hours later. All results are expressed as the mean cpm incorporated ± SD of triplicate wells.
IL-2 bioactivity of anti-Id–IL-2 fusion proteins. (A) HT-2 cells were incubated with various concentrations of S5A8, chS5A8, chS5A8–IL-2, scFv5A8–IL-2 or recombinant mouse IL-2. (B) Irradiated 38C13 cells were incubated with 10 μg/mL of various anti-Id Abs and fixed with paraformaldehyde. HT-2 cells were then incubated with 1 × 104 or 2 × 104 Ab-coated cells. Proliferation was measured by 3H-thymidine uptake 16 to 24 hours later. All results are expressed as the mean cpm incorporated ± SD of triplicate wells.
The activity of the fusion proteins could also be inhibited by the anti–IL-2 Ab, indicating that the cytokine activity was specific (data not shown). Both forms of the anti-Id–IL-2 fusion proteins were further tested for functional duality by binding to irradiated 38C13 tumor cells and then using the Ab-coated tumor cells to stimulate the proliferation of HT-2 cells. The results shown in Fig 4B clearly demonstrated that the tumor cell-bound chS5A8–IL-2 and scFvS5A8–IL-2 efficiently stimulated the growth of HT-2 cells. In contrast, S5A8- or chS5A8-coated tumor cells did not cause any significant proliferation of HT-2 cells.
Pharmacokinetic analysis of anti-Id–IL-2 fusion proteins.
The pharmacokinetics of the various anti-Id Abs were examined in normal BALB/c mice after IV injection. Sera were collected at various time points after injection, and the specific Ab concentration in each sample was determined by ELISA for the anti-Id determinant. S5A8 and the mouse-human chimeric chS5A8 had similar clearance rates. The α phase (distribution of the protein from the blood to extravascular space) half-life of S5A8 was 5.6 hours and the β phase (elimination of the protein from the body) half-life was 80.2 hours, whereas α phase and β phase half-lives of chS5A8 were 3.2 and 90.5 hours, respectively (Table 1). The chS5A8–IL-2 fusion protein was more rapidly cleared from the circulation compared with the free anti-Id Abs. This was mainly due to the rapid clearance rate of the β phase (17.6 hours) of chS5A8–IL-2, because its α phase half-life (2.4 hours) was only slightly different from that of the free Abs. The scFvS5A8–IL-2 fusion protein was most rapidly cleared from the circulation among the anti-Id Abs tested, with an α phase half-life of only 5 minutes and a β phase half-life of 49 minutes.
Blood Clearance Half-Life of the Free Anti-Id Abs and the Anti-Id–IL-2 Fusion Proteins
| Ab . | t1/2 . | |
|---|---|---|
| α . | β . | |
| S5A8 | 5.6 h | 80.2 h |
| chS5A8 | 3.2 h | 90.5 h |
| chS5A8–IL-2 | 2.4 h | 17.6 h |
| scFvS5A8–IL-2 | 5 min | 49 min |
| Ab . | t1/2 . | |
|---|---|---|
| α . | β . | |
| S5A8 | 5.6 h | 80.2 h |
| chS5A8 | 3.2 h | 90.5 h |
| chS5A8–IL-2 | 2.4 h | 17.6 h |
| scFvS5A8–IL-2 | 5 min | 49 min |
BALB/c mice were injected IV with 25 μg of the various anti-Id Abs and blood samples were collected at various time points after injection. The specific Ab concentration in each sample was determined by ELISA for anti-Id determinant. The α phase (distribution) and the β phase (elimination) half-life values were determined as described in Materials and Methods.
Cell-mediated cytotoxicity is enhanced by anti-Id–IL-2 fusion proteins.
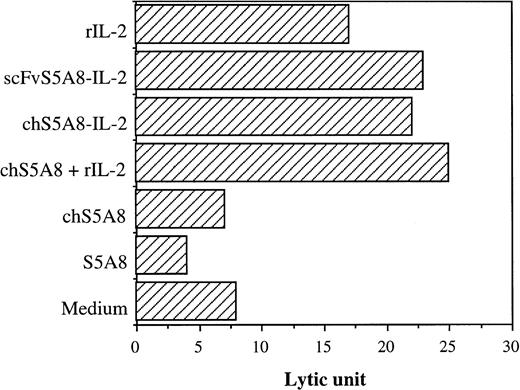
The various S5A8-derived anti-Id Abs were evaluated for their ability to mediate cytolysis of 38C13 target cells using 125IUdR release assay.38 Spleen cells stimulated with anti-CD3 Ab and recombinant IL-2 were used as a source of effector cells. The cytotoxicity was expressed in LU, which was defined as the number of effectors required to give 30% specific lysis for 5 × 103 target cells. At a concentration of 100 ng/mL, S5A8 and chS5A8 did not increase specific lysis of target cells compared with the medium alone control (Fig 5). In contrast, the lytic potential of CD3-activated blasts was significantly enhanced by the addition of either chS5A8–IL-2 or scFvS5A8–IL-2 fusion proteins, which was equally as effective as an equivalent amount of chS5A8 and recombinant IL-2 in potentiating tumor cell lysis. When the CD3-activated blasts were incubated with IL-2 alone, the specific lysis of tumor cells was also enhanced, indicating that the increased cytotoxicity mediated by chS5A8–IL-2 and scFvS5A8–IL-2 was mainly due to the IL-2 biological activity of the fusion proteins. The enhanced tumor cell lysis by the anti-Id–IL-2 fusion proteins was only observed with CD3- and IL-2 activated blasts but not with splenocytes without previous activation (data not shown).
Effect of anti-Id–IL-2 fusion proteins on cell-mediated cytolytic activity. CD3-activated blasts were incubated with125IUdR-labeled 38C13 cells with tested Abs for 20 hours. Values of specific lysis are represented in LU.
Effect of anti-Id–IL-2 fusion proteins on cell-mediated cytolytic activity. CD3-activated blasts were incubated with125IUdR-labeled 38C13 cells with tested Abs for 20 hours. Values of specific lysis are represented in LU.
Antitumor activity of anti-Id–IL-2 fusion proteins.
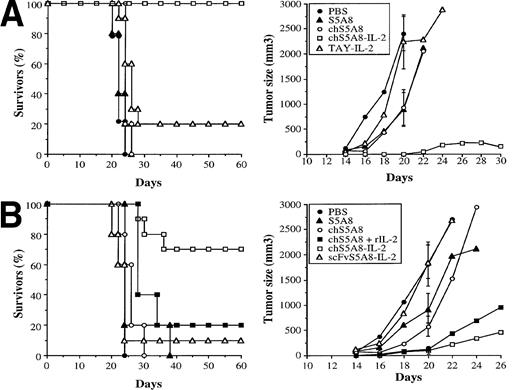
To determine the in vivo antitumor activity of the anti-Id–IL-2 fusion proteins, syngeneic animals were inoculated SC with 1 × 103 38C13 tumor cells and treated beginning on the following day by IV administration of 10 μg per day of S5A8, chS5A8, or chS5A8–IL-2 over a period of 5 days. Mice treated with PBS alone or the nonspecific TAY–IL-2 fusion protein were included as controls. The percentage of survivors and the tumor volume progression curves are shown in Fig 6A. Compared with mice receiving PBS alone, treatment with S5A8 or chS5A8 anti-Id Abs led to tumor suppression to some extent; however, no long-term survivors were observed. Treatment with the nonspecific TAY–IL-2 fusion protein did not show any inhibition of tumor growth and resulted in only 20% long-term survivors (P > .05), which was not statistically different from PBS controls. In contrast, the specific chS5A8–IL-2 fusion protein produced 100% long-term survivors (P < .0001). One of the 10 mice initially exhibited a small tumor nodule that eventually regressed. In a separate experiment, the chimeric IgG and the scFv anti-Id–IL-2 fusion proteins were compared for their in vivo antitumor activity. As shown in Fig 6B, chS5A8–IL-2 significantly suppressed tumor growth and resulted in 70% long-term survivors (P < .001). Treatment with the scFvS5A8–IL-2 fusion protein did not show any inhibition of tumor growth and led to only 10% long-term survivors (P > .1). The chS5A8–IL-2 fusion protein also proved to be more proficient than a combined therapy with equivalent doses of chS5A8 and recombinant IL-2. The combined therapy was effective in delaying tumor growth but resulted in only 20% long-term survivors (P > .05).
Effect of anti-Id–IL-2 therapy on the in vivo growth of 38C13 tumors. (A) and (B) represent data of two independent experiments. Syngeneic mice C3H/HeN (n = 10) were inoculated SC with 1 × 103 tumor cells and then treated on the following day by IV injection of various Abs over a period of 5 days. Tumor growth was measured 3 times a week. The percentage of tumor-free animals and the mean tumor volume were calculated. SDs (bars) are only given at day 20 for clarity.
Effect of anti-Id–IL-2 therapy on the in vivo growth of 38C13 tumors. (A) and (B) represent data of two independent experiments. Syngeneic mice C3H/HeN (n = 10) were inoculated SC with 1 × 103 tumor cells and then treated on the following day by IV injection of various Abs over a period of 5 days. Tumor growth was measured 3 times a week. The percentage of tumor-free animals and the mean tumor volume were calculated. SDs (bars) are only given at day 20 for clarity.
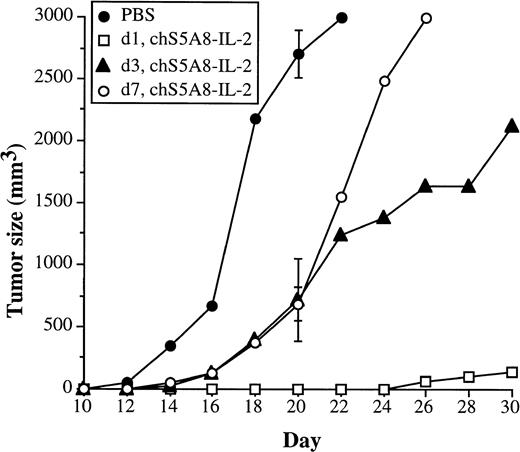
We then performed a more stringent experiment with more established tumors to assess the power of the anti-Id–IL-2 fusion protein in the treatment of B-cell lymphoma. Syngeneic animals were similarly implanted with 38C13 tumor cells and treatment was started on days 1, 3, or 7 after tumor cell inoculation. Compared with the PBS control group, objective tumor growth suppression could be observed in all treated groups (Fig 7). By day 20, the last day before the control group required sacrifice, mice treated with chS5A8–IL-2 on days 3 and 7 after tumor cell inoculation had smaller mean tumor volumes (716 ± 332 mm3 and 753 ± 310 mm3, respectively), as compared with 2,706 ± 189 mm3 in the control group. On day 20, the day-1–treated group had no measurable tumors in any of the mice. There were no long-term survivors in the control group as well as the day-3– and day-7–treated group, whereas mice treated with chS5A8–IL-2 on day 1 resulted in 80% long-term survivors (data not shown).
Effect of anti-Id–IL-2 therapy on animals with more established tumors. Syngeneic mice C3H/HeN (n = 10) were inoculated SC with 1 × 103 tumor cells. Treatment with the chS5A8–IL-2 fusion protein was started 1, 3, or 7 days after tumor inoculation. Animals treated with PBS on day 1 were included as controls. The mean tumor volume of each group was calculated. SDs (bars) are only given at day 20 for clarity.
Effect of anti-Id–IL-2 therapy on animals with more established tumors. Syngeneic mice C3H/HeN (n = 10) were inoculated SC with 1 × 103 tumor cells. Treatment with the chS5A8–IL-2 fusion protein was started 1, 3, or 7 days after tumor inoculation. Animals treated with PBS on day 1 were included as controls. The mean tumor volume of each group was calculated. SDs (bars) are only given at day 20 for clarity.
DISCUSSION
The use of cytokines as adjuvants has been applied in different forms of cancer therapy. Many attempts have been made to target cytokines to the tumor site to achieve their optimal biological effects. These include direct intratumor injection of cytokines13,43 or ex vivo transfection of different cytokine genes into tumor cells for use as cancer vaccines.15,16 Cytokine genes, including IL-2, IL-6, and interferon-γ, have also been delivered intratumorally using a particle-mediated gene gun technology and resulted in significant antitumor effects.44 One of us (M.-H.T.), together with others, has previously reported that, by fusing a cytokine (granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-2 or IL-4) to a nonimmunogenic tumor Id protein, it can be converted to a strong immunogen capable of inducing Id-specific Abs without other carrier proteins or adjuvants and of protecting recipient animals from challenge with an otherwise lethal dose of tumor cells.30 31
In this study, we made anti-Id–IL-2 fusion proteins to use the unique targeting ability of the anti-Id Ab to direct IL-2 to the tumor site to locally boost the host immune response. Two forms of the anti-Id–IL-2 fusion proteins were constructed: an intact IgG fusion protein (chS5A8–IL-2) and an scFv fusion protein (scFvS5A8–IL-2). A monovalent scFv Ab approaches the minimum necessary structural component for antigen binding. Compared with the whole Ab molecule, a smaller scFv showed deeper penetration into tumors, faster blood clearance, and a higher ratio of tumor/normal tissues.45 46In addition, the monovalent structure of scFv Ab might have the advantage of avoiding antigen modulation. SDS-PAGE analysis showed that chS5A8–IL-2 was assembled into a complete Ab structure with MW of 212 kD (Fig 1). The scFvS5A8–IL-2 fusion protein was displayed as a single band of 51 kD on SDS-PAGE (Fig 1), comprising only one fourth the MW of chS5A8–IL-2. Both forms of the anti-Id–IL-2 fusion proteins retained specific recognition for the 38C13 Id and were able to bind to 38C13 but not to the Id-negative variant tumors (Fig 3).
We compared the in vivo antitumor activity of the chimeric IgG and the scFv anti-Id–IL-2 fusion proteins in syngeneic animals under conditions in which free anti-Id Abs were only minimally effective. We showed that the chS5A8–IL-2 fusion protein can effectively suppress in vivo growth of 38C13 tumor cells inoculated 1 day before treatment (Fig6A and B). The majority of treated animals survived more than 6 months and were apparently free of residual or dormant tumor cells, as demonstrated by in vitro culture and in vivo transfer of splenocytes (data not shown). For animals with more established tumors, treatment with the chS5A8–IL-2 fusion protein was less effective, resulting in transient inhibition of tumor growth but no long-term survivors (Fig7). We also demonstrated that chS5A8–IL-2 was more proficient in suppressing tumor growth than a combined therapy with equivalent doses of free anti-Id Ab and IL-2 (Fig 6B). Treatment with a structurally similar but nonspecific TAY–IL-2 fusion protein did not show any therapeutic effect (Fig 6A), indicating that the tumor-targeting ability of chS5A8–IL-2 was important for the observed antitumor effects. In contrast to the impressive antitumor effects accomplished by chS5A8–IL-2, treatment with scFvS5A8–IL-2 offered no protection against 38C13 challenge.
Compared with the chimeric anti-Id–IL-2 fusion protein, scFvS5A8–IL-2 was fourfold less active on a molar basis, as evidenced in a biological assay using the IL-2–dependent cell line HT-2 (Fig 4A). Tumor cells coated with the scFvS5A8–IL-2 fusion protein were capable of stimulating T-cell growth (Fig 4B), demonstrating the ability of scFvS5A8–IL-2 to activate host immunologic effector mechanisms in the tumor area. In vitro cytolytic analysis also showed that the scFvS5A8–IL-2 fusion protein was equally as effective as chS5A8–IL-2 in potentiating tumor cell lysis by CD3-activated blasts. Other scFv Ab–IL-2 fusion proteins previously reported were also shown to maintain the IL-2 biological activity.47 48
Except for a slight variation in IL-2 activity, there were more profound distinctions between scFvS5A8–IL-2 and chS5A8–IL-2 that may explain their dramatically different antitumor effects. First of all, scFvS5A8–IL-2 was 30- to 40-fold lower in its Id-binding activity than its bivalent counterpart (Fig 2). The inferior binding activity of scFvS5A8–IL-2 was not only due to its monovalent structure but also due to its decreased Id-binding affinity. This was apparent when compared with the chemically prepared monovalent S5A8 Fab. Calculations based on concentration required for 50% inhibition in a competition assay demonstrated that S5A8 Fab was about 12-fold better in its Id-binding activity than the scFvS5A8–IL-2 fusion protein. The decreased Id-binding affinity of scFvS5A8–IL-2 was most likely due to the presence of the carboxy terminal IL-2 molecule that was separated from the VL-VH by a short 4-amino-acid linker and might thus interfere with the correct folding of the Ab domains. Another feature to distinguish the chimeric IgG and scFv anti-Id–IL-2 fusion proteins is their pharmacokinetic properties. The scFvS5A8–IL-2 fusion protein exhibited a very rapid plasma clearance in mice, with an α (distribution) phase half-life of 5 minutes and a β (elimination) phase half-life of 49 minutes. In contrast, chS5A8–IL-2 had pharmacokinetic properties that were greatly improved over that of scFvS5A8–IL-2, with an α phase half-life of 2.4 hours and a β phase half-life of 17.6 hours. The greater serum half-life of the chS5A8–IL-2 fusion protein compared with that of the scFv construct would be expected to help generate a higher concentration of IL-2 in tumor microenvironment. Finally, the chS5A8–IL-2 fusion protein was constructed to contain the human IgG1 isotype that was proved to be effective in mediating complement-dependent hemolysis and ADCC.41 In contrast, the scFvS5A8–IL-2 fusion protein would not be expected to possess any of these effector functions, because it does not contain the Ig constant domains. A previous study using the same 38C13 tumor model demonstrated that the synergistic effect of IL-2 in monoclonal anti-Id therapy might be due to the activation of asialo-GM1–positive NK cells.26 We also showed that a population of asialo-GM1–positive NK cells is induced in the peripheral blood after administration of chS5A8–IL-2 (Liu et al, manuscript in preparation). NK cells express both Fcγ receptors and the intermediate-affinity IL-2 receptors.49,50 Another cell population that might be activated by Ab–IL-2 fusion proteins is T lymphocytes that also express IL-2 receptors but lack Fcγ receptors. It has been previously reported that Ab–IL-2 fusion proteins can enhance in vitro tumor cell killing by activated T cells51 or by a tumor-infiltrating lymphocyte line.20 We are currently working to dissect the possible cellular mechanisms involved in the antitumor response of the chS5A8–IL-2 fusion protein.
Several groups have also reported the construction of Ab-cytokine fusion proteins and their application in preclinical cancer therapy. In one study, the Ab–IL-2 fusion proteins were shown to be able to completely inhibit the growth of hepatic and pulmonary metastases of human neuroblastoma and melanoma in SCID mice previously reconstituted with human lymphokine-activated killer cells, whereas treatment with combinations of the corresponding Abs and IL-2 achieved only minimal antitumor effect.52,53 The efficacy of Ab-targeted IL-2 therapy has also been demonstrated in a syngeneic murine melanoma model.54,55 Interestingly, the effector cell population responsible for the observed antitumor responses was identified as CD8+ T cells but not NK cells. Another interesting feature of Ab–IL-2 fusion proteins that may help in cancer treatment is their ability to increase tumor vascular permeability and enhance Ab uptake.56 Lymphotoxin (LT) and GM-CSF were also reported to be constructed as Ab-cytokine fusion proteins. The Ab–GM-CSF fusion protein was able to enhance ADCC against its target tumors,57 whereas the Ab-LT fusion protein has a direct cytotoxic effect on the tumor cells via the induction of apoptosis.58 In vivo animal studies have demonstrated that the Ab-LT fusion protein can effectively inhibit growth of disseminated melanoma metastases by mechanisms that involve B cells and asialo-GM1–positive NK cells.58
In summary, we show that a chimeric IgG anti-Id–IL-2 fusion protein is more effective for the treatment for B-cell lymphoma than anti-Id Ab alone or a combination of anti-Id Ab and IL-2, whereas the scFv anti-Id–IL-2 fusion protein offered no protection against tumor challenge. We believe that the general approach of fusing a cytokine to monoclonal anti-Id Abs may be applicable to generate immunotherapeutic reagents for the treatment of B-cell malignancies.
Supported by Grants No. NSC 83-0203-B-001-102 and 84-2331-B-001-031 from the National Science Council, Taiwan, ROC.
Address reprint requests to Mi-Hua Tao, PhD, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan 11529; e-mail:bmtao@ccvax.sinica.edu.tw.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal