Abstract
Mobilized CD34+ cells from human peripheral blood (PB) are increasingly used for hematopoietic stem-cell transplantation. However, the mechanisms involved in the mobilization of human hematopoietic stem and progenitor cells are largely unknown. To study the mobilization of human progenitor cells in an experimental animal model in response to different treatment regimens, we injected intravenously a total of 92 immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice with various numbers of granulocyte colony-stimulating factor (G-CSF) –mobilized CD34+ PB cells (ranging from 2 to 50 × 106cells per animal). Engraftment of human cells was detectable for up to 6.5 months after transplantation and, depending on the number of cells injected, reached as high as 96% in the bone marrow (BM), displaying an organ-specific maturation pattern of T- and B-lymphoid and myeloid cells. Among the different mobilization regimens tested, human clonogenic cells could be mobilized from the BM into the PB (P= .019) with a high or low dose of human G-CSF, alone or in combination with human stem-cell factor (SCF), with an average increase of 4.6-fold over control. Therefore, xenotransplantation of human cells in NOD/SCID mice will provide a basis to further study the mechanisms of mobilization and the biology of the mobilized primitive human hematopoietic cell.
MOBILIZED HUMAN peripheral blood (PB) CD34+ cells are increasingly used in clinical protocols as a source of hematopoietic stem cells for allogeneic and autologous transplantation.1, 2 More recently, this alternative source of stem cells is being used as a target for genetic modification in somatic cell gene therapy trials.3 Compared with bone marrow (BM), the use of PB stem cells in patients undergoing autologous transplantation has resulted in a significantly accelerated engraftment.4-7 However, the mechanisms involved in the mobilization of these cells, and the quality and biological characteristics of the stem-cell subsets in the PB products are largely unknown.8 Other clinically important issues include the safety of the mobilizing regimens, the timing of mobilization and collection, the amount of stem and progenitor cells that can be harvested, and, ultimately, the adequacy of the product with respect to the speed, level, and stability of engraftment. Moreover, with the emergence of new candidate molecules for the mobilization of primitive hematopoietic cells, such as interleukin-8, macrophage inhibitory protein (MIP)-1α, and Flt3 ligand (as recently reviewed by To et al8), there is a great need for an animal model in which the mobilization of human cells can be studied in vivo.
Mouse hematopoietic stem cells have functionally been defined as cells that have the ability to engraft upon transplantation, proliferate, and sustain multilineage hematopoiesis in vivo for an extended period. In contrast, primitive human hematopoietic cells have only been systematically studied using clonogenic assays or long-term BM cultures.9,10 These assays have proven to be valuable in estimating the quality and frequency of human hematopoietic cells with extensive proliferative capacity. However, evidence from gene-marking studies in human clinical trials suggests that primitive hematopoietic cells cannot be adequately studied using in vitro assays, as no correlation could be found between the expression of transgenes in vitro and after transplantation in vivo.11-14 Similarly, when comparing retroviral gene transfer in human long-term culture-initiating cells (LTC-IC) and cells that are capable of engrafting immunodeficient mice, LTC-IC but not in vivo repopulating cells could be readily transduced.15 Although some investigators have reported significant overlap between LTC-IC and cells that give rise to engraftment in immunodeficient mice,16 others have described these subsets to be biologically distinct; differing in frequency,17phenotype,18 and the ability to be maintained on human BM stromal cells in vitro.19
To study the engraftment and differentiation of primitive human hematopoietic cells in vivo, various models of immunodeficient mice have been developed (see Wermann et al20 for a recent review). These models include the classical C.B-17 scid/scid severe combined immunodeficiency (SCID) mouse,21 the beige athymic nude X-linked (bnx) immunodeficient mouse,22 the humanized SCID (SCID-hu) mouse, in which human (fetal) hematopoietic tissues such as liver, thymus, and bone fragments, are surgically transplanted,23-25 and a transgenic SCID mouse that expresses the genes for human IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and stem-cell factor (SCF).26 Recently, a new mouse strain (NOD/LtSz-scid/scid, hereafter referred to as NOD/SCID) was developed by crossing SCID mice with nonobese diabetic (NOD/Lt) mice.27 It has been reported that the engraftment level of human cells in the NOD/SCID model, after transplantation of splenocytes,28 PB blood mononuclear cells,29BM, or cord blood cells,15,26,27 30-32 was fivefold to 10-fold higher than could be achieved in the classical SCID mouse.
In the present paper we used the NOD/SCID mouse to provide a detailed study of the engraftment and maturation of human mobilized PB CD34+ cells in the mouse and to investigate whether engrafted animals can be used as a model to study the mobilization of human hematopoietic progenitor cells in vivo. Our results show that NOD/SCID mice can be highly engrafted by PB CD34+ cells with differentiation along multiple lineages in a microenvironment-specific fashion, without supplemental human cytokines. In addition, using various regimens that have been previously shown to induce the mobilization of clonogenic cells in humans or primates, our data show for the first time that human progenitor cells can be mobilized from the BM of the NOD/SCID transplanted with PB stem cells by treatment of the animals for 4 to 6 days with G-CSF, or G-CSF and SCF. This model will enable future studies directed at investigating the biological mechanisms that modulate the mobilization of human progenitor cells in vivo.
MATERIALS AND METHODS
Animals.
A breeding colony of NOD/LtSz-scid/scid (NOD/SCID) mice27 was established at the Laboratory Animal Research Center at the Indiana University School of Medicine (Indianapolis, IN) from breeding pairs kindly provided by Dr Leonard D. Shultz (The Jackson Laboratory, Bar Harbor, ME). Animals were housed in a positive airflow ventilated rack (Lab Products, Maywood, NJ) and bred and maintained in microisolators under specific pathogen-free conditions. Animals were tested for the absence of mouse (CD4 + CD3+) T cells in the PB. Typically, T cells were undetectable (<1%, in lymphocyte light-scatter gate) in 50% to 60% of the animals (12-16 weeks of age). About 30% to 40% of the mice had a low level of circulating T cells (1%-5%), whereas 10% of the mice contained 14% ± 6% T cells. Before transplantation, 10- to 15-week-old male or female NOD/SCID mice received a sublethal dose of 300 cGy total-body irradiation (TBI) at 86 cGy/minute using a GammaCell 40 (Nordion International Inc, Ontario, Canada) equipped with two opposing 137Cesium sources. Radiation-associated mortality was not observed at this dose. In one specific experiment, animals were splenectomized 4 weeks before transplantation. All animal experiments were performed in accordance with institutional guidelines approved by the Animal Care Committee of the Indiana University School of Medicine.
Transplantation of human cells.
Using protocols approved by the Institutional Review Board of the Indiana University School of Medicine, healthy adult human volunteers were treated for 5 days with human granulocyte colony-stimulating factor (hG-CSF) (Filgrastim, Neupogen; Amgen, Thousand Oaks, CA; 10 μg/kg/d, subcutaneously). White blood cells were collected by apheresis and CD34+ cells were isolated by immunomagnetic methods using the Isolex 300i cell selection device (Baxter Immunotherapy, Irvine, CA). For these experiments, cell selection kits and disposables were kindly provided by Baxter Immunotherapy. The average purity of the CD34+ cells was 89% ± 7% with an overall recovery of 53% ± 19% from a total of 18 apheresis products containing 3.9 ± 1.7 × 1010nucleated cells (mean ± standard deviation [SD]). After separation, CD34+ cells were washed twice and injected into the lateral tail vein of preirradiated NOD/SCID mice at 2 to 50 × 106 cells/animal in 0.5 mL Hanks' Balanced Salt Solution (HBSS), 25 mmol/L HEPES, 20 U/mL heparin. In one specific experiment CD34− cells were collected, irradiated with 20 Gy, depleted of adherent cells by plastic-adherence for 30 minutes at 37°C, and injected intravenously (25 × 106cells/animal) 4 hours before injection with CD34+ cells.
Flow cytometric analysis.
At 6 to 8 weeks after transplantation, tissues were harvested for analysis. Blood (0.5 mL-1.0 mL) was collected from the subclavian vessels after the animals were anaesthetized with tri-bromo-ethyl alcohol (Sigma, St Louis, MO) and injected intravenously with 20 U of heparin sodium (Fujisawa USA, Deerfield, IL). Erythrocytes were depleted from the blood by a 2-minute incubation in 155 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA at 4°C. In addition, single-cell suspensions were prepared from the BM, spleen, and thymus. For flow cytometric analysis, cells were preincubated for 30 minutes at 4°C in phosphate-buffered saline (PBS) containing 0.1% (wt/vol) bovine serum albumin (BSA), 10% mouse serum (Caltag, South San Francisco, CA), and 10% rat serum (Caltag). Cells were then incubated with monoclonal antibodies (MoAbs) [specific or isotype controls] conjugated to either fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PerCP (at 0.5 μg/0.5-1.0 × 106 cells) for 30 minutes at 4°C, washed and analyzed for three-color fluorescence on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Routinely, 40,000 events per sample were collected. The lack of cross-reactivity of human-specific antibodies with mouse cells was confirmed in every experiment by staining BM cells from a nontransplanted irradiated control animal. In addition, every analysis included isotype control antibodies to assess the level of background fluorescence. Engraftment of human cells was defined by the presence of at least 1% nucleated cells showing expression of CD45 over the background. FITC- and PE-conjugated mouse (IgG1 and IgG2b) and rat (IgG2a) isotype control antibodies were purchased from Caltag, and PerCP-conjugated mouse IgG1 was obtained from Becton Dickinson. The IgG2a rat anti-mouse CD18 (mCD18)-FITC antibody was purchased from Caltag. All other antibodies were specific for human cell-surface antigens. CD10-FITC, CD19-PE, and CD38-PE (all IgG1 isotype) were purchased from Caltag; anti-human IgM-FITC (IgG1) and HLA-DR-FITC (IgG2b) were purchased from Pharmingen (San Diego, CA); goat anti-human IgD-PE was purchased from Southern Biotechnology (Birmingham, AL); Kappa-FITC and Lambda-FITC were bought from Kallestad (Redmond, WA); CD11b-FITC (IgG2b), CD16-FITC, and Glycophorin A (Gly A)-PE (both IgG1) were purchased from Immunotech (Westbrook, ME); and CD34-FITC, CD8-FITC, CD4-PE, CD7-PE, CD33-PE, CD20-PerCP, CD3-PerCP, CD45-PerCP (all IgG1 isotype), were bought from Becton Dickinson.
Mobilization.
In six individual experiments, groups of animals were treated with the following mobilizing regimen: Cyclophosphamide (Cytoxan; Mead Johnson, Princeton, NJ) at 200 mg/kg intraperitoneally followed 2 days later by 4 days of hG-CSF (Filgrastim Neupogen, Amgen) at 250 μg/kg/d subcutaneously33; hG-CSF at 25 μg/kg/d subcutaneously or 250 μg/kg/d subcutaneously for 4 days34; or hG-CSF at 25 μg/kg/d subcutaneously or 250 μg/kg/d subbcutaneously together with pegylated-human stem-cell factor (PEG-hSCF; Amgen) at 20 μg/kg/d subcutaneously for 4 to 6 days.34 35 Animals were individually weighed before injection. Cyclophosphamide was diluted in PBS and administered once intraperitoneally, PEG-hSCF was administered once-a-day subcutaneously in HBSS containing 25 mmol/L HEPES and 0.1% BSA, and hG-CSF was admistered twice-a-day subcutaneously in the morning and evening diluted in 0.9% NaCl with 5% fetal calf serum (FCS) at pH 4.5 (to ensure stability of the protein). For analysis, tissues and PB were harvested the day after the last injection.
Cultures.
PB and BM from transplanted animals were tested for the presence of human progenitor cells in semi-solid tissue culture medium consisting of Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY), 25% FCS, 10% human plasma, 2% Pen/Strep, 5 × 10-5 mol/L β-mercapto-ethanol, 11 ng/mL human IL-3 (Peprotech, Rocky Hill, NJ), 100 ng/mL hSCF (Amgen), 4 U/mL human erythropoietin (Amgen), and 0.8% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada). Triplicate plates with 3 × 104 to 3 × 105 cells/dish were cultured for 14 days at 37°C in a humidified environment with 5% CO2. The number of colonies was determined by microscope.
Histology.
In selected experiments, tissues from transplanted animals and controls were fixed and kept in 8% formaldehyde, 15% (wt/vol) sucrose in PBS. For histopathological examination, mouse humeri were decalcified, embedded in paraffin, and sectioned. The slides were stained with hematoxylin and eosin using standard procedures.
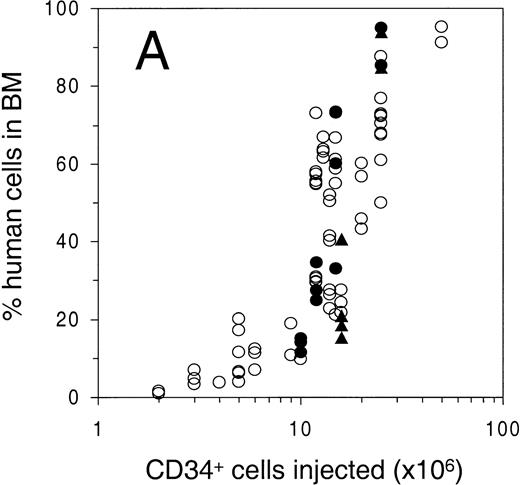
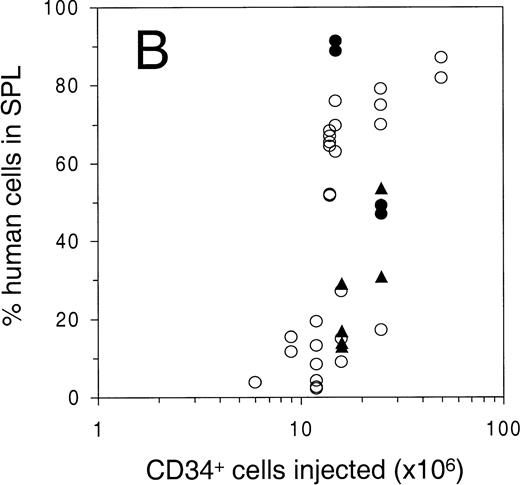
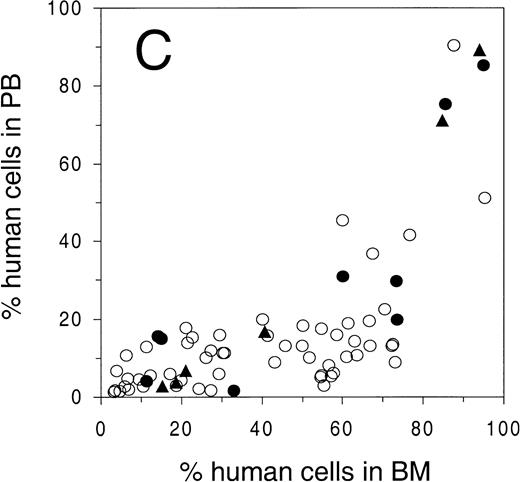
Engraftment of G-CSF–mobilized human peripheral blood CD34+ cells in sublethally irradiated NOD/SCID mice. The percentage of human cells in (A) BM, (B) spleen, and (C) PB was determined by flow cytometry at 6 to 8 weeks after transplantation. Open circles represent untreated animals, closed circles represent animals treated with human G-CSF, triangles indicate animals treated with G-CSF and SCF (see Materials and Methods for dosage). Number of animals depicted in (A) through (C) is 81, 38, and 75, respectively. (D) Mean level of bone marrow engraftment (± SD) in five individual experiments spanning the range of the number of CD34+cells injected. Number of animals: in experiments 1 and 2, n = 6; in experiments 3 through 5, n = 4.
Engraftment of G-CSF–mobilized human peripheral blood CD34+ cells in sublethally irradiated NOD/SCID mice. The percentage of human cells in (A) BM, (B) spleen, and (C) PB was determined by flow cytometry at 6 to 8 weeks after transplantation. Open circles represent untreated animals, closed circles represent animals treated with human G-CSF, triangles indicate animals treated with G-CSF and SCF (see Materials and Methods for dosage). Number of animals depicted in (A) through (C) is 81, 38, and 75, respectively. (D) Mean level of bone marrow engraftment (± SD) in five individual experiments spanning the range of the number of CD34+cells injected. Number of animals: in experiments 1 and 2, n = 6; in experiments 3 through 5, n = 4.
Statistical analysis.
Statistical variation in the text is indicated by the SD, whereas differences between groups and data in figures are expressed as the mean ± standard error of the mean (SEM). Differences between percentages were calculated using the Wilcoxon test, whereas differences between other groups were compared using either a Student's t-test or analysis of variance (ANOVA). The frequency of the NOD/SCID-repopulating cell was estimated using a generalized additive statistical model36 to describe the relationships between the number of CD34+ cells injected, the purity of the CD34+ cells, and engraftment. Specifically, the logit transformed percentage of human cells was modeled as the sum of smoothing spline and loess functions of the number of input CD34+ and non-CD34+ cells, using the SAS (SAS Institute, Cary, NC) statistical package. Finally, differences in mobilization between growth-factor treated and control groups were calculated based on a generalized linear model37 using SAS.
RESULTS
Conditioning of NOD/SCID mice.
The SCID mutation has been described to affect radiation-induced DNA repair.38 In initial experiments we monitored the survival of 10- to 15-week old NOD/SCID mice after various doses of TBI. After a dose of 450 cGy or higher all animals died between 1 and 2 weeks after irradiation. At 350 cGy to 400 cGy a late mortality (between 35 and 50 days after irradiation) was observed in about 20% of the animals, whereas after 300 cGy all animals survived for at least 8 weeks. In addition, in pilot studies we transplanted groups of 300 cGy, 200 cGy, and 100 cGy irradiated animals and one group of nonirradiated animals (3 animals per group) with 12 × 106 human G-CSF–mobilized PB CD34+ cells per mouse. At 6 weeks after transplantation no human cells could be detected in the BM of the nonirradiated animals, but chimerism in the 300 cGy, 200 cGy, and 100 cGy groups was 63.9%, 56.0%, and 36.5% (± 11.3%, pooled SD), respectively. Based on these results we established 300 cGy as a safe and adequate radiation dose for our subsequent transplantation experiments.
Engraftment of human hematopoietic cells in BM, spleen, and PB of NOD/SCID.
To study the level of engraftment of human PB cells in various hematopoietic organs of the NOD/SCID mouse, sublethally (300 cGy) irradiated animals were intravenously injected with increasing numbers of CD34+ cells. At 6 to 8 weeks after transplantation, PB, BM, and spleen were harvested and analyzed by flow cytometry for the presence of human cells (>1% of all nucleated cells) using antibodies against human pan-leukocyte marker CD45 and mouse CD18 (Fig 1A, B, C). Open circles indicate untreated animals, solid circles and triangles indicate animals that were treated for 4 to 6 days with G-CSF or G-CSF and SCF, respectively, which was part of a mobilization study that will be described below. The level of engraftment in BM and spleen (as determined by the percentage CD45+ mCD18− cells among all nucleated cells) showed a dose response with respect to the number of cells infused, and reached as high as 95% in the BM and 90% in the spleen (Fig 1A and 1B). No statistically significant change in engraftment was observed when animals were either preinjected with irradiated CD34− cells (25 × 106cells/animal), or were splenectomized 2 weeks before transplantation (Table 1). In contrast to the gradual dose-dependent increase in engraftment in BM and spleen (Fig 1A and 1B), there appeared to be a higher level of human cells in the PB only when high numbers of cells were infused (Fig 1C). More specifically, the level of human cells in the PB in animals with less than 60% human chimerism in the BM, stayed below 25%, whereas a high level of chimerism could only be observed in highly (>80% in BM) engrafted animals. In contrast to the overall variation in engraftment as observed in Fig 1A, the engraftment between animals in individual experiments (Fig 1D) has been shown to be highly reproducible.
Engraftment of CD34+ Cells in Animals Preinjected With Irradiated CD34− Cells and in Splenectomized Animals as Compared to Controls
| Experiment-150 . | Input . | % CD45-151 BM . | % CD45-151 Spleen . | % CD45-151 PB . |
|---|---|---|---|---|
| I | CD34− | <1.0 | <1.0 | <1.0 |
| CD34+ | 18.2 ± 11.2 | 8.5 ± 4.7 | 3.7 ± 2.0 | |
| CD34− and CD34+ | 14.8 ± 4.1 | 13.5 ± 1.9 | 2.6 ± 0.2 | |
| II | CD34+ | 26.3 ± 1.2 | nd | nd |
| CD34+ Spl-X | 19.4 ± 15.4 | — | nd |
| Experiment-150 . | Input . | % CD45-151 BM . | % CD45-151 Spleen . | % CD45-151 PB . |
|---|---|---|---|---|
| I | CD34− | <1.0 | <1.0 | <1.0 |
| CD34+ | 18.2 ± 11.2 | 8.5 ± 4.7 | 3.7 ± 2.0 | |
| CD34− and CD34+ | 14.8 ± 4.1 | 13.5 ± 1.9 | 2.6 ± 0.2 | |
| II | CD34+ | 26.3 ± 1.2 | nd | nd |
| CD34+ Spl-X | 19.4 ± 15.4 | — | nd |
Abbreviations: BM, bone marrow; PB, peripheral blood; nd, not done.
Two independent experiments in which sublethally irradiated NOD/SCID mice were injected with CD34+ cells from a G-CSF–mobilized apheresis product. In experiment I, animals were either injected with 20 Gy irradiated monocyte-depleted (by plastic adherence for 30 minutes at 37°C) CD34− cells (25 × 106 per animal) alone, with CD34+ cells alone (9 × 106 per animal), or with CD34− cells followed by CD34+ cells four hours later (n = 2 per group). In experiment II, 12 × 106 CD34+cells were injected in splenectomized or nonsplenectomized animals (n = 2 per group).
Percentage human cells was determined by flow cytometry using an antibody specific for human CD45.
To calculate the frequency of the NOD/SCID-repopulating cell (SRC)17 in mobilized PB cells and to study the relationship between the number of human cells injected and level of engraftment in various organs, the data in Fig 1A were analyzed using a generalized additive statistical model.36 Using a logit-transformation, we found a linear relationship between the engraftment in the BM (expressed as logit-transformed percentage of human cells) and the log of the number of CD34+ cells injected (P < .0001; Fig 2A). The logit transformation of percentages of human cells is used to transform the S-shaped curve shown in Fig 1A to linearity, which is required for the statistical model to assure a constant variance over the range of numbers of cells injected. Based on these data, the frequency of the NOD/SCID-repopulating cell (SRC) in mobilized PB cells could be calculated, and was estimated to be one in 1.7 × 106CD34+ cells, with a 95% confidence interval ranging from 0.9 to 3.1 × 106 CD34+ cells. The calculation was based on the level of engraftment in animals that were not treated with growth factors.
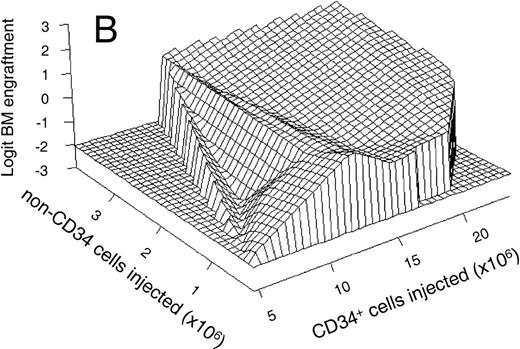
(A) Mean linear relationship between the natural log of the number of CD34+ cells injected (in millions) and the logit-transformed percentage of human cells in the BM (n = 63); and (B) mean estimated relationship (in 50 animals) between the number of CD34+ cells and CD34− cells injected (linear scale, horizontal axes) and the logit-transformed percentage of human cells in the BM (vertical axis). Data are based on a generalized additive statistical model calculated by the SAS statistical package. Both (A) and (B) only included animals that were not treated with growth factors. In (B), not every combination of CD34+and CD34− cells was available for analysis. The areas outside the data range are indicated by zero engraftment in the far-left and front-right corners of the graph. The graph was plotted using the S-Plus (Mathsoft) statistics package.
(A) Mean linear relationship between the natural log of the number of CD34+ cells injected (in millions) and the logit-transformed percentage of human cells in the BM (n = 63); and (B) mean estimated relationship (in 50 animals) between the number of CD34+ cells and CD34− cells injected (linear scale, horizontal axes) and the logit-transformed percentage of human cells in the BM (vertical axis). Data are based on a generalized additive statistical model calculated by the SAS statistical package. Both (A) and (B) only included animals that were not treated with growth factors. In (B), not every combination of CD34+and CD34− cells was available for analysis. The areas outside the data range are indicated by zero engraftment in the far-left and front-right corners of the graph. The graph was plotted using the S-Plus (Mathsoft) statistics package.
In addition to the relationship between engraftment and the number of CD34+ cells injected, the statistical model also showed a linear relationship between engraftment and the number of coinjected CD34− cells (P = .0027; not shown). This contrasts with the data noted above on preinjection of irradiated CD34− cells. The numbers of CD34+ and CD34− cells were deducted from the purity of the apheresis products used for transplantation in each individual experiment. The estimated 3-dimensional response surface for the relationship between the level of engraftment and the number of CD34+ and CD34− cells injected, is plotted in Fig 2B. The engraftment in the BM is shown on the vertical axis (again expressed as the logit-transformed percentage of human cells) as a function of the number of CD34+ and CD34− cells injected per animal (on a linear scale on the horizontal axes). The areas in the far-left and front-right corners of the graph show no engraftment due to the absence of data as not every combination of CD34+ and CD34−cells was available for analysis. The plot shows that the level of engraftment in the BM correlates with both the number of CD34+ and CD34− cells injected (P< .0001). Although the variables involved in the effect of CD34− cells on engraftment are unknown, the plot does show that these cells affect engraftment in the BM to a greater extent when low numbers of CD34+ are injected.
In transplanted NOD/SCID mice, mature human hematopoietic cells, as well as colony-forming cells (2091 ± 902 [SEM] colony-forming unit–granulocyte-macrophage [CFU-GM] per femur, 555 ± 397 erythroid burst forming unit [BFU-E] per femur) could be detected as long as 6.5 months after transplantation. Specifically, three animals were analyzed after 5 months, showing 60%, 63%, and 48% CD45+ cells in BM, whereas in another experiment three mice were analyzed after 6.5 months, showing 21%, 33%, and 74% CD45+ cells, respectively. No animals were found to be negative for human cells. The extent of hematopoietic differentiation found in these animals was similar to the differentiation described below. The longevity of hematopoietic chimerism, and the observed multilineage differentiation, are both indicative of the engraftment of a primitive human hematopoietic cell.
One highly engrafted animal was chosen for histological examination (Fig 3). Flow cytometric analysis showed high levels of human (CD45+ mCD18−) cells in both BM (88%, Fig 3A) and PB (92%, Fig 3C), whereas engraftment in the spleen was remarkably lower (15%, Fig 3B). Interestingly, the humerus of this animal appeared pale as compared with the humerus of an irradiated nontransplanted animal (Fig 3D). Confirming this observation at a histological level, examination of the humerus showed the presence of a solid mass of mostly undifferentiated cells and the apparent absence of erythropoiesis (Fig 3F) as compared with an irradiated nontransplanted control (Fig 3E). Despite the marked reduction in BM erythropoiesis, the animal was not anemic (not shown) but did have an enlarged spleen (with a cellularity of 17 × 106cells; control spleens contained 7.4 × 106 cells). As detected by flow cytometry, the spleen contained mouse as well as human (glycophorin A+) erythrocytes. In the five animals studied that had more than 80% human cells in the BM, the average spleen cellularity was 2.3 ± 0.6-fold higher (P < .05) than in control animals. In other human-mouse xenotransplants, the presence of an enlarged spleen has been attributed to compensatory mouse hematopoiesis39 40 due to the replacement of endogenous hematopoiesis in the BM by the xenotransplant.
Flow cytometric analysis of (A) BM, (B) spleen, and (C) PB of an animal transplanted with 25 × 106G-CSF–mobilized human peripheral blood CD34+ cells. The percentage of human cells (CD45+ and mCD18−) is indicated in the graphs. The spleen of this animal was enlarged as compared with control (17 × 106v 5.4 × 106 cells). In (D), humerus of this animal (top) as compared with the humerus of an irradiated nontransplanted control animal (bottom). In (E) and (F), histology (hematoxylin and eosin stain) of the bone marrow of control and transplanted animal, respectively.
Flow cytometric analysis of (A) BM, (B) spleen, and (C) PB of an animal transplanted with 25 × 106G-CSF–mobilized human peripheral blood CD34+ cells. The percentage of human cells (CD45+ and mCD18−) is indicated in the graphs. The spleen of this animal was enlarged as compared with control (17 × 106v 5.4 × 106 cells). In (D), humerus of this animal (top) as compared with the humerus of an irradiated nontransplanted control animal (bottom). In (E) and (F), histology (hematoxylin and eosin stain) of the bone marrow of control and transplanted animal, respectively.
Overall distribution and maturation of myeloid and B-lymphoid lineages in the hematopoietic organs.
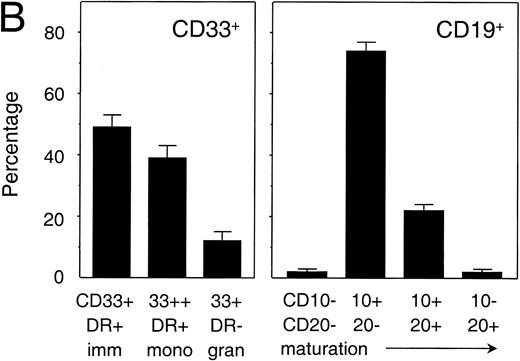
To investigate the capacity of human PB stem cells in NOD/SCID mice to differentiate into multiple lineages, and to compare the differentiation of human cells in different hematopoietic organs, cells in BM, spleen, and PB of transplanted animals were tested for the expression of the human myeloid-specific marker CD33 and B lymphoid-specific marker CD19 by flow cytometry.41 The distribution of CD33+ and CD19+ cells showed that the majority of human cells in the BM (n = 50) and spleen (n = 10) had differentiated into the B-lymphoid lineage (Fig 4A). On the other hand, in the PB (n = 50) myeloid and lymphoid cells were equally represented. When comparing BM and spleen, the percentage of myeloid cells in the BM (28.2 ± 16.4, mean ± SD, n = 50) was significantly higher (P = 0.3) than in the spleen (16.0 ± 10.3, n = 10), indicating that myelopoiesis preferentially took place in the BM. In animals that were treated with either G-CSF or G-CSF and SCF in our mobilization study (described below), the percentage of human myeloid cells in the BM had increased to 39.1% ± 17.0% (n = 18). This is significantly higher than the percentage in untreated animals (P = .02), which indicated that the treatment had triggered human myelopoiesis. In addition, in highly engrafted animals (with more than 70% human cells) the percentage of myeloid cells in the BM (56.1% ± 9.2% CD33+, n = 9) was significantly higher than in animals engrafted at lower levels (32.8% ± 18.8% CD33+, n = 41; P< .001), suggesting that the graft itself contributed to the microenvironment in supporting myelopoiesis. A more detailed flow cytometric analysis of the cells engrafted in the BM, which was based on the expression of CD33 and HLA-DR for myeloid cells and CD10 and CD20 for B-lymphoid cells (Fig 4B), showed that the majority of both myeloid and B-lymphoid cells had an immature phenotype. Although in the myeloid lineage mature monocytes and granulocytes could be identified based on the level of expression of CD33 and HLA-DR, maturation in the B-lymphoid lineage in the BM appeared to be incomplete as judged by the absence of the more mature CD20+ CD10−cells.
Analysis of myelopoiesis and B lymphopoiesis in transplanted NOD/SCID. (A) Overall distribution of myeloid (CD33+) and B-lymphoid (CD19+) lineages among human cells in spleen (n = 10), bone marrow (n = 50), and peripheral blood (n = 50). Differences between SPL and BM were significant (P < .05), differences between SPL and BM, and BM and PB were highly significant (P < .001). Bars indicate mean ± SEM. (B) Relative distribution of various myeloid and B-lymphoid subsets in the bone marrow of four representative animals (containing 84.5%, 76.6%, 78.9%, and 73.5% CD45+cells in the BM, respectively). Immature cells and monocytes both express HLA-DR+, whereas monocytes also express high levels of CD33. Granulocytes express CD33 but are negative for HLA-DR (left panel). All B cells express CD19 of which the most immature B cells lack both CD10 and CD20, with differentiation expression of CD10 is followed by expression of CD20 and, ultimately, loss of CD10 on the mature B cell (right panel). Animals included in this analysis had not been treated with a mobilizing regimen. Bars represent mean ± SEM (n = 4).
Analysis of myelopoiesis and B lymphopoiesis in transplanted NOD/SCID. (A) Overall distribution of myeloid (CD33+) and B-lymphoid (CD19+) lineages among human cells in spleen (n = 10), bone marrow (n = 50), and peripheral blood (n = 50). Differences between SPL and BM were significant (P < .05), differences between SPL and BM, and BM and PB were highly significant (P < .001). Bars indicate mean ± SEM. (B) Relative distribution of various myeloid and B-lymphoid subsets in the bone marrow of four representative animals (containing 84.5%, 76.6%, 78.9%, and 73.5% CD45+cells in the BM, respectively). Immature cells and monocytes both express HLA-DR+, whereas monocytes also express high levels of CD33. Granulocytes express CD33 but are negative for HLA-DR (left panel). All B cells express CD19 of which the most immature B cells lack both CD10 and CD20, with differentiation expression of CD10 is followed by expression of CD20 and, ultimately, loss of CD10 on the mature B cell (right panel). Animals included in this analysis had not been treated with a mobilizing regimen. Bars represent mean ± SEM (n = 4).
Flow cytometric analysis of various lineages in bone marrow and spleen.
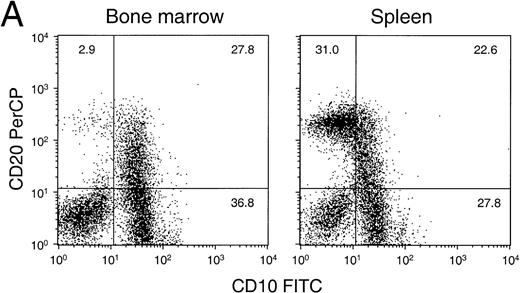
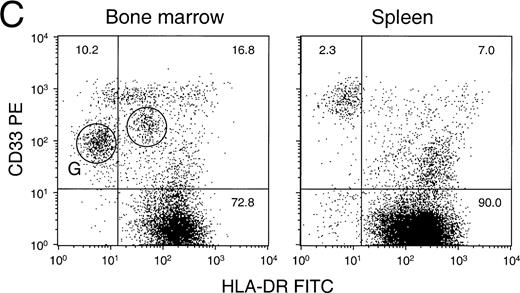
A detailed flow cytometric analysis of the human hematopoietic cells in BM and spleen of a representative animal is shown in Fig 5A through E. The differentiation of human B lymphocytes can be characterized by an increase in expression of CD20 on CD10+ cells, followed by a subsequent loss of CD10 from the cells expressing high levels of CD20.41 We show that both BM and spleen contained immature CD10+20− and CD10+20+ cells, whereas mature CD10−20+ cells were present only in the spleen (Fig 5A). Consistent with this observation, expression of IgM and IgD heavy chain as well as kappa and lambda light-chain immunoglobulins on CD19+ B cells was substantially greater in the spleen than the BM (Fig 5B). In contrast, differentiation into the granulocytic lineage could be observed in the BM but not in the spleen. Granulocytes could be identified by the intermediate expression of CD33 and low or medium expression of activation-marker HLA-DR (Fig5C, circled subsets). This observation was confirmed by analyzing BM and spleen for the expression of CD11b (alpha subunit of beta 2-integrin Mac-1) and CD16 (Fc gamma receptor IIIb), of which the level of CD16 on CD11b+ cells increases with granulocyte maturation (Fig 5D). Again, granulocytes could only be identified in the BM but not in the spleen. In contrast, monocytes (as defined by a high expression of CD33) could be found in both BM and spleen (Fig 5C), although monocytes in the spleen were mostly nonactivated as indicated by the low expression of HLA-DR.42 CD34 could be detected on human cells in both BM and spleen. On average, 14.2% ± 4.9% (mean ± SD, n=39) of human cells in the BM expressed CD34, whereas the percentage in the spleen was found to be approximately threefold lower (not shown). Finally, human erythroid cells could also be detected in both BM and spleen but only at a relatively low level (ranging from 4% to less than 1% of all cells). When gated for CD45− cells, a clear population of human Glycophorin A (Gly A)-positive (mouse CD18−) cells could be identified in the BM (18% of gated cells) and the spleen (3% of gated cells) (Fig 5E). The vast majority of Gly A+ cells had a moderate to high forward light scatter (not shown), supporting the notion that the in vivo maturation of erythroid progenitor cells into erythrocytes (which have a low forward light scatter) may have been impaired. Finally, a low level of platelet antigen (CD41 or CD61) could be detected in the BM, but was always associated with a high expression of CD33 (not shown), indicating the possible presence of human platelets on the surface of monocytes. In the PB, platelets could not be detected.
Detailed flow cytometric analysis of the various hematopoietic lineages in bone marrow and spleen of engrafted NOD/SCID. Plots are from a representative experiment in which animals were transplanted with 15 × 106 CD34+ cells and were analyzed after 6 to 8 weeks. (A) Distribution of B-cell markers CD10 and CD20 on all nucleated cells. (B) Distribution of heavy and light-chain cell-surface immunoglobulin on CD19+ human B cells (mean ± SEM of n = 4 to n = 7). All differences between BM and SPL were highly significant (P < .001). (C) Distribution of HLA-DR and CD33 (circles indicate activated HLA-DR+and resting HLA-DR− granulocytes) among CD45+ cells, (D) expression of CD11b and CD16 on CD45+ cells, and (E) expression of human Glycophorin A (Gly A) on CD45− nucleated and enucleated cells. 40,000 events per analysis were recorded.
Detailed flow cytometric analysis of the various hematopoietic lineages in bone marrow and spleen of engrafted NOD/SCID. Plots are from a representative experiment in which animals were transplanted with 15 × 106 CD34+ cells and were analyzed after 6 to 8 weeks. (A) Distribution of B-cell markers CD10 and CD20 on all nucleated cells. (B) Distribution of heavy and light-chain cell-surface immunoglobulin on CD19+ human B cells (mean ± SEM of n = 4 to n = 7). All differences between BM and SPL were highly significant (P < .001). (C) Distribution of HLA-DR and CD33 (circles indicate activated HLA-DR+and resting HLA-DR− granulocytes) among CD45+ cells, (D) expression of CD11b and CD16 on CD45+ cells, and (E) expression of human Glycophorin A (Gly A) on CD45− nucleated and enucleated cells. 40,000 events per analysis were recorded.
Flow cytometric analysis of human cells in the thymus.
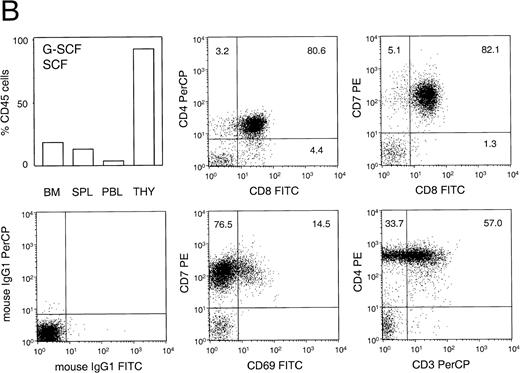
Human T lymphocytes, as defined by the expression of CD4 and CD3, could never be detected in BM, spleen, or PB of engrafted animals. However, human thymocytes could be detected in the thymus of highly engrafted animals (Fig 6A and B). Statistical analysis showed a positive correlation between the level of engraftment in the BM and the presence of human cells in the thymus (Fig 6A, left panel; P < .05). Of the 13 animals analyzed, thymocytes could be detected in 5 out of 6 animals that had been engrafted in the BM with at least 40% human cells. Interestingly, human thymocytes could also be detected in the thymus of animals that had only 25% human cells in the BM, but which had previously been treated for 6 days with human G-CSF and PEG-SCF (as part of a mobilization experiment), whereas the thymus of untreated animals contained virtually no human cells (Fig6A, right panel). Phenotypic analysis of these thymocytes, as shown for a representative animal in Fig 6B, showed a large percentage of CD4+ CD8+ double-positive cells and cells that coexpress CD8 and CD7. In addition, the majority of CD4+8− single-positive cells expressed high levels of CD3 (not shown), whereas some CD7+ cells also expressed the activation marker CD69. In the engrafted thymus, expression of CD33, CD20, or HLA-DR could not be detected. This phenotype was similar in all animals examined and is consistent with normal human T-cell lymphopoiesis.
Percentage and phenotype of human cells in the thymus of transplanted NOD/SCID. (A, left panel) Relationship between the percentage of human thymocytes in the thymus and level of engraftment in the BM (n = 13). Animals in individual experiments are indicated by the same symbol. (A, right panel) Mean engraftment in BM and thymus (THY) of animals that were either untreated (n=3) or treated (n=4) for 6 days with human G-CSF (250 μg/kg/d, subcutaneously) and pegylated human SCF (20 μg/kg/d, subcutaneously). Bars represent mean ± SEM. Differences between untreated and G-CSF/SCF-treated animals in the thymus were significant (P < .05) whereas chimerism in the BM was not significantly different. (B) Phenotypic analysis of the thymus of a representative animal. This animal was engrafted with human PB CD34+ cells and was treated with G-CSF and SCF. The left top panel shows the distribution of chimerism in various organs. Cells were analyzed for the expression of the human T-cell markers CD3, CD7, CD4, CD8, and activation marker CD69, or were incubated with isotype control antibodies.
Percentage and phenotype of human cells in the thymus of transplanted NOD/SCID. (A, left panel) Relationship between the percentage of human thymocytes in the thymus and level of engraftment in the BM (n = 13). Animals in individual experiments are indicated by the same symbol. (A, right panel) Mean engraftment in BM and thymus (THY) of animals that were either untreated (n=3) or treated (n=4) for 6 days with human G-CSF (250 μg/kg/d, subcutaneously) and pegylated human SCF (20 μg/kg/d, subcutaneously). Bars represent mean ± SEM. Differences between untreated and G-CSF/SCF-treated animals in the thymus were significant (P < .05) whereas chimerism in the BM was not significantly different. (B) Phenotypic analysis of the thymus of a representative animal. This animal was engrafted with human PB CD34+ cells and was treated with G-CSF and SCF. The left top panel shows the distribution of chimerism in various organs. Cells were analyzed for the expression of the human T-cell markers CD3, CD7, CD4, CD8, and activation marker CD69, or were incubated with isotype control antibodies.
Mobilization of mouse progenitors in nontransplanted NOD/SCID.
To study mobilization, we first established that mouse progenitor cells could be mobilized in a normal nontransplanted NOD/SCID. To test whether preirradiation interfered with this ability we also included a group of animals that had been sublethally irradiated 6-weeks before treatment. Both nonirradiated and sublethally irradiated animals were administered a single injection of cyclophosphamide (at 200 mg/kg interperoneally) followed 2-days later by 4 days of G-CSF (at 250 μg/kg/d). This mobilization protocol has previously been used by Neben et al33 to mobilize cells in C57BL/6 mice. Animals were sacrificed on day 6 after cyclophosphamide treatment and progenitor cells in both PB and BM were enumerated in methylcellulose cultures (Fig 7A). The results show that mouse progenitor cells could be efficiently mobilized in unirradiated as well as irradiated animals (1,120- and 300-fold increase in PB progenitors, respectively). The difference between unirradiated and irradiated animals was not statistically significant (P = .07). As expected, the increase in progenitor cells was accompanied by an increase in spleen weight (Fig 7B) and number of white cells in the blood (Fig 7C). These results indicate that the NOD/SCID mouse allowed murine progenitor cells to be mobilized into the periphery after an appropriate stimulus, and that circulating progenitor cells were present in sufficient numbers to be detected by standard colony assays even though the animals had not been splenectomized.
Mobilization of mouse progenitor cells in NOD/SCID. (A) Progenitor cells in femur and blood of animals unirradiated or preirradiated with 300 cGy, untreated or after treatment with cyclophosphamide (200 mg/kg interperitoneally at day 6) followed by 4 days of G-CSF (250 μg/kg/d, subcutaneously). Bars indicate mean ± SEM (n = 2 per group). The difference between irradiated and nonirradiated animals was not significant (P = .07). (Note: left axis has a log scale, right axis has a linear scale.) (B) Spleen weight and (C) white blood cell (WBC) counts of the same animals (mean ± SEM, n = 2). In nonirradiated animals, the increase in both spleen weight and WBC counts is highly significant (P < .01). Increase of WBC in irradiated animals as compared with control is also highly significant (P < .01) whereas spleen weight did not change.
Mobilization of mouse progenitor cells in NOD/SCID. (A) Progenitor cells in femur and blood of animals unirradiated or preirradiated with 300 cGy, untreated or after treatment with cyclophosphamide (200 mg/kg interperitoneally at day 6) followed by 4 days of G-CSF (250 μg/kg/d, subcutaneously). Bars indicate mean ± SEM (n = 2 per group). The difference between irradiated and nonirradiated animals was not significant (P = .07). (Note: left axis has a log scale, right axis has a linear scale.) (B) Spleen weight and (C) white blood cell (WBC) counts of the same animals (mean ± SEM, n = 2). In nonirradiated animals, the increase in both spleen weight and WBC counts is highly significant (P < .01). Increase of WBC in irradiated animals as compared with control is also highly significant (P < .01) whereas spleen weight did not change.
Mobilization of human progenitors in transplanted NOD/SCID.
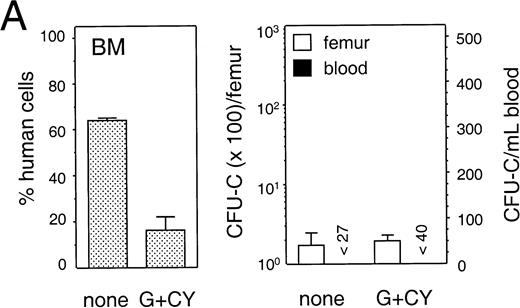
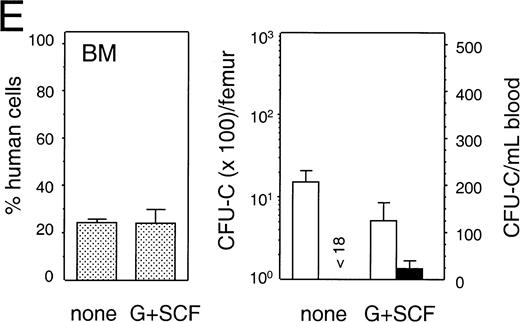
To detect mobilization of human progenitor cells we used a tissue culture medium containing the human-specific growth factors SCF and IL-3 plus Epo, that selectively supported the growth of human but not mouse progenitor cells (data not shown). For mobilization, NOD/SCID mice were treated with various regimens that have previously been shown to mobilize primitive hematopoietic cells (see Materials and Methods). Treatment was initiated 6 to 8 weeks after transplantation and PB and BM cells were harvested the morning after the last treatment day. The distribution of human cells among PB, spleen, and BM of engrafted animals, in treated and untreated groups of five individual experiments, is shown in Table 2. Figure 8 (A through E) shows the average percentage of human cells in the BM (left panel), and the number of human progenitor cells detected in BM and PB (right panel, log scale, and linear scale, respectively) of the same experiments. In animals treated with G-CSF, the average number of human cells in the PB was significantly increased as compared with control (P < .01 and P< .05, respectively; Table 2, B and C). In addition, human progenitor cells were found to be mobilized in animals treated with a low or high dose of G-CSF (Fig 8B and 8C, respectively), alone or in combination with SCF (Fig 8D and 8E, respectively). More specifically, the number of progenitor cells in the PB increased from 1.3-fold in an animal treated with a high-dose G-CSF and SCF (Fig 8E) to 7.9-fold in two animals treated with a high dose of G-CSF (Fig 8C). For statistical analysis a generalized linear model37 was used to account for the volume of blood collected, the number of cells injected per animal, the number of colonies counted, and whether or not animals had been treated with hematopoietic growth factors. When combining the results of individual experiments, an estimated 4.6-fold increase in progenitors could be found in the PB of animals treated with either G-CSF or G-CSF and SCF as compared with control (Table 3; P = .019), with a 95% confidence interval of (1.24, 17.0). The total number of nucleated cells and number of mouse cells in the BM or PB of animals treated with G-CSF or G-CSF and SCF had not changed (P > .05, not shown). The mobilized PB progenitor cells, as well as the progenitor cells in the BM of untreated and growth-factor treated animals, were predominantly CFU-GM (92.0% ± 4.2% (SEM; n = 9); 84.1% ± 4.3% (n = 17); and 85.6% ± 7.1% (n = 15), respectively), with only a minority being BFU-E (10%-20%) and less than 1% being CFU-Mix. In contrast to the mobilization described above, no progenitor cells could be detected in the PB of animals treated with cyclophosphamide and G-CSF (Fig 8A; Table 3, group I). In addition to the lack of mobilization, the average number of human cells in PB and BM had decreased significantly (Table 2; experiment A). In summary, our experiments show that human hematopoietic progenitor cells can be induced to egress from the BM space into the PB after stimulation with human G-CSF or a combination of human G-CSF and pegylated-SCF, albeit with a low frequency.
Distribution of Human Cells Among Hematopoietic Organs in Untreated and Mobilized NOD/SCID Mice
| Experiment . | CD34+ Cells Injected per Mouse (×106) . | n . | Treatment . | Human Cells per mL Blood (×105) . | Human Cells per Femur (×106) . | Human Cells per Spleen (×106) . |
|---|---|---|---|---|---|---|
| A | 13 | 4 | Control | 3.1 ± 0.8 | 7.7 ± 1.2 | nd |
| 5 | CY + G-CSF | 0.7 ± 0.2‡ | 0.7 ± 0.2‡ | nd | ||
| B | 15 | 3 | Control | 6.3 ± 0.9 | 8.7 ± 0.7 | 10.7 ± 2.3 |
| 3 | G-CSF | 18.5 ± 0.2† | 9.5 ± 0.3 | 9.7 ± 0.1 | ||
| C | 10 | 2 | Control | 1.7 ± 0.7 | 0.3 ± 0.2 | nd |
| 3 | G-CSF | 12.5 ± 2.7* | 0.8 ± 0.1 | nd | ||
| D | 25 | 2 | Control | 20.2 ± 4.9 | 5.7 ± 2.8 | 1.7 ± 1.2 |
| 2 | G-CSF | 29.1 ± 0.8 | 8.5 ± 1.0 | 5.9 ± 1.2 | ||
| 2 | G-CSF + SCF | 36.0 ± 6.9 | 9.0 ± 0.6 | 5.2 ± 1.6 | ||
| E | 16 | 3 | Control | 1.3 ± 1.0 | 3.4 ± 0.8 | 1.3 ± 0.3 |
| 4 | G-CSF + SCF | 1.1 ± 0.3 | 3.2 ± 0.9 | 2.6 ± 0.7 |
| Experiment . | CD34+ Cells Injected per Mouse (×106) . | n . | Treatment . | Human Cells per mL Blood (×105) . | Human Cells per Femur (×106) . | Human Cells per Spleen (×106) . |
|---|---|---|---|---|---|---|
| A | 13 | 4 | Control | 3.1 ± 0.8 | 7.7 ± 1.2 | nd |
| 5 | CY + G-CSF | 0.7 ± 0.2‡ | 0.7 ± 0.2‡ | nd | ||
| B | 15 | 3 | Control | 6.3 ± 0.9 | 8.7 ± 0.7 | 10.7 ± 2.3 |
| 3 | G-CSF | 18.5 ± 0.2† | 9.5 ± 0.3 | 9.7 ± 0.1 | ||
| C | 10 | 2 | Control | 1.7 ± 0.7 | 0.3 ± 0.2 | nd |
| 3 | G-CSF | 12.5 ± 2.7* | 0.8 ± 0.1 | nd | ||
| D | 25 | 2 | Control | 20.2 ± 4.9 | 5.7 ± 2.8 | 1.7 ± 1.2 |
| 2 | G-CSF | 29.1 ± 0.8 | 8.5 ± 1.0 | 5.9 ± 1.2 | ||
| 2 | G-CSF + SCF | 36.0 ± 6.9 | 9.0 ± 0.6 | 5.2 ± 1.6 | ||
| E | 16 | 3 | Control | 1.3 ± 1.0 | 3.4 ± 0.8 | 1.3 ± 0.3 |
| 4 | G-CSF + SCF | 1.1 ± 0.3 | 3.2 ± 0.9 | 2.6 ± 0.7 |
Distribution of the mean number (±SEM) of human cells in the bone marrow, spleen, and peripheral blood of mobilized and untreated NOD/SCID mice transplanted with mobilized human peripheral blood CD34+ cells. Experiments A through E are the same as those described in Fig 8. For treatment protocols, see legend of Fig 8. Numbers in control and treated animals have been statistically compared using the Student's t-test and the level of significance has been indicated.
P < .05.
P < .01.
P < .001.
Mobilization of human hematopoietic progenitors. Untransplanted and engrafted animals (at 6-8 weeks post-transplantation) were treated with various regimens to mobilize hematopoietic cells. In each case, the left hand panel shows the percentage of human cells in the BM, whereas the right hand panel shows the number of human progenitor cells per femur and per mL of peripheral blood. Bars indicate mean ± SEM. (A) Control (n = 4) and treated (n = 5) with a single injection of cyclophosphamide (200 mg/kg) followed by 4 days of hG-CSF (250 μg/kg/d); (B) control (n = 3) and treated (n = 3) with hG-CSF (25 μg/kg/d) for 4 days; (C) control (n = 2) and treated (n = 3) with hG-CSF (250 μg/kg/d for 4 days); (D) control (n = 2) and treated with hG-CSF (25 μg/kg/d) with or without pegylated-hSCF (20 μg/kg/d) for 4 days (both n = 2); and (E) control (n = 3) and treated (n = 4) with hG-CSF (250 μg/kg/d) and pegylated-hSCF (20 μg/kg/d) for 6 days.
Mobilization of human hematopoietic progenitors. Untransplanted and engrafted animals (at 6-8 weeks post-transplantation) were treated with various regimens to mobilize hematopoietic cells. In each case, the left hand panel shows the percentage of human cells in the BM, whereas the right hand panel shows the number of human progenitor cells per femur and per mL of peripheral blood. Bars indicate mean ± SEM. (A) Control (n = 4) and treated (n = 5) with a single injection of cyclophosphamide (200 mg/kg) followed by 4 days of hG-CSF (250 μg/kg/d); (B) control (n = 3) and treated (n = 3) with hG-CSF (25 μg/kg/d) for 4 days; (C) control (n = 2) and treated (n = 3) with hG-CSF (250 μg/kg/d for 4 days); (D) control (n = 2) and treated with hG-CSF (25 μg/kg/d) with or without pegylated-hSCF (20 μg/kg/d) for 4 days (both n = 2); and (E) control (n = 3) and treated (n = 4) with hG-CSF (250 μg/kg/d) and pegylated-hSCF (20 μg/kg/d) for 6 days.
Mobilization of Human Progenitors in NOD/SCID Mice
| Group . | No. of Animals . | Treatment* . | n-Fold Increase Over Control† . | 95% Confidence Limits . | P Value‡ . |
|---|---|---|---|---|---|
| I | 5 | CY + G-CSF | nd | — | — |
| II | 13 | pooled | 4.59 | (1.24, 17.0) | .019 |
| 8 | G-CSF | 5.60 | (1.63, 19.17) | .007 | |
| 5 | G-CSF + SCF | 1.82 | (0.45, 7.45) | .46 |
| Group . | No. of Animals . | Treatment* . | n-Fold Increase Over Control† . | 95% Confidence Limits . | P Value‡ . |
|---|---|---|---|---|---|
| I | 5 | CY + G-CSF | nd | — | — |
| II | 13 | pooled | 4.59 | (1.24, 17.0) | .019 |
| 8 | G-CSF | 5.60 | (1.63, 19.17) | .007 | |
| 5 | G-CSF + SCF | 1.82 | (0.45, 7.45) | .46 |
NOD/SCID mice were treated with various mobilization regimens 6 to 8 weeks after transplantation. Treatment regimens in (I): Cyclophosphamide (200 mg/kg) followed 2-days later by 4 days of G-CSF (250 μg/kg/day); in (II): treatment with G-CSF for 4 days (at 25 or 250 μg/kg/day), or with G-CSF (at 25 or 250 μg/kg/day) and pegylated-SCF (20 μg/kg/day) for 4 to 6 days.
Abbreviation: nd, not detected.
Group I shows the result of an individual experiment. In group II results have been combined. The group pooled includes all animals that received human G-CSF or SCF, whereas the other two groups show the combined result of all animals that were treated with either G-CSF alone or with G-CSF and SCF (at low or high dose) among the four experiments performed.
Differences between groups were statistically analyzed using a generalized linear model. Fold-increase over control indicates the mean fold-increase of the number of human progenitors found in the peripheral blood in animals treated with a mobilizing regimen as compared with the number of progenitors in untreated animals (n = 15).
The P value is based on the result of the generalized linear model and indicates the level of significance of the difference between treatment groups and control, adjusted for the number of cells injected per animal, the treatment regimen, and volume of blood collected.
DISCUSSION
The mechanisms of mobilization of progenitor cells from the BM into the blood are still poorly understood. A systematic study of the physiologic or therapeutic stimuli that induce mobilization, as well as the components that modulate the extent and timing of mobilization, requires the development of an in vivo transplantation model. In the present study we developed such a model by transplanting a total of 92 sublethally irradiated NOD/SCID mice with increasing numbers of CD34+ cells harvested from adult G-CSF–mobilized healthy donors. At 6 to 8 weeks after transplantation, the hematopoietic organs were analyzed for the presence of differentiated human hematopoietic cells using multiparameter flow cytometry, and groups of engrafted animals were treated with various regimens to study the level of mobilization of human hematopoietic progenitors into the circulation.
Using statistical modeling, a dose-response relationship could be shown between the number of cells infused and the level of engraftment in BM and spleen. In contrast, the level of human cells in the PB generally stayed below 25%, except in highly engrafted animals, whereas engraftment in the spleen was always lower than the engraftment in the BM, indicating the complex interactions between the endogenous mouse hematopoietic compartment and the xenotransplanted human cells. The model also showed a positive correlation between the impurity of the graft (number of CD34− cells injected) and the level of engraftment in BM, spleen, and PB, suggesting the presence of an NOD/SCID engraftment-facilitating cell among human-mobilized PB cells. Interestingly, other investigators have proposed the existence of a hematopoietic stem cell that lacks the expression of CD34.43 Whether the positive effect of CD34− cells on the engraftment in this model is related to the presence of a facilitating cell or a true CD34− engrafting cell remains to be investigated.
The frequency of the SRC was estimated to be one in 1.7 × 106 CD34+ cells, or approximately one in 2.2 × 108 total cells (based on an average of 0.76% CD34+ cells among the nucleated cells in the apheresis product). This result contrasts with a previously published estimate of the frequency of the SRC in human-mobilized PB cells of one in 6 × 106 total cells.17 In these experiments, the SRC frequency was estimated using Poisson statistics by limiting dilution analysis, in which as little as 0.1% of human cells could be detected by Southern hybridization. Apart from the method used for the estimate, and the difference in sensitivity between flow cytometry and Southern hybridization, a number of other factors may have played a role in the difference between our estimate and the previously reported SRC frequency. These may include: the health status of the NOD/SCID colony; the radiation dose, 375 cGy to 400 cGy as opposed to 300 cGy in our experiments, allowing higher levels of engraftment; the addition of human SCF, IL-3, and GM-CSF in the reported experiments whereas our animals did not receive additional human cytokines; and finally, the coinjection of CD34− cells, as our current data suggest that these cells enhance engraftment. This illustrates that the reported frequency should only be used for relative comparisons as the variables involved in the engraftment of human hematopoietic cells in NOD/SCID mice have not sufficiently been characterized.
As has previously been reported, the frequency of the SRC in CD34+ cells purified from G-CSF–mobilized PB is much lower than in umbilical cord blood.17,44 When our results are compared with a study in which recipients of umbilical cord blood CD34+ cells were conditioned with 350 cGy to 400 cGy, approximately 50-fold less cord blood cells were needed to reach the same level of engraftment in the BM.45 In NOD/SCID mice, it has been shown that both the engraftment of umbilical cord blood CD34+ cells, as well as mobilized PB CD34+ cells, is independent of the addition of human growth factors.40 45 The size of the graft necessary for the engraftment of PB cells in NOD/SCID mice, as compared with that of umbilical cord blood, may be caused by an intrinsic difference in SRC frequency. Alternatively, this may be due to a difference in the biological properties of CD34+ cells derived from fetal or adult hematopoietic tissue.
The engraftment pattern of PB CD34+ cells in NOD/SCID mice was multilineage and could be observed for at least 6.5 months after transplantation. When comparing the different hematopoietic organs, distinct differences were observed in the differentiation and maturation of human hematopoietic cells. Human myelopoiesis was favored in the BM, in which different maturation stages of granulopoiesis could be identified by flow cytometry. In contrast, whereas resting human monocytes could be detected in the spleen, granulocytes appeared to be absent. As has been reported earlier,44,45 our data show that the microenvironment of BM and spleen in NOD/SCID mice differ in their support of human B-cell maturation. Although immature B cells were present in large quantities in the BM, human B-cell maturation, as defined by the loss of CD10 expression on CD19+CD20+ and expression of cell-surface immunoglobulin (IgM, IgD, kappa and lambda), took place primarily in the spleen. Finally, our data confirm a previous report44that the BM of the NOD/SCID was the preferred site of human hematopoiesis because it contained the highest number of CD34+ cells as well as the highest number of in vitro clonable myelo-erythroid progenitors (J.C.M.v.d.L. and D.A.W., unpublished data, January 1998).
The engraftment of human T cells in the thymus of SCID or NOD/SCID mice transplanted with umbilical cord blood CD34+ cells has been reported to be inconsistent.31,45 In the present study we have found a dose-response relationship between the number of cells transplanted and the presence of human thymocytes. Because human thymocytes could only be found in moderately to highly engrafted animals, we speculate that the thymus was seeded with a T-cell precursor from the BM. This may be a rare event, explaining the lack of thymocytes in animals showing lower levels of human engraftment. On the other hand, the data may also suggest a defect in the recruitment and mobilization of human T-cell precursors from the BM or a defect in the seeding of the murine thymus. It has been shown that human T-cell precursors could egress from the BM of SCID mice transplanted with human fetal BM or fetal liver cells, migrate to the implanted human thymus, and give rise to mature T cells that populated the PB.46
In our mobilization studies, we could also detect human thymocytes in animals that were engrafted at low levels but only if animals had been treated with G-CSF and SCF. This finding supports the notion of mobilization of a T-cell precursor from the BM that might have been induced by G-CSF or SCF. Alternatively, treatment with G-CSF or SCF could also have stimulated a dormant T-cell precursor that had seeded the thymus directly at the time of transplantation as SCF is a growth factor that has been described to play an important role in the expansion of primitive thymocytes in vivo.47 As has been elegantly shown by gene marking of transplanted cells in bnxmice,48 and in SCID mice implanted with a human thymus,46 a shared retroviral integration pattern in both lymphocytic and myelocytic lineages would ultimately provide proof of a multilineage precursor in this model. Our results are in agreement with the finding that human thymocytes can be generated in the mouse thymus, as was shown in vitro using fetal thymus organ cultures seeded with human BM or umbilical cord blood cells.49 The fact that no human T cells could be detected in either the BM, spleen, or PB suggests that there may be a defect in either the maturation of human T cells grown in the xenograft microenvironment in vivo, or in their ability to egress from the thymus.
In both patients and experimental animals it has been shown that progenitor cells as well as primitive hematopoietic cells can be induced to mobilize from the BM into the blood stream by a variety of different stimuli (see review8). These stimuli include treatment with cytostatics, such as cyclophosphamide, with or without a subsequent course of hematopoietic growth factors33; hematopoietic growth factors alone or in combination, of which G-CSF or a combination of G-CSF and SCF have been most widely studied1,35,50-52; and infusion of antibodies that interfere with the binding of hematopoietic cells to either the endothelium or components of the BM extracellular matrix.53However, despite the potential for clinical application, the mechanisms involved in the mobilization of hematopoietic cells are still poorly understood. Some stimuli, such as IL-8, have been reported to mobilize cells within 30 minutes wheras G-CSF needs to be administered for several days.8 Moreover, the emergence of new regulators of hematopoiesis such as chemokines underscore the need for a model in which mobilization of human hematopoietic cells can be studied. In this study we present such a model and show that human hematopoietic cells can be induced to mobilize from the BM into the PB.
We have investigated several methods that have been reported to induce mobilization by presumably distinct mechanisms. Engrafted NOD/SCID mice were treated with either cyclophosphamide followed by high doses of human G-CSF,33 or G-CSF alone or G-CSF in combination with human (pegylated) SCF. Our results show for the first time that human progenitor cells can be mobilized from the BM of the NOD/SCID mouse into the PB after treatment with G-CSF or G-CSF and SCF, albeit at a low level. These results, and the generally low level of circulating human cells in the blood of nonmobilized animals, both seem to indicate that human cells do not egress normally from the BM into the blood stream. The reasons for this may be related to the lack of the appropriate human stimuli in the microenvironment, or the inability of human hematopoietic cells to physically negotiate the mouse endothelial and adventitial cell barrier.54
In summary, we have shown that human G-CSF–mobilized PB stem cells are able to induce long-term engraftment of NOD/SCID mice without exogenously added human cytokines. Together with previously published data,44 45 our observations show that the engraftment of human cells in NOD/SCID mice recapitulates the anatomical distribution of normal hematopoiesis in humans as well as in mice. Our results show that human G-CSF mobilized PB CD34+ cells can reliably engraft sublethally irradiated NOD/SCID mice, home to the appropriate sites of hematopoiesis, differentiate into multiple lineages, and mature in specific hematopoietic organs. In engrafted animals, all human hematopoietic lineages were represented, including cells of the T-cell lineage, and the data show that the mouse hematopoietic microenvironment played an important role in the maturation of specific lineages. In addition, we report for the first time that human hematopoietic cells can be induced to mobilize from the BM into the PB of NOD/SCID mice transplanted with human PB CD34+ cells. Therefore, we postulate that the xenotransplanted NOD/SCID mouse may be an excellent model to further study the mechanisms involved in the mobilization of human hematopoietic cells.
ACKNOWLEDGMENT
The authors thank the members of the Stem Cell Laboratory of Indiana University Medical School for the processing, purification, and analyses of the apheresis products and Dr Attilio Orazi for processing and reviewing the histology. We acknowledge Dr Leonard D. Shultz for providing the founders of our NOD/LtSz-scid/scid colony, Amgen for the gift of pegylated human SCF, and Baxter Immunotherapy (Irvine, CA) for providing us with preclinical Isolex immunomagnetic CD34-selection kits. In addition, we thank Dr John E. Dick and the members of the Wells Center for critically reviewing the manuscript.
The Wells Center for Pediatric Research is a Center of Excellence in Molecular Hematology funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P50 DK 49218) which supports the NOD/SCID colony and the Stem Cell laboratory.
Address correspondence to David A. Williams, MD, Herman B Wells Center for Pediatric Research, James Whitcomb Riley Hospital for Children, Cancer Research Building, 1044 W Walnut St, Rm 406C, Indianapolis, IN 46202; email: dwilliam@iupui.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.















This feature is available to Subscribers Only
Sign In or Create an Account Close Modal