The transcription factor, NF-κB, is important for T-cell activation, B-cell maturation, and human immunodeficiency virus transcription and plays a role in alternatively mediating and protecting against apoptosis in a variety of cell types. However, a role for NF-κB in human CD34+ bone marrow cells has not been described. We provide evidence here that virtually all human CD34+ bone marrow cells express NF-κB that can be activated by exposure to phorbol 12-myristate 13-acetate and a variety of cytokines, eg, tumor necrosis factor , interleukin-3, and granulocyte-macrophage colony-stimulating factor. In addition, we demonstrate that NF-κB may be required for human CD34+bone marrow cell clonogenic function and survival. These results offer insight into a new role for NF-κB in maintaining survival and function in hematopoietic stem and progenitor cells and suggest that proposed strategies involving inhibition of NF-κB activation as an adjunct to cancer chemotherapy should be approached with caution.
NF-κB IS A MEMBER of the mammalian Rel family of transcriptional activators that plays a central role in the activation and regulation of immune response.1 The family consists of 5 discrete DNA binding proteins (p50, p52, p65, c-rel, and RelB) that share partial homology, including a common DNA binding domain, dimerization domains, and a nuclear localization region.2 Rel family members form homodimers or heterodimers that bind to a specific DNA sequence called the κB motif.3-8 NF-κB is a heterodimer consisting of the 50-kD (p50) and 65-kD (p65) subunits9 that, in its inactive state, is located in the cytoplasm bound to an inhibitory protein, IκBα.10 Upon activation, NF-κB disassociates from IκBα, translocates to the nucleus11 and binds to DNA to regulate gene expression (for review see Thanos and Maniatis2 and Siebenlist et al12). Additionally, complex binding patterns among various members of the Rel family may account for differential gene activation with or without interaction with other transcriptional regulatory proteins.2
NF-κB is required for the expression of several gene products relevant to hematopoiesis including interleukin-1β (IL-1β),13 tumor necrosis factor-α (TNF-α),14 IL-6,15 macrophage colony-stimulating factor (M-CSF),16 granulocyte-macrophage colony-stimulating factor (GM-CSF),17 granulocyte colony-stimulating factor (G-CSF),18 erythropoietin (EPO),19 interferon-γ (INF-γ),20c-myc,21 and c-myb.22 In addition, NF-κB is activated by cytokines known to regulate hematopoiesis such as TNF-α,23 IL-1α and IL-1β,24INF-γ,25 leukemia inhibitory factor,26M-CSF,27 GM-CSF,27 transforming growth factor-β1 (TGF-β1),28 and IL-3.29 The role of NF-κB as a secondary messenger for cytokine response, as well as the hematopoiesis-specific genes that are responsive to NF-κB, suggest that it may be important in human CD34+ cell survival and/or differentiation. Studies with disruption of the various Rel family proteins support this observation. The disruption of therelA locus (p65) leads to embryonic lethality at 15 to 16 days of gestation with massive liver degeneration.30 Mice with homozygous disruption of relB exhibit multifocal, mixed inflammatory cell infiltration in several organs, myeloid hyperplasia, splenomegaly due to extramedullary hematopoiesis, a reduced number of thymic dendritic cells, and impaired cellular immunity.31Mice lacking p50 show no developmental abnormalities, but have impaired B-cell responses.32 Mice with constitutive NF-κB activation due to the disruption of IκBα expression show enhanced granulopoiesis and die within 8 days after birth.33 In addition, both apoptosis and anti-apoptotic functions can be mediated via NF-κB activation in different cells.34-36
NF-κB was originally described as a DNA-binding activity that recognized a sequence 5′-GGGGACTTTCC-3′ in the Ig k light chain gene enhancer in mature B cells.37 Subsequently, NF-κB has been found in all mature hematopoietic cell lineages (eg, T cells,10 monocytes,38granulocytes,39 mast cells,40 and dendritic cells41) and has been recently reported to be involved in erythropoiesis.42 However, the expression and role of NF-κB in human CD34+ bone marrow cells are currently unknown. Therefore, we investigated whether NF-κB is present in CD34+ bone marrow cells and whether NF-κB is required for colony formation. The results of these studies demonstrate that virtually all human CD34+ bone marrow cells contain NF-κB and that NF-κB is required for human CD34+ bone marrow cell clonogenic function and survival.
MATERIALS AND METHODS
Cell purification.
All protocols were approved by the University of Colorado Health Sciences Center Internal Review Board, and samples were taken with informed consent from normal adult volunteers. Human bone marrow and blood were obtained by aspiration from the posterior iliac crest and venapuncture, respectively. Mononuclear cells were isolated using Histopaque-1077 (Sigma, St Louis, MO), and purification of individual cell subpopulations was achieved using a high magnetic gradient MiniMACS purification system (Miltenyi, Sunnyvale, CA). Because NF-κB is known to be expressed in B lymphocytes,43 we used the pan–B-cell antigen CD19 to isolate CD34+ bone marrow cells devoid of B cells (CD34+CD19−) for use in these studies. CD19+ cells were removed and detected by using a fluorescein isothiocynate (FITC)-conjugated anti-CD19 monoclonal antibody followed by anti-FITC MicroBeads (Miltenyi). CD34+CD19− cells were then obtained by using the CD34 isolation kit. CD4+ T cells were purified using CD4 Microbeads. The purity of isolated cells was determined by flow cytometry analysis (Epics 752; Coulter Electronics, Hialeah, FL).
Electrophoretic mobility shift assay (EMSA).
Human CD34+CD19− bone marrow cells were either used directly after purification or for selected experiments cultured at 0.4 × 106 cells/mL for 18 hours at 37°C with 5% CO2 in complete media (RPMI-1640, 10% fetal bovine serum, 100 mg/mL streptomycin, 100 U/mL penicillin, and 2 mmol/L L-glutamine) with and without 25 ng/mL phorbol 12-myristate 13-acetate (PMA), cytokines, or NF-κB nuclear localization-sequence (NLS) peptides before use in the EMSA. Cytokine concentrations used in these experiments were identical to those used in the colony-forming assays and TNF-α was used at 50 ng/mL. Initial experiments were performed with NF-κB NLS peptides purchased from Biomol (Plymouth Meeting, PA); however, quality control issues required us to synthesize these peptides based on the published sequences.44 The NF-κB NLS peptides were used at 100 μg/mL. Nuclear protein was extracted using a modified Dignam protocol45 from 0.5 to 1 million cells per treatment group. Protein concentrations were determined using a BCA protein kit (Pierce, Rockford, IL) and the nuclear extracts were frozen at −80°C until used. An NF-κB probe was made and labeled as described.46 For super-shift samples, cellular extracts were preincubated with appropriate specific antibodies for 1 hour to overnight at 4°C. Antihuman p50, p52, p65, and rel B antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and antihuman c-rel and a second antihuman p65 antibody were purchased from Rockland (Gilbertsville, PA). Nuclear protein (4 μg) was incubated on ice for 10 minutes with 1 μg dl-dC, 4 μL binding buffer (20 mmol/L HEPES [pH 7.9] 40 mmol/L KCl, 10% glycerol, 0.05 mmol/L EDTA, 1.6 mmol/L MgCl2) 1 mmol/L DTT, and deionized water for a total volume of 19 μL. One microliter of 32P-labeled probe (∼50,000 cpm) was added, and the binding reaction was continued for 30 minutes at 22°C. After complex formation, 2 μg of loading buffer (250 mmol/L Tris HCl [pH 7.5], 0.2% bromophenol blue, 0.2% xylene cyanol, 40% glycerol) was added to the DNA-protein complexes, and the sample was analyzed by electrophoresis on a prerinsed 6% polyacrylamide gel. The gels were dried and exposed at −80°C to Fuji x-ray film (Tokyo, Japan). All reported experiments were repeated at least 3 times.
Measuring NF-κB by flow cytometry.
Cells were exposed to 25 ng/mL PMA and 1 μg/mL ionomycin or buffer for 1 hour at 37°C in RPMI-1640 containing 1% bovine serum albumin (BSA). The cells were then washed, followed by permeabilization in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.2, with 0.1% saponin and 0.01 mol/L HEPES for 10 minutes at room temperature. The cells were labeled with 2 μg/mL anti–NF-κB antibody (IgG3; Boerhinger Mannheim, Mannheim, Germany) or a nonspecific IgG3 antibody (isotype control). The cells were then labeled with biotin-conjugated goat antimouse IgG3, washed 2 times, and subsequently labeled with allophyocyanin (APC)-conjugated strepavidin. After the final wash, the cells were fixed with 1% paraformaldehyde and stored at 4°C until flow cytometric analysis. Flow cytometry was performed using a MoFlo system (Cytomation, Fort Collins, CO). These experiments were repeated at least 3 times.
Colony-forming assays.
These were performed as previously described.47 Briefly, CD34+ bone marrow cells were purified and plated in 35-mm culture dishes at a concentration of 3 to 4.5 × 103cells/mL in 1 mL of modified Iscove’s medium containing 10% fetal bovine serum, 100 mg/mL streptomycin, 100 U/mL penicillin, 2 mmol/L L-glutamine, 50 μmol/L 2-mercaptoethanol, 1.2% (wt/vol) methyl cellulose, and recombinant cytokines. Each cytokine was used at concentrations experimentally determined to produce maximal colony formation (eg, 5 ng/mL GM-CSF, 25 ng/mL IL-3, 25 ng/mL G-CSF, 25 ng/mL M-CSF, 25 ng/mL stem cell factor [SCF], and 5 U/mL EPO). All chemicals were mixed with the methyl cellulose media before the addition of cells, which preceded the addition of the cytokines. All cultures were maintained at 37°C in 5% CO2 and scored on day 14 of culture. Five plates were scored for each treatment group, and results are expressed as the mean ± 1 standard error of the mean (SEM). Significant differences (P ≤ .05) between groups were determined using the Student’s t-test (Excel 4.0; Microsoft Corp, Redmond, WA). All reported experiments were repeated at least 3 times.
Apoptosis assay.
Purified CD34+CD19− bone marrow cells were exposed to NF-κB NLS fusion peptide, control peptide, or buffer in RPMI-1640 media supplemented with 10% fetal bovine serum in 5% CO2 at 37°C for 16 hours. Apoptosis was measured by flow cytometric analysis of deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL; In Situ Cell Death Detection Kit; Boerhinger Mannheim) for each sample. All reported experiments were repeated at least 3 times.
RESULTS
Induction of NF-κB–specific DNA-binding activity in human CD34+CD19− bone marrow cells.
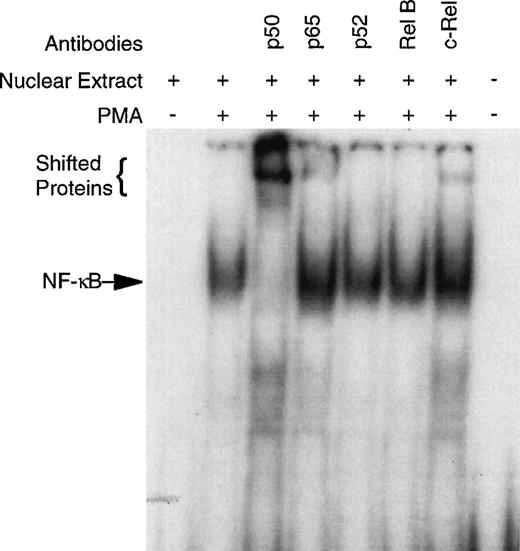
CD34+ bone marrow cells devoid of B cells (CD34+CD19−) were used in these studies, because NF-κB is known to be expressed in B lymphocytes (see Materials and Methods).43CD34+CD19− bone marrow cells were purified and cultured overnight with and without PMA. EMSA of the nuclear extracts demonstrate no detectable NF-κB specific DNA-binding in the unstimulated cells; however, PMA exposure induced considerable binding (Fig 1). Super-shift EMSA using antibodies specific for the Rel family members demonstrated the presence of p50, p65, and c-rel by the appearance of shifted bands (Fig 1). Although antibodies specific for p52 and rel B did not cause a supershift, the possibility that any of these Rel family members are present cannot be completely excluded due the heterogeneity of the CD34+cells. The unreactive antibodies serve as an Ig control demonstrating the specificity of the p50, p65, and c-rel antibodies.
PMA induces NF-κB specific DNA-binding in human CD34+CD19− bone marrow cells. To confirm specificity and identify which Rel family members may be binding to the NF-κB consensus sequence, super-shift EMSA were performed with antibodies specific for human p50, p65, p52, rel B, and c-rel. The CD34+CD19− cells were 96.8% pure for this representative experiment.
PMA induces NF-κB specific DNA-binding in human CD34+CD19− bone marrow cells. To confirm specificity and identify which Rel family members may be binding to the NF-κB consensus sequence, super-shift EMSA were performed with antibodies specific for human p50, p65, p52, rel B, and c-rel. The CD34+CD19− cells were 96.8% pure for this representative experiment.
NF-κB distribution in human CD34+CD19− bone marrow cells.
We evaluated the distribution of activated NF-κB in both PMA-stimulated and unstimulated human CD34+CD19− bone marrow and CD4+ T cells by flow cytometry using a monoclonal antibody that recognizes an epitope within the NF-κB nuclear localization region that is exposed upon activation.48 Using this antibody, we confirmed the results of the EMSA and determined that activated NF-κB is present in virtually all human CD34+CD19− cells (Fig2).
Activated NF-κB measured by flow cytometry in human CD4+ peripheral T cells ([A] unstimulated, [B] stimulated) and CD34+CD19− bone marrow cells ([C] unstimulated, [D] stimulated). Stimulated cells were incubated with 25 ng/mL PMA and 1 μg/mL ionomycin for 1 hour at 37°C before NF-κB analysis. Activated NF-κB is a black line and the background isotype antibody binding is a gray line for each sample. The monoclonal antibody used in these experiments has been shown to be specific for the NF-κB nuclear localization region, which is detected only if NF-κB is activated. The CD4+ T cells and CD34+CD19− bone marrow cells were more than 97% and more than 99% pure, respectively.
Activated NF-κB measured by flow cytometry in human CD4+ peripheral T cells ([A] unstimulated, [B] stimulated) and CD34+CD19− bone marrow cells ([C] unstimulated, [D] stimulated). Stimulated cells were incubated with 25 ng/mL PMA and 1 μg/mL ionomycin for 1 hour at 37°C before NF-κB analysis. Activated NF-κB is a black line and the background isotype antibody binding is a gray line for each sample. The monoclonal antibody used in these experiments has been shown to be specific for the NF-κB nuclear localization region, which is detected only if NF-κB is activated. The CD4+ T cells and CD34+CD19− bone marrow cells were more than 97% and more than 99% pure, respectively.
NF-κB is required for CD34+ cell colony formation.
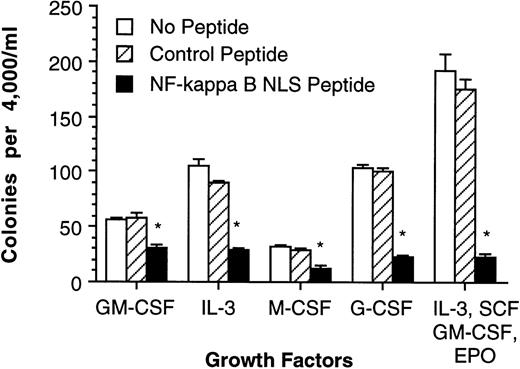
To determine if NF-κB is necessary for colony formation, a fusion peptide containing the NF-κB NLS and a membrane-permeable hydrophobic region that has been previously demonstrated to specifically prevent NF-κB nuclear translocation44 was added to the media in a methyl cellulose colony-forming assay. After activation of NF-κB, its NLS associates with a specific NLS-receptor, resulting in transport of NF-κB in to the nucleus. This can be blocked by occupying the NF-κB NLS-receptors with peptides possessing the NF-κB NLS. NF-κB NLS peptide and control peptide (an inactive NF-κB NLS via specific amino acid substitutions) were used to evaluate the effects of NF-κB inhibition on colony-forming unit (CFU) formation using human CD34+ bone marrow cells stimulated by either GM-CSF, IL-3, M-CSF, G-CSF, or a mixture of IL-3, SCF, GM-CSF, and EPO (Fig 3). These results suggest that NF-κB is either required for cell proliferation and/or required for clonogenic cell survival.
Blocking NF-κB nuclear translocation inhibits human CD34+ bone marrow cell colony formation. Human CD34+ bone marrow was purified (>96% pure) and cultured in methyl cellulose medium containing various cytokines and (1) 200 μg/mL NF-κB NLS peptide, (2) 200 μg/mL control peptide, and (3) no peptides. Error bars indicate 1 standard error of mean (SEM) for 5 cultures and are omitted when smaller than the data symbol. *Significant decrease compared with cultures containing the control peptide (P ≤ .05).
Blocking NF-κB nuclear translocation inhibits human CD34+ bone marrow cell colony formation. Human CD34+ bone marrow was purified (>96% pure) and cultured in methyl cellulose medium containing various cytokines and (1) 200 μg/mL NF-κB NLS peptide, (2) 200 μg/mL control peptide, and (3) no peptides. Error bars indicate 1 standard error of mean (SEM) for 5 cultures and are omitted when smaller than the data symbol. *Significant decrease compared with cultures containing the control peptide (P ≤ .05).
GM-CSF, IL-3, and TNF-α, but not M-CSF and G-CSF, activate NF-κB in human CD34+CD19− bone marrow cells.
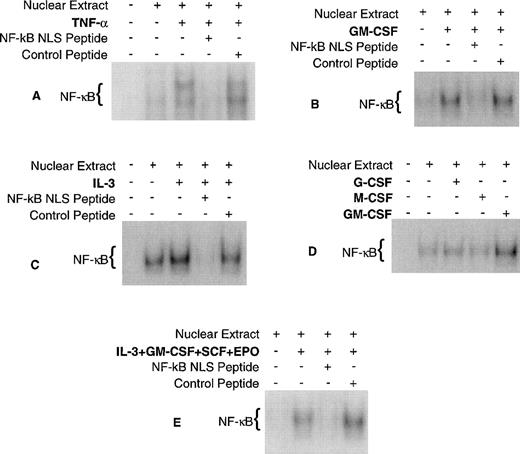
EMSA were performed to determine if CFU formation/inhibition (Fig 3) correlates with NF-κB activation/inhibition, respectively, using the cytokines and NF-κB fusion peptides previously described. As a positive control, EMSA was also performed using TNF-α, because it is a well-documented activator of NF-κB in a variety of cell types. The results of these experiments demonstrate that GM-CSF, IL-3, and TNF-α activate NF-κB and that NF-κB nuclear localization is blocked by the active fusion peptide (Fig 4) for each stimulus. G-CSF and M-CSF did not activate NF-κB in these studies. These results are consistent with previous findings using other cell types except for M-CSF, which has been shown to activate NF-κB in macrophages.27 28 NF-κB activation by cytokines does not correlate with CFU formation, because there are NF-κB activators (PMA and TNF-α) that do not induce CFU formation and there are cytokines (G-CSF and M-CSF) that stimulate CFU formation but do not activate NF-κB. In addition, there were several experiments in which there was a baseline NF-κB activity (Fig 4A, C, and D) suggesting that NF-κB activity may be constitutive. Although NF-κB activation does not correlate with CFU formation, NF-κB inhibition does correlate with CFU inhibition. Taken together, these data suggest that NF-κB may be required for cell survival rather than proliferation.
Cytokine activation of NF-κB in CD34+CD19− bone marrow cells. Human CD34+CD19− bone marrow was purified and incubated for 18 hours at 37°C with each cytokine/peptide combination and EMSA was performed. (A) TNF-, (B) GM-CSF, (C) IL-3, and (E) a combination of GM-CSF, IL-3, SCF, and EPO activated NF-κB and its nuclear translocation could be blocked by the NF-κB NLS peptide. (D) M-CSF and G-CSF did not activate NF-κB. The purity of the CD34+CD19− cells for experiments (A) through (E) was 99.1%, 98.7%, 96.8%, 95.6%, and 98.6%, respectively.
Cytokine activation of NF-κB in CD34+CD19− bone marrow cells. Human CD34+CD19− bone marrow was purified and incubated for 18 hours at 37°C with each cytokine/peptide combination and EMSA was performed. (A) TNF-, (B) GM-CSF, (C) IL-3, and (E) a combination of GM-CSF, IL-3, SCF, and EPO activated NF-κB and its nuclear translocation could be blocked by the NF-κB NLS peptide. (D) M-CSF and G-CSF did not activate NF-κB. The purity of the CD34+CD19− cells for experiments (A) through (E) was 99.1%, 98.7%, 96.8%, 95.6%, and 98.6%, respectively.
NF-κB is required for human CD34+CD19− cell survival.
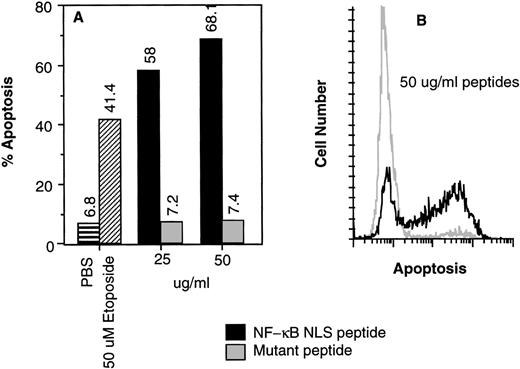
To test whether NF-κB is required for human CD34+ cell survival, apoptosis was measured using the TUNEL assay in human CD34+CD19− cells incubated for 16 hours with NF-κB NLS fusion active or control peptide. Cells were also exposed to 50 μmol/L etoposide as a positive control for apoptosis. Inhibition of NF-κB nuclear translocation using 50 μg/mL peptide resulted in 68.1% of the cells undergoing apoptosis (Fig5). These results suggest that NF-κB is necessary for human CD34+CD19− bone marrow cell survival.
(A) Inhibition of NF-κB nuclear translocation induced apoptosis of human CD34+CD19− human bone marrow cells. The deoxynucleotidyl TUNEL assay was performed on human CD34+CD19− bone marrow cells (>98% pure) after (A) 16 or (B) 18 hours of incubation with various agents.19 The controls were incubated with buffer only (▤) or 50 μmol/L etoposide (▨). (A) NF-κB NLS peptide (▪); control mutant peptide (▩). (B) Flow cytogram comparison of apoptosis induced by 50 μg/mL NF-κB NLS peptide (black line) and control mutant peptide (gray line) shown in (A).
(A) Inhibition of NF-κB nuclear translocation induced apoptosis of human CD34+CD19− human bone marrow cells. The deoxynucleotidyl TUNEL assay was performed on human CD34+CD19− bone marrow cells (>98% pure) after (A) 16 or (B) 18 hours of incubation with various agents.19 The controls were incubated with buffer only (▤) or 50 μmol/L etoposide (▨). (A) NF-κB NLS peptide (▪); control mutant peptide (▩). (B) Flow cytogram comparison of apoptosis induced by 50 μg/mL NF-κB NLS peptide (black line) and control mutant peptide (gray line) shown in (A).
DISCUSSION
We demonstrate here that NF-κB is present in human CD34+CD19− bone marrow cells and may play a role in their survival. Virtually all CD34+CD19− human bone marrow cells contain NF-κB DNA-binding activity that can be activated by PMA exposure, and a variety of cytokines (eg, GM-CSF, IL-3, and TNF-α) are able to activate NF-κB in these cells. NF-κB specificity was verified by antibody recognition in both the supershift EMSA and flow cytometric assays and by competitive inhibition of nuclear translocation using a peptide specific for the NF-κB NLS. In addition, inhibition of NF-κB nuclear translocation induced both apoptosis and loss of clonogenic function in human CD34+ cells. These findings are consistent with the established role of NF-κB as a secondary messenger for GM-CSF,27 IL-3,29 and TNF-α23 signal transduction as measured in other cell types. Contrary to other studies,27,28 M-CSF did not activate NF-κB in our system. It is possible that the proportion of the CD34+CD19− bone marrow cells that expressed M-CSF receptors and are therefore capable of responding to M-CSF was below the threshold of NF-κB detection in the EMSA. Another explanation is that the M-CSF signal transduction pathway in mature or peripheral mononuclear phagocytes uses NF-κB,27 whereas CD34+CD19− bone marrow cells used in our experiments have an NF-κB–independent M-CSF signal transduction pathway. Differences in NF-κB activation have been observed for IL-3 signaling, depending on the cell type. Oster et al27 did not detect NF-κB activation after IL-3 exposure using human peripheral mononuclear phagocytes, but Besançon et al29 did observe IL-3–induced NF-κB activation in a pro-B IL-3–dependent cell line (Ba/F3). In addition, these investigators demonstrated that NF-κB was required for survival in these cells. Our results indicate both NF-κB activation by IL-3 and a requirement for NF-κB in CD34+CD19−bone marrow cell survival.
It has been reported that Rel family members are sequentially expressed during B-cell development and that ordered expression may help regulate genes that are involved in any one stage of differentiation.43 Flow cytometric analysis of p65 distribution in CD34+ cells suggests that p65 is present in virtually all CD34+ cells, suggesting a role for NF-κB in supporting cell survival rather than in the regulation of differentiation-specific genes. The distribution of c-rel and its possible role in stage-specific differentiation of human CD34+ bone marrow cells remains unknown.
A number of different mechanisms may explain the role of NF-κB in the survival of CD34+ cells. Inhibition of NF-κB has been demonstrated to induce apoptosis in a variety of cell types, and in B lymphocytes, inhibition of NF-κB is reported to be associated with a decline in c-myc expression.29,49 NF-κB has also been shown to transactivate c-myb as well,22 which is also required for hematopoietic cell survival.50 In addition, NF-κB is a positive regulator for GM-CSF production,51and GM-CSF has been shown to play an autocrine role in colony formation.52
The inhibition of NF-κB by a variety of molecules, such as glucocorticoids, has been proposed as a means of inducing apoptosis in target cell populations.34 35 The data presented here indicate that NF-κB activation is necessary for general clonogenic response and protects against apoptosis in CD34+ bone marrow cells. Based on these results, proposed strategies involving inhibition of NF-κB activation as an adjunct to anticancer therapeutic paradigms should be approached with caution.
In addition to the potential role for the NF-κB in regulating hematopoiesis, these proteins are also proto-oncogenes6,53,54 and may play a role in the transformation process in several types of cancer. The avian homologue of c-rel, v-rel, causes the tumorigenicity of the avian reticuloendotheliosis virus (REV-T).55 Human T-lymphotrophic virus-I (HTLV-I)–mediated T-cell leukemia is thought to involve constitutive NF-κB activation induced by the tax protein.56 P50/p65 NF-κB has also been reported to be activated by the p210 BCR-ABL fusion protein that mediates transformation in chronic myelogenous leukemia (CML),57 and NF-κB is reportedly a critical downstream element of Ha-Ras signaling and may mediate its oncogenic potential.58
In conclusion, our findings demonstrate that NF-κB (p50, p65, c-rel) is present in human CD34+CD19− bone marrow cells and suggest that it is required for colony formation and cell survival. These results suggest that NF-κB plays an important role in human CD34+ cell signal transduction, gene expression, and transformation.
ACKNOWLEDGMENT
The authors thank D. Som and C. Hodge for their technical assistance and K. Helm for technical assistance with flow cytometry.
Supported by Grant No. ES06258 from the National Institute of Environmental Health Sciences, National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. This publication was also made possible by the cooperation and support of University of Colorado Cancer Center Flow Cytometry Core (Grant No. 2 P30 CA 46934-09) and the Clinical Investigation Core.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard D. Irons, PhD, D.A.B.T., University of Colorado Health Sciences Center, 4200 E 9th Ave, Box C238, Denver, CO 80262; e-mail:Richard.Irons@UCHSC.edu.

![Fig. 2. Activated NF-κB measured by flow cytometry in human CD4+ peripheral T cells ([A] unstimulated, [B] stimulated) and CD34+CD19− bone marrow cells ([C] unstimulated, [D] stimulated). Stimulated cells were incubated with 25 ng/mL PMA and 1 μg/mL ionomycin for 1 hour at 37°C before NF-κB analysis. Activated NF-κB is a black line and the background isotype antibody binding is a gray line for each sample. The monoclonal antibody used in these experiments has been shown to be specific for the NF-κB nuclear localization region, which is detected only if NF-κB is activated. The CD4+ T cells and CD34+CD19− bone marrow cells were more than 97% and more than 99% pure, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3302.410a38_3302_3308/5/m_blod41038002x.jpeg?Expires=1763885409&Signature=btoovkyU2mHEtM33EQZ8560vlsxGt~K~m4xGC-WM2X1HwMjPpeFmA0R1yJUUPIVujrj5krfuqyqMdefRZTzOZWah3-Q6EOQ90~1AD80lhb5snfoC7ZM1fg4j-~MEyqZTkFhJUYFei-069iOrUKxEZZMbfDd4rsbBDyUQh2usznNwNBH4miKIAV8GI6aC2GPswznLGOj0k-Ar2YxBHrl9aakSeEN~460pO9K~qZd1IMqklUd3N3MQlVXYEdhOQamfK3-FAe8UCP3F~OqXimUlkqdUP~LQTSAg~7vYxqGBsiFDZhK1ZMfGgrvj6f0x50pB4MllrnvC6RhmdyBrkYQHMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal