Abstract
The hallmark of chronic myelogenous leukemia (CML) is the Philadelphia (Ph) chromosome that fuses genetic sequences of the BCR gene on chromosome 22 with c-ABL sequences translocated from chromosome 9. BCR/ABL fusion proteins have a dysregulated protein tyrosine kinase (PTK) activity exerting a key role in malignant transformation. Targeting the tyrosine kinase activity of BCR/ABL or using agents capable of triggering apoptosis might represent attractive therapeutic approaches for ex vivo purging. AG957, a member of the tyrphostin compounds, exerts a selective inhibition of p210BCR/ABLtyrosine phosphorylation. We report here that preincubation of CML or normal CD34+ cells with graded concentration of AG957 (1 to 100 μmol/L) resulted in a statistically significant, dose-dependent suppression of colony growth from multipotent, erythroid, and granulocyte-macrophage progenitors as well as the more primitive long-term culture-initiating cells (LTC-IC). However, AG957 doses causing 50% inhibition (ID50) of CML and normal progenitors were significantly different for multilineage colony-forming units (CFU-Mix; 12 v 64 μmol/L; P = .008), burst-forming unit-erythroid (BFU-E; 29 v 89 μmol/L;P = .004), colony-forming unit–granulocyte-macrophage (CFU-GM; 34 v 85 μmol/L; P = .004), and LTC-IC (43 v 181 μmol/L; P = .004). In 5 of 10 patients, analysis of BCR/ABL mRNA on single progenitors by reverse transcription-polymerase chain reaction showed that AG957 at 50 μmol/L significantly reduced the mean (±SD) percentage of BCR/ABL-positive progenitors (92% ± 10% v 33 ± 5%;P = .001). Because AG957 treatment resulted in significantly higher percentages of apoptotic cells (30% v9%) in the BCR/ABL-transfected 32DLG7 cells as compared with 32D-T2/93 cells (BCR/ABL-negative), we investigated the combined effects of AG957 with the anti-Fas receptor (Fas-R) monoclonal antibody CH11 that triggers apoptosis. As compared with AG957 alone, the sequential treatment of CML CD34+ cells with AG957 (1 μmol/L) and CH11 (1 μg/mL) increased CFU-Mix, BFU-E, and CFU-GM growth inhibition by 1.6-fold, 3-fold, and 4-fold, respectively. In contrast, the treatment of normal CD34+ cells with AG957 and CH11 failed to enhance AG957-induced colony growth inhibition. We conclude that (1) AG957 inhibits in a dose-dependent manner CML CD34-derived colony formation by both primitive LTC-IC as well as committed CFU-Mix, BFU-E, and CFU-GM; (2) this growth inhibition is associated with the selection of a substantial amount of BCR/ABL-negative progenitors; and (3) the antiproliferative effect of AG957 is dramatically increased by combining this compound with the anti–Fas-R antibody CH11. These data may have significant therapeutic applications.
CHRONIC MYELOGENOUS leukemia (CML) is associated with a specific chromosomal abnormality known as the Philadelphia (Ph) chromosome that results from a reciprocal translocation between chromosomes 9 and 22 and fuses genetic sequences of the BCR gene on chromosome 22 with c-ABL sequences translocated from chromosome 9.1,2 Depending on the breakpoint in BCR, the BCR/ABL chimeric gene generates one of three types of fusion proteins: p210BCR/ABL is detectable in 95% of patients with CML and also occurs in approximately one third of BCR/ABL-positive acute lymphoblastic leukemia (ALL); p190BCR/ABL is detectable in two thirds of BCR/ABL-positive ALL; and p230BCR/ABL is associated with the rare Ph-positive chronic neutrophilic leukemia.3,4 As compared with the native ABL protein (p145ABL), BCR/ABL fusion proteins have dysregulated protein tyrosine kinase (PTK) activity,5 transforming activity for hematopoietic cells,6 and the ability to cause CML-like myelopoiesis in mice.7 Dysregulated PTK activity of BCR/ABL fusion proteins and the subsequent changes in the phosphorylation pattern of regulatory proteins play a key pathogenetic role in CML.8,9 Several signal transduction substrates, including p21RAS,10p120GAP,11 and p62DOK,12 are directly involved in BCR/ABL-dependent leukemogenesis. The Src homology 2 (SH2) and Src homology 3 (SH3) domain-containing Grb-2 protein links tyrosine kinases to Ras signaling.13,14 Binding of Grb-2 to BCR/ABL is mediated by the direct interaction of the Grb-2 SH2 domain with a phosphorylated tyrosine within the BCR first exon.15BCR/ABL tyrosine kinases also phosphorylate Shc proteins on tyrosine, inducing the formation of an Shc-Grb-2 complex that also has the potential to stimulate Ras.16 Another important substrate is the SH2/SH3 domain-containing CRKL protein, which is phosphorylated by and also forms specific complexes with p210BCR/ABL.17
Treatment options in CML are still limited, because only a minority of patients are eligible for allogeneic bone marrow transplantation, which represents the only curative treatment for CML.18 Based on experimental and clinical findings, autologous stem cell transplantation (ASCT) is currently considered as a strategy to be included in the therapeutic management of CML.19-21 ASCT leads to 5% long-term cytogenetic remission, with leukemic relapse being the main cause of treatment failure.21 Although cure of disease has not yet been achieved using autografting, long-term survival is possible and indeed approaches the survival rate for allogeneic related donor transplant recipients.20 To improve the therapeutic index of ASCT, both purging of the leukemic stem cells or selecting for the nonleukemic stem cells have been explored.22 These strategies have focused on either in vivo purging with single or double autograft23,24 or in vitro purging with chemical, biological, or immunological techniques.25-32 Targeting the PTK activity of BCR/ABL has been proposed as an attractive therapeutic strategy due to the potential for malignant transformation of this kinase activity.33
Inhibition of the BCR/ABL PTK activity has been obtained with nonselective compounds, such as genistein, as well as with selective compounds, such as herbimycin A.34,35 More recently, a selective inhibition of the BCR/ABL tyrosine kinase activity has been demonstrated with the 2-phenylaminopyrimidine derivative CGP57148B.36,37 Tyrphostins represent an additional family of PTK inhibitors acting as competitive inhibitors of protein substrate and/or ATP binding.38 Because of their chemical design, tyrphostins are slightly hydrophobic, low molecular weight, nonpeptidic compounds with high biological stability and cell permeability.38 In K562 cells, a selective inhibition of p210BCR/ABL tyrosine phosphorylation without inhibition of total protein phosphorylation has been obtained with the tyrphostin AG95739 that resulted in 13-fold more potent blocking of the kinase activity of p210BCR/ABL than blocking of the kinase activity of p140ABL.40Recently, AG957 has been shown to restore integrin-mediated adhesion in CML progenitors.41
The use of agents capable of triggering apoptosis can also be considered in the context of in vitro purging strategies aimed at counteracting the inhibition of the apoptotic machinery induced by BCR/ABL gene product.42 Fas receptor (Fas-R), also termed CD95 or Apo-1, is a transmembrane glycoprotein belonging to the tumor necrosis factor receptor family expressed in a variety of tissues, including thymus, heart, lung, and liver, as well as hematopoietic cells, such as CD34+ cells, T cells, B cells, and monocytes.43,44 The characterized function of Fas-R on normal CD34+ cells involves triggering of apoptosis upon specific binding with the natural Fas ligand (Fas-L) or with agonistic monoclonal antibodies (eg, CH11) against CD95.45 CML CD34+ marrow cells constitutively express Fas-R at significantly higher levels than do normal CD34+ marrow cells.46 Despite a partial resistance of CML cells to apoptosis, priming with interferon-α (IFN-α) before Fas-R triggering is associated with apoptosis induction and decreased BCR/ABL protein level.47
Therefore, it was the aim of the present study to investigate the differential effects of graded concentration of AG957 on the in vitro growth of CML and normal hematopoietic progenitors. The capability of AG957 to select in vitro for BCR/ABL-negative progenitors was analyzed by detecting the BCR/ABL mRNA on single progenitors by reverse transcription-polymerase chain reaction (RT-PCR). In addition, the combined effects of AG957 and the anti–Fas-R monoclonal antibody CH11 on CML and normal progenitor cells were investigated.
MATERIALS AND METHODS
Patients.
Ten patients (5 men and 5 women) with median age of 51 years (range, 40 to 64 years) and a diagnosis of Ph-positive CML in chronic phase were included in this study. The main clinical characteristics of the patients are summarized in Table 1. Seven patients were studied at diagnosis and before any treatment, whereas 3 patients had received hydroxyurea and/or IFN-α therapy for 4 to 16 months before being studied. All patients were 100% Ph-positive at direct cytogenetic analysis. Eight patients showed a b3a2 BCR/ABL junction, and 2 cases showed a b2a2 junction when analyzed by RT-PCR.
Clinical Characteristics of the Patients at the Time of the Study
| Case . | Age/Sex . | Clinical Status . | Peripheral Blood . | Ph+/Total Metaphases* . | BCR-ABL Junction . | Time From Diagnosis (mos) . | Previous Therapy . | |
|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Plt (×109/L) . | |||||||
| 1 | 49/M | CP | 43 | 442 | 25/25 | b3a2 | 0 | — |
| 2 | 44/M | CP | 18 | 478 | 25/25 | b3a2 | 4 | HU |
| 3 | 58/F | CP | 12 | 349 | 20/20 | b3a2 | 12 | HU |
| 4 | 64/F | CP | 13 | 463 | 22/22 | b3a2 | 0 | — |
| 5 | 40/F | CP | 40 | 207 | 25/25 | b3a2 | 16 | HU, IFN-α |
| 6 | 59/F | CP | 67 | 519 | 15/15 | b3a2 | 0 | — |
| 7 | 40/M | CP | 162 | 264 | 20/20 | b3a2 | 0 | — |
| 8 | 49/M | CP | 69 | 305 | 30/30 | b2a2 | 0 | — |
| 9 | 54/M | CP | 87 | 555 | 20/20 | b3a2 | 0 | — |
| 10 | 64/F | CP | 54 | 456 | 30/30 | b2a2 | 0 | — |
| Case . | Age/Sex . | Clinical Status . | Peripheral Blood . | Ph+/Total Metaphases* . | BCR-ABL Junction . | Time From Diagnosis (mos) . | Previous Therapy . | |
|---|---|---|---|---|---|---|---|---|
| WBC (×109/L) . | Plt (×109/L) . | |||||||
| 1 | 49/M | CP | 43 | 442 | 25/25 | b3a2 | 0 | — |
| 2 | 44/M | CP | 18 | 478 | 25/25 | b3a2 | 4 | HU |
| 3 | 58/F | CP | 12 | 349 | 20/20 | b3a2 | 12 | HU |
| 4 | 64/F | CP | 13 | 463 | 22/22 | b3a2 | 0 | — |
| 5 | 40/F | CP | 40 | 207 | 25/25 | b3a2 | 16 | HU, IFN-α |
| 6 | 59/F | CP | 67 | 519 | 15/15 | b3a2 | 0 | — |
| 7 | 40/M | CP | 162 | 264 | 20/20 | b3a2 | 0 | — |
| 8 | 49/M | CP | 69 | 305 | 30/30 | b2a2 | 0 | — |
| 9 | 54/M | CP | 87 | 555 | 20/20 | b3a2 | 0 | — |
| 10 | 64/F | CP | 54 | 456 | 30/30 | b2a2 | 0 | — |
Abbreviations: WBC, white blood cell counts; Plt, platelet counts; CP, chronic phase; HU, hydroxyurea.
Number and type of bone marrow metaphases obtained after routine cytogenetic analysis of freshly aspirated bone marrow at the time of study.
Cell separation procedures.
CML marrow cells were obtained by aspiration from the posterior iliac crest. Normal cells were obtained from healthy donors undergoing peripheral blood progenitor cell mobilization. All patients and normal individuals provided informed consent for these studies. Mononuclear cells (MNCs) were separated by centrifugation on a Ficoll-Hypaque gradient (density = 1.077 g/mL).28 MNCs were washed and suspended in RPMI-1640 (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Stem Cell Technologies, Vancouver, British Columbia, Canada). CD34+ cells were enriched according to a magnetic cell sorting methodology (MACS; Miltenyi Biotec, Bergish Gladbach, Germany).48 Briefly, MNCs were labeled with a haptenized CD34 antibody (QBEND/10) that was then magnetically labeled in a second-step reaction with an antihapten antibody coupled to super-paramagnetic microbeads. Labeled cells were then separated using a high gradient magnetic separation column placed in a strong magnetic field. The magnetically stained cells were retained in the column while unstained cells passed through. When the column was removed from the magnetic field, the magnetically retained cells were eluted. Purity of CML and normal CD34+ cell fractions ranged from 63% to 97% and 75% to 86%, respectively.
Cell lines.
32D-T2/93 (BCR/ABL-negative) and 32DLG7 (BCR/ABL-positive) cell lines14 (kindly provided by Dr A. Santucci, Hematology Department, Bologna, Italy) were used to investigate the apoptotic-inducing effect of AG957. Both cell lines were cultured in RPMI-1640 supplemented with FBS (10% vol/vol) and L-glutamine (2 mmol/L). Culture medium for 32D-T2/93 cells was also supplemented (10% vol/vol) with a conditioned medium of the WEHI-3 cell line as a source of murine interleukin-3 (IL-3).
Multilineage colony-forming units (CFU-Mix), burst-forming unit-erythroid (BFU-E), and colony-forming unit–granulocyte-macrophage (CFU-GM) assay.
The assay for CFU-Mix, BFU-E, and CFU-GM was performed as described elsewhere.28 Briefly, 1 × 103CD34+ cells were plated in 35-mm Petri dishes in 1-mL aliquots of Iscove’s modified Dulbecco’s medium (IMDM; Seromed, Berlin, Germany) containing 30% FBS (Stem Cell Technologies), 10−4 mol/L 2-mercaptoethanol (GIBCO), and 1.1% (wt/vol) methylcellulose. Cultures were stimulated with stem cell factor (SCF; 50 ng/mL; Amgen Inc, Thousand Oaks, CA), IL-3 (10 ng/mL; Sandoz, Basel, Switzerland), granulocyte colony-stimulating factor (G-CSF; 10 ng/mL; Amgen Inc), granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL; Sandoz), and erythropoietin (Epo; 3 U/mL; Amgen Inc). After incubation (37°C, 5% CO2) for 14 to 18 days in a humidified atmosphere, progenitor cell growth was evaluated according to previously published criteria.28
Long-term culture-initiating cell (LTC-IC) assay.
The LTC-IC assay was performed according to Sutherland et al.49 Briefly, test cell suspensions (1 × 104 CD34+ cells) were resuspended in complete medium consisting of α-medium (GIBCO) supplemented with FBS (12.5%), horse serum (12.5%; Stem Cell Technologies), L-glutamine (2 mmol/L), 2-mercaptoethanol (10−4 mol/L), inositol (0.2 mmol/L), folic acid (20 μmol/L), and freshly dissolved hydrocortisone (10−6 mol/L). Test cells were seeded into cultures containing a feeder layer of irradiated (8,000 cGy) murine M2-10B4 cells (3 × 104/cm2; kindly provided by Dr C. Eaves, Terry Fox Laboratory, Vancouver, British Columbia, Canada) engineered by retroviral gene transfer to produce human IL-3 and human G-CSF.50 Cultures were fed weekly by replacement of half of the growth medium containing half of the nonadherent cells with fresh complete medium. After 5 weeks in culture, nonadherent and adherent cells harvested by trypsinization were pooled, washed, and assayed together for clonogenic cells in standard methylcellulose cultures stimulated with IL-6 (10 ng/mL; Sandoz), SCF, IL-3, G-CSF, GM-CSF, and Epo. The total number of clonogenic cells (ie, CFU-Mix plus BFU-E plus CFU-GM) present in 5-week-old LTC provides a relative measure of the number of LTC-IC originally present in the test suspension.19 Absolute LTC-IC values were calculated by dividing the total number of clonogenic cells by 4, which is the average output of clonogenic cells per LTC-IC, according to limiting dilution analysis studies reported by others.19
Cytogenetic analysis.
Cytogenetic analysis and standard GTG- or QFQ-banding techniques were performed according to standard methods.51 To exclude that AG957 could induce false-negative results by blocking BCR/ABL gene expression from otherwise Ph-positive colonies, in two experiments individual colonies were aspirated, divided into two aliquots, and analyzed both at the cytogenetic and molecular level.48
Detection of BCR/ABL mRNA in individual progenitors.
Colonies were individually removed under an inverted microscope and transferred into microcentrifuge tubes containing 40 μL phosphate-buffered saline (PBS).48 After adding guanidinium thiocyanate (40 μL), colonies were frozen at −70°C until nested RT-PCR was performed. Briefly, after adding 4 μg of MS2 phage RNA (Boehringer Mannheim, Mannheim, Germany) as a carrier, RNA was extracted using TRIzol (GIBCO), precipitated with isopropanol, washed with ethanol, dried, and redissolved in RNAse-free water.52Total RNA from each colony was reverse transcribed into cDNA in a 20 μL reaction primed with random hexamers. Total cDNA from each colony was divided into two aliquots for detection of the BCR/ABL rearrangement and the ABL sequence, respectively. PCR amplifications were performed in 45 μL reactions containing 10 μL of cDNA, 0.25 mmol/L dNTP, 0.57 mmol/L of each primer (for BCR/ABL: sense 5′-GAA GAA GTG TTT CAG AAG CTT CTC CC-3′ and antisense 5′-GAC CCG GAG CTT TTC ACC TTT AGT T-3′; for ABL: sense 5′-TTC AGC GGC CAG TAG CAT CTG ACT T-3′ and antisense 5′-GAC CCG GAG CTT TTC ACC TTT AGT T-3′), and 2 U Taq polymerase (GIBCO) in PCR buffer (20 mmol/L Tris-HCl and 1.5 mmol/L MgCl2).53 Forty-five cycles, each consisting of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds, were performed using a Perkin-Elmer Cetus DNA Thermal Cycler (Perkin-Elmer Cetus, Norwalk, CT). One microliter of the first PCR product for BCR/ABL was reamplified with internal nested primers (sense 5′-GTG AAA CTC CAG ACT GCT CAC AGC A-3′ and antisense 5′-TCC ACT GGC CAC AAA ATC ATC ATA CAG T-3′) under slightly modified conditions (35 cycles, each consisting of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds). Twenty microliters of each PCR product was electrophoresed through a 2% agarose gel stained with ethidium bromide and photographed under UV light. The sizes of the BCR/ABL fragments obtained from nested PCR were 272 bp and 197 bp, depending on the position of junction point within M-BCR. The expected product generated by PCR for ABL was 185 bp. To adequately control that RNA was correctly reverse transcribed and that the PCR product was generated by amplifying cDNA and not genomic DNA eventually contaminating an RNA sample, ABL sequence amplification was performed with two primers located on exon 2 and exon 3 of the cDNA sequence, respectively. Only colonies positive for ABL amplification were considered evaluable. To eliminate the possibility of false-positive results, a blank control for RNA extraction and a negative BCR/ABL control consisting of RNA isolated from normal marrow-derived CFU-GM were used.54 Each PCR amplification also included a control reaction without template cDNA (H2O blank) and, as a positive control, RNA extracted from K562 colonies.
DNA fragmentation.
To investigate the capability of AG957 to trigger apoptosis, nuclear DNA fragmentation was detected by terminal deoxynucleotidyl transferase (TdT) assay.34 55 Briefly, cells were fixed in PBS containing 4% paraformaldehyde, washed, and permeabilized with 0.1% Triton X-100. Cells were then resuspended in 50 μL of a solution containing 0.1 mol/L sodium cacodylate, 1 mmol/L CoCl2, 0.1 mmol/L dithiothreitol, 0.05 mg/mL bovine serum albumine, 10 U TdT, and 0.5 nmol fluorescein isothiocyanate (FITC)-conjugated biotin-16-deoxyuridine triphosphate. All chemicals and nucleotides were purchased from Boehringer Mannheim. The cells were incubated for 60 minutes at 37°C and analyzed by flow cytometry on a FACSort (Becton Dickinson, Mountain View, CA).
AG957 treatment.
AG957 was diluted in dimethyl sulfoxide (DMSO) to prepare 1,000-fold concentrated solutions. To evaluate the effect of AG957 as a single agent, CD34+ cells (1 × 105/mL) were exposed (30 minutes at 37°C in 5% CO2) to either control medium (IMDM, 10% FBS) or medium containing increasing doses of AG957 (1 to 100 μmol/L). At the end of the incubation period, the cells were washed three times and cultured to quantitate CFU-Mix, BFU-E, CFU-GM, and LTC-IC. For each experiment, appropriate controls with vehicle alone (DMSO at 1 μL/dish) were set up. To evaluate the combined effects of AG957 and anti–Fas-R monoclonal antibody, untreated and AG957-treated (1 to 50 μmol/L for 30 minutes) CD34+ cells were resuspended in serum-free medium (StemPro-34 SFM; GIBCO) and exposed (2 hours at 37°C) to the anti–Fas-R antibody CH11 (1 μg/mL; Immunotech, Marseilles, France). At the end of the incubation, CD34+ cells were incorporated in a standard methylcellulose assay to quantitate hematopoietic progenitors. For each experiment, appropriate controls with cells exposed to either CH11 alone or an irrelevant monoclonal antibody was set up.
Statistical analysis.
Four plates were scored for each data point per experiment and the results were expressed as the mean ± 1 standard error of the mean (SEM). Statistical analysis was performed with the statistical package Statview (BrainPower Inc, Calabasas, CA) run on a Macintosh 6300 personal computer (Apple Computer Inc, Cupertino, CA). The Student’st-test for paired or unpaired data (two-tail) or the Wilcoxon signed-rank test were used where appropriate to test the probability of significant differences between samples. AG957 concentrations resulting in 50% inhibition (ID50) of colony formation were calculated for each experiment by extrapolating from a least square linear regression line relating AG957 concentration to the percentage of colony growth inhibition.
RESULTS
Effect of AG957 ± CH11 on CML and normal progenitors.
In preliminary experiments, the time-dependent effect of AG957 was investigated. Preincubation of CML cells with a single dose of AG957 (10 μmol/L) resulted in a maximal inhibition of colony formation after 30 minutes of exposure (data not shown). This length of time was therefore used in subsequent experiments aimed at exploring the dose-dependent effect of AG957.
As shown in Fig 1, preincubation of CML CD34+ cells (n = 10) with AG957 (1 to 100 μmol/L) followed by extensive washings resulted in a statistically significant (CFU-Mix: P = .04 at 1 μmol/L; BFU-E: P = .01 at 1 μmol/L; CFU-GM: P = .04 at 5 μmol/L), dose-dependent suppression of colony growth from multipotent (Fig 1A), erythroid (Fig1B), and granulocyte-macrophage (Fig 1C) progenitors. Regression analysis showed that inhibition was linearly related (CFU-Mix:r = .73, P = .04; BFU-E: r = .87, P = .01; CFU-GM: r = .91, P = .01) to AG957 concentration over the range tested. Although individual CML samples exhibited variable sensitivity to the inhibitory effects of AG957, no one failed to respond to this agent. The degree of colony suppression was not related to colony number in control cultures.
Effect of AG957 on CML (▪) and normal (○) CD34+-derived CFU-Mix (A), BFU-E (B), and CFU-GM (C). Each data point represents the mean (±SEM) percentage of inhibition from separate experiments using 10 CML and 6 normal samples. For CML, control colonies per 1 × 103 CD34+ cells ranged from 2 to 7 for CFU-Mix, 39 to 80 for BFU-E, and 31 to 180 for CFU-GM. For normal samples, control colonies 1 × 103CD34+ cells ranged from 4 to 8 for CFU-Mix, 24 to 96 for BFU-E, and 40 to 64 for CFU-GM. When compared with control cultures (Wilcoxon signed-rank test), the inhibitory effect of AG957 on CML progenitors was statistically significant at the dose of 1 μmol/L for CFU-Mix (P = .04) and BFU-E (P = .01) and of 5 μmol/L for CFU-GM (P = .04). The inhibitory effect of AG957 on normal progenitors was statistically significant at the dose of 5 μmol/L for CFU-Mix (P = .02), and BFU-E (P = .04) and 10 μmol/L for CFU-GM (P = .03).
Effect of AG957 on CML (▪) and normal (○) CD34+-derived CFU-Mix (A), BFU-E (B), and CFU-GM (C). Each data point represents the mean (±SEM) percentage of inhibition from separate experiments using 10 CML and 6 normal samples. For CML, control colonies per 1 × 103 CD34+ cells ranged from 2 to 7 for CFU-Mix, 39 to 80 for BFU-E, and 31 to 180 for CFU-GM. For normal samples, control colonies 1 × 103CD34+ cells ranged from 4 to 8 for CFU-Mix, 24 to 96 for BFU-E, and 40 to 64 for CFU-GM. When compared with control cultures (Wilcoxon signed-rank test), the inhibitory effect of AG957 on CML progenitors was statistically significant at the dose of 1 μmol/L for CFU-Mix (P = .04) and BFU-E (P = .01) and of 5 μmol/L for CFU-GM (P = .04). The inhibitory effect of AG957 on normal progenitors was statistically significant at the dose of 5 μmol/L for CFU-Mix (P = .02), and BFU-E (P = .04) and 10 μmol/L for CFU-GM (P = .03).
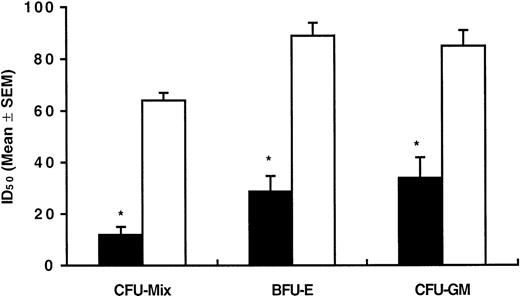
Colony formation by normal CD34+ cells (n = 6) exposed to AG957 was also inhibited in a dose-dependent manner (Fig 1A through C). However, normal cells were suppressed at a significantly lower extent than CML cells, with a relevant toxic effect being evident only when AG957 doses in excess of 50 μmol/L were used. As shown in Fig 2, AG957 doses causing 50% inhibition (ID50) of CML and normal progenitor cell growth were significantly different for CFU-Mix (12 v 64 μmol/L;P = .008), BFU-E (29 v 89 μmol/L; P = .004), and CFU-GM (34 v 85 μmol/L; P = .004).
Mean (±SEM) concentrations of AG957 inducing 50% inhibition (ID50) of CML (▪) and normal (□) colony formation. ID50 values were calculated for each experiment by extrapolating from a least square linear regression line relating AG957 concentration to the percentage of colony growth inhibition. *Statistically significant (P = .004, at least) when compared with normal samples (Student’s t-test for unpaired data).
Mean (±SEM) concentrations of AG957 inducing 50% inhibition (ID50) of CML (▪) and normal (□) colony formation. ID50 values were calculated for each experiment by extrapolating from a least square linear regression line relating AG957 concentration to the percentage of colony growth inhibition. *Statistically significant (P = .004, at least) when compared with normal samples (Student’s t-test for unpaired data).
The effect of AG957 was also assayed by plating CML and normal CD34+ cells on irradiated murine M2-10B4 cells engineered by retroviral gene transfer to produce human IL-3 and human G-CSF. After 5 weeks, cultures were evaluated for the production of secondary clonogenic cells in the nonadherent supernatant and the adherent stromal layers. After exposure to increasing doses of AG957, a statistically significant and dose-dependent suppression of CML (r = .94, P = .005) and normal (r = .89,P = .01) LTC-IC growth was observed (Table 2). Again, normal LTC-IC were significantly less inhibited than CML LTC-IC, as demonstrated by statistically different ID50 values (181 ± 8 v43 ± 5 μmol/L; P = .004).
Effect of AG957 on CML and Normal LTC-IC
| AG957 (μmol/L) . | CML . | Normal . |
|---|---|---|
| 0 | 0 | 0 |
| 1 | −18 ± 15 | 1 ± 7 |
| 5 | 21 ± 8 | 10 ± 4 |
| 10 | 22 ± 17 | 16 ± 14 |
| 50 | 76 ± 15 | 19 ± 10 |
| 100 | 100 | 29 ± 12 |
| AG957 (μmol/L) . | CML . | Normal . |
|---|---|---|
| 0 | 0 | 0 |
| 1 | −18 ± 15 | 1 ± 7 |
| 5 | 21 ± 8 | 10 ± 4 |
| 10 | 22 ± 17 | 16 ± 14 |
| 50 | 76 ± 15 | 19 ± 10 |
| 100 | 100 | 29 ± 12 |
Results are the percentage of inhibition and are expressed as the mean (±SEM) values of five and three separate experiments for CML and normal, respectively.
Fas-R is constitutively expressed at significantly higher levels on CML than on normal CD34+ cells.46,56 Fas-R triggering by the natural Fas-L or agonistic monoclonal antibodies results in apoptosis, reduced colony growth, and a decrease in the BCR/ABL protein level.46,47,56 To investigate whether Fas-R triggering could have enhanced AG957-induced colony suppression, the effect of a sequential treatment with AG957 (0 to 50 μmol/L for 30 minutes) and CH11 (1 μg/mL for 120 minutes) was investigated. In agreement with already reported data,45-47 treatment of CD34+ cells with CH11 alone suppressed the growth CFU-Mix, BFU-E, and CFU-GM by both CML (59%, 54%, and 42%, respectively) and normal (28%, 19%, and 33%, respectively) CD34+ cells.
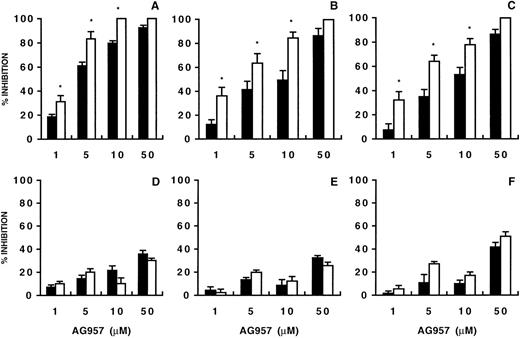
The sequential treatment of CML CD34+ cells with AG957 and CH11 significantly increased growth suppression (Fig 3A through C). When incubation with AG957 concentrations as low as 1 μmol/L was followed by CH11 exposure, CFU-Mix, BFU-E, and CFU-GM growth inhibition was increased by 1.6-fold, 3-fold, and 4-fold, respectively (Fig 3A through C). In contrast, the sequential treatment of normal CD34+ cells with AG957 and CH11 failed to enhance AG957-induced colony growth inhibition (Fig 3D through F).
Effect of AG957 alone (▪) or AG957 plus CH11 (□) on CML (upper panels) and normal (lower panels) progenitors. The effects on CFU-Mix (A and D), BFU-E (B and E), and CFU-GM (C and F) are shown. To evaluate the combined effects of AG957 and CH11, untreated and AG957-treated (1 to 50 μmol/L for 30 minutes) CD34+cells (1 × 105/mL) were resuspended in serum-free medium and exposed (2 hours at 37°C) to CH11 (1 μg/mL). At the end of the incubation, CD34+ cells were incorporated in a standard methylcellulose assay to quantitate hematopoietic progenitors. Each histogram represents the mean (±SEM) percentage of inhibition from separate experiments using CML (n = 4) and normal (n = 2) CD34+ cells. For CML, control colonies per 1 × 103 CD34+ cells ranged from 2 to 7 for CFU-Mix, 39 to 80 for BFU-E, and 31 to 180 for CFU-GM. For normal samples, control colonies 1 × 103 CD34+cells ranged from 2 to 6 for CFU-Mix, 53 to 96 for BFU-E, and 97 to 154 for CFU-GM. When compared with cultures treated with AG957 alone (Wilcoxon signed-rank test), the inhibitory effect of AG957 plus Fas-L on CML progenitors was significantly different at the dose of 1 μmol/L for CFU-Mix (P = .04), BFU-E (P = .01), and CFU-GM (P = .04). For normal samples, no statistically significant difference was detected by comparing the inhibitory effects of AG957 or AG957 plus Fas-L. *Statistically significant when compared with samples treated with AG957 alone (Student’s t-test for unpaired data).
Effect of AG957 alone (▪) or AG957 plus CH11 (□) on CML (upper panels) and normal (lower panels) progenitors. The effects on CFU-Mix (A and D), BFU-E (B and E), and CFU-GM (C and F) are shown. To evaluate the combined effects of AG957 and CH11, untreated and AG957-treated (1 to 50 μmol/L for 30 minutes) CD34+cells (1 × 105/mL) were resuspended in serum-free medium and exposed (2 hours at 37°C) to CH11 (1 μg/mL). At the end of the incubation, CD34+ cells were incorporated in a standard methylcellulose assay to quantitate hematopoietic progenitors. Each histogram represents the mean (±SEM) percentage of inhibition from separate experiments using CML (n = 4) and normal (n = 2) CD34+ cells. For CML, control colonies per 1 × 103 CD34+ cells ranged from 2 to 7 for CFU-Mix, 39 to 80 for BFU-E, and 31 to 180 for CFU-GM. For normal samples, control colonies 1 × 103 CD34+cells ranged from 2 to 6 for CFU-Mix, 53 to 96 for BFU-E, and 97 to 154 for CFU-GM. When compared with cultures treated with AG957 alone (Wilcoxon signed-rank test), the inhibitory effect of AG957 plus Fas-L on CML progenitors was significantly different at the dose of 1 μmol/L for CFU-Mix (P = .04), BFU-E (P = .01), and CFU-GM (P = .04). For normal samples, no statistically significant difference was detected by comparing the inhibitory effects of AG957 or AG957 plus Fas-L. *Statistically significant when compared with samples treated with AG957 alone (Student’s t-test for unpaired data).
DNA fragmentation.
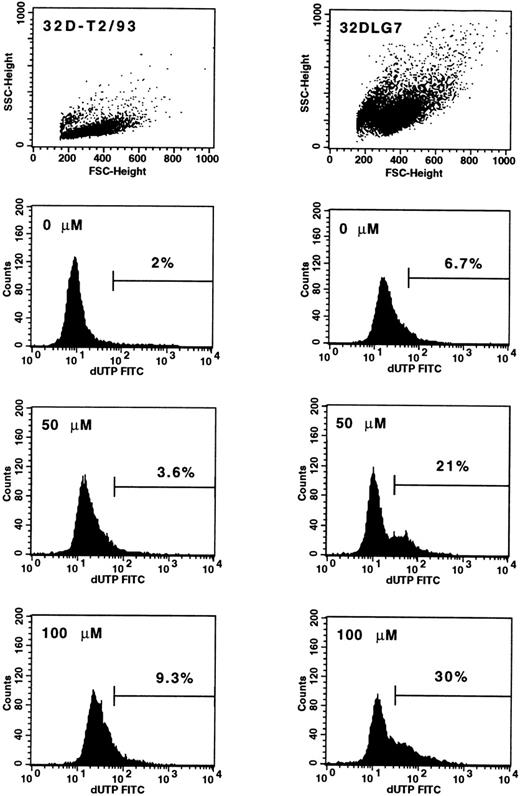
Data obtained by AG957 preincubation suggest that the effect of the drug does involve toxicity. To examine whether apoptosis is involved in AG957-induced inhibition of progenitor cell growth, two cell lines, namely 32D-T2/93 and 32DLG7, were treated with AG957 (0 to 100 μmol/L for 12 hours) and apoptosis was analyzed by the TdT assay. Upon AG957 exposure, 9% of 32D-T2/93 cells (BCR/ABL-negative) were in a progressive stage of apoptosis, whereas virtually no apoptotic cells were detected in the control (Fig 4, left panels). AG957 treatment of the BCR/ABL-transfected 32DLG7 cells resulted in significantly higher percentages of apoptotic cells (Fig 4, right panels).
Detection of apoptotic cells by means of TdT assay in untreated and AG957-treated 32D-T2/93 (BCR/ABL-negative) and 32DLG7 (BCR/ABL-positive) cell lines. Mean (±SEM) percentages of apoptotic cells in the live gated cell populations are indicated for 32DT2/93 (left panels) and 32DLG7 (right panels) cells exposed to graded concentrations of AG957 for 12 hours.
Detection of apoptotic cells by means of TdT assay in untreated and AG957-treated 32D-T2/93 (BCR/ABL-negative) and 32DLG7 (BCR/ABL-positive) cell lines. Mean (±SEM) percentages of apoptotic cells in the live gated cell populations are indicated for 32DT2/93 (left panels) and 32DLG7 (right panels) cells exposed to graded concentrations of AG957 for 12 hours.
BCR/ABL mRNA expression on single colonies.
Quantitative differences in CML and normal colony growth suppression argue for an antiproliferative effect of AG957 that is specifically related to BCR/ABL inhibition. To investigate whether AG957 could affect CML colony formation not only quantitatively but also qualitatively, CML colonies were individually harvested and analyzed by RT-PCR for the expression of hybrid BCR/ABL mRNA. At the time of the study, all patients were 100% Ph-positive by standard cytogenetics. Table 3 shows that preincubation with 1 to 10 μmol/L of AG957 failed to show any antileukemic effect. However, AG957 at 50 μmol/L significantly reduced the mean (±SD) percentage of BCR/ABL-positive progenitors (92% ± 10% v33% ± 5%; P = .001).
PCR Analysis of Individual Bone Marrow CFU-GM
| Case No. . | AG957 (μmol/L) . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 5 . | 10 . | 50 . | |
| 1 | 100 | NE | 100 | 94 | 33 |
| 2 | 100 | 100 | 75 | 53 | 38 |
| 3 | 100 | NE | 100 | 89 | NE |
| 4 | 75 | NE | 75 | 67 | 25 |
| 5 | 100 | 100 | 75 | 100 | NE |
| 6 | 100 | 100 | 75 | 100 | NE |
| 7 | 83 | 89 | 70 | 70 | NE |
| 8 | 81 | NE | NE | 89 | 33 |
| 9 | 85 | 86 | 70 | 60 | 35 |
| 10 | 100 | 100 | 95 | 85 | NE |
| Mean ± SD | 92 ± 10 | 96 ± 7 | 82 ± 13 | 81 ± 17 | 33 ± 53-150 |
| Case No. . | AG957 (μmol/L) . | ||||
|---|---|---|---|---|---|
| 0 . | 1 . | 5 . | 10 . | 50 . | |
| 1 | 100 | NE | 100 | 94 | 33 |
| 2 | 100 | 100 | 75 | 53 | 38 |
| 3 | 100 | NE | 100 | 89 | NE |
| 4 | 75 | NE | 75 | 67 | 25 |
| 5 | 100 | 100 | 75 | 100 | NE |
| 6 | 100 | 100 | 75 | 100 | NE |
| 7 | 83 | 89 | 70 | 70 | NE |
| 8 | 81 | NE | NE | 89 | 33 |
| 9 | 85 | 86 | 70 | 60 | 35 |
| 10 | 100 | 100 | 95 | 85 | NE |
| Mean ± SD | 92 ± 10 | 96 ± 7 | 82 ± 13 | 81 ± 17 | 33 ± 53-150 |
Values are the percentage that is BCR-ABL positive. A minimum of 20 CFU-GM was analyzed for each data point.
Abbreviation: NE, not evaluable.
Statistically different (P ≤ .001) as compared with control.
Because the combined treatment with AG957 and CH11 was associated with a significant increase of the antiproliferative effect of the tyrphostin, in four experiments colonies generated by CD34+cells sequentially exposed to low doses of AG957 (1 and 5 μmol/L) and CH11 were analyzed for BCR/ABL expression. Indeed, the combined AG957/CH11 treatment failed to improve the selection of nonleukemic progenitors (data not shown).
To rule out the possibility that AG957 could affect BCR/ABL transcription, thus inducing the growth of Ph-positive progenitors with nonfunctional BCR/ABL gene (BCR/ABL negative), in two experiments (cases no. 2 and 5) colonies were individually harvested and split into two aliquots, one for cytogenetics and the other for RT-PCR. In both cases, no difference was observed in the percentages of Ph-positive and BCR/ABL-positive colonies generated by AG957-treated cultures (data not shown), thus confirming that AG957-induced increase in the percentage of nonleukemic progenitors was not related to suppression of BCR/ABL transcription.
DISCUSSION
The increasing knowledge of transmembrane and intracellular signal transduction phenomena now allows us to manipulate cell growth by altering signaling pathways.9,33 Because PTKs participate in the establishment and progression of several malignant diseases, inhibitors of PTKs represent attractive antiproliferative agents.33-37 AG957 prevents the phosphorylation of p210BCR/ABL,39,40 thus downregulating the activation of regulatory proteins that stimulate Ras and play a crucial role in the pathogenesis of CML.9,10,14-16 In addition, AG957 restores integrin-mediated adhesion and proliferation inhibition in CML progenitors.41
In the present study, we demonstrate that AG957 inhibits in a dose-dependent manner the growth of CML-derived LTC-IC, CFU-Mix, BFU-E, and CFU-GM. The inhibitory effect of AG957 on highly purified CD34+ cells indicates that the antiproliferative action of AG957 does not involve accessory cells. A significant growth inhibition is seen after exposure to AG957 concentrations as low as 1 μmol/L and a nearly complete inhibition (>80%) is detected at 50 μmol/L.
Inhibition of CML progenitor cell growth to 50% of control values is achieved with doses of AG957 that are 2.5-fold to 5-fold lower than those required for normal progenitors, suggesting a differential effect of AG957 on normal and CML cells. However, exposure of normal CD34+ cells to AG957 concentrations ≥50 μmol/L is associated with a relevant toxicity on committed (CFU-Mix, BFU-E, and CFU-GM) but not primitive (LTC-IC) progenitors. The toxicity of AG957 on Ph-negative cells is also demonstrated by detection of nuclear DNA fragmentation of the BCR/ABL-negative 32D-T2/93 cell line. However, once again, the quantitative evaluation of apoptotic cells by the TdT assay suggests a more effective action of AG957 on Ph-positive rather than Ph-negative cells. The loss of selectivity of AG957 may be intrinsic to the mechanism of action of tyrphostins that are competitive inhibitors not only of protein substrate but also of ATP binding.33 In fact, the ATP antagonism is likely to mediate inhibition of tyrosine kinases other than BCR/ABL, including normal c-ABL, that are essential for the proliferation of normal colony-forming cells.
Analysis of BCR/ABL mRNA expression in individual progenitors demonstrates that exposure of CML cells to 10 μmol/L of AG957 fails to enrich BCR/ABL-negative progenitors, whereas a substantial depletion of leukemic colonies is observed with AG957 at 50 μmol/L, a dose suppressing the growth of CML and normal LTC-IC by 76% and 19%, respectively. The limited, nonspecific toxic effect on normal LTC-IC associated with a selective antileukemic action supports the use of AG957 for purging malignant progenitors from CML marrow. Indeed, when CD34+ cells were treated with 50 μmol/L AG957, only half of the patients generated sufficient numbers of colonies to be analyzed at the molecular level. BCR/ABL-negative progenitors were detected in 4 patients studied at diagnosis and 1 patient who had received hydroxyurea for a short period, whereas we were unable to detect significant levels of nonleukemic progenitors in 2 patients analyzed after ≥12 months of therapy as well as in 3 additional patients analyzed at diagnosis. These results are in keeping with previous studies demonstrating a progressive reduction of the BCR/ABL-negative compartment in chronic-phase CML.57,58 The failure of AG957 to select nonleukemic colonies may be explained by the rarity of BCR/ABL-negative progenitors that could therefore be underscored when a limited number of colonies (on average, 20 to 30) is analyzed. Alternatively, the presence of additional mutations in a subset of progenitors would allow cell proliferation independently of BCR/ABL. Finally, individual progenitors expressing p190BCR/ABL at high levels might not be efficiently suppressed by AG957.40
Analysis of individual colonies by both cytogenetics and RT-PCR showed similar percentages of Ph-negative and BCR/ABL-negative colonies generated by AG957-treated samples, thus ruling out that AG957 acts by suppressing BCR/ABL transcription, as has been described for IFN-α.59 This is further supported by the evidence of Kaur et al,39 who have shown that preincubation of K562 cells with the tyrphostin AG957 inhibits cell growth, p210BCR/ABL tyrosine kinase activity, and DNA synthesis as early as 2 hours, a time at which RNA and protein synthesis were not affected.
BCR/ABL signals to cause transformation through several mechanisms, including activation of mitogenic signaling pathways,8,60induction of anchorage-independent growth,61 and suppression of apoptosis.42,62 Inhibition of BCR/ABL tyrosine kinase activates a death program, as shown by apoptosis of 32DLG7 cells upon exposure to AG957. Because recent findings demonstrate that, after priming with IFN-α, Fas-R triggering on CML CD34+ cells is associated with dose-dependent decrease of colony formation, apoptosis, and decrease in the BCR/ABL protein level, we hypothesized that Fas-R triggering after AG957 treatment could have enhanced the antiproliferative action of the tyrphostin. In agreement with previously reported results,45 46 exposure to CH11 alone results in an antiproliferative effect on both CML and normal progenitors. Under our experimental conditions, CH11 inhibits CML progenitors more than normal progenitors. This finding is likely to be related either to different levels of Fas-R expression by CML and normal CD34+ cells or the use of normal CD34+cells that were not preincubated with inducers of Fas-R expression.
As compared with AG957 alone, the combined AG957/CH11 treatment resulted in a dramatic increase of CML, but not normal, growth inhibition. The different behavior of CML and normal progenitors in response to the sequential AG957/CH11 treatment might be due to different levels of Fas-R expression. These data suggest that AG957-induced inhibition of BCR/ABL tyrosine kinase enhances the response of CML cells to Fas-L and allow us to hypothesize the existence of a link between reduced PTK activity of BCR/ABL and apoptosis triggering by the Fas-R/Fas-L system. Although we were not able to demonstrate a better antileukemic effect by sequentially combining low doses of AG957 with CH11, the marked antiproliferative effect seen on CML progenitors in the absence of an additive toxicity on normal progenitors may be of therapeutic relevance and is currently under investigation in an animal model. In fact, the combined AG957/CH11 treatment could allow the use of the tyrphostin at low dose, thereby reducing the risk of nonspecific toxicity on normal progenitors.
The in vitro selection for normal hematopoietic stem and progenitor cells from within CML marrow and the potential for using these cells as leukemia-free autografts has been the topic of increasing discussion.19,25,28 30-32 Data reported here demonstrate the possibility to select nonclonal CML progenitors by means of a simple incubation with the PTK inhibitor AG957, thus suggesting that it may be feasible to select a population of benign progenitors from CML marrow that could be used for autografting patients without suitable allogeneic bone marrow donors. Further investigations are also required to explore the therapeutic potential of PTK inhibitors in combination with other agents, including biological response modifiers and apoptosis-inducing molecules.
Supported in part by grants from “Ministero dell’Università e della Ricerca Scientifica e Tecnologica” (MURST-40% & 60%), “Associazione Italiana per la Ricerca sul Cancro” (AIRC), and “Associazione Italiana Leucemie (AIL)-Trenta Ore per la Vita.” D.G. is supported by a grant from the Azienda Ospedaliera di Parma. E.R. is a recipient of an AIRC fellowship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Carmelo Carlo-Stella, MD, UnitàTrapianto di Midollo, Istituto Nazionale Tumori, Via Venezian, 1, 20133 Milano, Italy; e-mail: ccs@ipruniv.cce.unipr.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal