Interferon γ (IFNγ) induces apoptosis in purified human erythroid colony-forming cells (ECFC) and inhibits cell growth. Fas (APO-1; CD95) and Fas ligand (FasL) mediate apoptosis induced by IFNγ, because Fas is significantly upregulated by IFNγ, whereas Fas ligand is constitutively present in the ECFC and neutralization of FasL greatly reduces the apoptosis. Because conversion of caspases from their dormant proenzyme forms to active enzymes has a critical role in transducing a cascade leading to apoptosis, we performed further studies of the expression and activation of caspases in normal human and IFNγ-treated day-6 ECFC to better understand the mechanism of IFNγ action in producing this cell death. RNase protection assays showed that the caspase-1, -2, -6, -8, and -9 mRNAs were upregulated by IFNγ, whereas the caspase-5 and -7 mRNAs were not increased. Western blots showed that FLICE/caspase-8 was upregulated and activated by 24 hours of incubation with IFNγ. FADD was not similarly altered by incubation with IFNγ. Western blots of ICE/caspase-1, which might be required for amplification of the initial FLICE activation signal, showed that pro-ICE expression significantly increased after treatment with IFNγ for 24 hours and cleavage of pro-ICE also increased. CPP32/apopain/caspase-3, responsible for the proteolytic cleavage of poly (ADP) ribose polymerase (PARP), was also studied and treatment of ECFC with IFNγ resulted in an increased concentration of caspase-3 by 24 hours and a clear induction of enzyme activation by 48 hours, which was identified by the appearance of its p17-kD peptide fragment. The cleavage of PARP was demonstrated by an obvious increase of the 89-kD PARP cleavage product, which was observed at almost the same time as caspase-3 activation in the IFNγ-treated cells, whereas untreated ECFC showed little change. Peptide inhibitors of the caspase proteins, DEVD-fmk, DEVD-cho, YVAD-cho, and IETD-fmk, were incubated with the ECFC to obtain further evidence for the involvement of caspases in IFNγ-induced apoptosis. The activation of FLICE/caspase-8 and CPP32/caspase-3 and cleavage of PARP clearly were inhibited, but the reduction of cell growth due to apoptosis, induced by IFNγ, was only partially blocked by the presence of the inhibitors. These results indicate that IFNγ acts on ECFC not only to upregulate Fas, but also to selectively upregulate caspases-1, -3, and -8, which are activated and produce apoptosis, whereas the concentrations of FasL and FADD are not demonstrably changed.
INTERFERON γ (IFNγ) is secreted by activated T lymphocytes and natural killer cells. It induces the activation and differentiation of mononuclear phagocytes producing profound antiviral and immunomodulatory activities.1 Many investigators have reported that IFNγ also inhibits hematopoiesis in vitro, including both granulocytic and erythroid progenitors.1-9 Experiments in our laboratory have shown that IFNγ inhibited erythroid colony formation, cell proliferation, and differentiation of highly purified human day-3 to day-6 burst-forming units-erythroid (BFU-E) in a dose-dependent manner and also produced profound erythroblast apoptosis that was demonstrated by nuclear condensation and fragmentation plus flow cytometry after in situ end-labeling.9
Apoptosis, or programmed cell death, has a major role in normal development, tissue homeostasis, defense against viral invasion, immune modulation, and, when dysregulated, modulation of autoimmune and clonal or neoplastic diseases.10,11 It is characterized by cell shrinkage, chromatin condensation, internucleasomal DNA cleavage, membrane blebbing, and the formation of apoptotic bodies that are phagocytosed by other cells.12,13 Many factors, such as the ligation of the Fas (APO-1; CD95) or tumor necrosis factor (TNF) receptors, growth factor withdrawal, toxic or chemotherapeutic chemicals, viruses, radiation, and heat shock, trigger apoptosis.10 The Fas/Fas ligand (Fas L) system constitutes a very important cellular pathway responsible for the initiation of apoptosis. Fas is a 45-kD membrane glycoprotein belonging to the TNF receptor family and is widely distributed among diverse tissues and cells.8,10,11,14 Fas contains a 70 amino acid cytoplasmic domain, designated as a death domain, that is necessary for transduction of the apoptotic signal,15,16 whereas FasL is a 40-kD type II protein member of the TNF family. In contrast to Fas, FasL was initially thought to be relatively restricted in its cell and tissue distribution,17,18 but recent investigations have shown FasL to be present in thyroid and erythroid cells.11 14
Ligation of Fas results in aggregation of the intracellular death domains, leading to the recruitment of a set of signaling proteins and the formation of the death-inducing signaling complex (DISC), which includes Fas, FasL, an adopter molecule, Fas-associated protein with death domain (FADD), and FLICE (FADD-like ICE).19-21 FADD is recruited to Fas upon its ligation and binds to Fas via interactions between the death domains. The N-terminal region, termed the death effector domain (DED), is responsible for downstream signal transduction. FLICE (caspase-8) contains two N-terminal DED domains through which it binds FADD19-21 and belongs to a family of cysteine proteases (caspases). After binding to FADD, FLICE can produce an activation of proteolytic activity and trigger the interleukin-1β converting enzyme (ICE)-like protease cascade.22,23 At least 10 caspases have been identified, including caspase-1 (ICE), caspase-2 (Nedd2, ICH-1), caspase-3 (CPP32, Yama, apopain), caspase-4 (ICH-2, TX/ICErel II), caspase-5 (ICErel III, TY), caspase-6 (Mch2), caspase-7 (Mch3, ICE-LAP3, CMH-1), caspase-8 (FLICE, MACH, Mch5), caspase-9 (Mch6, ICE-LAP6), and caspase-10 (Mch4), and this family of proteases plays a critical role in the biochemical events governing apoptosis.24,25 The activated ICE-like members cleave a variety of substrates, such as poly(ADP)-ribose polymerase (PARP), lamins, fodrin, and protein kinase C, as well as producing morphologic alterations of the cells and nuclei.24
FasL is constitutively present in human erythroid progenitors as they mature from BFU-E to colony-forming unit-erythroid (CFU-E).14 This has been demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) and flow cytometric analysis. Whereas only a small percentage of normal human erythroid progenitors express Fas, and this is at very low level, the addition of IFNγ markedly increases the percentage of erythroid colony-forming cells (ECFC) expressing Fas on the surface of erythroid progenitors as well as the intensity of Fas expression and this increase is associated with the inhibitory effect of IFNγ on cell proliferation and the production of ECFC apoptosis.14 In addition, IFNγ downregulates the erythropoietin (EPO) and stem cell factor (SCF) receptors,26 whose ligands and activities protect human ECFC from apoptosis.27 In this study, the effect of IFNγ on the expression and activation of several caspases and the cleavage of PARP has been studied in highly purified human erythroid progenitor cells. Additionally, the effects of caspase inhibitors on the activation of these caspases and protection of the cells from apoptosis have also been examined.
MATERIALS AND METHODS
Purification and expansion of human blood ECFC.
Four hundred milliliters of blood was obtained from normal donors after receiving informed consent approved by the Vanderbilt University and Department of Veterans Affairs Medical Center Institutional Review Boards. BFU-E were first isolated by sequential density gradient centrifugation, depletion of lymphocytes and adherent cells, and further negative selection of contaminant cells with CD2, CD11b, CD16, and CD45 monoclonal antibodies (MoAbs), as previously described.9 28 The BFU-E were suspended in 20 mL Iscove’s modified Dulbecco’s medium (IMDM) containing 20% fetal calf serum (FCS), 5% pooled human AB serum, 1% bovine serum albumin (BSA), 5 × 10−5 mol/L 2-mercaptoethanol (2-ME), 10 μg/mL insulin, 2 U/mL EPO (Amgen, Inc, Thousand Oaks, CA), 50 U/mL interleukin-3 (IL-3), 50 ng/mL SCF (Amgen Inc), penicillin at 500 U/mL, and streptomycin at 40 μg/mL in polystyrene flasks to generate ECFC. After incubation at 37°C in 5% CO2/95% humidified air for 4 days (day-5 cells) or 5 days (day-6 cells), the cells were collected and further enriched by centrifugation through 10% BSA and over Ficoll-Hypaque, as necessary. The cells were then incubated in liquid culture containing 20% FCS, 5% pooled AB serum, 1% BSA, 10 μg/mL insulin, 2 U/mL EPO, and penicillin/streptomycin. IFNγ (4.75 × 107 U/mg; Genzyme Corp, Cambridge, MA) and/or SCF were added, as further delineated, in some cultures. The cells were collected after incubation for the indicated times to perform protein or RNA extractions, and plasma clot cultures were made to measure the number of ECFC.
Plasma clot culture of ECFC.
Cells were plated at a concentration of 103/mL in an IMDM mixture containing 20% FCS, 5% pooled human AB serum, 1% deionized BSA, 10 μg/mL insulin, 2 U/mL EPO, penicillin, streptomycin, 2 mg/mL fibrinogen (Sigma, St Louis, MO), and 0.2 U/mL bovine thrombin (Parke-Davis, Morris Plains, NJ). In some experiments, IFNγ was added at the indicated concentrations. The clots were fixed on day 15 and stained with 3,3′ demethoxybenzidine and hematoxylin. Colonies of two or more hemoglobinized cells were scored as ECFC. ECFC purity was determined by the plating efficiency and data were expressed as the means ± SD with significance calculated using the t-test. The average purity of the ECFC in our experiments was 61% ± 8% (day 6), 68% ± 9% (day 7), 86% ± 5% (day 8), 90% ± 4% (day 9), and 89% ± 3% (day 10). Because the cell number and colony-forming capacity decreased after incubation of the cells with IFNγ for 72 hours or longer,9 we used an equal amount of RNA or protein from control and IFNγ-treated cells for RNA protection assays and Western blot analyses.
RNase protection assay.
Day-5 cells were incubated with or without 500 U/mL of IFNγ for 72 hours and total RNA was isolated using ULTRASPEC (Bioticx Laboratories, Inc, Houston, TX). Twenty-microgram RNA samples were analyzed for the presence of transcripts of mRNAs related to apoptosis. An hApo-1 Multi-Probe Template Set including probes for caspases-1, -2, -5, -6, -7, -8, and -9 and granzyme B was purchased from PharMingen (San Diego, CA). L32 and GAPDH were included as internal controls. RNA protection assays were performed with the MAXIscript and RPA II Ribonuclease Protection Assay Kit (Ambion, Austin, TX), according to the manufacturer’s recommendations. Protected transcripts were separated by denaturing polyacrylamide gels and quantified by autoradiography.
Western blot analysis.
Cell extracts were prepared by lysing 107 cells in 100 μL lysis buffer containing 1% Triton X-100, 20 mmol/L Tris-HCl, pH 7.5, 10% glycerol, 140 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L EDTA, 2 mmol/L vanadate, 0.2 mmol/L phenylmethylsulfonyl fluoride, and 0.15 U/mL aprotinin at 4°C. Insoluble materials were removed by centrifugation for 20 minutes at 14,000g and 4°C.29 The samples were quantitated using a Bio-Rad protein assay kit II (Bio-Rad, Hercules, CA) and were boiled for 5 minutes in a sodium dodecyl sulfate (SDS) sample buffer before 8% or 13% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of protein per cell was nearly identical between normal and IFNγ-treated cells. After SDS-PAGE, the proteins were transferred to nitrocellulose. The membranes were blocked by incubation in Tris-buffered saline with 0.05% Tween-20 (TBST) and 5% nonfat milk powder at 4°C overnight. After a brief rinse, the blots were incubated for 2 hours at room temperature in TBST-5% milk powder with one of the following antibodies.
Primary antibodies were diluted as follows: MoAb for FasL (Transduction Laboratories, Lexington, KY) had a dilution of 1:1,000; MoAb for FADD (Transduction Laboratories) had a dilution of 1:250; MoAb for caspase-3 (Transduction Laboratories) had a dilution of 1:1,500; polyclonal rabbit antibody for caspase-3 (a gift from Dr Donald Nicholson, Merck Frosst Canada Inc, Quebec, Canada), which can recognize both the proenzyme and its fragments, had a dilution of 1:10,000; polyclonal goat antibodies for caspase-8, p10, and p20 (Santa Cruz Inc, Santa Cruz, CA) had dilutions of 1:200; polyclonal rabbit antibody for caspase-1 and p10 (Santa Cruz) had a dilution of 1:200; and MoAb for PARP (Enzyme Systems Products, Livermore, CA) had a dilution of 1:10,000.
Immunoblotting was followed by washing in TBST and 2 hours of incubation at 26°C with antimouse (Amersham Corp, Arlington Heights, IL), antirabbit, (Amersham), or antigoat (Santa Cruz) IgG-peroxidase conjugate (1:2,000) in TBST-5% milk powder. After washing 5 times for 5 minutes each time, the immunoblots were developed using the ECL method (Amersham). Some blots were stripped by incubation in a buffer containing 2% SDS, 62.5 mmol/L Tris-HCl for 30 minutes at 50°C (and were then reblocked and reprobed). Density scanning was performed using an LKB 2222-010 UltraScan XL Laser Densitometer (LKB Produkter AB, Bromma, Sweden). Caspase-3 inhibitor DEVD-fmk and caspase-8 inhibitor IETD-fmk were purchased from Enzyme Systems Products, whereas YVAD-cho and DEVD-cho were purchased from BIOMOL (Plymouth Meeting, PA).
RESULTS
Caspases-1, -2, -6, -8, and -9 mRNAs are upregulated by IFNγ.
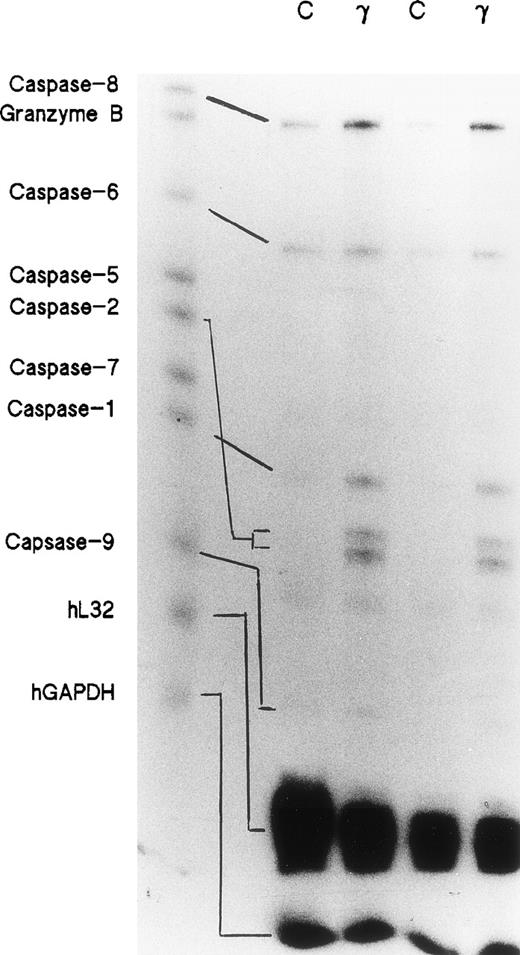
Day-5 cells were treated with or without 500 U/mL of IFNγ for 72 hours and the total RNAs were isolated. RNase protection assays were performed using a multiprobe set including 7 members of the caspase family and granzyme B, as well as two housekeeping genes, L32 and GAPDH, which delineate RNA loading on the gels. Two separate experiments demonstrated that the expressions of caspase-1, -2, -6, -8, and -9 mRNAs were significantly increased in the cells incubated with IFNγ. Caspase-5 and -7 mRNAs were not changed by incubation with IFNγ (Fig 1).
Effects of IFNγ on caspase mRNAs. Day-5 cells were incubated for 72 hours with or without 500 U/mL of IFNγ, and RNase protection assays were performed for members of the caspase family. Two separate experiments were performed (lanes 1/2 and lanes 3/4). Twenty-six micrograms of total RNA from each cell group was analyzed with the hApo-1 Multi-Probe Template Set (Pharmingen) to detect caspases-1, -2, -5, -6, -7, -8, and -9 and granzyme B mRNAs.
Effects of IFNγ on caspase mRNAs. Day-5 cells were incubated for 72 hours with or without 500 U/mL of IFNγ, and RNase protection assays were performed for members of the caspase family. Two separate experiments were performed (lanes 1/2 and lanes 3/4). Twenty-six micrograms of total RNA from each cell group was analyzed with the hApo-1 Multi-Probe Template Set (Pharmingen) to detect caspases-1, -2, -5, -6, -7, -8, and -9 and granzyme B mRNAs.
IFNγ upregulates and activates caspase-8/FLICE but not FADD.
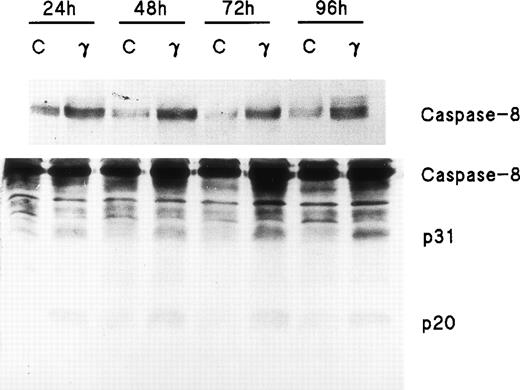
Because FADD and caspase-8, the most receptor-proximal ICE-like protease, are associated with the CD95 DISC and the DISC is essential for induction of apoptosis by Fas,22 we studied the effect of IFNγ on FADD and caspase-8. Human day-6 ECFC were treated with IFNγ (500 U/mL) for 24 to 96 hours and immunoblots were made of the cell protein extracts. This concentration of IFNγ has previously been shown to be optimum in this system.9,14 These studies showed that FADD is constitutively expressed in human erythroid progenitors from day 6 to day 10 and that the amount of FADD is not altered by incubation with IFNγ (Fig 2). FasL expression in the ECFC was similarly examined and also demonstrated constitutive expression without any quantitative change after incubation with IFNγ, similar to the pattern previously demonstrated by flow cytometric analysis.14 In contrast, procaspase-8 was upregulated 30% to 150% by IFNγ after as little as 24 hours of incubation and remained upregulated through 96 hours (Fig 3). Proteolytically cleaved forms of caspase-8 (p31, p20) were clearly observed in our cells after IFNγ treatment, and this cleavage occurred also at and after 24 hours of incubation.
FADD and FasL protein expression in human ECFC is constituitive and not affected by IFNγ. Day-6 cells were cultured in medium with or without 500 U/mL IFNγ for 24 to 96 hours and then whole cell protein lysates were prepared at each time for immunoblot analysis
FADD and FasL protein expression in human ECFC is constituitive and not affected by IFNγ. Day-6 cells were cultured in medium with or without 500 U/mL IFNγ for 24 to 96 hours and then whole cell protein lysates were prepared at each time for immunoblot analysis
Caspase-8/FLICE is upregulated and activated by IFNγ. In two separate experiments, day-6 cells were cultured in medium with or without 500 U/mL of IFNγ for 24 to 96 hours. Cell protein lysates were prepared and immunoblot analysis was performed with anti–caspase-8 antibodies. Each lane contained 150 μg of total protein. The top panel, using antibody to Mch5 p10 and a 3-minute exposure, shows that pro–caspase-8 increased after incubation with IFNγ for 24 hours. The bottom panel shows the result with antibody to Mch5 p20 and 15 minutes of exposure. Activation products of caspase-8, p31, and p20 are clearly observed in the IFNγ-treated cells by 24 hours.
Caspase-8/FLICE is upregulated and activated by IFNγ. In two separate experiments, day-6 cells were cultured in medium with or without 500 U/mL of IFNγ for 24 to 96 hours. Cell protein lysates were prepared and immunoblot analysis was performed with anti–caspase-8 antibodies. Each lane contained 150 μg of total protein. The top panel, using antibody to Mch5 p10 and a 3-minute exposure, shows that pro–caspase-8 increased after incubation with IFNγ for 24 hours. The bottom panel shows the result with antibody to Mch5 p20 and 15 minutes of exposure. Activation products of caspase-8, p31, and p20 are clearly observed in the IFNγ-treated cells by 24 hours.
IFNγ upregulates and activates caspase-1/ICE.
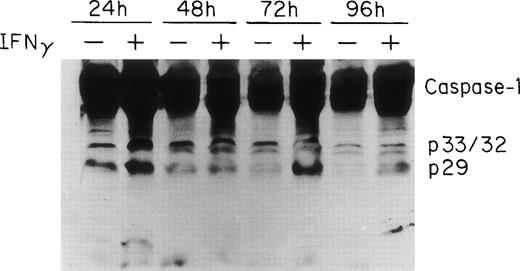
Two groups have reported that STAT1 is required for efficient constitutive expression of caspase-1 in some human cell lines and that caspase-1 is essential for IFNγ-induced apoptosis.30 31Previous experiments in this laboratory have shown that IFNγ induces a strong STAT1 activation (C.H. Dai, unpublished data). To observe the expression and activation of caspase-1 in ECFC, the cells were cultured in medium with or without IFNγ for 24 to 96 hours and the total cell lysates from each time point were prepared for immunoblot analysis using anti–caspase-1 antibody. The results are shown in Fig 4 and demonstrate that procaspase-1 expression significantly increased after incubation with IFNγ for 24 hours. Two additional experiments, with lesser exposure, clearly confirmed this (data not shown). In addition, the cleavage of caspase-1 was also increased by 24 hours of incubation with IFNγ and persisted for 96 hours. A slight decrease in control caspase-1 with time of incubation also was evident.
ICE/caspase-1 is upregulated and activated by IFNγ in human ECFC. Day-6 cells were incubated in liquid medium or medium containing 500 U/mL of IFNγ for 24 to 96 hours and immunoblot analysis was performed using whole cell lysates and antibody to caspase-1. An increase in caspase-1 and its activation fragments was observed in IFNγ-treated cells by 24 hours.
ICE/caspase-1 is upregulated and activated by IFNγ in human ECFC. Day-6 cells were incubated in liquid medium or medium containing 500 U/mL of IFNγ for 24 to 96 hours and immunoblot analysis was performed using whole cell lysates and antibody to caspase-1. An increase in caspase-1 and its activation fragments was observed in IFNγ-treated cells by 24 hours.
Caspase-3/CPP32/ apopain is upregulated and activated by IFNγ.
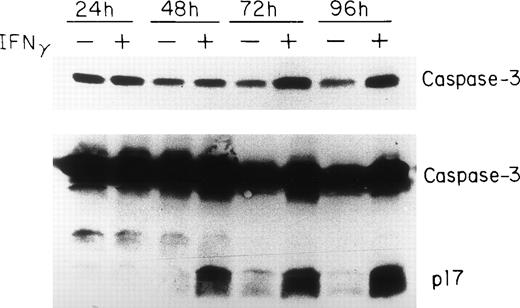
Because the activation of caspase-3 to either of its catalytically active p17 or p12 subunits has been demonstrated in cells undergoing apoptosis,32-34 we analyzed caspase-3 expression in human erythroid progenitors at various times after IFNγ treatment. These experiments indicate that caspase-3 is very highly expressed in human erythroid progenitors and increases from day 7 to day 10 of cell incubation with IFNγ (Fig 5). An increase of the proenzyme level of caspase-3 was observed after 24 hours of IFNγ treatment using two different anti-CPP32 antibodies. The mean increase in concentration of procaspase-3 at 24, 48, 72, and 96 hours of incubation with IFNγ, compared with the controls, in four experiments, as detected by scanning, was 15%, 31%, 145%, and 134%, respectively. Activation of caspase-3, identified by the appearance of the 17-kD peptide fragment, occurred at and after 48 hours of IFNγ stimulation (Fig 5).
Upregulation and delayed activation of caspase-3/CPP32/apopain in human ECFC after incubation with IFNγ. Day-6 cells were cultured in liquid medium or medium containing 500 U/mL of IFNγ for 24 to 96 hours and the cell proteins were analyzed by immunoblotting in three separate experiments. The top panel shows intact caspase-3 expression using MoAb that only reacts with the proenzyme. A polyclonal antibody reacting to both the intact enzyme and its fragments was used in the bottom panel. The proenzyme is significantly increased and a clear cleavage of caspase-3 occurs by 48 hours after incubation with IFNγ.
Upregulation and delayed activation of caspase-3/CPP32/apopain in human ECFC after incubation with IFNγ. Day-6 cells were cultured in liquid medium or medium containing 500 U/mL of IFNγ for 24 to 96 hours and the cell proteins were analyzed by immunoblotting in three separate experiments. The top panel shows intact caspase-3 expression using MoAb that only reacts with the proenzyme. A polyclonal antibody reacting to both the intact enzyme and its fragments was used in the bottom panel. The proenzyme is significantly increased and a clear cleavage of caspase-3 occurs by 48 hours after incubation with IFNγ.
PARP is cleaved in the presence of IFNγ.
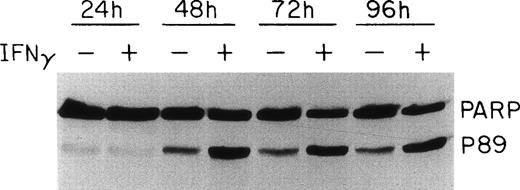
PARP, one of the proteolytic substrates of the caspases during apoptosis, has been reported to be cleaved to 24- and 89-kD fragments, which represent the N-terminal DNA binding domain and the C-terminal catalytic domain of the enzyme, respectively.35 Whereas most of the caspases can cleave PARP at high concentrations in vitro, it appears that caspase-3 and -7 are primarily responsible for PARP cleavage during apoptosis.36 Because caspase-3 was activated in the presence of IFNγ, we further investigated whether this activation, as well as the activation of other caspases, would lead to the cleavage of PARP. As shown in Fig 6, an increase of the 89-kD PARP cleavage product was observed at 48 hours of IFNγ incubation, approximately the same time as caspase-3 activation. A comparison of the intact PARP band in control and IFNγ-treated samples showed that a decrease of the inactive form of PARP of up to 60% accompanied the formation of the activation fragment after 48 to 96 hours of incubation with IFNγ. In the control cultures not treated with IFNγ, a smaller cleavage of PARP also was noted.
Effect of IFNγ on PARP cleavage. Day-6 cells were incubated with or without 500 U/mL of IFNγ for the indicated times and whole cell protein lysates were prepared for immunoblotting. The IFNγ-treated cells showed an enhanced cleavage of PARP at 48 hours, denoted by the appearance of the p89 activation fragment.
Effect of IFNγ on PARP cleavage. Day-6 cells were incubated with or without 500 U/mL of IFNγ for the indicated times and whole cell protein lysates were prepared for immunoblotting. The IFNγ-treated cells showed an enhanced cleavage of PARP at 48 hours, denoted by the appearance of the p89 activation fragment.
Effects of caspase inhibitors on caspase activation and apoptosis.
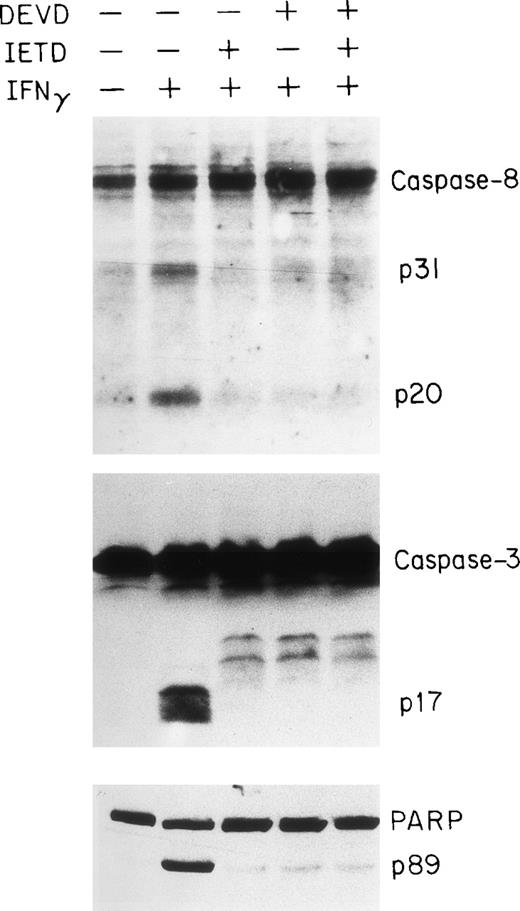
To obtain further evidence for the involvement of the caspase system in IFNγ-induced apoptosis of human ECFC, we tested the capacities of peptide inhibitors of the caspase family to inhibit caspase activities in vitro and reduce apoptosis. DEVD is a relatively potent inhibitor of caspases-1, -3, and -8, whereas YVAD is a potent and selective inhibitor of caspase-1 and IETD is known to inhibit caspase-8.37 38 Day-6 cells were treated with or without the inhibitor(s), in the absence or presence of IFNγ, for 96 hours and then cell lysates were prepared for immunoblot analysis. As shown in Fig 7, either IETD-fmk or DEVD-fmk effectively inhibited the cleavage of caspase-3 and -8 as well as the PARP substrate.
Inhibition of caspase-8 and -3 and PARP cleavage by DEVD and IETD. Day-6 cells were cultured in medium with or without 500 U/mL of IFNγ for 96 hours in the presence or absence of DEVD-fmk and IETD-fmk at 40 μmol/L. The cell protein lysates were electrphoresed using a 13% acrylamide gel to separate caspase-8 and -3 and an 8% gel for PARP.
Inhibition of caspase-8 and -3 and PARP cleavage by DEVD and IETD. Day-6 cells were cultured in medium with or without 500 U/mL of IFNγ for 96 hours in the presence or absence of DEVD-fmk and IETD-fmk at 40 μmol/L. The cell protein lysates were electrphoresed using a 13% acrylamide gel to separate caspase-8 and -3 and an 8% gel for PARP.
To observe the effects of the inhibitor on the reduction of cell growth induced by IFNγ, two classes of caspase inhibitors, -fmk and -cho, were used. These experiments indicated that the addition of either DEVD or IETD to IFNγ-treated ECFC for 96 hours partially blocked the reduction in cell growth induced by IFNγ (Table 1). The addition of similar concentrations of IETD-fmk or DEVD-fmk to IFNγ-treated plasma clot cultures of day-6 ECFC reduced the number of erythroid colonies with cellular nuclear fragmentation, indicative of apoptosis, by 33% and 38%, respectively (data not shown).
Effect of Inhibitors on Reduction of Cell Growth Induced by IFNγ
| Inhibitors–fmk . | Inhibitors–cho . | ||||
|---|---|---|---|---|---|
| Treatment . | % . | PValue . | Treatment . | % . | P Value . |
| Control | 100 ± 10 | Control | 100 ± 6 | ||
| IFNγ | 56 ± 4 | v Control <.01 | IFNγ | 66 ± 6 | v Control <.01 |
| IFNγ + IETD | 71 ± 3 | v IFNγ <.01 | IFNγ + YVAD | 87 ± 4 | v IFNγ <.01 |
| IFNγ + DEVD | 82 ± 3 | v IFNγ <.01 | IFNγ + DEVD | 88 ± 7 | v IFNγ <.01 |
| Inhibitors–fmk . | Inhibitors–cho . | ||||
|---|---|---|---|---|---|
| Treatment . | % . | PValue . | Treatment . | % . | P Value . |
| Control | 100 ± 10 | Control | 100 ± 6 | ||
| IFNγ | 56 ± 4 | v Control <.01 | IFNγ | 66 ± 6 | v Control <.01 |
| IFNγ + IETD | 71 ± 3 | v IFNγ <.01 | IFNγ + YVAD | 87 ± 4 | v IFNγ <.01 |
| IFNγ + DEVD | 82 ± 3 | v IFNγ <.01 | IFNγ + DEVD | 88 ± 7 | v IFNγ <.01 |
Two experiments with -fmk and three with -cho inhibitors were performed. Day-6 cells were preincubated for 2 hours with 40 μmol/L IETD-fmk or DEVD-fmk or 1 μmol/L YVAD-cho or DEVD-cho before adding 100 U/mL of IFNγ. After incubation for 96 hours, the cells were collected and counted. The number of cells in the group not treated with IFNγ was taken as 100% and the other groups were then compared with this control.
DISCUSSION
Previous experiments have demonstrated that IFNγ produces apoptosis of erythroblasts and reduces the number and size of erythroid colonies as well as the degree of differentiation.9 IFNγ downregulates the EPO and SCF receptors of ECFC and upregulates Fas mRNA and protein, whereas FasL remains constitutively produced without apparent change as the cells mature.14 To further investigate the manner in which the Fas/FasL pathway is involved in human erythroid progenitor cell apoptosis induced by IFNγ, we examined the expression and activation of several members of the caspase family that have been reported to mediate apoptosis in many cells of different origin.
Although several models for apoptosis-induced by IFNγ through the Fas/FasL system have been proposed,39 the data presented here seem most consistent with the model presented by Fraser and Evan.40 First, ligation of Fas recruits other proteins, such as FADD, and forms the CD95 DISC.19-21 Caspase-8/FLICE is a third component of the DISC and is the first caspase in a cascade of ICE-like proteases activated by CD95. Upon binding to FADD, through the DED, caspase-8 is converted to its active subunits, which are released to the cytosol and can activate a cascade of ICE-like preteases.22 Hence, caspase-8 is called an initiator protease. Overexpression of caspase-8 resulting in apoptosis has been reported.24 When we measured FADD levels, no major change was evident in IFNγ-treated human ECFC. In contrast, procaspase-8 was upregulated and cleavage of caspase-8 was detected in the cells by 24 hours of incubation with IFNγ. The kinetics of caspase-8 expression were similar to the kinetics of Fas upregulation that occurs after incubation of ECFC with IFNγ.14
Although the precise role of caspase-1/ICE in apoptosis is still uncertain, ectopic expression of ICE in some cells has resulted in apoptosis41 and a reduction of apoptosis in ICE−/− cells provides evidence for a requirement of ICE in IFNγ-induced cell death. Because ICE appears to be activated near the apex of an ICE-related protease cascade, ICE has been called an amplifier protease, which amplifies the initial caspase-8 activation signal.21 40 Both increases in the expression of ICE and the cleavage of ICE, demonstrated by the appearance of activation fragments, were detected at and after 24 hours of incubation with IFNγ. These observations indicate that ICE is activated in IFNγ-treated cells and that activation of ICE may precede activation of other caspase, such as caspase-3.
Caspase-3/CPP32/apopain has been called an executioner protease, and it appears to have a critical role during apoptosis. It has been shown to be responsible either partially or totally for the proteolytic cleavage of many key proteins, such as PARP, DNA-PK, and U1-70 kD.24Activation of caspase-3 has been reported in human hematopoietic cells during apoptosis induced by EPO deprivation as well as IL-3 deprivation.42 43 In this study, an increased expression of intact caspase-3 was observed in the human ECFC at and after 24 hours of treatment with IFNγ. A distinct activation of caspase-3 and its substrate, PARP, demonstrated by the appearance of proteolytically fragments, was detected 24 hours later, as compared with caspase-8 and caspase-1. This correlates with the appearance of apoptotic changes induced by IFNγ and occurs later than the increase in FAS, caspase-8, and caspase-1.
It has been reported that IFNγ enhanced the activity of several apoptosis-related genes in HT-29 cells, a human colon adenocarcinoma cell line, including Fas, and caspases-1, -3, -4, -7, -8, and -10, but not caspases-2 and -6.44 Some gene mRNAs were induced or upregulated by 1 hour of IFNγ treatment, such as those for Fas and caspases-7, -8, and -10. Caspase-3 and -4 mRNAs were weakly induced, whereas others, such as those for Fas and caspases-1, -8, and -10, were strongly induced by IFNγ.42 However, no studies were reported on the presence, activation, or changes in concentration of the caspase proteins.
To further determine whether caspases had an intermediary role in the induction of apoptosis in human ECFC by IFNγ, two classes of caspase inhibitors were tested. Both prevented at least 90% of the IFNγ induced cleavage of caspases-8 and -3, as well as PARP. However, addition of the inhibitors to the IFNγ-treated cells only partially prevented apoptosis. This suggests that other, alternative pathways for apoptosis also may be activated by IFNγ, or that the inhibitors may be active for a limited time during the 96-hour cell incubations.
In conclusion, FADD and FasL were constitutively present in human ECFC, whereas Fas expression was strongly upregulated by IFNγ. Caspase-1, -3, and -8 were not only upregulated but also activated when these cells were induced to undergo apoptosis by IFNγ. Cleavage of PARP appeared at nearly the same time as caspase-3 activation and both were delayed compared with the activation times for caspase-8 and -1. The caspase inhibitors, IETD and DEVD, very efficiently blocked the activation of caspase-8 plus -3 and the cleavage of PARP, but only reversed the inhibition of cell growth induced by IFNγ by approximately 30%. Therefore, the Fas/FasL pathway may be only partially responsible for production of ECFC apoptosis by IFNγ.
ACKNOWLEDGMENT
The authors are very grateful to Dr Donald Nicholson and Merck Frosst Canada, Inc for their gift of polyclonal antibody to caspase-3.
Supported by a Veterans Health Administration Merit Review Grant and Grants No. DK-15555 and 5 T32-DK07186 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sanford B. Krantz, MD, Department of Medicine-Hematology/Oncology, Vanderbilt University Medical School, 547 Medical Research Bldg II, Nashville, TN 37232-6305.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal