To the Editor:
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired disease resulting from somatic mutations in the PIG-A gene involving primitive hematopoietic stem cells. The PNH clones may have growth or survival advantages relative to normal clones that may promote their expansion, resulting in the development of overt PNH. However, little is known about how PNH clones gain growth advantage. Recent studies demonstrated preferential hematopoiesis by PNH clones in vivo.1,2 However, proliferation may be affected similarly in PIG-A–deficient clones and in normal clones,3suggesting that PIG-A abnormalities alone may not be sufficient to confer a growth advantage on PNH clones.
A proportion of PNH patients terminate in severe pancytopenia with dysplasia, ie, myelodysplastic syndrome (MDS), and rarely progress to acute leukemia. We previously reported in BLOOD that specificp15INK4B gene inactivation by promoter hypermethylation may be associated with the development of MDS,4 because it may confer a growth advantage on cells. One overt leukemia patient analyzed in this study in whom PNH evolved through MDS (PNH/MDS-OL) showed intense hypermethylation of thep15INK4B gene. Surface marker analysis of his leukemic blasts showed low levels of expression of CD59, suggesting that leukemic blasts were derived from the PNH clone. So, to clarify at what point the p15INK4B gene was densely methylated and inactivated in this PNH/MDS-OL patient and whether this p15INK4B gene methylation is related to the expansion of PNH clones, we analyzed this patient and an additional 17 PNH patients.
We obtained clinical samples after receiving informed consent from a total of 18 patients (4 men and 14 women) who were positive for sugar water test and/or acidified serum (Ham) test and were diagnosed as PNH based on clinical manifestations. They included 12 female patients (unique patient no. [UPN] 1 through 12) analyzed in our previous study in which we demonstrated the existence of monoclonal populations with PNH phenotype by clonality analysis using X-chromosome inactivation and assessment of expression of glycophosphatidylinositol-anchored proteins by flow cytometry.5 We extracted DNA from polymorphonuclear cells (PMNCs) and mononuclear cells (MNCs) (or T lymphocytes), as described previously,5 and analyzed the methylation status of thep15INK4B gene by the simple and sensitive methylation-specific polymerase chain reaction (MSP) method.6 Because MSP can detect 10−3methylated alleles among unmethylated alleles,6 changes of the methylation status in PNH clones could be detected even if they are present at a very low incidence. After bisulfite modification, we amplified their DNAs with each primer set specific for unmethylated and methylated DNA in a thermal cycler (TAKARA, Kyoto, Japan).
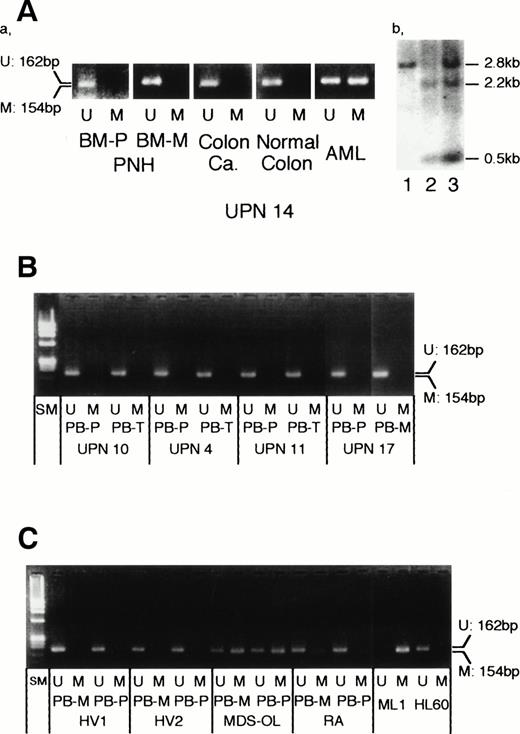
DNA from the PNH/MDS-OL patient (UPN 14) in the leukemic state showed densely methylated p15INK4B gene, consistent with the results of Southern blotting. However, thep15INK4B gene in both his PMNCs and MNCs obtained in the PNH state was not methylated (Fig1A). The p15INK4Bgene of PMNCs in which we showed the monoclonal PNH clone in our previous report5 was also not methylated (Fig 1B, UPN 10 and UPN 11). The strong methylation band of PMNCs similar to that of MNCs in another MDS-OL patient (Fig 1C) supported our previous Southern blotting result, indicating that thep15INK4B gene methylation is not limited to leukemic blasts but is also present in PMNCs of MDS origin.4 Therefore, the methylation band may be able to be detected in the PMNC population of PNH ifp15INK4B gene methylation occurs in PNH clones. Finally, we could not detect methylation bands in PMNC or MNC populations in any patient with PNH. This sensitive method also detected no methylated band in healthy volunteers (Fig 1C).
Methylation status of the p15INK4Bgene in patients with PNH. (A) Methylation status of thep15INK4B gene in a patient (UPN 14) with acute myelogenous leukemia (AML) evolved from PNH. (a) Unmethylated DNA-specific and methylated DNA-specific MSP primers produced 162-bp and 154-bp products, respectively. Bone marrow (BM) samples at PNH (BM-P, polymorphonuclear cells; BM-M, mononuclear cells), his colon cancer, and normal colon tissue resected 1 year after PNH presentation showed unmethylated p15INK4B gene. In contrast, leukemic blasts showed hypermethylation of thep15INK4B gene. (b) Southern blotting also showed the methylated status in leukemic blasts4; lanes 1 and 2, control B lymphocytes. Lane 3, patient’s leukemic blasts. (B) Methylation status of the p15INK4B gene in patients with PNH. All samples obtained from peripheral blood (PB) showed unmethylated pattern. PB-P, polymorphonuclear cells; PB-M, mononuclear cells; PB-T, T lymphocytes; SM, size marker (ØX174/HaeIII). UPNs are common to those in our previous report.5 (C) Methylation status of the p15INK4B gene in control samples. Two healthy volunteers (HV) showed unmethylated pattern. A patient with overt leukemia evolved from MDS (MDS-OL) showed intense methylation in both PB-M and PB-P populations, whereas a patient with refractory anemia (RA) showed faint methylation. ML1 and HL60 were completely methylated and unmethylated, respectively, as previously reported.4
Methylation status of the p15INK4Bgene in patients with PNH. (A) Methylation status of thep15INK4B gene in a patient (UPN 14) with acute myelogenous leukemia (AML) evolved from PNH. (a) Unmethylated DNA-specific and methylated DNA-specific MSP primers produced 162-bp and 154-bp products, respectively. Bone marrow (BM) samples at PNH (BM-P, polymorphonuclear cells; BM-M, mononuclear cells), his colon cancer, and normal colon tissue resected 1 year after PNH presentation showed unmethylated p15INK4B gene. In contrast, leukemic blasts showed hypermethylation of thep15INK4B gene. (b) Southern blotting also showed the methylated status in leukemic blasts4; lanes 1 and 2, control B lymphocytes. Lane 3, patient’s leukemic blasts. (B) Methylation status of the p15INK4B gene in patients with PNH. All samples obtained from peripheral blood (PB) showed unmethylated pattern. PB-P, polymorphonuclear cells; PB-M, mononuclear cells; PB-T, T lymphocytes; SM, size marker (ØX174/HaeIII). UPNs are common to those in our previous report.5 (C) Methylation status of the p15INK4B gene in control samples. Two healthy volunteers (HV) showed unmethylated pattern. A patient with overt leukemia evolved from MDS (MDS-OL) showed intense methylation in both PB-M and PB-P populations, whereas a patient with refractory anemia (RA) showed faint methylation. ML1 and HL60 were completely methylated and unmethylated, respectively, as previously reported.4
Our results indicated that p15INK4B gene inactivation is not related to the growth advantage of PNH clones and may not play any role in their expansion. These observations also support our4 and other investigator’s7suggestion that p15INK4B gene methylation promotes the progression of MDS to overt leukemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal