Abstract
Hemangioblastomas are highly vascular tumors of the central nervous system that overexpress the hypoxia-inducible gene, vascular endothelial growth factor (VEGF), as a consequence of mutational inactivation of the von Hippel-Lindau tumor suppressor gene (VHL). Previous reports showed that hemangioblastomas can also express erythropoietin (Epo), which is also hypoxia-inducible. However, Epo expression in hemangioblastomas was observed only in individual cases, and the analyses were mainly based on indirect determination of erythropoiesis-stimulating activity. Therefore, we analyzed a series of 11 hemangioblastomas for Epo, VEGF, and VHL expression by Northern blot analysis and compared the results with normal brain and glioblastomas. Surprisingly, we observed Epo mRNA expression in all hemangioblastoma specimens analyzed, but in none of four glioblastomas. In contrast, VEGF mRNA was expressed in all hemangioblastomas and all glioblastomas. In situ hybridization revealed neoplastic stromal cells as Epo- and VEGF-producing cells in hemangioblastomas. These results suggest that in the nonhypoxic microenvironment of hemangioblastoma, Epo, similar to VEGF, might be negatively regulated by the VHL gene product.
© 1998 by The American Society of Hematology.
ERYTHROPOIETIN (Epo) is a glycoprotein hormone that acts as major regulator of erythropoiesis. The site of Epo production switches during development from fetal liver to adult kidney, with low-level expression remaining in adult liver.1 It is well established that Epo gene expression is controlled by oxygen tension. Hypoxic and anemic conditions result in elevated Epo expression in mammals.2 Until recently, erythroid precursor cells have been thought to be the exclusive target site for Epo. However, recent reports suggest that Epo may also be involved in neuronal functions. Epo gene expression was detected in human, monkey, and murine brain by quantitative reverse transcriptase–polymerase chain reaction,3 and Epo protein was found in cerebrospinal fluid.4 Epo binding sites were localized in defined areas of the mouse brain.5Furthermore, in the brain of hypoxic rodents and primates, increased Epo mRNA levels were observed.3 6
Epo production has also been reported in a variety of human tumors, particularly renal cell carcinomas and cerebellar hemangioblastomas. Cerebellar hemangioblastomas are highly vascularized cystic tumors typically consisting of blood-filled capillaries separated by intervascular stromal cells. The histologic origin of stromal cells in hemangioblastomas is not defined, but it has been shown that stromal cells are the neoplastic cell type in hemangioblastomas.7,8Current evidence suggests that hemangioblastoma formation is dependent on mutational inactivation of the von Hippel-Lindau tumor suppressor gene (VHL) because (1) hemangioblastomas are the most frequent manifestations of germline VHL mutations (eg, hereditary von Hippel-Lindau disease9) and (2) sporadic nonhereditary hemangioblastomas carry somatic mutations in VHL.10 More recently, VHL has been shown to control tumor angiogenesis by negative regulation of vascular endothelial growth factor (VEGF) expression.11-13
VEGF is upregulated as much as 50-fold in certain types of brain tumors, and exceptionally high levels have been found in the highly vascularized hemangioblastomas and in glioblastomas.14,15In hemangioblastomas, VEGF is expressed in almost all stromal cells,14,16 whereas in glioblastomas, VEGF expression is restricted to tumor cells surrounding necrotic areas.17,18Immunohistochemical studies and in situ hybridization demonstrated that hypoxia is a microenvironmental stimulus for upregulation of VEGF in glioblastomas.17,19 However, in hemangioblastomas, it is unlikely that hypoxia is the major trigger of VEGF expression, since these tumors are highly vascularized and show no signs of necrosis. Interestingly, hypoxia-induced regulation of VEGF and Epo synthesis is based on similar regulatory mechanisms involving transcriptional activation and increased mRNA stability.20-22 Besides VEGF, other hypoxia-inducible genes seem to be negatively regulated by the VHL gene product11 and can thus be activated by a hypoxia-independent mechanism. Although Epo has been suggested to be a possible target gene for VHL regulation, extensive studies of Epo expression in hemangioblastomas are missing. Therefore, we investigated a series of capillary hemangioblastomas by Northern blot analysis for expression of Epo. In comparison to normal cerebrum and normal cerebellum, all analyzed hemangioblastomas showed upregulation of Epo mRNA levels. In situ hybridization revealed coexpression of Epo with VEGF in stromal cells, suggesting that Epo expression may be under control of the VHL protein.
MATERIALS AND METHODS
Clinical data.
All clinical data were obtained from patient records at the Neurocenter, Freiburg University Medical School, Freiburg, Germany.
Specimens.
All tumor specimens (11 hemangioblastomas and four glioblastomas) were obtained from the brain tumor bank of the Department of Neuropathology, Freiburg University Medical Center, at random. Tumor specimens were immediately snap-frozen after removal and stored at −70°C. As a control, normal cerebellum and normal cerebrum from two patients without neurologic disease were collected postmortem and snap-frozen in liquid nitrogen.
RNA extraction and Northern analysis.
Total RNA was isolated using the guanidinium thiocyanate method.23 Aliquots of 20 μg total RNA were separated in 1% agarose gel containing 0.66 mol/L formaldehyde in 1× MOPS buffer (40 mmol/L 3-(N-morpholino)propanesulfonic acid, 10 mmol/L sodium acetate, and 1 mmol/L EDTA, pH 7.2) and transferred onto a nylon membrane (Duralon, Stratagene, La Jolla, CA) by capillary blotting in 20× SSC (1× SSC is 150 mmol/L NaCl plus 15 mmol/L sodium citrate). For hybridizations, a human Epo cDNA (pe49f; kindly provided by Dr C. Shoemaker, Boston, MA), a human VEGF cDNA (kindly provided by Dr H. Weich, Braunschweig, Germany), and a human VHL cDNA (kindly provided by Dr B. Zbar, Frederick, MD) were labeled with32P-dCTP using the random priming labeling kit (Stratagene). β-Actin cDNA was used for control hybridizations as described previously.7
In situ hybridization.
The technique used for in situ hybridization was essentially as described by Breier et al.24 RNA probes were generated by in vitro transcription of the plasmid pBShEPO3, a partial human Epo cDNA clone. This plasmid was obtained by subcloning a 433-bpKpn I-Stu I fragment spanning exon 2 to exon 5 of the human Epo cDNA clone pe49f in BluescriptSK+. Single-strand antisense or sense RNA probes were generated using 100 μCi 35S-UTP and T3 or T7 RNA polymerases as described by the manufacturer (Stratagene). Snap-frozen hemangioblastomas embedded in tissue tec (Sakura, Torrance, CA) were sectioned in a cryostat (Zeiss, Oberkochen, Germany). Ten-micrometer sections, two on each slide, were melted on 3-Aminopropyl-trimethoxysilan–coated (Fluka, Bucks, Switzerland) glass slides. Sections were incubated in 2× SSC at 70°C, digested with pronase (40 μg/mL), fixed in 4% paraformaldehyde, and acetylated with acetic anhydride diluted 1:400 with 0.1 mol/L triethanolamine. Hybridization was performed in buffer containing 50% formamide, 10% dextran sulfate, 10 mmol/L Tris hydrochloride (pH 7.5), 10 mmol/L sodium phosphate (pH 6.8), 2× SSC, 5 mmol/L EDTA, 150 μg/mL yeast tRNA, 0.1 mmol/L UTP, 1 mmol/L ADPβS, 1 mmol/L ATPγS, 10 mmol/L dithiothreitol, 10 mmol/L 2-mercaptoethanol, and 2.5 to 5 × 104 cpm/mL35S-labeled RNA probe overnight at 48°C. The slides were washed in 2× SSC/50% formamide at 37°C for 4 hours, digested with RNase (20 μg/mL) for 15 minutes, washed again with 2× SSC/50% formamide overnight, and dehydrated in graded ethanol. They were then coated with Kodak NTB-2 emulsion (Eastman Kodak, Rochester, NY) diluted 1:1 in water and exposed for 3 to 4 weeks. The slides were developed and counterstained with toluidine blue, air-dried, and mounted.
For identification of mast cells in hemangioblastomas, paraffin sections were stained with toluidine blue according to standard protocols.
RESULTS
Clinical Data
We analyzed seven hemangioblastomas from six patients with clinically confirmed VHL disease and four hemangioblastomas from four patients without clinical evidence of VHL disease. Four glioblastomas from four patients without evidence of VHL disease were used for comparison. Preoperative hemoglobin and hematocrit levels were available in 9 of 10 hemangioblastoma patients and in all glioblastoma patients (Table1). Hemoglobin and hematocrit levels in the patients were within the normal range, with the exception of one hemangioblastoma patient who presented with a slightly decreased hemoglobin level. However, in general, hemoglobin and hematocrit levels were higher in hemangioblastoma versus glioblastoma patients (hemoglobin, 14.7 ± 1.5 v 13.0 ± 1.1 g/L; hematocrit, 43.7% ± 3.6% v 39.2% ± 2.5%, respectively).
Clinical Data of Patients With Hemangioblastomas and Glioblastomas
| Lane No./Diagnosis . | Age (yr)/ Sex . | Tumor Location . | Hemoglobin (g/L) . | Hematocrit (%) . | VHL Syndrome . |
|---|---|---|---|---|---|
| 3/Hemangioblastoma | 45/M | Cerebellum | NA | NA | Yes-150 |
| 4/Hemangioblastoma | 25/F | Cerebellum | NA | NA | No |
| 5/Hemangioblastoma | 38/F | Cerebellum | 14.8 | 43.3 | No |
| 6/Hemangioblastoma | 56/M | Spinal | 13.1 | 39.9 | No-151 |
| 7/Hemangioblastoma | 26/F | Cerebellum | 14.8 | 45.9 | Yes |
| 8/Glioblastoma | 70/F | Temporal | 12.2 | 37.2 | No |
| 9/Glioblastoma | 72/F | Parietal | 12.1 | 36.9 | No |
| 12/Hemangioblastoma | 40/M | Cerebellum | 14.6 | 42.1 | Yes |
| 13/Hemangioblastoma | 24/M | Medulla | 14.6 | 42.9 | Yes |
| 14/Hemangioblastoma | 34/F | Cerebellum | 11.8 | 37.6 | Yes |
| 15/Hemangioblastoma | 44/F | Cerebellum | 16.2 | 47.7 | No |
| 16/Hemangioblastoma | 19/M | Cerebellum | 15.7 | 45.5 | Yes |
| 17/Hemangioblastoma | 49/M | Cerebellum | 16.5 | 48.5 | Yes-150 |
| 18/Glioblastoma | 37/F | Parietal | 14.1 | 41.6 | No |
| 19/Glioblastoma | 61/M | Occipital | 13.8 | 41.0 | No |
| Lane No./Diagnosis . | Age (yr)/ Sex . | Tumor Location . | Hemoglobin (g/L) . | Hematocrit (%) . | VHL Syndrome . |
|---|---|---|---|---|---|
| 3/Hemangioblastoma | 45/M | Cerebellum | NA | NA | Yes-150 |
| 4/Hemangioblastoma | 25/F | Cerebellum | NA | NA | No |
| 5/Hemangioblastoma | 38/F | Cerebellum | 14.8 | 43.3 | No |
| 6/Hemangioblastoma | 56/M | Spinal | 13.1 | 39.9 | No-151 |
| 7/Hemangioblastoma | 26/F | Cerebellum | 14.8 | 45.9 | Yes |
| 8/Glioblastoma | 70/F | Temporal | 12.2 | 37.2 | No |
| 9/Glioblastoma | 72/F | Parietal | 12.1 | 36.9 | No |
| 12/Hemangioblastoma | 40/M | Cerebellum | 14.6 | 42.1 | Yes |
| 13/Hemangioblastoma | 24/M | Medulla | 14.6 | 42.9 | Yes |
| 14/Hemangioblastoma | 34/F | Cerebellum | 11.8 | 37.6 | Yes |
| 15/Hemangioblastoma | 44/F | Cerebellum | 16.2 | 47.7 | No |
| 16/Hemangioblastoma | 19/M | Cerebellum | 15.7 | 45.5 | Yes |
| 17/Hemangioblastoma | 49/M | Cerebellum | 16.5 | 48.5 | Yes-150 |
| 18/Glioblastoma | 37/F | Parietal | 14.1 | 41.6 | No |
| 19/Glioblastoma | 61/M | Occipital | 13.8 | 41.0 | No |
Lane numbers refer to Fig 1. All hemoglobin and hematocrit values represent measurements prior to tumor resection. The diagnosis of VHL syndrome is based on clinical data alone.
Abbreviations: F, female; M, male; NA, not available.
Patient with multiple cerebellar hemangioblastomas with surgery at 2 different time points.
Patient with recurrent spinal hemangioblastomas but no other stigmata of VHL syndrome.
Northern Analysis
Expression of VEGF mRNA.
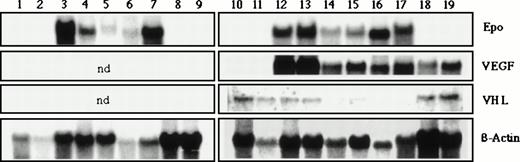
We investigated some of the tumor and control specimens for expression of the hypoxia-inducible angiogenesis factor VEGF (Fig1). We observed a strong upregulation of VEGF expression in all hemangioblastomas (lanes 12 to 17) and glioblastomas (lanes 18 and 19) analyzed, consistent with our previous findings and reports from other groups. Under the hybridization conditions we used, no VEGF mRNA was detectable in normal cerebellum and normal cerebrum (lanes 10 and 11).
Northern blot analysis of total RNA from different human brain tissues: lanes 1 and 10, normal cerebrum; 2 and 11, normal cerebellum; 3-7 and 12-17, eleven different capillary hemangioblastomas; 8, 9, 18, and 19, four different glioblastomas. The membranes were hybridized with 32P-labeled human Epo cDNA and partially reprobed with human VEGF and VHL probes. Hybridization with β-actin served as a loading control (ND, not determined).
Northern blot analysis of total RNA from different human brain tissues: lanes 1 and 10, normal cerebrum; 2 and 11, normal cerebellum; 3-7 and 12-17, eleven different capillary hemangioblastomas; 8, 9, 18, and 19, four different glioblastomas. The membranes were hybridized with 32P-labeled human Epo cDNA and partially reprobed with human VEGF and VHL probes. Hybridization with β-actin served as a loading control (ND, not determined).
Expression of Epo mRNA.
We analyzed 11 surgically removed hemangioblastomas, four surgically removed glioblastomas, and four normal brain specimens obtained postmortem for expression of Epo mRNA by Northern blot analysis using a human Epo cDNA as the hybridization probe (Fig 1). Epo mRNA (size, 1.6 kb) could be detected in all hemangioblastomas tested, albeit at different levels. High amounts of Epo mRNA were present in six of 11 hemangioblastomas (lanes 3, 7, 12, 13, 16, and 17), four samples exhibited intermediate levels (lanes 4, 6, 14, and 15), and only one of 11 hemangioblastomas showed a weak signal (lane 5). In contrast, Epo mRNA expression was not detectable either in normal brain tissues (cerebellum, lanes 2 and 11; cerebrum, lanes 1 and 10) or in the four different glioblastomas tested (lanes 8, 9, 18, and 19).
Expression of VHL mRNA.
In hemangioblastomas, VHL mRNA was detected in three of six specimens (Fig 1, lanes 12, 13, and 15). The lack of VHL expression in three hemangioblastomas might reflect VHL inactivation by transcriptional silencing due to gene hypermethylation, which has previously been described to occur in a proportion of VHL loss of function–associated tumors, including hemangioblastomas.25 In hemangioblastomas that expressed VHL mRNA, the protein is most likely mutated.10 In all normal brain specimens, VHL mRNA was expressed at similar levels. Glioblastomas also expressed VHL mRNA. We assume that both normal brain and glioblastomas express wild-type VHL, since glioblastomas are not associated with VHL disease and somatic mutations, to our knowledge, have not been described. Blots were rehybridized with a β-actin probe as the loading control.
In Situ Hybridization
To determine the nature of Epo-producing cells in hemangioblastomas, we performed in situ hybridization and compared the results with our previous findings on VEGF mRNA in situ hybridization.7,16 A strong hybridization signal was observed in stromal cells of hemangioblastomas (Fig 2B and E), whereas endothelial cells remained negative. Compared with VEGF mRNA, which we found to be highly expressed in almost all stromal cells, Epo mRNA showed a weaker expression level in stromal cells. In addition, Epo mRNA was not expressed in all stromal cells. However, by morphologic analysis, we were unable to observe differences between stromal cells that expressed Epo and those that did not. Since, by immunohistochemistry, expression of Epo in hemangioblastomas has been described in stromal cells and mast cells,26 we stained tissue sections with toluidine blue. Within a given tumor, the number of Epo mRNA–expressing cells significantly exceeded the number of mast cells, suggesting that Epo mRNA–expressing cells are stromal cells. Control hybridization using a sense Epo probe did not show significant staining (Fig 2C and F). In glioblastoma cells, no Epo mRNA expression was observed (not shown). In normal cerebellum specimens, a weak Epo mRNA signal was observed in Purkinje cells (not shown).
In situ hybridization for Epo mRNA in a human hemangioblastoma using 35S-labeled antisense and sense cRNA probes. Sections were counterstained with toluidine blue. Epo mRNA was detected in intervascular stromal cells. A-C, 100× original magnification; D-F, 400× original magnification; A and D, bright field illumination; B and E, dark field illumination; C and F, sense (control) hybridization and dark field illumination.
In situ hybridization for Epo mRNA in a human hemangioblastoma using 35S-labeled antisense and sense cRNA probes. Sections were counterstained with toluidine blue. Epo mRNA was detected in intervascular stromal cells. A-C, 100× original magnification; D-F, 400× original magnification; A and D, bright field illumination; B and E, dark field illumination; C and F, sense (control) hybridization and dark field illumination.
DISCUSSION
Previous reports on Epo expression in hemangioblastomas of the nervous system were mainly based on the measurement of erythropoiesis-stimulating activity in the fluid of cysts and tumor extracts.27,28 Although hemangioblastomas are known for the capability to induce erythrocytosis, data on mRNA and protein expression in tumor tissues are sparse. Therefore, we examined 11 surgically removed and snap-frozen hemangioblastomas for expression of Epo mRNA by Northern blot analysis. Surprisingly, Epo mRNA could be detected in all hemangioblastomas tested, whereas Epo mRNA expression was not present either in normal brain or in four different glioblastomas. These findings suggest that expression of Epo is intrinsic to all hemangioblastomas and might be of importance for hemangioblastoma development and progression. In contrast to hemangioblastomas, glioblastoma development and progression appear to be independent from Epo production, since Epo mRNA could not be detected. This observation is consistent with the finding that glioblastomas are not known to induce erythrocytosis. To investigate the mechanism responsible for selective Epo upregulation in hemangioblastomas, we analyzed a portion of the tissue specimens for expression of the hypoxia-inducible angiogenesis factor VEGF. By Northern analysis, we observed a strong upregulation of VEGF expression in all hemangioblastomas and glioblastomas analyzed, whereas in normal cerebellum and normal cerebrum, no VEGF mRNA was detectable. These findings are compatible with different regulatory mechanisms of VEGF and Epo expression in hemangioblastomas and glioblastomas. Current evidence suggests that VEGF expression in glioblastomas is driven by hypoxia, leading to activation of VEGF gene transcription and an increase in mRNA stability.19,22 Epo is also hypoxia-inducible, but was not expressed in glioblastomas. Failure of glioblastoma cells to produce Epo might reflect a highly cell-specific expression pattern of Epo, inhibiting Epo expression in glioblastoma cells even under hypoxic conditions, although in vitro normal astrocytes have been shown to be a source of Epo.3,29 In contrast to glioblastomas, hemangioblastomas are nonhypoxic tumors but also express high levels of VEGF. The underlying mechanism of high VEGF expression in hemangioblastomas has recently been identified, since VEGF gene transcription and mRNA stability in VHL-deficient tumor cells are increased in a hypoxia-independent manner.11-13 We therefore examined VHL expression in all tissue specimens. We observed similar amounts of VHL mRNA in normal brain and all glioblastomas by Northern blot analysis. However, only three of six hemangioblastomas expressed VHL mRNA. In addition, hemangioblastomas consistently contain mutations in the VHL gene, whereas glioblastomas express wild-type VHL. Given the common regulatory pathway of Epo and VEGF expression by hypoxia21 and the finding that VHL inactivation leads to upregulation of hypoxia-inducible genes, it is tempting to speculate that VHL loss of function in hemangioblastomas leads to upregulation of Epo. Indeed, we observed coexpression of VEGF and Epo in hemangioblastoma stromal cells. Moreover, previous reports identified stromal cells as VEGF-producing cells in hemangioblastomas,14,16 which have also been shown to selectively carry VHL mutations.8 These findings suggest that both VEGF and Epo are upregulated in hemangioblastoma stromal cells due to VHL loss of function. However, coexpression of VEGF mRNA and Epo mRNA was not observed in all stromal cells, since VEGF-expressing cells outnumbered Epo-expressing cells. One possible explanation is that in hemangioblastomas, there are subsets of stromal cells with different capacities to express Epo.
The precise role of Epo expression in hemangioblastomas remains speculative. Tumor-induced upregulation of Epo mRNA can result in secondary erythrocytosis, which was described in some tumors of the central nervous system, for example, hemangioblastoma30 and meningioma.31 In capillary hemangioblastomas, secondary erythrocytosis has been reported in up to 20% of patients.32 None of our hemangioblastoma patients showed erythrocytosis clinically. However, interestingly, hemoglobin and hematocrit levels were higher in hemangioblastoma versus glioblastoma patients. We assume that in hemangioblastomas, the blood-brain barrier is leaky due to overexpression of VEGF, which induces vascular permeability, and Epo is able to enter the systemic circulation. However, the amount of Epo may not be sufficient to induce clinically overt erythrocytosis, instead leading to an insignificant increase of hemoglobin and hematocrit levels. In addition to these systemic effects, Epo expression in hemangioblastomas may have functions that are confined to the tumor microenvironment. In a detailed study of 26 hemangioblastomas, foci of extramedullary hematopoiesis characterized by islands of normoblasts were detected in four tumors.33These islands were found mostly within or adjacent to capillaries and within areas of stromal cells. One possible function of Epo expression in hemangioblastomas could be to act as a locally produced stimulus for erythroid differentiation. In this context, it is noteworthy that hemoglobin, a marker for erythroid differentiation, was found to be present in neurons, where it may be involved in oxygen homeostasis.34
There are also reports suggesting that Epo might play a role in angiogenesis. Epo receptors have been shown to be expressed on rat brain capillary endothelial cells35 and on human endothelial cells, and Epo can induce cell proliferation and migration of endothelial cells.36,37 Furthermore, Epo has been shown to stimulate angiogenesis on rat aortic rings in vitro.38However, since we have been unable to detect Epo receptor expression in hemangioblastomas by Northern analysis (not shown), evidence for a role of Epo in tumor angiogenesis is still lacking.
An important question is whether Epo expression, similar to VEGF expression, is under control of the VHL protein. Experiments to solve this problem are hampered by the fact that VHL-deficient hemangioblastoma cell lines are not available and that most available VHL-deficient renal cell carcinoma cell lines lack Epo expression. However, by Northern blot analysis, human renal clear cell carcinomas were also shown to produce Epo39 and high amounts of VEGF.40 Interestingly, renal clear cell carcinomas are also known to be affected by mutations of the VHL gene and are associated with hereditary VHL disease.41
In this study, we have shown strong Epo expression in hemangioblastomas and identified stromal cells as Epo-producing cells. In concordance with previous observations that stromal cells also express VEGF and mutant VHL, these findings suggest that loss of function of the VHL gene product may also upregulate Epo expression in hemangioblastomas.
ACKNOWLEDGMENT
We thank Dr C. Shoemaker (Boston, MA) for providing human Epo cDNA, Dr B. Zbar (Frederick, MD) for human VHL cDNA, Dr H. Weich (Braunschweig, Germany) for human VEGF cDNA, and Richard Haas (Freiburg, Germany) for technical assistance.
Supported by Grant No. C4 from the Center for Clinical Research I, Freiburg University Medical School, Grant No. PL 158-3/1 from the Deutsche Forschungsgemeinschaft (K.H.P.), and in part by fellowships from the Swiss National Science Foundation (Forschungskommission der Universität Zürich) and the Schweizerische Stiftung für Medizinisch-Biologische Stipendien (H.H.M.).
Address reprint requests to Karl H. Plate, MD, Neurozentrum, Abteilung Neuropathologie, Breisacherstr. 64, 79106 Freiburg/Brsg; e-mail: plate@nz11.ukl.uni-freiburg.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal