Abstract
Dendritic cells (DCs), which phagocytose antigens and subsequently proliferate and migrate, may be the most powerful antigen-presenting cells that activate naive T cells. To determine their role in the immune response to tumors, we used WEHI-3B murine leukemia cells transduced with adenovirus vectors expressing cytokines. We found that mixtures of irradiated cells expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) plus those expressing interleukin-4 (IL-4) or tumor necrosis factor (TNF) protected mice against WEHI-3B–induced leukemias. When bone marrow mononuclear cells (BMMNCs) obtained from mice that had been injected with irradiated, cytokine-expressing tumor cells were injected into tumor-bearing mice, the survival of the latter was significantly prolonged; the longest survival was observed in mice receiving BMMNCs containing an increased number of DCs from animals injected with a mixture of tumor cells expressing GM-CSF with those expressing IL-4. Assay for antileukemic effects in spleen of the latter animals showed specific antitumor cytotoxicity against WEHI-3B, suggesting that DCs from donor mice activate specific T cells in the tumor-bearing recipients. These results suggest that the infusion of syngeneic BMMNCs stimulated with cytokine-expressing tumor cells may be effective in treating certain types of tumors.
THE INTRODUCTION OF genes encoding cytokines, costimulatory factors, or antigen-presenting molecules into tumor cells can enhance the host’s antitumor immunity and may be useful in treating patients with cancer.1-5 Vaccination of using animals with cytokine-expressing tumor cells has shown that, of the cytokines assayed, the most potent, specific, and long-lasting inducer of immune responses against non–gene-modified tumor cells is granulocyte-macrophage colony-stimulating factor (GM-CSF).2 3 These findings suggested that GM-CSF induces the production of antigen-presenting cells (APCs), primarily dendritic cells (DCs), in these hosts. In addition, a high peritumoral level of GM-CSF, induced by an enhanced localized immune response, can also attract immune cells (DCs, T lymphocytes, natural killer [NK] cells, neutrophils, and macrophages) to the tumor site without causing systemic cytotoxicity.
It has been hypothesized that functional immature DCs in the bone marrow (BM) may take up tumor-associated antigens6 and, after migrating into the peripheral tissues, such as the lymph nodes or spleen, may develop into mature DCs.7 These, in turn, may activate killer T cells, which exhibit antitumor activity in syngeneic leukemia-bearing mice. DCs can be induced from BM of humans and mice by either tumor necrosis factor α (TNFα) or interleukin-4 (IL-4) in combination with GM-CSF.8-12 To determine the combination of cytokines that effectively induces the in vivo activation of T cells by DCs, we used a murine monocytic leukemic cell line, WEHI-3B, infected with an adenoviral vector expressing murine GM-CSF, TNFα, or IL-4. We tested the ability of these irradiated, cytokine-expressing WEHI-3B cells, individually or in combination, to suppress the induction of tumors by wild-type WEHI-3B cells. We also assayed the ability of BM cells from mice injected with cytokine-expressing WEHI-3B cells to suppress the growth of pre-existing leukemic cells in syngeneic mice.
MATERIALS AND METHODS
Cell lines, reagents, and mice.
The murine monocytic leukemic cell line, WEHI-3B, was obtained from the Institute for Fermentation (Osaka, Japan) and A20 was obtained from American Type Culture Collection (ATCC TIB-208; ATCC, Rockville, MD). Hamster antimouse antibody CD11c (N418) was purchased from PharMingen (San Diego, CA) and goat antihamster IgG FITC were purchased from Southern Biotechnology Associates (Birmingham, AL). Antimouse CD80- and CD86-fluorescein isothiocyanate (FITC) were purchased from Immunotech (Marseille, France). Rat antimouse monoclonal antibody (MoAb) against CD4, CD8, Mac-1, B220, Gr-1, goat antirat MoAb-FITC all were purchased from PharMingen. DEC205 (NDLC-145) was kindly provided by Dr Ralph Steinman (The Rockefeller University, New York, NY). Female BALB/c mice, 7 to 8 weeks old, were obtained from SLC (Shizuoka, Japan) and housed under specific pathogen-free conditions in the Kumamoto University Animal Center (Kumamoto, Japan).
Generation of recombinant adenoviruses.
Murine GM-CSF, TNFα, and IL-4 cDNA were each subcloned into a plasmid, pCAGGS, that consists of a cytomegalovirus enhancer, a chicken β-actin promoter, cDNA cloning sites, and an anti–(rabbit β-globin) poly(A) signal sequence. Each expression cassette was subcloned into the Swa I site of pAdex1cw, a 42-kb cosmid containing the 31-kb adenovirus type 5 genome that lacks the E1A, E1B, and E3 genes. Each cosmid was transfected into 293 cells (ATCC; CRL1573), and positive clones were picked using standard techniques.13
Transduction of adenovirus vector into WEHI-3B.
Exponentially growing WEHI-3B cells in complete medium (CM), consisting of Dulbecco’s modified Eagle’s medium (DMEM) containing 100 U/mL penicillin, 100 μg/mL kanamycin, 2 mmol/L L-glutamine, and 10% fetal calf serum (FCS), were transduced for 1 hour with a recombinant adenovirus expressing murine GM-CSF, TNFα, or IL-4, at a multiplicity of infection (moi) of 0 to 4,000 with frequent gentle shaking; these cells, designated WEHI-3B–GM-CSF, WEHI-3B–TNFα, and WEHI-3B–IL-4 cells, respectively, were subsequently incubated for 48 hours at 37°C in CM in a 5% CO2 incubator. Cells transduced with recombinant adenovirus-lacZ (Ad-lacZ) and donated WEHI-3B–lacZ cells were used as a control in all experiments. Efficiency of transduction was verified by fluorescent-activated cell sorting (FACS) of β-galactosidase–stained cells. Briefly, cells were fixed with 1% glutaraldehyde for 5 minutes, rinsed once with phosphate-buffered saline, and stained for 8 hours in X-Gal buffer containing 5 mmol/L K4Fe(CN)6, 5 mmol/L K3Fe(CN)6, 2 mmol/L MgCl2, and 1 mg/mL 5-bromo-4-chloro-3-indolyl β-galactoside (X-Gal). The number of blue cells was scored visually and analyzed with a FluoReporterlacZ Flow Cytometry Kit (Molecular Probes, Eugene, OR). The fluorescence intensity of individual cells was measured as relative fluorescence unit(s) (FU).14
Cytokine production by modified WEHI-3B cells.
One million WEHI-3B cells in 10 mL CM were infected with adenovirus expressing GM-CSF, TNFα, or IL-4 at an moi of 40, 400, or 4,000. The medium was removed after 48 hours and cytokine expression was measured by enzyme-linked immunosorbent assay (ELISA) in a 96-well microtiter plate (Nunc, Roskilde, Denmark), using murine GM-CSF, TNFα, and IL-4 ELISA testing kits (Amersham, Little Chalfont, Buckinghamshire, UK).
Vaccination with irradiated, modified WEHI-3B cells.
To assess whether tumor cells expressing cytokines can induce systemic immunity, BALB/c mice were injected with WEHI-3B–lacZ cells or with WEHI-3B–GM-CSF cells, alone or in combination with WEHI-3B–TNFα or with WEHI-3B–IL-4 cells, before injection of wild-type WEHI-3B cells. Briefly, adenovirus-transduced WEHI-3B cells were irradiated to 70 Gy by a 137Cs source, and 5 × 105 of these cells were injected into the tail vein of each mouse. Seven days later, 5 × 105 wild-type WEHI-3B cells were injected into each tail vein for measurement of survival in the first group (Fig 1A and B). On the same day, a second group of 5 animals in each group were killed, and the B cells, T cells, and DC content in the BM of each mouse were analyzed by flow cytometry.
Schematic diagrams of in vivo experiments in leukemic mice. (A) Mice were injected with wild-type WEHI-3B cells and survival was measured. (B) Mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF, WEHI-3B–GM-CSF plus WEHI-3B–IL-4, or WEHI-3B–GM-CSF plus WEHI-3B–TNF cells. One week later, mice were injected with wild-type WEHI-3B cells and survival was measured. (C) One group of mice was injected with wild-type WEHI-3B cells. A second group of mice was injected with irradiated, modified cells as described in (B), above. After 7 days, the latter mice were killed and BMMNCs from these animals were injected into the first group of mice.
Schematic diagrams of in vivo experiments in leukemic mice. (A) Mice were injected with wild-type WEHI-3B cells and survival was measured. (B) Mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF, WEHI-3B–GM-CSF plus WEHI-3B–IL-4, or WEHI-3B–GM-CSF plus WEHI-3B–TNF cells. One week later, mice were injected with wild-type WEHI-3B cells and survival was measured. (C) One group of mice was injected with wild-type WEHI-3B cells. A second group of mice was injected with irradiated, modified cells as described in (B), above. After 7 days, the latter mice were killed and BMMNCs from these animals were injected into the first group of mice.
Effect of BM mononuclear cells (BMMNCs) from mice injected with irradiated, modified WEHI-3B cells on syngeneic mice previously inoculated with wild-type WEHI-3B cells.
Wild-type WEHI-3B cells (5 × 105/mouse) were inoculated into one group of 10 mice. On the same day, other groups of 10 mice each were injected with 5 × 105 WEHI-3B–lacZ or WEHI-3B–GM-CSF cells or with WEHI-3B–GM-CSF cells plus WEHI-3B–TNFα or WEHI-3B–IL-4 cells, irradiated as described above. After 7 days, these mice in the latter four groups were killed, and 5 × 105 or 106 BMMNCs were separated from each femur sample by centrifugation for 30 minutes at 1,500 rpm on a Lympholyte-M (Cedarlane Laboratories, Hornby, Ontario, Canada) and were injected into the tail vein of the first group of mice (Fig 1C). Survival was measured in one group of recipient mice.
Cell-mediated cytotoxicity assay.
WEHI-3B–lacZ or WEHI-3B–GM-CSF cells, or WEHI-3B–GM-CSF cells plus WEHI-3B–TNFα or WEHI-3B–IL-4 cells, irradiated as described above, were inoculated into one group of 3 mice (5 × 105/mouse). After 7 days, these mice were killed, and 106 BMMNCs were injected into the tail vein of the other group of mice. Moreover, after 7 days, these mice were killed for the preparation of flow cytometric analysis and cytotoxicity assay (see Fig6A). Cytotoxicity of tumor cells by spleen cells was measured in vitro using the lactate dehydrogenase (LDH) release assay and CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI). The spleen cells were tested for their cytotoxicity against WEHI-3B, A20, and BMMNC from the identical mice in an LDH assay at an effector:target (E:T) ratio of 1:1, 3:1, 5:1, 25:1, and 50:1. Target cells were cocultured with effector cells at various ratios for 6 hours in 96-well round-bottomed plates (Nunc) in phenol red-free RPMI (GIBCO BRL, Grand Island, NY) containing 0.5% FCS. Spontaneous release of effector and target cells was controlled by separate incubation of the respective populations. Maximal LDH enzyme release was measured after lysis of the target cells with lysis solution. Cell-free supernatants were incubated in a separate 96-well plate (Nunc) with LDH substrate for 30 minutes before measuring absorbance using a microplate reader (SOFTmax; Molecular Devices Corp, Sunnyvale, CA) at 490 nm with 650 nm reference. The percentage of cytotoxicity was calculated according to the following formula: % Cytotoxicity = ([E − St − Se]/[M − St]) × 100 (with E being the LDH release by effector-target coculture, St the spontaneous release by target cells, Se the spontaneous release by effector cells, and M the maximal release by target cells). Target cells are WEHI-3B, A20, and BMMNCs derived from the syngeneic mouse.
Statistical analysis.
Survival curves of mice were prepared by the Kaplan-Meier method, and the differences between the survival curves were evaluated using log-rank tests.
RESULTS
Transduction efficiencies and expression of cytokine genes in WEHI-3B cells.
Adenovirus constructs expressing lacZ and the three cytokines (GM-CSF, IL-4, and TNFα) were transduced into WEHI-3B cells. Each construct showed a similar transduction efficiency, as determined by β-galactosidase staining and flow cytometric analysis (Fig 2). When we assayed cytokine production in the culture supernatants, we found that transduction of cells with each construct led to the production of that cytokine, but not of the others, and that cytokine production was dependent on the moi of infection (Table 1). We also observed that irradiation of these cells did not markedly affect cytokine production, and the stability of cytokine’s secretion at these concentrations continued for approximately 5 days.
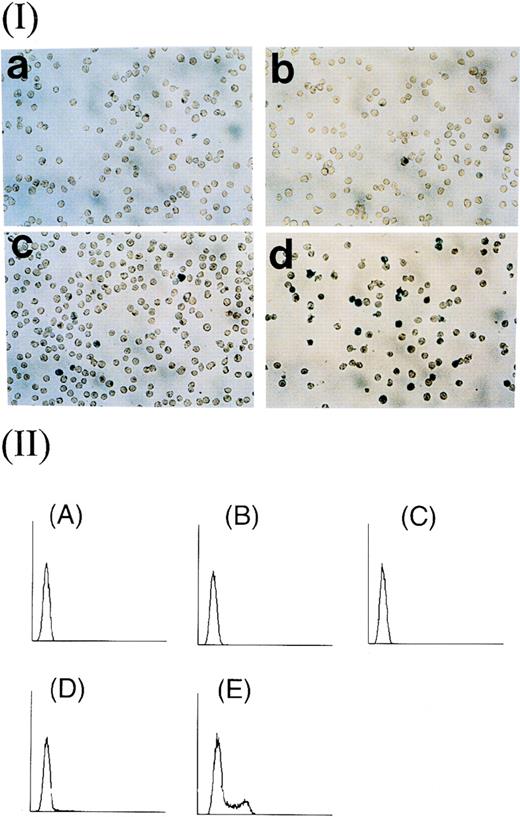
Efficiency of transduction of recombinant adenoviruses into WEHI-3B cells. (Panel I) β-gal staining of cells transduced with virus at moi of (a) 4, (b) 40, (c) 400, and (d) 4,000. (Panel II) Flow cytometric analysis of fluorescein-stained cells transduced with virus at moi of (A) 0, (B) 4, (C) 40, (D) 400, and (E) 4,000 and exposed to 20 mmol/L fluorescein di-β-D-galactopyranoside (FDG) at 37°C for 1 hour. The rate of transduction of cells at each moi was (A) 0.38%, (B) 0.55%, (C) 1.20%, (D) 3.74%, and (E) 29.69%.
Efficiency of transduction of recombinant adenoviruses into WEHI-3B cells. (Panel I) β-gal staining of cells transduced with virus at moi of (a) 4, (b) 40, (c) 400, and (d) 4,000. (Panel II) Flow cytometric analysis of fluorescein-stained cells transduced with virus at moi of (A) 0, (B) 4, (C) 40, (D) 400, and (E) 4,000 and exposed to 20 mmol/L fluorescein di-β-D-galactopyranoside (FDG) at 37°C for 1 hour. The rate of transduction of cells at each moi was (A) 0.38%, (B) 0.55%, (C) 1.20%, (D) 3.74%, and (E) 29.69%.
Cytokine Production in Adenovirus Vector-Transduced WEHI-3B Cells
| moi . | Amount of Respective Cytokine Secreted (pg/106 cells) Cytokine Gene Transduced . | ||
|---|---|---|---|
| GM-CSF . | TNFα . | IL-4 . | |
| 0 | 0 | 0 | 0 |
| 40 | 209.25 ± 0.38 | 62.61 ± 0.31 | 27.35 ± 0.15 |
| 400 | 4,323.00 ± 0.10 | 123.08 ± 6.09 | 484.83 ± 8.13 |
| 4,000 | 169,848.54 ± 1.49 | 7,662.63 ± 0.07 | 19,589.27 ± 0.74 |
| moi . | Amount of Respective Cytokine Secreted (pg/106 cells) Cytokine Gene Transduced . | ||
|---|---|---|---|
| GM-CSF . | TNFα . | IL-4 . | |
| 0 | 0 | 0 | 0 |
| 40 | 209.25 ± 0.38 | 62.61 ± 0.31 | 27.35 ± 0.15 |
| 400 | 4,323.00 ± 0.10 | 123.08 ± 6.09 | 484.83 ± 8.13 |
| 4,000 | 169,848.54 ± 1.49 | 7,662.63 ± 0.07 | 19,589.27 ± 0.74 |
WEHI-3B cells were transduced with adenovirus vector constructs expressing GM-CSF, TNFα, or IL-4 at various moi and the amount of cytokine secreted was measured by ELISA. Each value is the mean ± SD of culture supernatant measurements (per 106 cells/24 hours).
Immune system cells in BMMNCs involved in the response to cytokine-expressing WEHI-3B cells.
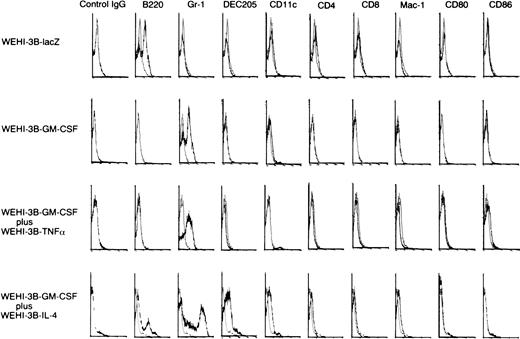
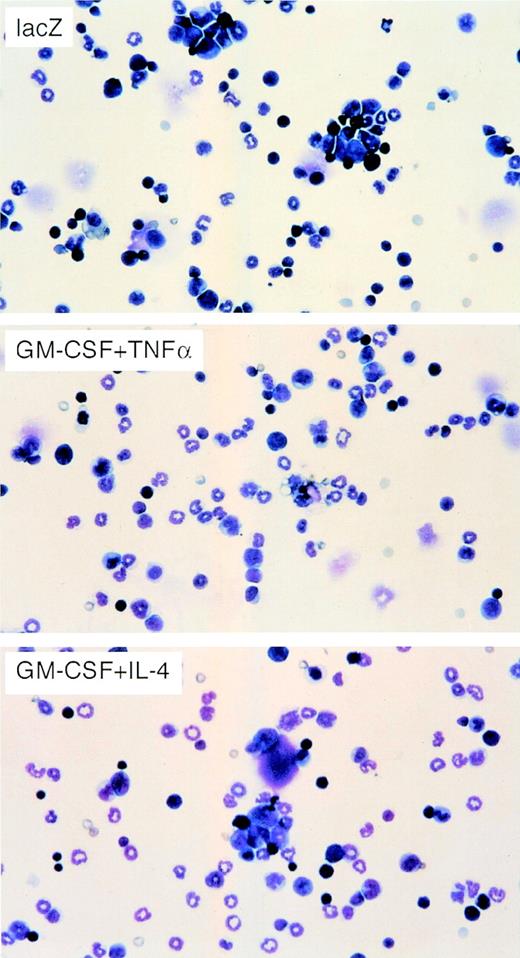
To determine the types of immune cells involved in the response to the injection of cytokine-gene modified WEHI-3B cells, we injected irradiated adenovirus-transduced WEHI-3B cells into BALB/c mice. Seven days later, we assayed the number of DCs and the number of CD4+ and CD8+ T lymphocytes in the BMMNCs by flow cytometry (Fig 3). The number of DCs in the BM, determined by measuring cells expressing DC marker, CD11c, and DEC205, was higher in mice injected with WEHI-3B–GM-CSF and WEHI-3B–IL-4 cells (4.12% of these cells were CD11c+, and 10.60% of these cells were DEC205+) than in mice injected with WEHI-3B–GM-CSF cells alone (3.15% of these cells were CD11c+, and 0.32% of these cells were DEC205+, respectively) or in combination with WEHI-3B–TNFα cells (1.74% of these cells were CD11c+, and 0.39% of these cells were DEC205+). However, they expressed little Mac-1, CD4, CD8, and mature DC marker, CD80 (B7-1) and CD86 (B7-2) (Fig 3). We showed May-Giemsa stain of the BMMNCs, indicating the proliferation in granulocytes owing to the effects of GM-CSF (Fig 4).
Analysis of immune cell phenotypes of BMMNCs in mice injected with WEHI-3B cells expressing exogenous cytokines. BALB/c mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF, WEHI-3B–GM-CSF plus WEHI-3B–TNF, or WEHI-3B–GM-CSF plus WEHI-3B–IL-4 cells. Seven days later, immune cell phenotypes were assayed by flow cytometry.
Analysis of immune cell phenotypes of BMMNCs in mice injected with WEHI-3B cells expressing exogenous cytokines. BALB/c mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF, WEHI-3B–GM-CSF plus WEHI-3B–TNF, or WEHI-3B–GM-CSF plus WEHI-3B–IL-4 cells. Seven days later, immune cell phenotypes were assayed by flow cytometry.
May-Giemsa stain of BMMNCs from mice injected with WEHI-3B cells expressing exogenous cytokines. BALB/c mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF plus WEHI-3B–TNF, or WEHI-3B–GM-CSF plus WEHI-3B–IL-4 cells. Seven days later, each BMMNC was analyzed by May-Giemsa stain.
May-Giemsa stain of BMMNCs from mice injected with WEHI-3B cells expressing exogenous cytokines. BALB/c mice were injected with irradiated WEHI-3B–lacZ, WEHI-3B–GM-CSF plus WEHI-3B–TNF, or WEHI-3B–GM-CSF plus WEHI-3B–IL-4 cells. Seven days later, each BMMNC was analyzed by May-Giemsa stain.
Effect of preinjection of cytokine-expressing WEHI-3B cells on tumorigenesis.
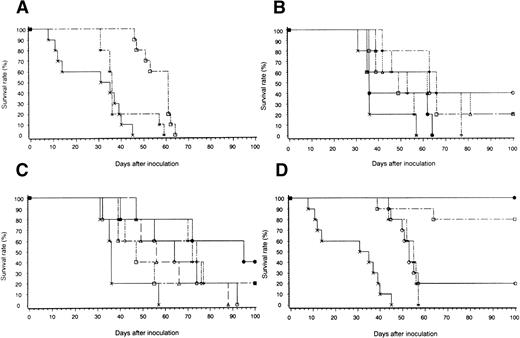
When we assayed the ability of transduced, irradiated cells to inhibit tumorigenesis in BALB/c mice, we found that mice injected with 5 × 105 untransduced or WEHI-3B–lacZ cells died 45 to 57 days after inoculation; these mice had a mean survival of 27.2 and 39.0 days, respectively (P > .05; Fig 5A). Mice inoculated with WEHI-3B–GM-CSF cells alone showed a significantly prolonged survival (mean, 56 days; P < .05; Fig 5A). To determine the optimal amounts of GM-CSF plus TNFα and of GM-CSF plus IL-4 needed to enhance survival, we injected mice with cells that secreted various concentrations of these cytokines. In mice injected with WEHI-3B–GM-CSF and WEHI-3B–TNFα cells, optimal survival was observed with cells secreting 10 ng/mL GM-CSF and 1 ng/mL TNFα (Fig5B). In mice injected with WEHI-3B–GM-CSF and WEHI-3B–IL-4 cells, optimal survival was conferred by cells secreting 500 ng/mL GM-CSF and 100 ng/mL IL-4 (Fig 5C). Both of these were significantly superior to control (mice injected with WEHI-3B–lacZ cells; P < .007). In these vaccine models, other statistical analysis showed that, in Fig5B, the group of mice injected with WEHI-3B–GM-CSF secreting 10 ng/mL and WEHI-3B–TNFα secreting 1 ng/mL (10 ng/mL GM-CSF-WEHI-3B plus 1 ng/mL TNFα-WEHI-3B) survived other groups with statistical significance (P < .05). In Fig 5C, statistically significant differences were shown between mice of wild-type and mice of 500 ng/mL GM-CSF-WEHI-3B plus 10 ng/mL IL-4-WEHI-3B, 500 ng/mL GM-CSF-WEHI-3B plus 100 ng/mL IL-4-WEHI-3B, and 10 ng/mL GM-CSF-WEHI-3B plus 100 ng/mL IL-4-WEHI-3B (P < .05); however, the mouse group of 500 ng/mL GM-CSF-WEHI-3B plus 10 ng/mL IL-4-WEHI-3B survived the groups of 100 ng/mL GM-CSF-WEHI-3B plus 10 ng/mL IL-4-WEHI-3B, 100 ng/mL GM-CSF-WEHI-3B plus 100 ng/mL IL-4-WEHI-3B, and 500 ng/mL GM-CSF-WEHI-3B plus 100 ng/mL IL-4-WEHI-3B, but without statistical significance (P > .05). However, 7 days after inoculation, we did not detect any increase in serum concentrations of the three cytokines in mice inoculated with cytokine-expressing cells (data not shown).
Survival of mice inoculated with irradiated WEHI-3B cells expressing exogenous cytokines. (A) Mice were injected with 5 × 105 wild-type WEHI-3B cells (X). Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ (x) or WEHI-3B–GM-CSF (□) cells and then with 5 × 105 wild-type WEHI-3B cells. (B) Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ cells (X) or with WEHI-3B–GM-CSF and WEHI-3B–TNF cells expressing 500 ng/mL GM-CSF and 10 ng/mL TNF (x); 10 ng/mL GM-CSF and 1 ng/mL TNF (○); 100 ng/mL GM-CSF and 1 ng/mL TNF (▵); 500 ng/mL GM-CSF and 1 ng/mL TNF (□); 10 ng/mL GM-CSF and 10 ng/mL TNF (◊); or 100 ng/mL GM-CSF and 10 ng/mL TNF (•). Seven days later, each mouse was injected with 5 × 105wild-type WEHI-3B cells. (C) Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ cells (X) or with WEHI-3B–GM-CSF and WEHI-3B–IL-4 expressing 10 ng/mL GM-CSF and 100 ng/mL IL-4 (x); 100 ng/mL GM-CSF and 10 ng/mL IL-4 (□); 10 ng/mL GM-CSF and 10 ng/mL IL-4 (▵); 500 ng/mL GM-CSF and 100 ng/mL IL-4 (◊); 100 ng/mL GM-CSF and 100 ng/mL IL-4 (○); or 500 ng/mL GM-CSF and 10 ng/mL IL-4 (•). Seven days later, each mouse was injected with 5 × 105 wild-type WEHI-3B cells. (D) Mice were injected with 5 × 105 wild-type WEHI-3B cells (X). Seven days later, they were injected with 5 × 105 BMMNCs from syngeneic mice inoculated with 5 × 105 irradiated WEHI-3B–LacZ (x) or cytokine transfectants expressing 10 ng/mL GM-CSF and 1 ng/mL TNF (○) or 500 ng/mL GM-CSF and 10 ng/mL IL-4 (□) or with 106 BMMNCs from mice inoculated with 5 × 105 WEHI-3B–GM-CSF and WEHI-3B–IL-4 cells expressing 500 ng/mL GM-CSF and 10 ng/mL IL-4 (•).
Survival of mice inoculated with irradiated WEHI-3B cells expressing exogenous cytokines. (A) Mice were injected with 5 × 105 wild-type WEHI-3B cells (X). Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ (x) or WEHI-3B–GM-CSF (□) cells and then with 5 × 105 wild-type WEHI-3B cells. (B) Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ cells (X) or with WEHI-3B–GM-CSF and WEHI-3B–TNF cells expressing 500 ng/mL GM-CSF and 10 ng/mL TNF (x); 10 ng/mL GM-CSF and 1 ng/mL TNF (○); 100 ng/mL GM-CSF and 1 ng/mL TNF (▵); 500 ng/mL GM-CSF and 1 ng/mL TNF (□); 10 ng/mL GM-CSF and 10 ng/mL TNF (◊); or 100 ng/mL GM-CSF and 10 ng/mL TNF (•). Seven days later, each mouse was injected with 5 × 105wild-type WEHI-3B cells. (C) Mice were injected with 5 × 105 irradiated WEHI-3B–lacZ cells (X) or with WEHI-3B–GM-CSF and WEHI-3B–IL-4 expressing 10 ng/mL GM-CSF and 100 ng/mL IL-4 (x); 100 ng/mL GM-CSF and 10 ng/mL IL-4 (□); 10 ng/mL GM-CSF and 10 ng/mL IL-4 (▵); 500 ng/mL GM-CSF and 100 ng/mL IL-4 (◊); 100 ng/mL GM-CSF and 100 ng/mL IL-4 (○); or 500 ng/mL GM-CSF and 10 ng/mL IL-4 (•). Seven days later, each mouse was injected with 5 × 105 wild-type WEHI-3B cells. (D) Mice were injected with 5 × 105 wild-type WEHI-3B cells (X). Seven days later, they were injected with 5 × 105 BMMNCs from syngeneic mice inoculated with 5 × 105 irradiated WEHI-3B–LacZ (x) or cytokine transfectants expressing 10 ng/mL GM-CSF and 1 ng/mL TNF (○) or 500 ng/mL GM-CSF and 10 ng/mL IL-4 (□) or with 106 BMMNCs from mice inoculated with 5 × 105 WEHI-3B–GM-CSF and WEHI-3B–IL-4 cells expressing 500 ng/mL GM-CSF and 10 ng/mL IL-4 (•).
Effect of BMMNCs from mice inoculated with cytokine-expressing cells on tumor-bearing mice.
Because cytokine-expressing WEHI-3B cells inhibited tumor growth when injected before wild-type WEHI-3B cells, we sought to determine whether the BMMNCs from mice injected with cytokine-expressing cells could inhibit pre-existing tumors. We therefore injected BMMNCs from mice inoculated with irradiated cytokine-secreting WEHI-3B cells into syngeneic mice that had received wild-type WEHI-3B cells 7 days before. Compared with the injection of BMMNCs from mice receiving irradiated WEHI-3B or WEHI-3B–lacZ cells (mean survival, 52.8 days), the injection of cells from mice receiving cytokine-expressing WEHI-3B led to a highly significant (P < .0001) enhancement of survival (Fig 5D). This was especially notable with BMMNCs from mice injected with WEHI-3B–GM-CSF and WEHI-3B–IL-4 cells: 80% of the animals inoculated with 5 × 105 BMMNCs and 100% of the animals receiving 106 BMMNCs survived more than 100 days (Fig 5D). There are some statistically significant differences between survival curves: infused mice group with modified BMMNCs (by WEHI-3B–GM-CSF and WEHI-3B–TNFα/IL-4) survived the infused group with BMMNCs (by WEHI-3B–lacZ) (P < .0009) or control group (P < .0001), whereas 1 × 106 cells infused mice group with BMMNCs (by WEHI-3B–GM-CSF and WEHI-3B–IL-4) survived the 5 × 105 cell infused mice group without statistical significance (P > .05), but apparently both groups significantly survived the infused mice group with modified BMMNCs (by GM-CSF-WEHI-3B and TNFα-WEHI-3B) (P < .0004). Simple BMMNCs (by WEHI-3B–lacZ) infused mice group survived the control group, but without statistical significance (P > .05).
Cell-mediated cytotoxicity assay.
BMMNCs and spleen cells of mice injected with irradiated cytokine gene-expressing leukemic cells showed weak cytotoxicity against WEHI-3B in vitro using CytoTox 96 (15.2% ± 3.3% and 25.5% ± 2.3% in BMMNCs and spleen cells at an E/T ratio of 50:1, respectively), and T-cell–depleted BMMNCs by negative selection using antimouse CD4 MoAb, antimouse CD8 MoAb, and antirat IgG MoAb-coated immunomagnetic beads (Dynal, Oslo, Norway) showed weak cytotoxicity (11.7% ± 0.10% at an E/T ratio of 50:1). BMMNCs from mice inoculated with BM cells from mice injected with cytokine gene transfectants also showed weak cytotoxicity (12.3% ± 3.1% at an E/T ratio of 50:1). However, spleen cells from mice administered BMMNCs from mice injected with a mixture of tumor cells expressing GM-CSF with those expressing IL-4 or those expressing TNFα cells expressed mature DC phenotypic marker, B7-1, and B7-2 (Fig 6B) as well as DC-specific marker DEC205, and CD11c (data not shown), and they could exhibit the specific cytotoxicity against WEHI-3B (62.9% ± 5.8% and 53.4% ± 4.9% at an E/T ratio of 50:1, respectively), whereas less than 12% of nonspecific lysis were observed at the same E/T ratio when A20 and syngeneic BMMNCs were used as targets. This suggests that 40% to 50% of killing activity was due to the activity of specific cytotoxic T lymphocytes (CTLs) against WEHI-3B (Fig 6C and D).
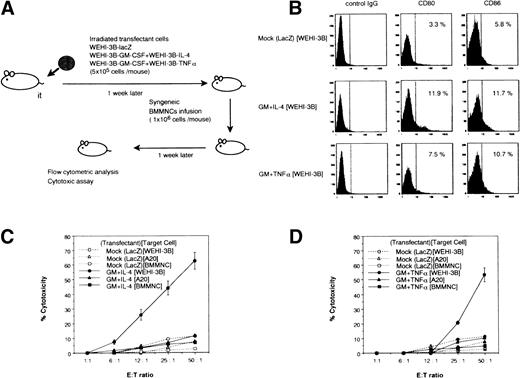
Flow cytometric analysis and cytotoxicity assay of spleen cells from mice receiving BMMNCs from animals injected with cytokine-expressing WEHI-3B cells. (A) Schematic diagrams of biological analysis of mice transfused with modified BMMNCs. (B) Expression of CD80 (B7-1) as well as CD86 (B7-2) in the spleen cells. Control (isotype IgG), mouse injected with modified BMMNCs by WEHI-3B–lacZ, mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–IL-4, and mouse injected with modified BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–TNF. (C) Cytotoxic activity by spleen cells of mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–IL-4. (D) Cytotoxic activity by spleen cells of mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–TNF.
Flow cytometric analysis and cytotoxicity assay of spleen cells from mice receiving BMMNCs from animals injected with cytokine-expressing WEHI-3B cells. (A) Schematic diagrams of biological analysis of mice transfused with modified BMMNCs. (B) Expression of CD80 (B7-1) as well as CD86 (B7-2) in the spleen cells. Control (isotype IgG), mouse injected with modified BMMNCs by WEHI-3B–lacZ, mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–IL-4, and mouse injected with modified BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–TNF. (C) Cytotoxic activity by spleen cells of mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–IL-4. (D) Cytotoxic activity by spleen cells of mouse injected with modified-BMMNCs by WEHI-3B–GM-CSF plus WEHI-3B–TNF.
DISCUSSION
We evaluated the effects of BM cells obtained from mice injected with irradiated cytokine gene-expressing leukemic cells on syngeneic tumor-bearing animals. The expansion of T-cell clones in recipient mice as well as their prolonged survival suggests that therapy with activated BMMNCs containing modified tumor cells (eg, cytokine gene-expressing cells) may be a promising approach to eliminate minimum residual disease (MRD) in leukemic patients.
Studies with vaccine models have indicated the efficacy of antitumor immunotherapy.15-19 The induction of DCs by tumor cells expressing GM-CSF has been found to lead to the activation of T cells,2,3,17 whereas a deficiency of DCs in tumor-bearing mice has been observed to underlie both the observed nonresponsiveness of T cells to tumor antigens and the resulting rapid progression of tumor.20 However, the stimulation of T cells by DCs generated by GM-CSF from hematopoietic precursors in tumor-bearing mice almost completely reversed this nonresponsiveness of the T cells, resulting in an arrest of tumor growth and a dramatic prolongation of survival.21
Tumor-specific immunity by tumor cells expressing GM-CSF has been shown to involve both CD4+ and CD8+ T cells,2 whereas CD4+ T cells and NK cells have an effect on MHC class I-negative tumors.22 In this study, CD4+ T cells and CD8+ T cells in spleen also performed a specific cytotoxicity against leukemic cells (Fig 6). However, the direct systemic administration of cytokines would probably meet with limited success for severe toxicities. The local production of GM-CSF may therefore be associated with the ability of this cytokine to induce the differentiation of DC precursors into dedicated APCs in the peritumor area.17,23 Because the combination of GM-CSF with IL-4 or of GM-CSF with TNFα is more efficient in inducing DCs from the hematopoietic stem cells than is GM-CSF alone in vitro,8-12 the localized production of the same combination of cytokines as described above may be more effective than GM-CSF alone in treating tumors in vivo (Fig 5).23
We performed a set of experiments in which WEHI-3B cells were transduced with GM-CSF gene or GM-CSF plus IL-4 genes or GM-CSF plus TNFα genes. In assaying the antitumor activity of cytokine-expressing cells, we found that the combination of cells that secreted GM-CSF and IL-4 was more effective than that of cells that expressed GM-CSF and TNFα (Figs 5 and 6). These findings suggest that GM-CSF and IL-4 may be superior to GM-CSF and TNFα in the in vivo generation of functional DCs from BMMNCs. Although 10 mice that were injected with irradiated cells secreting GM-CSF and IL-4 demonstrated an enhanced survival, there was a significant increase in the number of immature DCs (positive cells for DEC-205 are 10.6%; however, they express little CD80 and CD86) in BMMNCs (Fig 3). Even simply the BMMNC fraction as well as T-cell–depleted BMMNCs perform little cytotoxic effects, which suggests that the relevant cells in the BMMNC fraction, including slightly immature DCs, do not exert antileukemic effects. They may have the potential to induce cytotoxic activity against tumor cells and show effective proliferation in syngeneic mice (Figs 5 and6).
The development of a local immunological microenvironment during therapy may increase the immunogenicity of tumor cells and may enhance the effectiveness of the systemic immune response. The excellent immune response observed in BMMNCs from syngeneic mice that were inoculated with cytokine-expressing tumor cells suggests that the homing sites of modified tumor cells are important (ie, BM for leukemic cells and regional lymph nodes for melanoma cells). Alternatively, it may suggest that the importance of the migration of modified tumor cells into the BM may be associated with the production of immature DCs from BM hematopoietic stem cells. We demonstrated that the transduced tumor cells home to the BM at significant enough numbers to create a significant effect by GM-CSF with or without IL-4 or TNFα on local BM differentiation (Figs 3 and 4). These findings suggest that the presence of GM-CSF and IL-4 at the site of leukemic cells in the BM can lead to the production of DCs and to the attraction of immature DCs from the surrounding hematopoietic progenitor cells in the BM. The combination of leukemic cells and immature DCs in the BMMNC preparations we have used may induce the maturation of these functional DCs. This may occur partly in the BM, due to the uptake of tumor extracts in specific stages from precursors, but final maturation after uptake of exogenous antigens is thought to take place mainly in the peripheral tissues,24 including the spleen and lymph nodes, after the migration of the immature DCs to these organs. Mature DCs expressing B7-1 as well as B7-2 have been shown to enhance the induction of specific antitumor immune responses in both the spleen and lymph nodes (Figs 5 and 6).25
We then injected these cells into mice intravenously and showed that DCs from the BM can be transferred to third-party recipient mice and transfer antitumor immunity. These transfers putatively result in expansion of various Vβ TCR segments in the third-party recipient mice, suggesting that some of the proliferating T-lymphocyte clonotypes in the recipient mice may be involved in the recognition of leukemia-associated antigens (data not shown). In fact, in the spleens of mice receiving BMMNCs, the mature DCs (DEC205+B7-1+ cells of these cells are 15% in the control group, are 35% in the GM-CSF + IL-4 group, and are 30% in the GM-CSF + TNFα group) were proliferated and the T lymphocytes were activated, probably due to the ability of the immature DCs in these BMMNC preparations to migrate to the lymph nodes or spleen, expand, and interact with the T lymphocytes in the peripheral tissues. The generation of a specific antitumor response requires that these functional DCs encounter the relevant tumor-associated antigens and reflux into the peripheral tissues, thus providing these cells with the greatest potential for presenting the relevant antigens to the T lymphocytes that possess the appropriate T-cell receptors (data not shown).26,27 Host T lymphocytes should thus enhance the specific antitumor immune response, ie, they demonstrated the strong cytotoxicity assay against parental tumors with increasing the number of mature DCs expressing B7-1 and B7-2 and with presenting tumor antigens (Fig 6).25
Our results thus suggest that effective immunity may be induced by increasing the in vivo activity of functional DCs. For application of this approach to clinical study, we have further studied the vaccination using DCs following autologous transplantation models in which leukemic cells at de novo were cryopreserved and, after they were genetically engineered with cytokine genes and irradiated, they were infused to tumor-bearing animals as a vaccine therapy after autologous BM transplantation. Moreover, because this approach should be relatively general, efficacy may be observable with a variety of syngeneic tumors.
These findings also suggest that the infusion of syngeneic or autologous BM cells stimulated with cytokine-expressing tumor cells without chemotherapy may be a novel and promising immunotherapeutic strategy for managing patients with certain types of cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Shin-ichiro Fujii, MD, PhD, The Center for Bone Marrow Transplantation and Immunotherapy, Institute for Clinical Research, Kumamoto National Hospital, 1-5 Ninomaru, Kumamoto 860-0008, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal