Abstract
Members of the Myc and Jun/Fos gene families have been found to be expressed in late stages of cutaneous T-cell lymphoma (CTCL) and may be responsible for the transition from low-grade to high-grade tumors. The composition of these complexes is an important parameter, as the different homo- and heterodimeric jun and myc complexes can have gene transcription activating or suppressing activities. We determined the composition of the jun and myc DNA-binding complexes in three CTCL cell lines and malignant cells of seven Sézary patients by electrophoretic mobility shift assays (EMSAs) and “supershift” assays in which specific antibodies against the different members of the tested gene families were included in the binding reactions. Complexes containing JunD were found in three cell lines and two patients. The three cell lines and one patient contained also c-Myc/Max heterodimers. Because c-Myc/Max heterodimers are strong gene transcription activators and are necessary for cell-cycle progression, they may play a role in the progression of CTCL. JunD may also promote cell-cycle progression and influence the expression of cell death survival genes. Interleukin-7 (IL-7) and IL-15, which have been identified as growth factors for CTCL cells, stimulated the DNA binding of JunD and two novel c-Myc recognition site (E-box) binding proteins, but not the DNA binding of c-Myc/Max heterodimers.
CUTANEOUS T-CELL lymphoma (CTCL) is a heterogeneous group of lymphoproliferative disorders of the skin.1 The most frequent forms of CTCL are Mycosis fungoides (MF) and its leukemic counterpart the Sézary syndrome (SS). MF proceeds mostly very slowly (up to 5 to 10 years), but leads ultimately to death, which is often caused in the late phase of CTCL by blastic transformation with rapidly growing and ulcerating tumors and immune disorders.
Patients with SS show, besides skin tumors, generalized erythroderma and leukemic T cells in the blood. Their life expectancy is generally shorter (3 years) than that of patients who suffer from MF.
CTCLs are restricted to the skin during most of the time course of the disease and a systemic spread of malignant cells to other organs occurs only in the very last stages. This is also true for the leukemic variant, the SS, in which malignant cells also occur in the blood.
No data on jun and fos expression in CTCL are available and expression of myc genes in CTCL has only been found in one cell line (HUT782) and in the late stages of the disease.3 Several members of the myc, fos, and jun families have been implied in cancerogenesis, cell-cycle progression, and gene transcription. Therefore, they are supposed to be involved in the transition of CTCLs from low-grade to high-grade lymphoma. The individual members of these three gene families can form different dimers, which can act either as transcriptional enhancers or repressors. Until now, it has not been determined which members of these three oncogene families are involved in gene regulation in CTCL cells. Translocations and overexpression of the most prominent member of the myc gene family, c-myc, are involved in the cancerogenesis of Burkitt’s lymphoma,4plasmacytoma,5 and T-cell lymphoma.6 C-Myc cannot bind alone to DNA and it has to heterodimerize with the protein Max to bind to its DNA target sequence. The c-Myc/Max heterodimer is a potent transactivator7 and implied in the promotion of the cell-cycle and apoptosis, whereas Max/Max homodimers are transcriptional repressors. The proteins, Mad1, Mad2 (=Mxi), Mad3, and Mad4 compete with c-Myc for Max and antagonize the effects of c-Myc on gene transcription and cell-cycle progression. Consequently, they are involved in cell growth arrest and cell differentiation.8
Other members of the myc gene family are N-myc and L-myc, which are involved in the development of neuroblastomas,9 small cell lung cancer,10 and in hematopoietic malignancies.11 The genes, B-myc and S-myc, are less well studied and are in their wild-type forms probably antioncogenes.12-15
The products of the fos gene family (c-fos, fosB, fra-1, and fra-2) can only bind to DNA when they form heterodimers16 with members of the jun gene family (c-jun, junB, junD). In general, c-Fos, Fra-1, Fra-2, c-Jun, and JunD are activators of gene transcription, whereas FosB and JunB are transcription inhibitors or weak activators. The function of the single Jun and Fos proteins depends on the composition of the dimers and on the context of the respective enhancer or promoter region.17 The jun and fos genes are induced by extracellular signals (eg, ultraviolet B [UVB] and phorbol esters) via several mitogen-activated protein kinase (MAPK) cascades.18 Jun/Fos heterodimers and Jun/Jun homodimers, originally described as activation protein-1 (AP-1), bind to a 9-bp recognition sequence, which has been first identified as the TPA (= PMA = phorbolester 12-O-tetradecanoylphorbol-13-acetate) responsive element (TRE).19 Like the myc genes, the jun and fos genes also have an impact on cell-cycle progression.20 21
Interleukin-7 (IL-7) and IL-15 have been identified as B- and T-cell growth-stimulating cytokines,22-24 and they also enhance the growth factors of CTCL cells.25,26 Both are produced by keratinocytes in the skin25 27-29 and may be responsible for the epidermotropism of CTCL cells.
In this work, we investigated which members of the jun and fos family are active in CTCL and whether their activity can be stimulated further by IL-7 and IL-15.
MATERIALS AND METHODS
Cell culture.
The cell line HUT78 (SS) was obtained from ECACC (European Collection of Animal Culture Cells, Salisbury, UK). The cell lines, MyLa (MF) and SeAx (SS) were kind gifts of Dr Keld Kaltoft, University of Aarhus, Aarhus, Denmark.30 31 HUT 78 and MyLa and patient Sézary cells were grown in HEPES buffered RPMI 1640 medium with 2 mmol/L glutamine, supplemented with 10% fetal calf serum (FCS), 0.25 mg/mL amphotericin B, 100 U penicillin G, 100 U streptomycin, and 1 mmol/L pyruvate. SeAx cells were grown under the same conditions with the exception that 10% human serum (HS) instead of 10% FCS was used and that 5 ng/mL (10 U) of IL-7 (R&D Systems, Abingdon, UK) and 10 ng/mL (10 U) of IL-15 (PeproTech, London, UK) were added to the medium.
Blood and skin samples.
Skin biopsy specimens and blood samples from eight patients were taken primarily for diagnostic purposes with informed consent of the patients and the specimens used were surplus material available after all of the routine diagnostic procedures. The diagnoses are summarized in Table1 and were in accordance with standard criteria. The assignment to a certain stage was performed according to the recommendations of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group.32 Sézary cells from patients’ blood were isolated by ficoll gradient centrifugation and sorted by two-color fluorescence-activated cell sorting (FACS) using antibodies against CD 4 and the vβ region of their T-cell receptor,33 when the number of malignant T cells was below 50% of all blood lymphocytes (two patients).
Data of the Patients Analyzed
| Patient No. . | Sex . | Age* . | Diagnosis† . | Stage‡ . |
|---|---|---|---|---|
| 1 | F | 70 | Sézary | III |
| 2 | M | 88 | Sézary | III |
| 3 | F | 47 | Sézary | IVa |
| 4 | F | 63 | Sézary | III |
| 5 | F | 57 | Sézary | IVa |
| 6 | F | 68 | Sézary | III |
| 7 | F | 53 | Sézary | III |
| 8 | M | 40 | Sézary | III |
| Patient No. . | Sex . | Age* . | Diagnosis† . | Stage‡ . |
|---|---|---|---|---|
| 1 | F | 70 | Sézary | III |
| 2 | M | 88 | Sézary | III |
| 3 | F | 47 | Sézary | IVa |
| 4 | F | 63 | Sézary | III |
| 5 | F | 57 | Sézary | IVa |
| 6 | F | 68 | Sézary | III |
| 7 | F | 53 | Sézary | III |
| 8 | M | 40 | Sézary | III |
The average age was 60.75 ± 15.0 years.
Sézary, Sézary syndrome.
The assignment to a certain stage was done according to the recommendations of the EORTC Cutaneous Lymphoma Project Group.32
Electrophoretic mobility shift assay (EMSA) and “supershift” experiments.
EMSAs were performed following the method of Barberis et al.34 Double-stranded γ-32P adenosine-5′-triphosphate (ATP)-labeled oligonucleotides (30,000 cpm) containing the binding sites for c-Myc (5′ AACGACCAGCTGGTTTAC 3′), and AP-1 (Jun/Fos heterodimers or Jun/Jun homodimers, 5′ TACTTGATGACTCAGCTAGAG 3′) were incubated with 3 μg of nuclear extracts of the investigated cells in binding buffer consisting of 10 mmol/L HEPES pH 7.9, 60 mmol/L KCl, 4% Ficoll, 1 mmol/L 1,4 dithiothreitol (DTT) and 1 mmol/L EDTA pH 8. A total of 2 μg of poly [d (I-C)] (=poly-deoxy-inosinic-deoxy-cytidilic acid) was used as competitor for unspecific DNA binding activities. The total volume of the reaction was 30 μL. In the case of “supershift” experiments, 2 to 4 μg of the corresponding antibody (TransCruz gel supershift reagent; Santa Cruz Biotechnology, Santa Cruz, CA) was added. Nuclear extracts were prepared following the method of Gerber et al.35 The reaction mixture was incubated for 30 minutes at 4°C and then loaded on a 4% native polyacrylamide gel (0.25 × TBE [tris, borate, EDTA]). The electrophoresis was run at 4°C for 8 to 10 hours in 0.25 TBE buffer at 10 V/cm.
The oligonucleotides were synthesized by Microsynth, Balgach, Switzerland. Antibodies against the different Myc, Max, Mad, Jun, and Fos proteins were obtained from Santa Cruz Biotechnology.
Western blots.
For Western blotting, 15 to 30 μg protein of nuclear extracts or 30 to 60 μg of protein of cytoplasmatic extracts were loaded on a 7.5% to 9% sodium dodecyl sulfate (SDS) polyacrylamide gel and separated by polyacrylamide gel electrophoresis (PAGE). The percentage of polyacrylamide depended on the molecular weight of the protein to be investigated. The proteins were transferred to a nitrocellulose filter using a Mini Trans Blot Cell (Bio-Rad, Glattbrugg, Switzerland) following the instructions of the supplier. Unspecific antibody binding sites were blocked by incubation of the filter overnight at 4°C in tris-buffered saline (TBS), 0.3% Tween 20 and 2% milk powder. The filter was incubated with the first antibody (Santa Cruz Biotechnology; 1:1,000 dilution) 4 hours at room temperature in TBS, 0.3% Tween 20, and 1% milk powder. The incubation with the second antibody (Santa Cruz Biotechnology, 1:1,000 dilution) was performed in TBS, 0.3% Tween 30 for 4 hours at room temperature. The signal was detected by incubation with BM purple AP substrate (Boehringer Mannheim, Rotkreuz, Switzerland) following the instructions of the supplier.
RESULTS
Nuclear extracts of CTCL cells contain constitutive c-Myc–DNA complexes consisting of c-Myc/Max heterodimers.
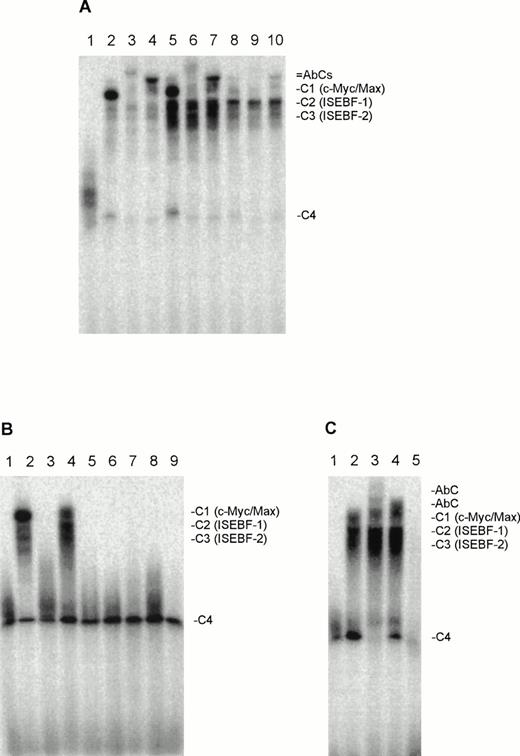
When we incubated nuclear extracts of the three CTCL cell lines, HUT78, MyLa, and SeAx, with a double-stranded radiolabeled oligonucleotide containing the binding sequence of Myc proteins, up to four different protein-DNA complexes per cell line could be detected (Fig 1A). These complexes could be efficiently competed with a 100-fold excess of the unlabeled myc-oligo, therefore all of the observed protein-DNA complexes must be made up by sequence-specific DNA binding proteins (Fig 1C). No such complexes were found in peripheral blood cells of healthy donors (lane 1). Coincubation with antibodies against c-Myc and Max “supershifted” the largest of the complex (C1) indicating that this complex is made up by c-Myc/Max heterodimers. The migration of C4 was also influenced by both antibodies indicating that this complex may contain Max and degradation products of c-Myc. Antibodies against N-Myc, L-Myc, Mad1, Mad2 (Mxi), Mad3, and Mad4, had no effect. The C1 complex is the most prominent one in HUT78 and MyLa cells, whereas complex 2 (C2) is the most prominent one in SeAx cells. Complex 2 (C2) is weak in MyLa cells and in HUT78 cells. The signal of complex C3 is weak in HUT78 and SeAx and relatively strong in MyLa cells. When we tested nuclear extracts of malignant cells from SS patients, similar DNA-protein complexes for the cell lines were detected in only one patient (Fig 1B). These complexes migrated at positions that correspond to those of C1, C2, and C3 (Fig1B). “Supershift” experiments with Myc and Max family antibodies showed that C1 of this patient consisted of c-Myc/Max heterodimers and thus confirmed that the complex C1 of the patient cells was identical to the C1 complexes found in the nuclear extracts of the three CTCL cell lines (Fig 1C). In EMSAs with nuclear extracts from malignant T cells from all patients, we observed a strong C4 complex, which could be disrupted completely by the anti-Max antibody (Fig 1C), indicating that this complex consists of Max/Max homodimers. Because the c-Myc antibody reduced the signal of C4, it may also contain degraded c-Myc, which may only consist of the Max- and DNA-binding region.
(A) Nuclear extracts of the CTCL cell lines HUT78, MyLa, and SeAx contain myc-oligo binding activities, which include c-Myc and Max. Lane 1, peripheral blood lymphocytes; lanes 2 through 4, HUT78 nuclear extracts; lanes 5 through 7, MyLa nuclear extracts; lanes 8 through 10, SeAx nuclear extracts. Lanes 3, 6, and 9, coincubation with Max antibody; lanes 4, 7, and 10, coincubation with c-Myc antibody. (B) Myc-oligo binding activities in the nuclear extracts of malignant cells of seven SS patients (lanes 3 to 9). Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract from HUT78 cells (positive control). (C) The myc-oligo–binding activities of patient 2 contain c-Myc and Max. Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract without antibody; lane 3, nuclear extract with Max antibody; lane 4, nuclear extract with c-Myc antibody; lane 5, competition experiment with a 100-fold excess of unlabeled myc-oligo in the absence of antibodies. The position of the complexes C1-C4 and the DNA-c-Myc/Max-antibody complexes (AbC) are indicated on the right.
(A) Nuclear extracts of the CTCL cell lines HUT78, MyLa, and SeAx contain myc-oligo binding activities, which include c-Myc and Max. Lane 1, peripheral blood lymphocytes; lanes 2 through 4, HUT78 nuclear extracts; lanes 5 through 7, MyLa nuclear extracts; lanes 8 through 10, SeAx nuclear extracts. Lanes 3, 6, and 9, coincubation with Max antibody; lanes 4, 7, and 10, coincubation with c-Myc antibody. (B) Myc-oligo binding activities in the nuclear extracts of malignant cells of seven SS patients (lanes 3 to 9). Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract from HUT78 cells (positive control). (C) The myc-oligo–binding activities of patient 2 contain c-Myc and Max. Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract without antibody; lane 3, nuclear extract with Max antibody; lane 4, nuclear extract with c-Myc antibody; lane 5, competition experiment with a 100-fold excess of unlabeled myc-oligo in the absence of antibodies. The position of the complexes C1-C4 and the DNA-c-Myc/Max-antibody complexes (AbC) are indicated on the right.
We also tested for the presence of the different myc and max/mad gene family members by Western blotting. Strong signals were detected only for the c-Myc and Max proteins (Fig 2). N-Myc may also present in CTCL cell nuclei in trace amounts (not shown). No signals were obtained for L-Myc and Mad1-4 (not shown).
(A) Expression of c-Myc in CTCL cell lines studied by Western blotting with a polyclonal c-Myc antiserum (Santa Cruz Biotechnology). C, positive control with a 65-kD recombinant c-Myc–Glutathione-S-transferase (GST) fusion protein (Santa Cruz Biotechnology). Lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The expected position of the differently modified c-Myc proteins (approximately 60 to 65 kD) is indicated on the right by an asterisk. The different bands in the area may result from differently phosphorylated forms of c-Myc. (B) Expression of Max in CTCL cell lines tested by a polyclonal Max antibody (Santa Cruz Biotechnology). M, marker; lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The positions of the two forms of the Max protein are indicated on the right.
(A) Expression of c-Myc in CTCL cell lines studied by Western blotting with a polyclonal c-Myc antiserum (Santa Cruz Biotechnology). C, positive control with a 65-kD recombinant c-Myc–Glutathione-S-transferase (GST) fusion protein (Santa Cruz Biotechnology). Lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The expected position of the differently modified c-Myc proteins (approximately 60 to 65 kD) is indicated on the right by an asterisk. The different bands in the area may result from differently phosphorylated forms of c-Myc. (B) Expression of Max in CTCL cell lines tested by a polyclonal Max antibody (Santa Cruz Biotechnology). M, marker; lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The positions of the two forms of the Max protein are indicated on the right.
Nuclear extracts of CTCL cell lines contain constitutive Jun-DNA complexes consisting mainly of JunD.
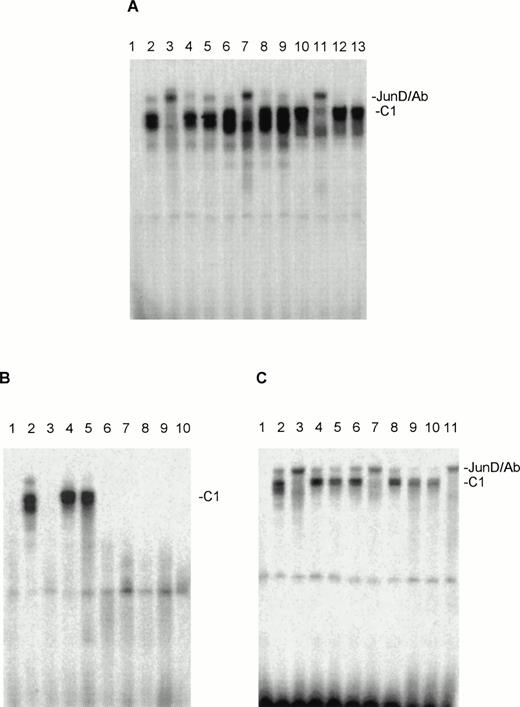
When nuclear extracts of the HUT78, MyLa, and SeAx cell lines were incubated with an oligonucleotide containing the AP-1 binding sites for Jun and Fos proteins protein-DNA one complex (C1) could be observed for HUT78, MyLa and SeAx cells (Fig 3A), which seemed to consist of a double band in all three cell lines. The migration behavior seems to vary a little bit in the three cell lines. “Supershift” experiments with the appropriate antibodies showed that in all three cell lines this complex contained almost only JunD (Fig 3A). No obvious “supershifts” were found when antibodies against c-Fos, c-Jun (Fig 3A), FosB, Fra-1, Fra-2, and JunB were used (not shown). In some experiments, a certain weakening of the C1 signal was observed, so that some trace amounts of other Fos and Jun proteins may be present in the C1 complex. Corresponding experiments with nuclear extracts from malignant T cells from eight SS patients showed that extracts from two of these patients contained AP-1 oligo-binding activities, which migrated at nearly the same position as C1 (Fig 3B). “Supershift” experiments showed that JunD is practically the only component of this complex (Fig 3C). Antibodies against other Jun and Fos family members weakened somewhat the strength of the C1 signal, as it has been observed with nuclear extract of the CTCL cell lines.
Nuclear extracts of the CTCL cell lines HUT78, MyLa, and SeAx contain AP-1–oligo binding activities, which include JunD. (A) Tests with antibodies against JunD (lanes 3, 7, and 11), c-Jun (lanes 4, 8, and 12), and c-Fos (lanes 5, 9, and 13). Lane 1, peripheral blood lymphocytes (negative control); lanes 2, 6, and 10, incubations without antibodies. Lanes 2 through 5, nuclear extracts from HUT78 cells; lanes 6 through 9, nuclear extracts from MyLa cells; lanes 10 to 13, nuclear extracts from SeAx cells. (B) AP-1–oligo binding activities in the nuclear extracts of malignant cells of eight SS patients (lanes 3 through 10). Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract from HUT78 cells (positive control). (C) The AP-1–oligo-binding activities of patient 2 (lanes 4 through 7) and 3 (lanes 8 through 11) contain JunD. Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extracts of HUT78 cells with (lane 3) and without (lane 2) JunD antibody (positive control); lanes 4 and 8, incubations without antibodies; lanes 5 and 9, nuclear extracts with c-Fos antibody; lanes 6 and 10, nuclear extracts with c-Jun antibody; lanes 7 and 11, nuclear extracts with JunD antibody. C1, JunD-DNA complex; JunD/Ab, JunD-DNA-antibody complex. The complex above C1 migrating at nearly the same position as JunD/Ab is probably unspecific, as it reacts with no antibody and is not seen in every experiment.
Nuclear extracts of the CTCL cell lines HUT78, MyLa, and SeAx contain AP-1–oligo binding activities, which include JunD. (A) Tests with antibodies against JunD (lanes 3, 7, and 11), c-Jun (lanes 4, 8, and 12), and c-Fos (lanes 5, 9, and 13). Lane 1, peripheral blood lymphocytes (negative control); lanes 2, 6, and 10, incubations without antibodies. Lanes 2 through 5, nuclear extracts from HUT78 cells; lanes 6 through 9, nuclear extracts from MyLa cells; lanes 10 to 13, nuclear extracts from SeAx cells. (B) AP-1–oligo binding activities in the nuclear extracts of malignant cells of eight SS patients (lanes 3 through 10). Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extract from HUT78 cells (positive control). (C) The AP-1–oligo-binding activities of patient 2 (lanes 4 through 7) and 3 (lanes 8 through 11) contain JunD. Lane 1, peripheral blood lymphocytes (negative control); lane 2, nuclear extracts of HUT78 cells with (lane 3) and without (lane 2) JunD antibody (positive control); lanes 4 and 8, incubations without antibodies; lanes 5 and 9, nuclear extracts with c-Fos antibody; lanes 6 and 10, nuclear extracts with c-Jun antibody; lanes 7 and 11, nuclear extracts with JunD antibody. C1, JunD-DNA complex; JunD/Ab, JunD-DNA-antibody complex. The complex above C1 migrating at nearly the same position as JunD/Ab is probably unspecific, as it reacts with no antibody and is not seen in every experiment.
Western blots of nuclear extracts of CTCL cells detected only JunD signals (Fig 4). Thus, junD seems indeed to be the only member of the jun and fos gene superfamily to be present in CTCL cells. In all three cell lines tested, the anti-JunD antibody detected two signals of 36 kD and 39 kD, which corresponds to the sizes of unphosphorylated and phosphorylated JunD, respectively (Fig 4).
Expression of JunD in CTCL cell lines studied by Western blotting with a polyclonal JunD antiserum (Santa Cruz Biotechnology). M, marker; lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The positions of the two forms of JunD (39 kD and 36 kD) are indicated on the right.
Expression of JunD in CTCL cell lines studied by Western blotting with a polyclonal JunD antiserum (Santa Cruz Biotechnology). M, marker; lanes 1 to 3, nuclear extracts from HUT78 (lane 1), MyLa (lane 2), and SeAx (lane 3) cells. The positions of the two forms of JunD (39 kD and 36 kD) are indicated on the right.
The effects of IL-7 and IL-15 on the DNA binding of JunD and c-Myc–like proteins.
To study the effects of IL-7 and IL-15 on E-box and AP-1 site binding proteins, we used the CTCL cell line, SeAx. This cell line was originally developed from malignant T cells of an SS patient as an IL-2–dependent cell line.30 Later experiments showed that IL-7 and IL-15 were 10 times more efficient as growth factors for this cell line than IL-2.26 SeAx cells can be grown in the absence of the ILs 2, 7, and 15 for 4 to 5 days before apoptosis occurs. To study the effects of IL-7 and IL-15, SeAx cells were kept without ILs for 2 days, and nuclear extracts were prepared 30 minutes after the readdition of IL-7 and IL-15. As a control, another aliquot of SeAx was kept for 30 minutes in the absence of ILs. Figures 5 and 6show that IL-7 and IL-15 increase the DNA-binding of E-box and AP-1 oligo-binding activities and these two ILs are more effective than IL-2 (Fig 5). The effect occurred within 30 minutes, indicating that the increase of the signals was due to the activation of preexisting DNA binding factors and not due to protein synthesis during a general recovery of the cells after the readdition of the ILs, as it may occur after 2 to 4 hours.
Effects of IL-7 (10 U), IL-15 (10 U), and IL-2 (100 U) on the DNA binding of c-Myc and Max to the E box in SeAx cells. Lanes 1 to 3, no IL, lanes 4 to 6, IL-7; lanes 7 to 9, IL-15; lanes 10 to 12, IL-2. The individual DNA binding proteins were identified by specific antibodies (see Max-Ab and c-Myc-Ab bands). C1, c-Myc/Max heterodimer; C2, IL-stimulated E-box binding factor-1 (ISEBF-1); C3, IL-stimulated E-box binding factor-2 (ISEBF-2). Lanes 1, 4, 7, 10, no antibody (Ab); lanes 2, 5, 8, 11, Max Ab; lanes 3, 6, 9, 12, c-Myc Ab.
Effects of IL-7 (10 U), IL-15 (10 U), and IL-2 (100 U) on the DNA binding of c-Myc and Max to the E box in SeAx cells. Lanes 1 to 3, no IL, lanes 4 to 6, IL-7; lanes 7 to 9, IL-15; lanes 10 to 12, IL-2. The individual DNA binding proteins were identified by specific antibodies (see Max-Ab and c-Myc-Ab bands). C1, c-Myc/Max heterodimer; C2, IL-stimulated E-box binding factor-1 (ISEBF-1); C3, IL-stimulated E-box binding factor-2 (ISEBF-2). Lanes 1, 4, 7, 10, no antibody (Ab); lanes 2, 5, 8, 11, Max Ab; lanes 3, 6, 9, 12, c-Myc Ab.
Influence of IL-2, IL-7 (10 U), and IL-15 (10 U) on the DNA binding of Jun and Fos proteins in SeAx cells. (A) Lane 1, unstimulated SeAx cells; lane 2, IL-2 (100 U)-stimulated SeAx cells; lane 3, IL-7 (10 U)-stimulated SeAx cells; lane 4, IL-15 (10 U)-stimulated SeAx cells. (B) The IL-stimulated AP-1 oligomer-binding protein is JunD. Lane 1, peripheral blood lymphocytes (PBLs, negative control). Lanes 2 to 5, no IL; lanes 6 to 9, IL-7; lanes 10 to 13, IL-15. The individual DNA binding proteins were identified by specific antibodies. Lanes 1, 2, 6, and 10, no Ab; lanes 3, 7, and 11, c-Jun Ab; lanes 4, 8, and 12, c-Fos Ab; lanes 5, 9, and 13, Jun-D Ab. JunD-Ab, JunD-DNA-antibody complex; C1, JunD-DNA complex.
Influence of IL-2, IL-7 (10 U), and IL-15 (10 U) on the DNA binding of Jun and Fos proteins in SeAx cells. (A) Lane 1, unstimulated SeAx cells; lane 2, IL-2 (100 U)-stimulated SeAx cells; lane 3, IL-7 (10 U)-stimulated SeAx cells; lane 4, IL-15 (10 U)-stimulated SeAx cells. (B) The IL-stimulated AP-1 oligomer-binding protein is JunD. Lane 1, peripheral blood lymphocytes (PBLs, negative control). Lanes 2 to 5, no IL; lanes 6 to 9, IL-7; lanes 10 to 13, IL-15. The individual DNA binding proteins were identified by specific antibodies. Lanes 1, 2, 6, and 10, no Ab; lanes 3, 7, and 11, c-Jun Ab; lanes 4, 8, and 12, c-Fos Ab; lanes 5, 9, and 13, Jun-D Ab. JunD-Ab, JunD-DNA-antibody complex; C1, JunD-DNA complex.
Figure 5 shows that IL-7 and IL-15 increase the signals of the complexes C2 and C3, but not of the c-Myc/Max heterodimer. Because the C2 and C3 forming proteins do not react with antibodies directed against the myc and max-mad gene family members and are stimulated by at least three ILs, we would like to call these two proteins provisionally ISEBF-1 and ISEBF-2 for IL-stimulated E-box binding factor 1 and 2. Supershift experiments with an antibody directed against the proteins E12 and E47, which have a DNA binding domain similar to Myc proteins, were also negative.
IL-7, IL-15, and also IL-2 stimulated the DNA-binding of JunD (Fig 6A) and IL-7 and IL-15 had the strongest effects. Figure 6B shows that the DNA binding both components of the double band is enhanced by both ILs and that also both components are supershifted by a JunD antibody, but not by antibodies directed against other jun/fos superfamily members.
DISCUSSION
Our results show that nuclear extracts of all three investigated CTCL cell lines and one SS patient contain several c-Myc-oligo binding activities. Because these complexes occur only in one of seven patients and the three cell lines, we conclude that these binding activities occur only late in CTCL tumor development, as cell lines can much more easily be established from late stage tumors. This interpretation is corroborated by immunohistochemical findings3 with CTCL biopsy material indicating that c-myc is only expressed in late CTCL stages. Because these complexes also occur in malignant cells of an SS patient, they cannot be interpreted as cell culture artefacts. “Supershift” experiments showed that complex C1 consisted of c-Myc/Max heterodimers. C1 is the predominant complex in the IL-7– and IL-15–independent cell lines, HUT78 and MyLa. The complex C2 (ISEBF-1) predominates in the IL-7– and IL-15–dependent cell line, SeAx, and the malignant T cells of one patient. IL-728 and IL-1526 have been shown to be growth factors for CTCL cells and therefore SeAx cells may represent an earlier CTCL stage. C2 (ISEBF-1) is upregulated by IL-7 and IL-15 in SeAx cells, therefore one can assume that ISEBF-1 plays an important role in CTCL genesis. Heterodimers of c-Myc/Max may take over the function of ISEBFs in late CTCL stages and CTCL tumors may thus become IL-7– and IL-15–independent, as we did not observe an effect of these ILs on the c-Myc/Max binding activity in these cells.
The less abundant complex C3 (ISEBF-2) is also regulated by IL-7 and IL-15 and may play a similar role as ISEBF-1. Both proteins may be members of the HLH (helix loop helix) transcription factor superfamily, which bind to DNA motifs that are very similar to the Myc-binding sequences (E-box). Experiments with an antibody against the E box-binding transcription factors, E12 and E47, showed that these two proteins are not part of ISEBF-1 or ISEBF-2 (J-Z. Qin, unpublished). Because the Myc-binding site we used is nearly identical to the optimal c-Myc binding site defined by Halazonetis and Kandil,36 but less similar to the optimal binding site of other E-box proteins, we suppose that ISEBF-1 and ISEBF-2 are quite near relatives to Myc and Max proteins. However, we cannot exclude the possibility that a totally different protein family has developed an alternative DNA binding region for Myc/Max target sequences. The nuclear extracts from all tested patients contained Max/Max homodimers. Max/Max homodimers are antagonists of Myc/Max heterodimers and inhibit Myc/Max heterodimer-directed gene activation. The malignant T cells of the patient with c-Myc/Max may be a transition state between the malignant T cells from patients with stable disease and the cell lines. They already contain c-Myc/Max heterodimers and the two ISEBFs, but also Max/Max homodimers, which, at least in part, may inhibit the c-Myc/Max heterodimers. In this state, the ISEBFs may be important for the transcription of c-Myc target genes.
The c-Myc/Max heterodimer is a strong transactivator of transcription. Besides gene transcription, c-Myc/Max heterodimers have been implied in cell-cycle progression and cell dedifferentiation.8 Targets of c-Myc/max are genes that are involved in DNA-synthesis (eg, ornithine decarboxylase [ODC]), translation (elongation factors elF-2α and elF-4E), and cell-cycle regulation.37 In the cell-cycle c-Myc/Max heterodimers inhibit the action of the cell-cycle blocking protein p27KIP1,38 and increase the expression of the cyclins A and E.39 They also stimulate the expression of cdc25A,40,41 a tyrosine phosphatase that promotes the progression of the cell cycle from the G1 to the S phase by activating several cyclin-dependent kinase (CDK) complexes. Overexpression of the c-myc and max genes could therefore lead to an increased growth of CTCLs, and indeed this was found in the patient who showed constitutive c-Myc and Max activities. Whether the activation of the c-myc gene in MyLa and SeAx cells and patients occurs by translocation of the c-myc gene as in HUT78 cells2 6 has to be established.
Initially, JunD has been considered to be an inhibitor of tumor formation,42,43 but later it has been reported that JunD can transform rat embryo fibroblasts in cooperation with Ras44 and that mutant forms of JunD have a transforming potential.45 JunD seems to be important for cell-cycle progression, as antibodies against JunD downregulate the transition from G1 to the S phase.20 Potential target genes for JunD are cyclin D1 and c-myb.46,47 The proto-oncogene, c-myb, is expressed during the transition from G1 to S phase and increases the expression of the antiapoptotic gene bcl-2.48 49 Cyclin D1 is the product of the bcl-1 oncogene and forms complexes with CDKs, which in turn, are targets of c-Myc/Max heterodimers. Therefore c-Myc, Max, and JunD could cooperate during the progressing transformation of CTCLs, and indeed, in one patient and the three cell lines, these three proteins are active at the same time. During the time course of our experiments, the number of leukemic cells in the blood of this patient increased markedly, but the number of malignant cells decreased when the DNA synthesis inhibitor, methotrexate, was given, indicating that methotrexate may be able to counteract the effects of c-Myc/Max heterodimers on DNA synthesis. The other patient whose malignant T cells also had constitutive JunD activity contained no c-Myc/Max heterodimers, but overexpressed bcl-2 (A. Salvekar and R. Dummer unpublished). Her tumors also grew quickly and were resistant to irradiation. Whether JunD can regulate or cooperate with Bcl-2 has not yet been established.
Whether the JunD molecules of the CTCL cells represent wild-type or mutant versions of this gene has to be investigated. From this point of view, it is noteworthy that the JunD complexes of the various investigated cell lines migrate somewhat differently on the gel.
Because IL-7 and IL-15 are growth factors of CTCL cells and increase the DNA binding of ISEBF-1, ISEBF-2, and JunD, these three molecules may at least in part mediate the effects of these ILs, eg, by increasing the growth rate via the c-Myc–like ISEBF factors and protecting CTCL cells from apoptosis by increased bcl-2 expression. At least the latter seems to be likely, as we found recently that IL-2, IL-7, and IL-15 increased c-myb and bcl-2 expression (unpublished data).
ACKNOWLEDGMENT
The authors thank F. Bonvin, A. Flace, H. Grundmann, and S. Manolio for their excellent technical assistance, and M. Johnson and M. Bär for the preparation of the photographs.
Supported by Grant No. KFS 275-1-1996 from the Swiss Cancer League (to G.B.), the Hartmann Müller Foundation, Grant No. 3100-43244.95/1 from the Swiss National Science Foundation (to G.B.), the United Bank of Switzerland (UBS), and the Kanton of Zürich.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to U. Döbbeling PhD, Department of Dermatology, University Hospital Zurich, Gloriastrasse 31, CH-8091 Zurich, Switzerland.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal