Abstract
Although it is well known that CD8+cytotoxic T lymphocytes (CTLs) play an important role in the suppression of cancer cell growth, the significance of CD4+ CTLs in resistance to cancer is obscure. In an attempt to elucidate the role of CD4+ CTLs in immunosurveillance of chronic myelogenous leukemia (CML), we examined the immunologic functions of bcr-abl b3a2 fusion peptide-specific CD4+ CTL clones. Seven CD4+ T-cell clones that responded to stimulation with b3a2 peptide, but not with b2a2 peptide or physiological counterparts bcr b3b4 and abl 1A-a2 peptides, were established from two healthy individuals. Restriction elements of these clones were HLA-DRB1*0901. These CD4+ T-cell clones exhibited b3a2 peptide-specific and HLA-DRB1*0901-restricted cytotoxicity and produced interleukin-3 (IL-3), IL-4, IL-10, interferon-γ, tumor necrosis factor–, and granulocyte-macrophage colony-stimulating factor in response to bcr-abl peptide stimulation, indicating they were Th0 clones. The numbers of HLA-DRB1*0901-positive b3a2, but not those of b2a2-positive or HLA-DRB1*0901-negative CML cell colonies increased when CML cells were cultured with b3a2-specific CD4+ CTL clones. These data suggest that bcr-abl–specific CD4+ CTLs recognize CML cells in an antigen-specific and HLA-DR–restricted manner, and that they do not inhibit, but in fact augment, CML cell growth.
© 1998 by The American Society of Hematology.
TRANSLOCATION BETWEEN two distinct genes resulting in formation of a new chimeric protein occurs specifically in various types of leukemia. The translocation t(9;22) (q34;q11) that gives rise to a hybrid bcr-abl gene encoding a 210-kD fusion protein is detected in over 95% of patients with chronic myelogenous leukemia (CML).1,2 Two major types of bcr-abl chimeric protein, ie, the products of b2a2 fusion between exon 2 of thebcr gene and exon 2 of the abl gene, and b3a2 fusion between exon 3 of the bcr gene and exon 2 of the ablgene have been found to be expressed in CML cells.3 Because chimeric protein is only expressed in leukemic cells, not in normal cells, the fusion sequence may act as a potential target for a T-cell–mediated immune response to leukemia.
Recently, synthetic peptides spanning the fusion point between bcr and abl have been shown to bind to certain types of HLA class I molecule and induce bcr-abl fusion peptide-specific CD8+ cytotoxic T lymphocytes (CTLs) in vitro.4-8 Induction of CD4+ T lymphocytes that proliferate specifically in response to stimulation with bcr-abl fusion peptide in an HLA class II–restricted manner has been also achieved by stimulating peripheral blood lymphocytes of healthy individuals with synthetic bcr-abl fusion peptide.9-12 Although these recent findings support the concept that the bcr-abl fusion peptide is immunogenic in the T-cell–mediated immune response, the majority of previous studies involved experiments using synthetic peptide-loaded antigen presenting cells (APCs) and whether T lymphocytes can recognize naturally processed bcr-abl fusion peptide in the context of major histocompatibility complex (MHC) molecules on CML cells is obscure.
There is no doubt that CTLs play an important role in protection against tumor development. It was thought that antigen-specific cytotoxicity was mediated solely by HLA class I–restricted CD8+ CTLs, but it is now well known that some CD4+ T lymphocytes also exert antigen-specific cytotoxicity in an HLA class II–restricted manner.13-16 Although it is well established that CD8+ CTLs play an important role in resistance to tumor cell growth, the significance of CD4+CTLs in immunosurveillance of tumors is obscure. The recent demonstration that infusing donor lymphocytes depleted of CD8+ T lymphocytes could induce remissions with a low rate of severe graft-versus-host disease in patients with CML who had relapsed after allogeneic bone marrow transplantation17suggests that CD4+, but not CD8+, T lymphocytes are essential for a graft-versus-leukemia effect in patients with CML.
In this study, we established bcr-abl b3a2-specific and HLA-DRB1*0901-restricted CD4+ CTL clones from two healthy individuals and investigated their immunologic functions by focusing on their effects on CML cell growth. Unexpectedly, our results showed that bcr-abl fusion peptide-specific CD4+ CTL clones did not inhibit, but actually augmented, CML cell colony formation in vitro in an HLA-DR–restricted fashion. These data strongly suggest that HLA class II–restricted cytotoxicity mediated by CD4+ CTL does not itself inhibit CML cell growth effectively and that CD4+ T lymphocytes can recognize the bcr-abl fusion peptide in the context of HLA class II molecules expressed on CML cells, resulting in the production of growth factors for CML cells.
MATERIALS AND METHODS
Generation of bcr-abl fusion peptide-specific T-cell clones.
Peptides were synthesized to a minimum of 90% purity using an automated peptide synthesizer (Model 432A Synergy; Applied Biosystems Inc, Foster City, CA) with the Fmoc procedure. The sequences of the 17-mer amino acid peptides synthesized (underlined amino acids indicate the breakpoints with a new amino acid) were as follows: bcr-abl b2a2, IPLTINKEEALQRPVAS; bcr-abl b3a2, ATGFKQSSKALQRPVAS; bcr b3b4, ATGFKQSSNLYCTLEVD; and abl 1A-a2, SSSSCYLEEALQRPVAS. Peripheral blood mononuclear cells (PBMCs) from healthy individuals suspended in RPMI 1640 medium supplemented with 10% heat-inactivated human AB type serum (referred to hereafter as the culture medium) and synthetic peptide at a concentration of 10 μg/mL were seeded in round-bottom microtiter plate wells at a concentration of 1 × 105 cells/0.2 mL. After 7 days in culture, half of the medium was exchanged for fresh culture medium and a second stimulation was performed by addition of 1 × 105autologous PBMCs treated with mitomycin C (MMC) as APCs and peptide at a concentration of 10 μg/mL. After a further 7 days, a third stimulation was performed as for the second stimulation. Four days after the third stimulation with peptide, human recombinant interleukin-2 (IL-2; Boehringer-Mannheim, Mannheim, Germany) was added to each well at a concentration of 10 U/mL. Then, the growing cells were transferred from the microtiter wells to 16-mm diameter wells and their proliferative responses were examined. Bulk cells showing a proliferative response to stimulation with bcr-abl fusion peptide were cloned by the limiting dilution method described previously.18 T-cell clones were cultured continuously in IL-2–containing culture medium and MMC-treated autologous PBMCs and bcr-abl fusion peptide were added to the wells every 2 weeks.
Proliferative response to synthetic peptide.
Proliferative response of T cells to stimulation with peptide was examined as described previously19 with a slight modification. Briefly, 2 × 104 T cells and 2 × 105 MMC-treated PBMCs or 3 × 104MMC-treated HLA-DR gene-transfected murine L cells as a source of APCs in 0.2 mL culture medium were seeded into flat-bottom microtiter wells, to which synthetic peptide was added. In preliminary experiments to determine the optimal concentration of peptide, bcr-abl b3a2 fusion peptide-specific T-cell clones proliferated maximally in the presence of over 10 μg/mL peptide. Therefore, we used synthetic peptide at a concentration of 10 μg/mL in this series of experiments. The culture was incubated at 37°C in a 5% CO2incubator for 72 hours. For the final 16 hours of incubation, 1 μCi of [3H]thymidine (3H-TdR; New England Nuclear, Boston, MA) was added to each well and the incorporation of3H-TdR was determined by liquid scintillation counting. To determine the restriction element(s) governing the interaction between T-cell clones and APCs, monoclonal antibody (MoAb) L243 (anti–HLA-DR; American Type Culture Collection, Rockville, MD), TÜ169 (anti–HLA-DQ; Pharmingen, San Diego, CA), or HI43 (anti–HLA-DP; Pharmingen) was added to each well at its optimal concentration, and the inhibitory effect of each MoAb on the proliferative response of the T-cell clones was examined as described previously.19
Cytotoxicity assays.
51Cr-release assays were performed as described previously.20 Briefly, 1 × 10451Cr (Na251CrO4; New England Nuclear) -labeled Epstein-Barr virus-transformed B lymphoblastoid cell lines (B-LCLs) suspended in 0.1 mL RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) (referred to hereafter as the assay medium) were seeded into round-bottom microtiter wells and incubated with or without synthetic peptide at a concentration of 50 μg/mL for 2 hours. Then, various numbers of effector cells suspended in 0.1 mL assay medium were added to each well. After incubation for 4 hours, 0.1 mL supernatant was collected from each well and its radioactivity was determined using a gamma counter. The percentage of specific 51Cr release was calculated as follows: (cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release) × 100. Each cytotoxicity assay was performed at least twice and yielded identical data.
Cytokine production.
Cloned T cells were collected from the wells and washed twice with RPMI 1640 medium to remove IL-2 and any other cytokines present in the culture medium. Then 5 × 105 T cells and 2 × 106 MMC-treated autologous PBMCs were suspended in 2 mL assay medium and cultured in 16-mm wells in the presence or absence of peptide. After 72 hours, the supernatant was collected from each well and assayed for the production of various cytokines by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN).
Detection of cytolytic mediator mRNA expression.
Expression of various cytolytic mediator mRNAs in the T-cell clones was investigated by reverse transcriptase-polymerase chain reaction (RT-PCR). The total RNA was extracted from each T-cell clone that had been stimulated with peptide 5 days previously and cDNA was synthesized by reverse transcription with Moloney murine leukemia virus RT, as described previously.21 Amplification of cDNAs by the PCR was performed using the following primers: perforin, 5′-ACCAGCAATGTGCATGTGTCTGTG-3′ and 5′-GAAGGAGGCCGTCATCTTGTGCTT-3′; granzyme B, 5′-TGCAGGAAGATCGAAAGTGCG-3′ and 5′-GAGGCATGCCATTGTTTCGTC-3′; Fas ligand, 5′-ATAGGATCCATGTTTCTGCT- CTTCCACCTACAGAAGGA-3′ and 5′-ATAGAATTCTGACCAAGA- GAGAGCTCAGATACGTTGAC-3′; tumor necrosis factor–α (TNF-α), 5′-TGAGCACTGAAAGCATGATC-3′ and 5′-TTATCTCTCAGCTCCACGCC-3′; and lymphotoxin-β, 5′-ATAAGCTTGATCAGGGAGGACTGGTAACGG-3′ and 5′-TAGGTACCTCGCACCACGCACTCATATTC-3′. The expected lengths of the amplified cytolytic mediator cDNAs were as follows: perforin, 459 bp; granzyme B, 180 bp; Fas ligand, 506 bp; TNF-α, 375 bp; and lymphotoxin-β, 635 bp.
Colony formation assay.
The effects of the T-cell clones on CML cell growth were examined by performing the colony formation assays described previously22 with a slight modification. Bone marrow cells were isolated from patients with chronic-phase CML when the Ph chromosome was detected in all bone marrow cells analyzed and had been cryopreserved until use. Cloned T cells and CML bone marrow cells were suspended in assay medium at an E:T ratio of 5:1, centrifuged at 1,000 rpm for 3 minutes to ensure close cell contact, and then coincubated in assay medium at 37°C for 4 hours. To examine the role of HLA-DR in the interaction between cloned T cells and CML cells, CML bone marrow cells were preincubated with anti–HLA-DR MoAb before centrifugation with cloned T cells. Control CML bone marrow cells were centrifuged and incubated without T-cell clone in the same manner. After incubation, 5 mL Iscove’s Modified Dulbecco’s medium containing 1% methylcellulose, 5% GCT conditioned medium, 1% bovine serum albumin, 30% FCS, 10−4 mol/L 2-mercaptoethanol, and 3 U/mL erythropoietin (Stem cell CFU Kit; Baxter, Deerfield, IL) was added to the cell pellet at the final CML cell concentration of 1 × 105 cells/mL. Then, each cell suspension was placed in triplicate 24-mm wells and cultured at 37°C for 12 to 14 days, after which the numbers of colony-forming unit granulocyte-macrophage (CFU-GM) and burst-forming unit erythroid (BFU-E) were counted using an inverted microscope. For the control assay, bone marrow cells were isolated from a HLA-DRB1*0901-positive patient with a nonhematopoietic disorder and cocultured with cloned T cells as described above. To examine the effect of soluble factors produced by T-cell clones on CML cell growth, the culture supernatants of T-cell clones that had been stimulated with peptide for 2 days were obtained, and then added to the CML bone marrow cells. The significance of differences between values for two groups was determined using the paired two-tailed Student’s t-test and those at P < .05 were considered significant.
RESULTS
Generation of T-cell clones directed against bcr-abl fusion peptide and analysis of HLA restriction.
PBMCs from two healthy individuals, MY and TO, whose HLA-DR types were DRB1*0901/*1406 and *0405/*0901, respectively, were stimulated repeatedly with b2a2 or b3a2 fusion peptide in microtiter wells, as described in Materials and Methods. Of a total of 192 wells per donor seeded with PBMCs, three and four CD4+ T-cell clones that proliferated in response to stimulation with b3a2 peptide in the presence of autologous APCs were generated from MY and TO, respectively. A CD4+ T-cell clone that showed a proliferative response to stimulation with b2a2 peptide in an HLA-DRB1*1406-restricted manner was also generated from PBMCs of MY, but this clone stopped growing during the study. Thus, seven CD4+ T-cell clones, designated MY-1, MY-2, MY-3, TO-1, TO-2, TO-3, and TO-4, that proliferated in response to stimulation with b3a2 fusion peptide were used for further experiments. Southern blot analysis of the T-cell receptor-β genes of these T-cell clones showed distinct rearrangement patterns (data not shown), indicating that these seven T-cell clones originated from different T cells. Because identical results were obtained with each of these seven T-cell clones, only the data for MY-1 and TO-1 are presented. MY-1 and TO-1 proliferated when stimulated with the 17-mer b3a2 peptide, but did not do so in response to stimulation with b2a2 or physiological 17-mer counterpart peptides bcr b3b4 or abl 1A-a2. The proliferative responses of MY-1 and TO-1 to b3a2 peptide were inhibited by adding the anti-DR MoAb to the culture medium, but not by adding the anti-DQ or anti-DP MoAb, suggesting that the proliferative responses of MY-1 and TO-1 were restricted by HLA-DR (Table 1, Exp. 1). The restriction elements of both MY-1 and TO-1 seemed to be HLA-DR9, as both clones proliferated in response to peptide stimulation in the presence of allogeneic APCs bearing HLA-DR9, but not in the presence of HLA-DR9–negative allogeneic APCs (data not shown). To examine this further, we used HLA-DRA and HLA-DRB1*0901gene-transfected murine L cells (L-DR9) as APCs. As shown in Table 1, Exp. 2, MY-1 and TO-1 proliferated in response to stimulation with the b3a2 peptide in the presence of L-DR9, but not in the presence ofHLA-DRA and DRB1*0401 gene-transfected L cells (L-DR4) or control L cells transfected with the selection markerNeor gene alone (L-Neo). These data show that the proliferative responses of MY-1 and TO-1 were restricted by HLA-DRB1*0901. In view of the results of a recent study on peptide motifs for the HLA-DR9 molecule,23 the fourth amino acid (F) and the seventh amino acid (S) may be binding motifs for HLA-DR9 positions 1 and 4, respectively.
HLA-DRB1*0901-Restricted Proliferative Responses of bcr-abl b3a2-Specific CD4+ T-Cell Clones
| Exp. 1-150 Clone . | APC . | MoAb Added . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|---|
| MY-1 | auto PBMC | None | None | 259 ± 37 |
| b3a2 | 26,771 ± 2,628 | |||
| b2a2 | 324 ± 28 | |||
| b3b4 | 332 ± 31 | |||
| 1A-a2 | 300 ± 29 | |||
| anti–HLA-DR | b3a2 | 449 ± 69 | ||
| anti–HLA-DQ | b3a2 | 26,678 ± 3,326 | ||
| anti–HLA-DP | b3a2 | 26,139 ± 4,559 | ||
| TO-1 | auto PBMC | None | None | 219 ± 24 |
| b3a2 | 31,309 ± 3,237 | |||
| b2a2 | 279 ± 102 | |||
| b3b4 | 145 ± 6 | |||
| 1A-a2 | 157 ± 11 | |||
| anti–HLA-DR | b3a2 | 308 ± 200 | ||
| anti–HLA-DQ | b3a2 | 25,664 ± 523 | ||
| anti–HLA-DP | b3a2 | 29,731 ± 2,632 |
| Exp. 1-150 Clone . | APC . | MoAb Added . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|---|
| MY-1 | auto PBMC | None | None | 259 ± 37 |
| b3a2 | 26,771 ± 2,628 | |||
| b2a2 | 324 ± 28 | |||
| b3b4 | 332 ± 31 | |||
| 1A-a2 | 300 ± 29 | |||
| anti–HLA-DR | b3a2 | 449 ± 69 | ||
| anti–HLA-DQ | b3a2 | 26,678 ± 3,326 | ||
| anti–HLA-DP | b3a2 | 26,139 ± 4,559 | ||
| TO-1 | auto PBMC | None | None | 219 ± 24 |
| b3a2 | 31,309 ± 3,237 | |||
| b2a2 | 279 ± 102 | |||
| b3b4 | 145 ± 6 | |||
| 1A-a2 | 157 ± 11 | |||
| anti–HLA-DR | b3a2 | 308 ± 200 | ||
| anti–HLA-DQ | b3a2 | 25,664 ± 523 | ||
| anti–HLA-DP | b3a2 | 29,731 ± 2,632 |
| Exp. 2-151 Clone . | APC . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|
| MY-1 | None | None | 210 ± 52 |
| b3a2 | 870 ± 212 | ||
| L-Neo | None | 214 ± 20 | |
| b3a2 | 702 ± 145 | ||
| L-DR9 | None | 248 ± 48 | |
| b3a2 | 24,208 ± 507 | ||
| L-DR4 | None | 254 ± 79 | |
| b3a2 | 799 ± 97 | ||
| TO-1 | None | None | 268 ± 30 |
| b3a2 | 936 ± 62 | ||
| L-Neo | None | 262 ± 34 | |
| b3a2 | 893 ± 22 | ||
| L-DR9 | None | 308 ± 50 | |
| b3a2 | 29,760 ± 2,554 | ||
| L-DR4 | None | 358 ± 27 | |
| b3a2 | 723 ± 54 | ||
| None | L-DR9 | None | 227 ± 25 |
| b3a2 | 367 ± 188 |
| Exp. 2-151 Clone . | APC . | Peptide Stimulation . | Proliferative Response (cpm) (mean ± SD) . |
|---|---|---|---|
| MY-1 | None | None | 210 ± 52 |
| b3a2 | 870 ± 212 | ||
| L-Neo | None | 214 ± 20 | |
| b3a2 | 702 ± 145 | ||
| L-DR9 | None | 248 ± 48 | |
| b3a2 | 24,208 ± 507 | ||
| L-DR4 | None | 254 ± 79 | |
| b3a2 | 799 ± 97 | ||
| TO-1 | None | None | 268 ± 30 |
| b3a2 | 936 ± 62 | ||
| L-Neo | None | 262 ± 34 | |
| b3a2 | 893 ± 22 | ||
| L-DR9 | None | 308 ± 50 | |
| b3a2 | 29,760 ± 2,554 | ||
| L-DR4 | None | 358 ± 27 | |
| b3a2 | 723 ± 54 | ||
| None | L-DR9 | None | 227 ± 25 |
| b3a2 | 367 ± 188 |
Abbreviation: Exp., experiment.
Incorporation of 3H-TdR into cloned T cells was determined in the presence of autologous APCs with and without various types of synthetic peptide and with and without anti-HLA MoAb.
Incorporation of 3H-TdR into cloned T cells was determined in the presence and absence of various types of APC with and without b3a2 peptide.
Cytotoxic reactivities of T-cell clones against peptide-loaded and -unloaded target cells.
Next, we examined the ability of T-cell clones to lyse b3a2 peptide-loaded target cells. Table 2 shows the cytotoxicities of MY-1 and TO-1 to autologous and various allogeneic B-LCLs in the presence and absence of the peptide. MY-1 and TO-1 were strongly cytotoxic to b3a2 peptide-loaded autologous B-LCL. As with their proliferative responses, the b3a2 fusion peptide-specific cytotoxicities of MY-1 and TO-1 were restricted by HLA-DRB1*0901, as allogeneic targets bearing HLA-DRB1*0901, but HLA-DRB1*0901-negative allogeneic cells were not lysed by MY-1 and TO-1.
Cytotoxicities of bcr-abl b3a2-Specific CD4+ T-Cell Clones
| Clone . | Target . | HLA-DRB1 . | Peptide . | % Cytotoxicity* . |
|---|---|---|---|---|
| MY-1 | auto-LCL | 0901/1406 | None | 0.1 |
| b3a2 | 49.9 | |||
| allo-LCL #1 | 0901/0405 | None | −0.4 | |
| b3a2 | 49.4 | |||
| allo-LCL #2 | 0901/1406 | None | 0.8 | |
| b3a2 | 48.0 | |||
| allo-LCL #3 | 1406/1502 | None | −2.4 | |
| b3a2 | 0.2 | |||
| TO-1 | auto-LCL | 0901/0405 | None | 1.2 |
| b3a2 | 55.6 | |||
| allo-LCL #1 | 0901/1406 | None | 1.9 | |
| b3a2 | 56.9 | |||
| allo-LCL #2 | 0901/1406 | None | −0.2 | |
| b3a2 | 54.8 | |||
| allo-LCL #3 | 1406/1502 | None | −0.1 | |
| b3a2 | 2.0 |
| Clone . | Target . | HLA-DRB1 . | Peptide . | % Cytotoxicity* . |
|---|---|---|---|---|
| MY-1 | auto-LCL | 0901/1406 | None | 0.1 |
| b3a2 | 49.9 | |||
| allo-LCL #1 | 0901/0405 | None | −0.4 | |
| b3a2 | 49.4 | |||
| allo-LCL #2 | 0901/1406 | None | 0.8 | |
| b3a2 | 48.0 | |||
| allo-LCL #3 | 1406/1502 | None | −2.4 | |
| b3a2 | 0.2 | |||
| TO-1 | auto-LCL | 0901/0405 | None | 1.2 |
| b3a2 | 55.6 | |||
| allo-LCL #1 | 0901/1406 | None | 1.9 | |
| b3a2 | 56.9 | |||
| allo-LCL #2 | 0901/1406 | None | −0.2 | |
| b3a2 | 54.8 | |||
| allo-LCL #3 | 1406/1502 | None | −0.1 | |
| b3a2 | 2.0 |
Abbreviations: auto, autologous; allo, allogeneic.
The cytotoxicities of MY-1 and TO-1 to various target cells in the presence and absence of b3a2 synthetic peptide were determined by 4-hour 51Cr release assays at an effector:target ratio of 5:1.
Cytokine production by bcr-abl–specific CD4+ T-cell clones.
MY-1 and TO-1 were cultured with autologous MMC-treated PBMCs as APCs in the presence or absence of peptide and the supernatants were analyzed for the production of IL-3, IL-4, IL-10, interferon-γ (IFN-γ), TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF). As shown in Table 3, MY-1 and TO-1 secreted all six cytokines after stimulation with b3a2 fusion peptide and, therefore, they were both classified as Th0 type CD4+ T-cell clones.
Cytokine Production by bcr-abl–Specific CD4+ T-Cell Clones
| Cells Cultured . | Peptide Stimulation . | Cytokine Production (pg/mL)* . | |||||
|---|---|---|---|---|---|---|---|
| IL-3 . | IL-4 . | IL-10 . | TNF-α . | IFN-γ . | GM-CSF . | ||
| MY-1 + auto APC | None | 0 | 0 | 5 | 39 | 57 | 115 |
| b3a2 | 131 | 504 | 1,324 | 953 | 2,756 | 1,786 | |
| MY auto APC alone | b3a2 | 0 | 0 | 16 | 84 | 4 | 11 |
| TO-1 + auto APC | None | 7 | 0 | 77 | 93 | 59 | 8 |
| b3a2 | 141 | 442 | 1,557 | 654 | 3,115 | 1,815 | |
| TO auto APC alone | b3a2 | 0 | 0 | 13 | 94 | 51 | 16 |
| Cells Cultured . | Peptide Stimulation . | Cytokine Production (pg/mL)* . | |||||
|---|---|---|---|---|---|---|---|
| IL-3 . | IL-4 . | IL-10 . | TNF-α . | IFN-γ . | GM-CSF . | ||
| MY-1 + auto APC | None | 0 | 0 | 5 | 39 | 57 | 115 |
| b3a2 | 131 | 504 | 1,324 | 953 | 2,756 | 1,786 | |
| MY auto APC alone | b3a2 | 0 | 0 | 16 | 84 | 4 | 11 |
| TO-1 + auto APC | None | 7 | 0 | 77 | 93 | 59 | 8 |
| b3a2 | 141 | 442 | 1,557 | 654 | 3,115 | 1,815 | |
| TO auto APC alone | b3a2 | 0 | 0 | 13 | 94 | 51 | 16 |
Abbreviation: auto, autologous.
T-cell clones were cultured in the presence of autologous APCs with and without b3a2 synthetic peptide for 72 hours, and the concentration of each cytokine in the culture supernatant was determined by ELISA.
Expression of cytolytic mediators by T-cell clones.
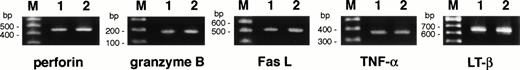
Because the mechanism underlying CD4+ CTL-mediated cytotoxicity is obscure, we examined the expression of cytolytic mediators reported to be important in cytotoxicity mediated by CD8+ and CD4+ CTLs and natural killer cells, namely perforin, granzyme B, Fas ligand, TNF-α, and lymphotoxin, using the RT-PCR. As shown in Fig 1, mRNAs for all cytolytic mediators examined were expressed in both MY-1 and TO-1. Flow cytometric analysis showed that TNF-α and lymphotoxin-α and -β were expressed on MY-1 and TO-1 as membrane-bound forms (data not shown).
Expression of cytolytic mediator mRNAs in bcr-abl–specific CD4+ T-cell clones. Expression of perforin, granzyme B, Fas ligand, TNF-, and lymphotoxin-β mRNAs was investigated by RT-PCR, as detailed in Materials and Methods. The mRNAs were extracted from MY-1 (lane 1) and TO-1 (lane 2) after stimulation with the b3a2 peptide and autologous APCs for 5 days. Lane M shows 100-bp ladder markers.
Expression of cytolytic mediator mRNAs in bcr-abl–specific CD4+ T-cell clones. Expression of perforin, granzyme B, Fas ligand, TNF-, and lymphotoxin-β mRNAs was investigated by RT-PCR, as detailed in Materials and Methods. The mRNAs were extracted from MY-1 (lane 1) and TO-1 (lane 2) after stimulation with the b3a2 peptide and autologous APCs for 5 days. Lane M shows 100-bp ladder markers.
Augmentation of CML cell colony formation by bcr-abl–specific CD4+ T-cell clones.
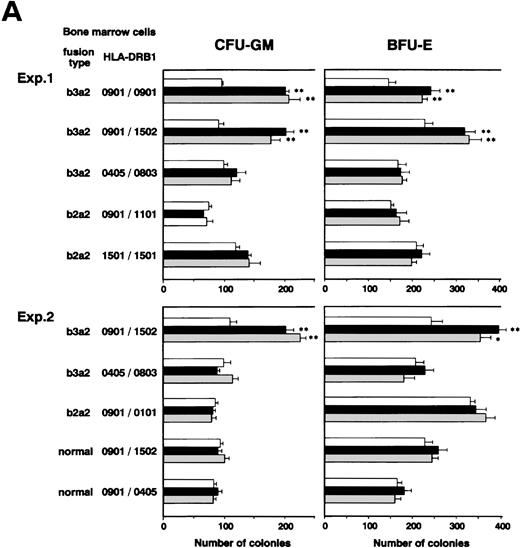
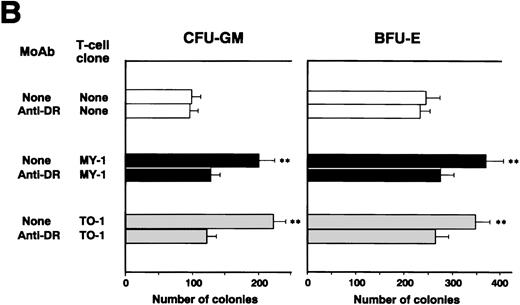
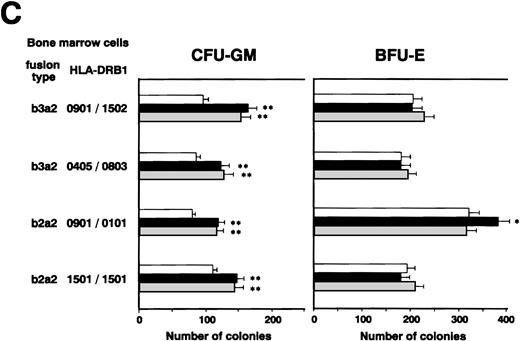
We investigated whether CD4+ CTLs can inhibit CML cell growth by examining the effects of b3a2-specific CD4+ CTL clones on colony formation by CML cells. Unexpectedly, as shown in Fig 2A, the numbers of CFU-GM and BFU-E generated from bone marrow cells of two patients with b3a2 CML who were HLA-DRB1*0901 positive were augmented significantly after coculture with MY-1 and TO-1. The augmentative effects of MY-1 and TO-1 on CML cell colony formation were antigen specific and HLA-DRB1*0901 restricted, as these clones had no effect on colony formation by b2a2 type or HLA-DRB1*0901-negative CML cells and HLA-DRB1*0901-positive normal bone marrow cells. In addition, augmentation of CML cell colony formation by MY-1 and TO-1 was inhibited by anti–HLA-DR MoAb (Fig 2B). The numbers of colonies formed by b2a2 as well as b3a2 CML cells increased when they were cultured in the presence of MY-1 and TO-1 culture supernatants, in comparison with those of CML cells grown in the absence of the culture supernatants (Fig 2C). These data strongly suggest that CD4+ CTLs are not cytotoxic against CML cells, but in fact augment CML cell growth by producing myelostimulatory cytokines, such as IL-3 and GM-CSF, through recognition of leukemia cells in an antigen-specific and HLA-restricted manner.
Augmentation of CML cell colony formation by bcr-abl–specific CD4+ T-cell clones. (A) The numbers of CFU-GM and BFU-E generated from bone marrow cells of various patients in the absence (□) and presence of b3a2-specific CD4+T-cell clones, MY-1 (▪), and TO-1 (▧) are shown. (B) The numbers of CFU-GM and BFU-E generated from bone marrow cells of a patient with HLA-DRB1*0901-positive b3a2 CML which had been untreated or treated with anti–HLA-DR MoAb and cultured without (□) or with MY-1 (▪) and TO-1 (▧) are shown. (C) The numbers of CFU-GM and BFU-E generated from bone marrow cells of various patients with CML in the absence (□) and presence of MY-1 (▪) and TO-1 (▧) culture supernatant are shown. Cells in triplicate wells were cultured and the data are expressed as mean colony counts ± standard deviation. *, P< .05; **, P < .01.
Augmentation of CML cell colony formation by bcr-abl–specific CD4+ T-cell clones. (A) The numbers of CFU-GM and BFU-E generated from bone marrow cells of various patients in the absence (□) and presence of b3a2-specific CD4+T-cell clones, MY-1 (▪), and TO-1 (▧) are shown. (B) The numbers of CFU-GM and BFU-E generated from bone marrow cells of a patient with HLA-DRB1*0901-positive b3a2 CML which had been untreated or treated with anti–HLA-DR MoAb and cultured without (□) or with MY-1 (▪) and TO-1 (▧) are shown. (C) The numbers of CFU-GM and BFU-E generated from bone marrow cells of various patients with CML in the absence (□) and presence of MY-1 (▪) and TO-1 (▧) culture supernatant are shown. Cells in triplicate wells were cultured and the data are expressed as mean colony counts ± standard deviation. *, P< .05; **, P < .01.
DISCUSSION
Recently, reports describing the immunogenicity of synthetic bcr-abl fusion peptide to CD4+ as well as CD8+ T lymphocytes of healthy individuals have been accumulating. Binding of b3a2 fusion peptides to HLA class I alleles A3, A11, B8, and B44 has been reported and these peptides have been shown to prime CD8+ CTLs in vitro.4-8 Furthermore, b3a2 peptides have been shown to induce HLA-DR1(DRB1*0101)-, DR2 (DRB1*1501)-, DR4 (DRB1*0401)-, and DR11 (DRB1*1101)-restricted proliferative responses of CD4+ T lymphocytes.9-12 In this study, we showed for the first time that b3a2 fusion peptide can also elicit a proliferative response of CD4+ T lymphocytes restricted by HLA-DR9 (DRB1*0901), the most frequent HLA-DR allele in the Japanese population. We also believe this study is the first in which b3a2-specific CD4+CTL clones have been established.
It is now well known that endogenous and exogenous antigens are processed and expressed on MHC class I and class II, respectively. However, recent studies have shown that endogenous proteins can also be expressed on MHC class II molecules and recognized by CD4+T lymphocytes.24-29 These reports suggest that bcr-abl–specific CD4+ T lymphocytes can distinguish CML and normal cells directly via recognition of the bcr-abl fusion peptide in the context of HLA class II expressed on leukemic cells. The recent study of ten Bosch et al10 showed that a b3a2-specific CD4+ T-cell line proliferated in response to stimulation with b3a2-positive CML blasts in an HLA-DR–restricted manner, which strongly suggests that CD4+ T lymphocytes can directly recognize bcr-abl fusion peptide that is naturally processed and expressed on CML cells. Although they observed a proliferative response of CD4+ T lymphocytes to CML cells, they did not show cytotoxic activity against CML blasts. In our study, although b3a2-specific CD4+ CTL clones exerted strong cytotoxicity against b3a2 peptide-loaded B-LCL, these clones did not inhibit, but actually augmented, colony formation by HLA-DR–matched CML cells. There are several possible explanations for the failure of CD4+ CTLs to inhibit CML cell growth. The first hypothesis is that the complexes of b3a2 peptide and HLA-DR molecules that CD4+ CTLs recognize are expressed only on some populations of chronic phase CML cells that have reached a certain differentiation level and that the CML progenitor cells that proliferate in in vitro assays do not express the bcr-abl fusion protein and/or HLA-DR. The second hypothesis is that CD4+ CTLs recognize bcr-abl fusion peptide in the context of the HLA class II expressed on CML cells, but the CML cells are resistant to CD4+ CTL-mediated cytotoxicity. MY-1 and TO-1 both seemed to express cytolytic mediators reported to be important for CTL- and natural killer cell–mediated cytotoxicity, namely perforin, granzyme B, Fas ligand, TNF-α, and lymphotoxin,30 but the main cytotoxic pathway of these CD4+ CTL clones is unknown. The Fas/Fas ligand system has been reported to account for the main pathway of CD4+CTL-mediated cytotoxicity in a murine system31-33 and CML cells have been reported to be sensitive to Fas-mediated apoptosis.34 We found that colony formation by CML cells was inhibited markedly by adding the agonistic anti-Fas MoAb to the assay medium (data not shown). In the light of these findings, the second hypothesis seems unlikely, but it cannot be excluded, because recently, we found that peptide-specific human CD4+ CTL clones lysed peptide-loaded Fas-deficient mutant target cells (manuscript in preparation). Therefore, the details of the cytotoxic mechanism mediated by bcr-abl fusion peptide-specific CD4+CTL clones and the sensitivities of CML cells to various cytolytic mediators need to be elucidated to resolve this issue. The third hypothesis, which we think is the most important, is that bcr-abl peptide cannot be expressed on CML cells as suggested by Pawelec et al,11 who showed that bcr-abl peptide-specific CD4+ T lymphocytes did not recognize CML cells. At present, the definitive reason for the lack of inhibition of CML cell growth by CD4+ CTLs is unknown and further studies to explore the above hypotheses are necessary.
Coculture of CML cells and bcr-abl peptide-specific CD4+T-cell clones resulted in increased numbers of CML cell colonies in comparison with those formed by CML cells cultured alone. These augmentative effects of CD4+ T-cell clones on CML cell growth were b3a2 specific and HLA-DRB1*0901 restricted, suggesting that bcr-abl–specific CD4+ T lymphocytes can recognize bcr-abl fusion protein in the context of HLA-DR molecules. There are two possible mechanisms whereby bcr-abl protein is recognized by CD4+ T lymphocytes. One is that CD4+ T lymphocytes may directly recognize the bcr-abl peptide and HLA-DR molecule complexes expressed on CML cells. The report by ten Bosch et al10 supports this hypothesis, as described above, but further studies are necessary to confirm this issue, as conflicting data have been also reported.11 The other mechanism is that bcr-abl protein released from CML cells as a result of cell death was processed and expressed on live CML cells or residual normal APCs. Evidence that bcr-abl peptide-specific CD4+ T lymphocytes can respond to peptides derived from purified whole bcr-abl fusion protein and cell lysates containing bcr-abl protein is accumulating. Murine CD4+ T lymphocytes specific for b3a2 peptide have been shown to proliferate in response to whole-fusion protein purified from a cell extract,35 and recently, human b3a2 peptide-specific CD4+ T lymphocytes were reported to respond to APCs exposed to b3a2-containing cell lysates.12Furthermore, the findings of Jiang et al36 that CML cells can process and present exogenous antigens suggests that CML cells themselves can present antigens to CD4+ T lymphocytes. These recent reports lend strong support to our observation that bcr-abl–specific CD4+ T lymphocytes responded to bcr-abl fusion peptide derived from CML cells.
In summary, we have reported the establishment and functional characterization of bcr-abl–specific CD4+ CTL clones. It should be noted that the present data do not mean that bcr-abl–specific CD4+ T lymphocytes are ineffective for immunotherapy of CML. In animal models, the adoptive transfer of immune tumor antigen-specific CD8+ T lymphocytes alone had an antitumor effect, but the combination of antigen-specific CD4+ and CD8+ T lymphocytes was generally more effective.37 Therefore, the development of combination adoptive therapy involving various types of immunocompetent cells, including bcr-abl–specific CD4+ and CD8+ T lymphocytes, and effective APCs, such as dendritic cells, is expected to lead to the development of new effective immunotherapy for CML.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan; the Mochida Foundation for Medical and Pharmaceutical Research; the Inamoro Foundation; and the Suzuken Memorial Foundation.
Address reprint requests to Masaki Yasukawa, MD, PhD, The First Department of Internal Medicine, Ehime University School of Medicine, Shigenobu, Ehime 791-0295, Japan; e-mail: yasukawa@m.ehime-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal