Abstract
Interleukin (IL)-15 regulates the proliferative activity of the CD8+ T-cell pool in human immunodeficiency virus (HIV)-infected patients, thereby contributing to the maintenance of the CD8+ T-cell–mediated immune response against HIV in extravascular tissues, including the lung. However, the effects of IL-15 on antigen-presenting cells (APC) during HIV infection are still unclear. In this study, we evaluated whether IL-15 regulates the macrophage stimulatory pathways governing inflammatory events that take place in the lung of patients with HIV infection. As a first step we evaluated the in vitro effects of IL-15 on lung macrophages retrieved from the respiratory tract of eight normal subjects. Although macrophages from uninfected individuals expressed the IL-15 binding proteins (IL-15R and the common γc) at resting conditions, they did not express IL-15 messenger RNA (mRNA). However, a 24-hour stimulation with IL-15 induced the expression of interferon-γ (IFN-γ) and IL-15 itself, suggesting a role for this cytokine in the activation of the pulmonary macrophage pool during inflammation. As a confirmation of the role of IL-15 in this setting, at resting conditions, alveolar macrophages of patients with HIV infection and T-cell alveolitis expressed IL-15, IFN-γ, and IL-15 binding proteins; showed an upmodulation of costimulatory molecules, B7 and CD72, which are involved in the APC of macrophages; and behaved as effective accessory cells because they elicited a strong proliferation of T cells. The accessory effect was inhibited by pretreatment with anti-CD72, anti-B7 (CD80 and CD86), and anti–IL-15 monoclonal antibodies (MoAb). We then investigated the relationship between IL-15 and the expression of costimulatory molecules by macrophages. A 24-hour stimulation of IL-15R+/γc+ macrophages with IL-15 upregulated the expression of CD80 and CD86. The evidence that IL-15 upregulates the expression of coligands that favor the contact between T cells and APC, per se, triggers T-cell activation and proliferation and acts as a chemoattractant for T cells, suggests that IL-15 plays a key role in Tc1-mediated defense mechanisms taking place in extravascular tissues of patients with HIV disease.
SEVERAL STUDIES HAVE shown the spread of human immunodeficiency virus (HIV) to the extravascular tissues and the appearance of local HIV-specific immune responses that attempt to eradicate the virus from the involved organs.1 Indeed, in striking contrast to the peripheral and tissue CD4 lymphopenia, HIV may cause a marked CD8+ T-cell infiltration in different tissues, including the lung, lymph nodes, liver, salivary glands, kidney, and bone marrow.2 We recently suggested that tissue macrophages are not bystander cells in this phenomenon, because they actively release mediators of inflammation that lead to the local activation and expansion of the cytotoxic T-lymphocyte (CTL) pool in organs affected by HIV infection.3 In particular, when a starvation of interleukin (IL)-2 occurs as a consequence of a progressive, quantitative impairment of CD4+ T cells, IL-15 plays a critical role in the CD8+ T-cell compartmentalization. For instance, in the lung IL-15 induces the proliferation of oligoclonal CD8+ CTL involved in the clearance of HIV-infected T cells within the alveolar space.4-6
The interaction between antigen-presenting cells (APC) and T cells is a critical factor in initiating the in loco T-cell activation and proliferation. Furthermore, the outcome of the interaction of a T cell with an APC on T-cell proliferation depends on the presence of a number of costimulatory molecules on the APC, including members of the B7 family (CD80 and CD86), some molecules of the tumor necrosis factor (TNF)-receptor superfamily (CD40 and CD27), and the CD5 coligand CD72.7 8 To investigate the molecular mechanisms governing IL-15–mediated activation of the CD8+ T-cell–mediated immune response we evaluated whether IL-15 modulates the expression of these costimulatory molecules in an extravascular tissue that is a common site of CD8+ T-cell infiltration, ie, the lung. Our results show that alveolar macrophages (AM) of HIV-infected patients express IL-15 and its receptor structure. In addition, IL-15 consistently upregulates the expression of costimulatory molecules involved in the APC/T-cell contact, the expression of cytokines involved in the accessory function of macrophages, and the proliferative activity of lung T cells that in most of our patients show a Tc1 cytokine pattern.
MATERIALS AND METHODS
Study populations.
Fourteen HIV-1 seropositive patients were analyzed (10 men and 4 women; average age 33.2 ± 4.1 years; risk category for HIV infection was high-risk heterosexual contact in 5 patients and intravenous drug use in 9 patients). At the time of the bronchoalveolar lavage (BAL) evaluation each patient underwent history, physical examination, and routine blood studies; HIV seropositivity was confirmed by both enzyme-linked immunoassay and Western blot analysis. According to the Centers for Disease Control (CDC) classification, 5 previously asymptomatic patients who during their follow-up showed clinical symptoms and signs of HIV infection other than, or in addition to lymphoadenopathy were reclassified in category B; they were all antiretroviral naive at the time of the BAL evaluation. Nine patients were classified in category C; 8 of these subjects had previously received therapy with zidovudine. In all patients, BAL was performed to obtain a specific diagnosis of opportunistic infections according to our study protocol for HIV patients with suspected pulmonary involvement.9 BAL samples were submitted for cytological studies, stained for Pneumocystis carinii, and stained and cultured for mycobacteria and fungi.9 On the basis of BAL analysis an opportunistic pulmonary infection (a Pneumocystis carinii pneumonia) was shown in 9 of the 14 patients.
Immunologic studies were restricted to HIV-seropositive selected patients in whom complete morphological and immunological analyses of BAL cellular components were available, including cell recovery, differential count of macrophages, lymphocytes, neutrophils, and eosinophils, and flow cytometry analysis of CD3, CD4, and CD8 BAL T-cell populations (Table 1). Patients included in the study had minimal prerequisite lung function status and arterial blood gases to minimize the risk factor for the development of adverse effects with BAL, a platelet count greater than 20,000/mL, and a prothrombin time greater than 50%.9 BAL specimens obtained from three HIV-infected patients with lymphocytic alveolitis were excluded from the study due to blood cell contamination of BAL specimens.
Summary of Cell Findings in Bronchoalveolar Lavage of 14 Patients With HIV Infection and CD8+ T-Cell Alveolitis
| Patients . | Cell Recovery ×103/mL . | Lymphocytes . | Alveolar Macrophages . | Neutrophils . | Eosinophils . | CD4 T Cells . | CD8 T Cells . | CD4/CD8 Ratio . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | |||
| HIV infection (n 14) | 294.7 ± 36.1 | 26.2 ± 5.9 | 76.4 ± 14.3 | 71.5 ± 6.2 | 206.5 ± 27.4 | 2.3 ± 0.8 | 5.4 ± 0.6 | 0.1 ± 0.2 | 0.2 ± 0.2 | 2.0 ± 3.4 | 1.5 ± 1.7 | 89.3 ± 8.7 | 67.8 ± 11.2 | 0.02 ± 0.08 |
| Controls (n 8) | 111.4 ± 23.9 | 5.5 ± 1.7 | 5.5 ± 1.3 | 94.0 ± 1.5 | 104.1 ± 23.7 | 0.1 ± 0.3 | 0.1 ± 0.7 | 0.1 ± 0.3 | 0.2 ± 0.2 | 47.1 ± 1.8 | 2.5 ± 1.0 | 21.9 ± 6.7 | 1.2 ± 0.6 | 1.97 ± 0.4 |
| HIV v C | 0.01 | 0.01 | 0.001 | 0.05 | 0.01 | NS | 0.05 | NS | NS | 0.001 | NS | 0.01 | 0.001 | 0.001 |
| Patients . | Cell Recovery ×103/mL . | Lymphocytes . | Alveolar Macrophages . | Neutrophils . | Eosinophils . | CD4 T Cells . | CD8 T Cells . | CD4/CD8 Ratio . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | % . | ×103/mL . | |||
| HIV infection (n 14) | 294.7 ± 36.1 | 26.2 ± 5.9 | 76.4 ± 14.3 | 71.5 ± 6.2 | 206.5 ± 27.4 | 2.3 ± 0.8 | 5.4 ± 0.6 | 0.1 ± 0.2 | 0.2 ± 0.2 | 2.0 ± 3.4 | 1.5 ± 1.7 | 89.3 ± 8.7 | 67.8 ± 11.2 | 0.02 ± 0.08 |
| Controls (n 8) | 111.4 ± 23.9 | 5.5 ± 1.7 | 5.5 ± 1.3 | 94.0 ± 1.5 | 104.1 ± 23.7 | 0.1 ± 0.3 | 0.1 ± 0.7 | 0.1 ± 0.3 | 0.2 ± 0.2 | 47.1 ± 1.8 | 2.5 ± 1.0 | 21.9 ± 6.7 | 1.2 ± 0.6 | 1.97 ± 0.4 |
| HIV v C | 0.01 | 0.01 | 0.001 | 0.05 | 0.01 | NS | 0.05 | NS | NS | 0.001 | NS | 0.01 | 0.001 | 0.001 |
Abbreviations: C, controls; NS, not significant.
Eight healthy adult controls were selected (five men and three women; average age 29.7 ± 5.9 years; three nonsmoking healthy persons and five subjects evaluated for complaints of cough without lung disease). They showed normal physical examinations, chest X-rays, lung function tests, and BAL cell numbers.
Preparation of cell suspensions.
The BAL was performed according to the technical recommendations and guidelines for the standardization of BAL procedures previously reported.10 Briefly, a total of 200 to 250 mL of saline solution was injected in 25 mL aliquots via fiberoptic bronchoscopy, with immediate vacuum aspiration after each aliquot. Immediately after the BAL, the fluid was filtered through gauze and the volume measured. A volume of 100 to 200 mL of BAL recovery and a sample of 50% of the instilled volume with a minimum of 50 mL were considered acceptable. The percentage of BAL recovery was 56.7% ± 3.4% and 58.1% ± 4.7% of the injected fluid in patients with HIV-1 infection and control subjects, respectively. Cells recovered from the BAL were washed three times with phosphate-buffered saline (PBS), resuspended in endotoxin tested RPMI 1640 (Sigma Chemical Co, St Louis, MO) supplemented with 20 mmol/L HEPES and L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS; ICN Flow, Costa Mesa, CA) and then counted. Macrophages, lymphocytes, neutrophils, and eosinophils were differentially counted in a total count of 300 cells according to morphological criteria in cytocentrifuged smears stained with Wright-Giemsa. Peripheral blood mononuclear cells were harvested from freshly heparinized blood specimens obtained from five control subjects by centrifugation on Ficoll-Hypaque (F/H) gradient as previously reported.3
Table 1 reports differential BAL cell counts in patients and controls. In all normal individuals the total cell recovery ranged from 6.5 to 8 × 106 BAL cells; less than 5% of BAL cells were lymphocytes. In normal subjects both CD4 helper-related and CD8 cytotoxic/suppressor-related cells were present in approximately the same proportions as in peripheral blood. In contrast, cell recovery was significantly higher in patients with HIV infection than in control subjects. With regard to the differential count of BAL cells, CD4+ T cells were less than 5% in all BAL specimens; all patients showed a T-cell alveolitis. As a consequence of the decrease in the CD4+ T-cell population and the increase in the absolute number of CD8+ T cells, the BAL CD4/CD8 ratio was dramatically decreased in all HIV-infected patients (Table 1).
Purification of AM and T cells.
AM were enriched from the BAL cell suspensions by rosetting with neuraminidase-treated sheep red blood cells (SRBC) followed by F/H–gradient separations.3 AM were further enriched by removing residual CD3+, CD16+, and CD56+ lymphocytes using high-gradient magnetic separation columns (Mini MACS, Miltenyi Biotec, Germany), as previously described.3 After this multistep selection procedure more than 95% of the above cells were viable, as judged by trypan blue exclusion test. The staining with monoclonal antibodies (MoAb) showed that more than 98% of BAL cells expressed the AM-associated CD68 antigen.
The cell suspension of peripheral blood mononuclear cells was depleted of adherent cells by incubation for 45 minutes in plastic Petri dishes at 37°C in an atmosphere of 95% air and 5% CO2. T cells were enriched from the resulting cell suspensions by rosetting with neuraminidase-treated SRBC followed by F/H–gradient separations, as previously described.3
MoAb.
The commercially available conjugated or unconjugated MoAb used belonged to the Becton Dickinson (Sunnyvale, CA), Immunotech (Marseille, France), and PharMingen (San Diego, CA) series and included: CD3, CD4, CD8, CD28, CD45R0, CD45RA, CD69 (FN50), CD72, CD80 (BB-1/B7-1), CD86 (B70/B7-2), CD152 (CTLA-4), and HLA-DR and isotype matched controls. Anti–IL-15 M110 (IgG1) and anti–IL-15 receptor (IL-15Rα)(IgG1) MoAb were kindly provided by Dr A. Troutt (Immunex Co, Seattle, WA); anti–TNF-α (MAB11), anti–IL-2 (MQ1-17H12), anti–IL-4 (8D4-8), and anti–interferon (IFN)-γ (4S.B3) MoAb were purchased from PharMingen. The frequency of BAL cells positive for the above reagents was determined by overlaying the flow cytometry histograms of the samples stained with the different reagents as previously described.3 Cells were scored using a FACScan analyzer (Becton Dickinson), and data were processed using the Macintosh CELLQuest software program (Becton Dickinson). The expression of cytoplasmic cytokine was evaluated after permeabilization of cell membranes using 1:2 diluted PermeaFix (Ortho, Raritan, NJ) for 40 minutes. After permeabilization procedures, anti–IL-15, anti–TNF-α, anti–IFN-γ, anti–IL-12, and anti–IL-2 MoAbs were added. Because pulmonary cells bore cytoplasmic cytokine in a unimodal expression pattern, indicating that the entire cell population exhibits relatively homogeneous fluorescence, the percentage of positive cells does not represent the most accurate way of enumerating positive cells. For this reason, the mean fluorescence intensity (MFI) was used to compare the positivity of these specific antigens on different cell populations. To evaluate whether the shift of the positive cell peak was statistically significant, the Kolmogorov-Smirnov test for analysis of histograms was used according to the Macintosh CELLQuest software user’s guide (Becton Dickinson).
For immunofluorescence analysis, control IgG1 and IgG2a and IgG2b were obtained from Becton Dickinson; control rat antiserum consisted of ascites containing an irrelevant rat IgG2b mAb (kindly provided by A. Rosato, Padova, Italy); control rabbit antiserum consisted of rabbit IgG myeloma (purified protein) purchased from Serotec (Serotec, UK); goat-anti-rabbit IgG and goat F(ab′)2 anti-rat IgG were obtained from Immunotech (Marseille, France).
RNA extraction, complementary DNA (cDNA) synthesis and polymerase chain reaction (PCR) amplification of IFN-γ, TNF-α, IL-15, and IL-15R complex (IL-15Rα, IL-2Rβ, IL-2Rγc).
Total cellular RNA was extracted using the Ultraspec I RNA isolation system (Biotecx Lab, Houston, Texas) from unstimulated and IL-15 stimulated AM. cDNAs were prepared from 2 μg of total cellular RNA by reverse transcription (RT) using a kit from Invitrogen Corp (San Diego, CA).
For the amplification of IFN-γ, TNF-α, IL-15, β-actin, and IL-15R complex, PCR was conducted in a 50 μL reaction using 2 μL of cDNA as template. The PCR mixture consisted of 1.5 mmol/L MgCl2, 50 mmol/l KCL, 10 mmol/l Tris-HCl, 0.2 mM/L concentrations of each deoxynucleotide triphosphate, 2.5 U of Taq polymerase (Perkin Elmer, Norwalk, CT) and 25 pmol/L of each specific primer. The following sense and antisense oligonucleotide primer sequences were used: for IFN-γ: 5′-AgT TAT ATC TTg gCT TTT CA, 3′-ACC gAA TAA TTA gTC AgC TT (expected size of 355 bp); for TNF-α: 5′-TCT CgA ACC CCg AgT gAC AA, 3′-TAT CTC TCA gCT CCA CAC CA (expected size of 124 bp); for IL-15: 5′-CTC gTC TAg AgC CAA CTg ggT gAA TgT AAT AAg, 3′-TAC TTA CTC gAg gAA TCA ATT gCA ATC AAg AAg Tg (expected size of 404 bp); for β-actin: 5′-gTg ggg CgC CCC Agg CAC CA, 3′-CTC CTT AAT gTC ACg CAC gAT TTC (expected size of 540 bp); for IL-15Rα: 5′-ggC gAC gCg ggg CAT CAC, 3′-TCg CTg Tgg CCC TgT ggA TA (expected size of 531 and 432 bp); for IL-2Rγ: 5′-TAT Agg ATC CgA AgA gCA AgC gCC ATg TTg AAg CC, 3′-AgA TTC TgC AgT TTT AgC ATC TgT gTg gCC (expected size of 451 bp). IL-2Rβ was amplified using the human IL-2 receptor β subunit kit (Clontech, Palo Alto, CA).
IFN-γ, TNF-α, and IL-2Rγc were amplified at the following conditions: 60 seconds melting at 94°C, 45 seconds annealing at 53°C for IFN-γ and 58°C for TNF-α and 120 seconds extension at 72°C for 35 cycles followed by a final extension for 7 minutes at 72°C in a Cetus/Perkin Elmer thermal cycler (Emeryville, CA). IL-15 was amplified at the following conditions: 30 seconds melting at 94°C, 30 seconds annealing at 55°C and 30 seconds extension at 72°C for 30 cycles followed by a final extension for 7 minutes at 72°C in a Cetus/Perkin Elmer thermal cycler. IL-15Rα was amplified at the following conditions: 30 seconds melting at 94°C, 45 seconds annealing at 62°C and 45 seconds extension at 72°C for 30 cycles followed by a final extension for 7 minutes at 72°C in a Cetus/Perkin Elmer thermal cycler.
For β-actin amplification conditions were 60 seconds melting at 94°C, 60 seconds annealing at 55°C and 120 seconds extension at 72°C for 30 cycles followed by a final extension for 7 minutes at 72°C. 10 μL of each PCR product was electrophoresed in a 2% agarose gel in Tris borate/EDTA buffer. Gels were stained with ethidium bromide and photographed.
Effect of IL-15 and other locally released cytokines on the expression of costimulatory molecules by pulmonary macrophages.
To assess the effects of IL-15 on macrophage activation, a time-course experiment of the effects of cytokines on the expression of CD72, CD80, and CD86 by AM was performed. AM at the concentration of 1 × 106 cells/mL were cultured in 24-well plates (Corning, New York, NY) for 24 hours at 37°C in 5% CO2 atmosphere. After 24 hours of incubation, plates were centrifuged and, after removal of supernatants, BAL cells were further cultured for 12 hours with medium alone, with IL-15 (100 ng/mL), or with IL-15 (100 ng/mL) and IFN-γ (100 UI/mL). The frequency of BAL cells positive for the above reagents was determined by flow cytometry, as described above. In particular, mean log fluorescence intensity (MFI) was obtained by subtracting the MFI of isotype control from the MFI of the positively stained sample. In this way, the values of MFI represent the relative increase in fluorescence over the background value reported as zero. Furthermore, to evaluate whether the differences between the peaks of cells were statistically significant with respect to controls, the Kolmogorov-Smirnov test for analysis of histograms was used, according to the CellQuest software user’s guide (Becton Dickinson).
Effect of IL-15 on the expression of cytokines by pulmonary macrophages.
We also evaluated whether IL-15 may induce IFN-γ expression on AM. The cytokine expression was evaluated by PCR analysis and by flow cytometry after permeabilization of cell membranes, as reported in detail above.
Evaluation of the involvement of costimulatory molecules in the accessory function of pulmonary macrophages.
Highly purified T cells at the concentration of 1 × 106 cells/mL were cultured in 96 round-bottom well plates for 72 hours at 37°C in 5% CO2 atmosphere with 12.5 × 103 AM in the presence of mitogens (ConA; 10 μg/mL, Sigma Chemical Co) as previously reported.3 In inhibition experiments, anti-CD80 (10 μg/mL), anti-CD86 (10 μg/mL) and anti-CD72 (20 μg/mL), anti-IL-15 (100 ng/mL) or control isotype-matched IgG1 were added at the beginning of the culture. Each experiment was carried out in quadruplicate. For the last 18 hours of culture, plates were pulsed with 1 μCi/well of (3H)thymidine (Dupont, Bruxelles, Belgium), as reported above.3
Statistical analysis.
Data were analyzed with the assistance of the Statistical Analysis System. Data are expressed as mean ±SD. Values were compared using the Anova test and the Spearman correlation test. AP value less than .05 was considered as significant.
RESULTS
The results of differential BAL cell counts and T-cell subpopulations retrieved from the lung of healthy patients and patients with HIV infection (Table 1) showed the presence of a discrete alveolitis. With regard to the differential count of BAL cells, the absolute number of lymphocytes and AM was significantly increased in patients with HIV infection with respect to control subjects (P < .001 and .01, respectively). Further characterization of lung lymphocytes showed that the alveolar lymphocytosis was sustained by CD8+ T cells. As a consequence of the marked increase in CD8+ T cells, the pulmonary CD4/CD8 ratio was significantly lower in HIV-infected patients than in controls (P < .001).
Pulmonary macrophages from uninfected individuals express IL-15 binding proteins, and IL-15 stimulation induces cytokine expression.
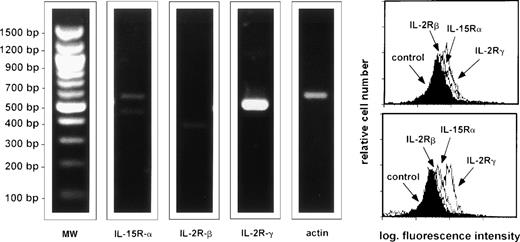
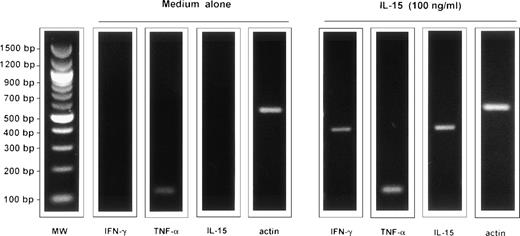
As a first step we evaluated the expression of IL-15 receptor on AM from healthy subjects. Figure 1 shows data from a representative subject, but consistent data were obtained in six normal subjects. At resting conditions AM from normal subjects expressed the IL-15Rα and the γc of the IL-2R, ie, the main molecules involved in the binding of IL-15. However, unstimulated AM from five normal subjects (Fig 2) did not express IFN- γ or IL-15, two cytokines which have been involved in local immune responses during interstitial lung disease; however, after a 24-hour stimulation, IL-15 was able to induce a strong expression of both cytokines (Fig 2). Concerning TNF-α, unstimulated AM expressed TNF-α messenger RNA (mRNA), but after incubation with IL-15, TNF-α signals significantly increased. In fact, in five consecutive normal subjects the optical density (OD) ratio of mRNA TNF-α/actin was 29.131 + 1.027 and 34.929 ± 1.866 in unstimulated AM and IL-15–stimulated AM, respectively (P < .02). These data suggest the putative role of IL-15 in the activation of the pulmonary macrophage pool.
RT-PCR analysis and flow cytometry profile of the expression of IL-15R, IL-2Rβ, and the γc by AM. The figure shows a representative healthy subject (subject #4) but a consistent pattern of expression was observed in six consecutively examined normal subjects. 94% of BAL cells of subject #4 were alveolar macrophages and 7% lymphocytes, as determined by morphological evaluation; the CD4/CD8 ratio was 1.9. AM were enriched as reported in the Materials and Methods section. AM bore high levels of IL-15R and γc whereas a faint expression of IL-2Rβ is detectable.
RT-PCR analysis and flow cytometry profile of the expression of IL-15R, IL-2Rβ, and the γc by AM. The figure shows a representative healthy subject (subject #4) but a consistent pattern of expression was observed in six consecutively examined normal subjects. 94% of BAL cells of subject #4 were alveolar macrophages and 7% lymphocytes, as determined by morphological evaluation; the CD4/CD8 ratio was 1.9. AM were enriched as reported in the Materials and Methods section. AM bore high levels of IL-15R and γc whereas a faint expression of IL-2Rβ is detectable.
RT-PCR analysis of the expression of the messages for IL-15, TNF-, and IFN-γ by AM of a representative healthy subject cultured for 24 hours in medium alone and in the presence of IL-15. The figure shows a representative healthy subject (subject #2) but a consistent pattern of expression was observed in five consecutively examined normal subjects. 96% of BAL cells of subject #2 were alveolar macrophages and 4% lymphocytes, as determined by morphological evaluation and the CD4/CD8 ratio was 2.1. AM were enriched as reported in the Materials and Methods section.
RT-PCR analysis of the expression of the messages for IL-15, TNF-, and IFN-γ by AM of a representative healthy subject cultured for 24 hours in medium alone and in the presence of IL-15. The figure shows a representative healthy subject (subject #2) but a consistent pattern of expression was observed in five consecutively examined normal subjects. 96% of BAL cells of subject #2 were alveolar macrophages and 4% lymphocytes, as determined by morphological evaluation and the CD4/CD8 ratio was 2.1. AM were enriched as reported in the Materials and Methods section.
BAL cells from patients with HIV infection are preactivated cells showing spontaneous expression of cytokines and an up-modulation of costimulatory molecules.
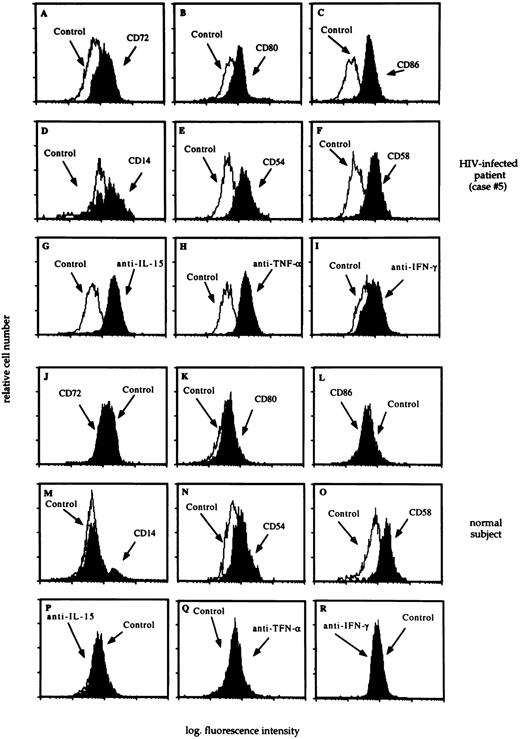
Profiles shown in Figures 3 and4 are representative of 14 HIV-infected patients with T-cell alveolitis and 6 uninfected, normal subjects. AM retrieved from patients with HIV infection and CD8+ T-cell alveolitis showed an up-modulation of costimulatory molecules, including members of the B7 family and the CD72 molecule (Fig 3, panels A, B, and C). Interestingly, the expression of CD72, CD80, CD86 by AM was associated with an increased cell surface density of activation antigens (Fig 3, panels D, E, and F) and cytokines, including IL-15, TNF-α, and IFN-γ (Fig 3, panels G, H, and I). As shown in Fig 5, the B7-2 molecule was found to be expressed at higher intensity than B7-1 by AM from patients with HIV infection; furthermore, there was an association between the expression of IL-15, the MFI of B7 family members and the degree of T-cell infiltration in the pulmonary tract. Taken together these data suggest that AM from patients with HIV infection are in an activation state with respect to normal AM. In fact, AM from normal subjects did not bear CD72, CD80, or CD86 (Fig 3, panels J, K, and L); less than 5% of normal AM were CD14+ (Fig 3, panel M). In addition, pulmonary macrophages from healthy subjects did not show membrane or cytoplasmic IL-15 expression (Fig 3, panel P) and less than 5% of mononuclear phagocytes isolated from the lung of healthy individuals showed cytoplasmic TNF-α (Fig 3, panel Q).
The flow cytometry profile of AM recovered from a representative HIV-infected patient with lymphocytic alveolitis (case #5) and an uninfected control subject. BAL analysis revealed the presence of a P. carinii pneumonia; 34% of BAL cells were lymphocytes, as determined by morphological evaluation and the CD4/CD8 ratio was 0.03. The profile of CD72, CD80, and CD86 molecules (panels A, B, C, J, K, and L), activation markers (panels D, E, F, M, N, and O), and cytoplasmic cytokines (panels G, H, I, P, Q, and R) was determined as reported in the Materials and Methods section. A consistent pattern of expression of costimulatory molecules and cytoplasmic cytokines was observed in all HIV-infected patients with lymphocytic alveolitis.
The flow cytometry profile of AM recovered from a representative HIV-infected patient with lymphocytic alveolitis (case #5) and an uninfected control subject. BAL analysis revealed the presence of a P. carinii pneumonia; 34% of BAL cells were lymphocytes, as determined by morphological evaluation and the CD4/CD8 ratio was 0.03. The profile of CD72, CD80, and CD86 molecules (panels A, B, C, J, K, and L), activation markers (panels D, E, F, M, N, and O), and cytoplasmic cytokines (panels G, H, I, P, Q, and R) was determined as reported in the Materials and Methods section. A consistent pattern of expression of costimulatory molecules and cytoplasmic cytokines was observed in all HIV-infected patients with lymphocytic alveolitis.
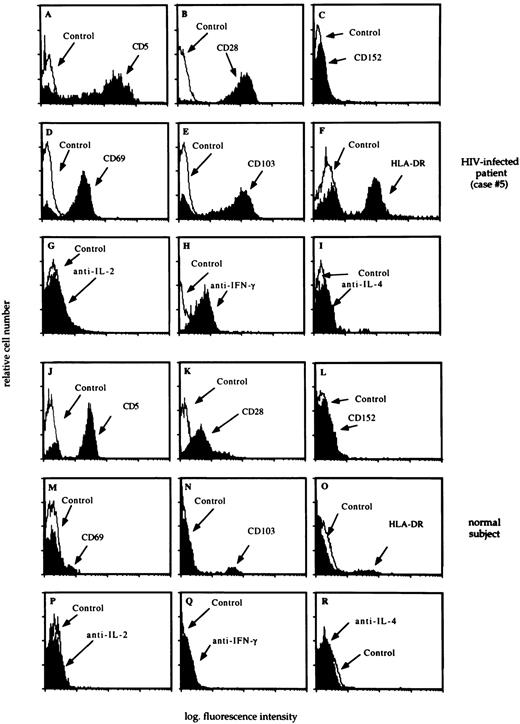
The flow cytometry profile of BAL T cells recovered from a representative HIV-infected patient with lymphocytic alveolitis (case #5) and an uninfected control subject. Main results of BAL analysis of case #5 are summarized in the legend of Fig 3. The profile of CD5, CD28, and CD152 molecules (panels A, B, C, J, K, and L), activation markers (panels D, E, F, M, N, and O), and cytoplasmic cytokines (panels G, H, I, P, Q, and R) was determined as reported in the Materials and Methods section. A consistent pattern of expression of costimulatory molecules and cytoplasmic cytokines was seen in all HIV-infected patients with lymphocytic alveolitis.
The flow cytometry profile of BAL T cells recovered from a representative HIV-infected patient with lymphocytic alveolitis (case #5) and an uninfected control subject. Main results of BAL analysis of case #5 are summarized in the legend of Fig 3. The profile of CD5, CD28, and CD152 molecules (panels A, B, C, J, K, and L), activation markers (panels D, E, F, M, N, and O), and cytoplasmic cytokines (panels G, H, I, P, Q, and R) was determined as reported in the Materials and Methods section. A consistent pattern of expression of costimulatory molecules and cytoplasmic cytokines was seen in all HIV-infected patients with lymphocytic alveolitis.
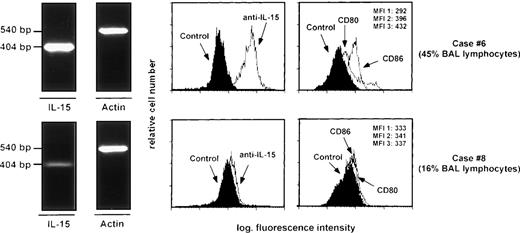
RT-PCR analysis of the expression of the messages for IL-15 and mean fluorescence intensity values of IL-15, CD80, and CD86 histograms shown by AM recovered from two patients with HIV infection. Specifically, case #6 was a symptomatic patient with AIDS-related complex whereas case #8 had full-blown AIDS (P. cariniipneumonia). The percentages of lymphocytes detected in the BAL are indicated on the right side of the fig. There was a strict association between the expression of IL-15, the MFI of IL-15, and B7 family members and the degree of T-cell infiltration, determined on the basis of BAL lymphocytes percentage. MFI 1: mean fluorescence intensity of control histogram; MFI 2: mean fluorescence intensity of CD80 histogram; MFI 3: mean fluorescence intensity of CD86 histogram.
RT-PCR analysis of the expression of the messages for IL-15 and mean fluorescence intensity values of IL-15, CD80, and CD86 histograms shown by AM recovered from two patients with HIV infection. Specifically, case #6 was a symptomatic patient with AIDS-related complex whereas case #8 had full-blown AIDS (P. cariniipneumonia). The percentages of lymphocytes detected in the BAL are indicated on the right side of the fig. There was a strict association between the expression of IL-15, the MFI of IL-15, and B7 family members and the degree of T-cell infiltration, determined on the basis of BAL lymphocytes percentage. MFI 1: mean fluorescence intensity of control histogram; MFI 2: mean fluorescence intensity of CD80 histogram; MFI 3: mean fluorescence intensity of CD86 histogram.
As reported in Table 1, T cells were markedly increased in the lung of patients with HIV infection. Flow cytometry analysis of BAL T lymphocytes showed that the alveolar lymphocytosis was sustained by CD8+/CD45R0+ T cells bearing CD5 and expressing CD28 molecules at high intensity but lacking CTLA-4 expression (Fig 4, panels A, B, and C, respectively). We also evaluated the activation state and the pattern of cytokine production by pulmonary CD8 T cells. In 11 out of the 14 HIV-infected patients most T cells accounting for the CD8 alveolitis were preactivated cells (Fig 4, panels D, E, and F) IFN-γ+ (Fig 4, panel H) but did not express IL-2 or IL-4 (Fig 4, panels G and I, respectively), a pattern which has been reported to be characteristic of Tc1 cells.11 In 3 patients CD8 T cells did not express cytoplasmic cytokines. Normal BAL T cells expressed CD5 antigen, and a percentage of lung lymphocytes ranging between 25.3% and 41.5% bore the CD28 molecule but not the CTLA-4 antigen (Fig 4, panels K and L, respectively); furthermore they did not express cytoplasmic cytokines (Fig 4, panels P, Q, and R).
IL-15 upregulates B7 family member expression on local APC.
Our phenotypic data showed that AM cells express IL-15, which is involved in the induction of T-cell inflammation in peripheral tissues, including the lung,3 and express a complete IL-15R complex. Because a number of cytokines are capable of regulating B7 family expression leading to APC activation, in a time-course experiment we evaluated whether soluble factors locally produced by AM (ie, IL-15, TNF-α, and IFN-γ) and T cells (IL-2 and IFN-γ) may modulate the expression of CD72, CD80, and CD86 ligands on highly purified AM from the lung of four patients with HIV infection. Table 2 shows the MFI values of CD72, CD80, and CD86 tested in the presence of medium and different cytokines. All the molecules under study showed a unimodal expression on cell surface, and the histogram was shifted to the right in relation to the intensity of antigen expression.
Mean Fluorescence Intensity (MFI) Values of CD80, CD86, and CD72 Histograms on AM and CD28 and CD152 Histograms on BAL T Cells in a Time Course Evaluation Test
| Alveolar Macrophages . | ||||||
|---|---|---|---|---|---|---|
| Time 0 | Medium Cultured | Stimulated | ||||
| TNF-α | IL-15 | IL-2 | IFN-γ | |||
| CD80 | 86.7 ± 14.1 | 10.7 ± 4.1 | 16.2 ± 5.1 | 84.2 ± 17.6 | 10.1 ± 2.2 | 80.1 ± 13.3 |
| CD86 | 142.6 ± 36.1 | 16.1 ± 5.0 | 18.8 ± 7.3 | 126.4 ± 22.2 | 14.6 ± 4.1 | 111.8 ± 33.6 |
| CD72 | 69.5 ± 6.5 | 19.3 ± 4.1 | 22.1 ± 4.4 | 20.9 ± 6.4 | 33.7 ± 4.9 | 29.0 ± 8.0 |
| Lung Lymphocytes | ||||||
| Time 0 | Medium Cultured | Stimulated | ||||
| TNF-α | IL-15 | IL-2 | IFN-γ | |||
| CD28 | 46.4 ± 14.1 | 17.1 ± 6.6 | 16.8 ± 7.0 | 36.6 ± 7.3 | 30.1 ± 12.2 | 38.9 ± 6.9 |
| CD152 | 6.1 ± 0.4 | 9.0 ± 1.1 | 3.9 ± 0.7 | 3.2 ± 0.7 | 6.9 ± 1.6 | 9.1 ± 4.1 |
| Alveolar Macrophages . | ||||||
|---|---|---|---|---|---|---|
| Time 0 | Medium Cultured | Stimulated | ||||
| TNF-α | IL-15 | IL-2 | IFN-γ | |||
| CD80 | 86.7 ± 14.1 | 10.7 ± 4.1 | 16.2 ± 5.1 | 84.2 ± 17.6 | 10.1 ± 2.2 | 80.1 ± 13.3 |
| CD86 | 142.6 ± 36.1 | 16.1 ± 5.0 | 18.8 ± 7.3 | 126.4 ± 22.2 | 14.6 ± 4.1 | 111.8 ± 33.6 |
| CD72 | 69.5 ± 6.5 | 19.3 ± 4.1 | 22.1 ± 4.4 | 20.9 ± 6.4 | 33.7 ± 4.9 | 29.0 ± 8.0 |
| Lung Lymphocytes | ||||||
| Time 0 | Medium Cultured | Stimulated | ||||
| TNF-α | IL-15 | IL-2 | IFN-γ | |||
| CD28 | 46.4 ± 14.1 | 17.1 ± 6.6 | 16.8 ± 7.0 | 36.6 ± 7.3 | 30.1 ± 12.2 | 38.9 ± 6.9 |
| CD152 | 6.1 ± 0.4 | 9.0 ± 1.1 | 3.9 ± 0.7 | 3.2 ± 0.7 | 6.9 ± 1.6 | 9.1 ± 4.1 |
Data are reported as mean ± SE of the MFI values after subtracting the MFI of isotype control MoAb from the MFI of positively stained samples. Bold numbers denote the significant values with respect to the medium cultured AMs and T lymphocytes.
Preliminary time course experiments showed that, after 24 to 36 hours of culture, unstimulated AM partially lose the expression of these costimulatory molecules (Table 2). Furthermore, the expression of activation markers and cytoplasmic cytokines by AM progressively drops in the absence of stimulation (data not shown). The progressive loss of the activation state was reversed by incubation with IL-15, because exposure to the cytokine reinduced the expression of B7 molecules on AM retrieved from the lung of HIV infected patients. As shown by the comparison of MFI levels, after IL-15 stimulation, AM showed enhanced levels of CD80 and CD86 expression with respect to the paired samples of medium-cultured AM (P < .001 as determined by the Kolmogorov-Smirnov analysis). Incubation with IFN-γ showed similar effects on B7 family member expression, whereas neither TNF-α nor IL-2 influenced the expression of accessory receptors. Furthermore, cytokine stimulation did not or only slightly influenced CD72 expression on AM (data not shown).
A similar phenomenon was observed by restimulating BAL T cells with the above cytokines (Table 2). Incubation with IL-15 and IFN-γ and, in some cases, with IL-2 enhanced the expression of CD28 on 36-hour cultured lung T cells. The Kolmogorov-Smirnov analysis showed that histograms of T cells cultured in a cytokine milieu were shifted with respect to histograms of unstimulated lymphocytes. TNF-α did not modify the expression of CD28. Furthermore, IL-2, IFN-γ , IL-15, and TNF-α stimulation did not induce CD154 expression.
Costimulatory molecules expressed by pulmonary macrophages following IL-15 exposure are involved in the regulation of the proliferative activity of T cells.
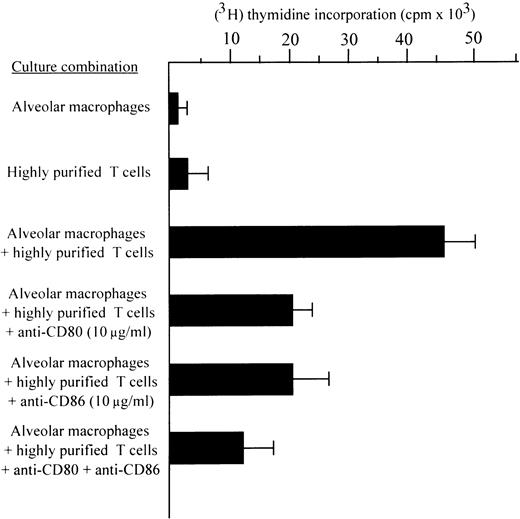
The possibility that the increased expression of B7 and CD72 coligands might account for the in situ proliferation of T cells was also investigated by an in vitro proliferation assay with highly purified allogeneic T cells (Fig 6). The purity of the T-cell populations used in the proliferation assays exceeded 99%, with virtually no detectable residual monocyte-macrophages. Highly purified T cells did not proliferate when stimulated with ConA unless accessory cells were added. However, T cells without accessory cells showed a discrete proliferative activity in the presence of 100 ng/mL of IL-15 (P < .001 with respect to highly purified ConA stimulated T cells without accessory cells).
The effects of anti-CD80, anti-CD86, and anti-CD72 MoAbs on accessory function of AM isolated from the BAL of nine patients with HIV infection (two patients with AIDS-related complex and seven patients with full-blown AIDS). The above quoted antibodies, when added to assays at the beginning of culture, significantly inhibited the mitogen-induced proliferation of highly purified T cells. AM and T cells were enriched as reported in the Materials and Methods section.
The effects of anti-CD80, anti-CD86, and anti-CD72 MoAbs on accessory function of AM isolated from the BAL of nine patients with HIV infection (two patients with AIDS-related complex and seven patients with full-blown AIDS). The above quoted antibodies, when added to assays at the beginning of culture, significantly inhibited the mitogen-induced proliferation of highly purified T cells. AM and T cells were enriched as reported in the Materials and Methods section.
Figure 6 also shows that AM induced a proliferation of highly purified T cells in the presence of mitogen; this data was not surprising given the known accessory function of pulmonary macrophages from patients with interstitial lung diseases (ILDs) in mitogen assays. The blocking effects of CD80 and CD86 MoAbs on the accessory function of AM are represented. When added to the mitogen assay at the beginning of culture, anti-CD72, anti-CD80, and anti-CD86 MoAbs inhibited the proliferation of T cells; there were no statistically significant differences in the blocking activity of CD80 and CD86 MoAbs, as determined by thymidine incorporation. The inhibitory ability of a control MoAb was always less than 2%; as previously reported,3 anti–IL-15 antibodies inhibited the accessory function of AM (data not shown). These data indicate the functional importance of the expression of these costimulatory molecules by AM in inducing both CD4 and CD8 T-cell immune response within the alveolar spaces.
DISCUSSION
In this study we show that IL-15 upregulates the expression of the CD72/CD5 and CD80, CD86/CD28 counterreceptors, suggesting a role for IL-15 in the regulation of costimulatory molecules that favor the APC-T cell contact and the compartmentalization of the CTL response within tissues involved by HIV infection.
The lung is a site of compartmentalization of the host immune response against HIV. In fact, we previously reported that lung involvement in HIV-infected patients is associated with the intraalveolar accumulation of CD8+ T cells that are phenotypically different from those in the peripheral blood12 and sometimes numerous T cells are still observed locally whereas the patient is severely lymphopenic in the peripheral blood.9 Mononuclear phagocytes resident within the lung compartment are not bystander cells in this phenomenon, because they release a number of factors that favor the accumulation of T cells.1 In particular, recent evidence from our laboratory suggests that macrophage-derived IL-15 favors the local cell proliferation in extravascular tissues involved by HIV infection3; in addition, we found that IL-15 binding proteins are expressed by pulmonary macrophages of patients with HIV infection,3 but we did not verify whether IL-15 exerts its effects on cells of the mononuclear phagocyte system. In this paper we show that IL-15 is part of the matrix of cytokines that regulates the accessory activity of AM at sites of inflammatory lesions in the pulmonary microenvironment. The addition of exogenous IL-15 and IFN-γ increased the expression of CD80 and, in particular, CD86 on pulmonary macrophages. Furthermore, exposure to IL-15 and IFN-γ augmented the expression of CD28 on pulmonary CD8 T cells, which in our system were CD8 Tc cells expressing activation markers and variable levels of IFN-γ. In contrast, IL-15 did not influence the expression of the CD5/CD72 costimulatory pathway. This observation suggests that additional signals may be important in regulating the accessory function of AM.
The maturation and expansion of pre-CTL to virus specific CTL occurs following a complex sequence of cellular interactions and cytokine-mediated activation signals. After T-cell receptor (TCR)–mediated engagement, costimulatory signals provided by the CD28/B7 interaction induce CTL maturation.13 A number of cytokines have the potential to regulate the expression of B7/CD28 molecules. CD80 and CD86 are expressed on circulating monocytes after IFN-γ activation14,15 whereas IL-10 blocks the expression of both antigens on peritoneal macrophages and downregulates B7-2 expression on dendritic cells.16 On the other hand, IL-2, IL-4, TNF-α, and IFN-γ act synergistically to increase CD28 expression.17-19 The present study claims that IL-15 serves as a potent inducer of an alternative pathway that may regulate B7 expression and thus CTL activation and growth in HIV disease. The putative regulatory properties of IL-15 in other viral diseases characterized by a CTL response are currently under investigation in our lab.
As shown by Kanai et al,20 IL-15 supports the in vitro generation of effector HIV-env–specific CTL in the absence of active IL-2. Furthermore, anti–IL-15 MoAb are able to block the generation and function of virus-specific CTL, suggesting that IL-15 may per se drive the CTL response. In our study, pulmonary CD4+ T cells were less than 5% in all patients. Because lung CD4+ T cells are the cell source of IL-2, it is conceivable that other soluble factors released by AM may surrogate IL-2 in maintaining the T-cell alveolitis when the number of CD4 T cells drops. In particular, IL-15, augmenting the set of costimulatory molecules on APC and signaling CD8+ T cells to proliferate, might drive the expansion of virus-specific CTL in the absence of functional CD4+ T lymphocytes.
Infection with HIV results in a generalized immunosuppression. The functional and quantitative impairment of the Th1 cell population makes patients with acquired immunodeficiency syndrome (AIDS) susceptible to opportunistic infections in the respiratory tract. The fact that IL-15 may contribute to the activation and growth of effector T cells not only against HIV but also against other infectious agents21-23 suggests an intriguing therapeutic potential for this cytokine in sustaining the pulmonary immune system in advanced HIV disease. An optimal host response against opportunists depends on the local accumulation and activation of effector immunocompetent cells, two phenomena that in normal subjects are keenly regulated by IL-2–producing CD4+ Th1 cells. The ability of IL-15 to enhance CTL and natural killer activity and induce T-cell chemotaxis might restore the defective Th1-dependent antimicrobial immunity, ie, a major factor that improves the prognosis in patients with HIV infection and pulmonary complications.
Because the mechanisms triggering the IL-15 production in the lung are not known, a major direction for future research will rest on the definition of molecular events that control IL-15 message translation in the lung of HIV-infected patients. Pulmonary macrophages are the first cell type shown to be infected by HIV; in fact, the HIV genome has been shown in lung macrophages of HIV-infected patients from the early phases of the disease. Therefore, it might be that HIV or HIV proteins may per se enhance IL-15 secretion by pulmonary macrophages, as recently suggested for peripheral blood monocytes.24 An alternative hypothesis could be that the initial contact between macrophages with APC and HIV-specific effector pulmonary T cells represents the event which triggers AM to synthesize IL-15, as previously reported for dendritic cells.25 It is also proposed that codependence mechanisms between cytokines that are locally released during HIV disease could set the stage for the IL-15 hyperproduction. For instance, the T-cell–induced synthesis of IFN-γ and TNF-α could activate macrophages to synthesize IL-15, which in turn could induce IFN-γ and TNF-α production, thus generating a positive feedback loop. IL-10 is another cytokine that might be relevant in the control of IL-15 production, because recent data indicate that IL-10 increases IL-15 mRNA.26 In this regard, we showed that IL-15 stimulation increases IL-10 expression on AM (data not shown). Non HIV-dependent mechanisms are likely to be equally important in inducing the production of this mitogenic and chemotactic factor, notably in patients with opportunistic infections. Because infection by human intracellular pathogens induces IL-15 expression,21 it is conceivable that IL-15 serves as an alarm cytokine that is secreted after the infection of macrophages by the several intracellular microorganisms that colonize the lung during HIV disease. In this regard, further studies are needed to verify whether recall antigens from pathogens of the respiratory tract (such as Pneumocystis carinii) may favor IL-15–dependent proliferation of pulmonary T cells.
In conclusion, this study supports the hypothesis that IL-15 augments T-cell activation in the lung by upregulating the expression of costimulatory ligands on alveolar macrophages. It has been suggested that the process of activation of pulmonary immune competent cells, supported by locally released cytokines, may paradoxically contribute to the spreading of HIV.1 With the advent of triple drug therapy with two nucleoside analogue reverse transcriptase inhibitors and a protease inhibitor (highly active antiretroviral therapy, HAART) it has been possible to obtain an impressive drop in HIV burden and, in most patients, a downregulation of immune activation of peripheral blood CD8+ T cells.27 The BAL provides an opportunity to evaluate the direct effect of HAART therapy on tissues. In particular, studies should be planned to evaluate whether combined therapy, which may lead to a reduction of HIV load in the lung (our preliminary data), is associated with a down- regulation of cytokine expression, including IL-15, and a return to the normal CD8+ T-cell activation status in pulmonary tissue.
ACKNOWLEDGMENT
The authors thank their colleagues from the Departments of Infectious Diseases and Pulmonary Medicine of the Padua Hospital, in particular Drs P. Cadrobbi and A. Cipriani, who contributed to this project by allowing the study of their patients and by performing the bronchoscopies. We also wish to thank Biogen, Cambridge, MA, for providing rIL-2; Knoll AG/BASF, Ludwigshafen, FRG, for providing TNF- α; Dr A. Troutt from Immunex Co for providing recombinant IL-15 and anti–IL-15 M110 MoAb; and Mr Martin Donach for his help in the preparation of the manuscript.
Supported by a Grant from the Ministero della Sanità—Istituto Superiore della Sanità—Progetto AIDS 1997 (Rome, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal