Abstract
We recently demonstrated that 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of de novo cholesterol synthesis, was a potential mediator of the biological effects of retinoic acid on human neuroblastoma cells. The HMG-CoA reductase inhibitor, lovastatin, which is used extensively in the treatment of hypercholesterolemia, induced a potent apoptotic response in human neuroblastoma cells. This apoptotic response was triggered at lower concentrations and occurred more rapidly than had been previously reported in other tumor-derived cell lines, including breast and prostate carcinomas. Because of the increased sensitivity of neuroblastoma cells to lovastatin-induced apoptosis, we examined the effect of this agent on a variety of tumor cells, including leukemic cell lines and primary patient samples. Based on a variety of cytotoxicity and apoptosis assays, the 6 acute lymphocytic leukemia cell lines tested displayed a weak apoptotic response to lovastatin. In contrast, the majority of the acute myeloid leukemic cell lines (6/7) and primary cell cultures (13/22) showed significant sensitivity to lovastatin-induced apoptosis, similar to the neuroblastoma cell response. Of significance, in the acute myeloid leukemia, but not the acute lymphocytic leukemia cell lines, lovastatin-induced cytotoxicity was pronounced even at the physiological relevant concentrations of this agent. Therefore, our study suggests the evaluation of HMG-CoA reductase inhibitors as a therapeutic approach in the treatment of acute myeloid leukemia.
MEVALONATE IS A CRITICAL component of a complex biochemical pathway whose products are vital for a variety of key cellular functions, including membrane integrity, cell signaling, protein synthesis, and cell cycle progression.1 Regulation of mevalonate synthesis is complex, involving multiple feedback mechanisms in which the endproducts of this pathway can regulate the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of this metabolic pathway.1 The endproducts of the mevalonate pathway include sterols, especially cholesterol, involved in membrane structure and steroid production; ubiquinone, involved in electron transport; farnesyl and geranylgeranyl isoprenoids involved in covalent binding of proteins such as ras to membranes; dolichol, which is required for glycoprotein synthesis; and retinoic acid precursors.1 2 This diverse array of critical metabolic endproducts strongly suggests that physiological regulation of HMG-CoA reductase is essential for the maintenance of cellular homeostasis.
Lovastatin is a specific, nonreversible competitive inhibitor of HMG-CoA reductase, whose ability to block this critical metabolic pathway has led to its extensive clinical use as a treatment for hypercholesterolemia.3,4 Lovastatin is also a potent inducer of growth arrest at the G1/S boundary in a wide variety of normal and tumor-derived cell lines. This effect can be reversed by the addition of mevalonate,5 the immediate endproduct of the reaction catalyzed by HMG-CoA reductase. The ability of lovastatin to cause growth arrest and its reversibility has led to its use as a cell-synchronizing agent in vitro.1,5 Prolonged exposure of various tumor-derived cell lines to relatively high concentrations of lovastatin (30 to 100 μmol/L) can lead to cellular apoptosis.3,5-7 The growth arrest and apoptotic properties of lovastatin has led to its evaluation as a potential therapeutic agent in the treatment of cancers that included breast and prostate carcinomas.8 The therapeutic dose for treatment of hypercholesterolemia is approximately a 1 mg/kg/d oral dose that produces serum levels in the order of 0.1 μmol/L lovastatin.8,9 In the phase I study of lovastatin as a therapeutic,8 doses up to 25 mg/kg/d were generally well tolerated; however, at these doses, the achievable peak serum levels of lovastatin were only in the range of 0.1 to 3.92 μmol/L and, as such, had little effect in reducing tumor load.8 Therefore, lovastatin did not appear to be a viable therapeutic alternative in the treatment of human cancer.
In a previous study, we identified HMG-CoA reductase as a potential mediator of the biological effects of retinoic acid on human neuroblastoma cells.10 Retinoic acid, derived from mevalonate metabolites,2 is a potent cell-differentiating and growth-inhibitory agent during embryogenesis that has demonstrated efficacy in the prevention and therapy of specific cancers.11,12 Exposure of a variety of neuroblastoma cell lines to lovastatin induced extensive apoptosis10 that was triggered with relatively low concentrations (<10 μmol/L) and rapid kinetics, compared with other tumor-derived cell lines previously reported.3,5-7 Therefore, a re-evaluation of lovastatin to induce apoptosis in tumor derived cell lines was undertaken. We included a variety of retinoic acid responsive cancers that had not been adequately surveyed previously. In this study, we evaluated the apoptotic response of acute myeloid leukemic (AML) and acute lymphocytic leukemic (ALL) cells, retinoic acid responsive12-14 and nonresponsive13 leukemias, respectively, to lovastatin.
MATERIALS AND METHODS
Cell culture.
The ALL cell lines B1, C1, W1, G2, KK, and NGR were derived from primary patient tumors from children with pre-B ALL at the Hospital for Sick Children (Toronto, Ontario, Canada) as previously described.15 The AML cell lines OCI-AML-1, OCI-AML-2, OCI-AML-3, OCI-AML-4, and OCI-AML-5 were established from patients from the Ontario Cancer Institute (Toronto, Ontario, Canada).16For presentation purposes, the OCI designation has been omitted when referring to these cell lines in this study. The AML cell lines NB-4 and HL60 were kindly provided by Dr E.A. McCulloch (Ontario Cancer Institute). Primary cultures were derived from the peripheral blood of the AML patients with informed consent. The fresh leukemic samples tested in this study were drawn from consecutive patients presenting at the Ontario Cancer Institute/Princess Margaret Hospital in Toronto. Normal bone marrow and cord blood cells were obtained with informed consent for the use of this material for research purposes and kindly provided by Drs H. Messner (Ontario Cancer Institute) and J.E. Dick (The Hospital for Sick Children), respectively. Mononuclear cell fractions from patient material were obtained by Ficoll-Hypaque centrifugation as previously described17 and regularly consisted of 95% to 98% blasts as determined by morphological examination. The ALL cell lines AML-2, AML-3, NB-4, and HL60 were maintained in α-minimal essential media (α-MEM; Princess Margaret Hospital Media Services) supplemented with 10% fetal calf serum (Sigma, St Louis, MO). AML-1, AML-4, and AML-5 cell lines as well as the bone marrow and the leukemic primary cultures were maintained in α-MEM supplemented with 10% fetal calf serum and 10% 5637 conditioned media.16 Cells were cultured in liquid suspension and treated with 1 to 150 μmol/L lovastatin (generously provided by Merck Research Laboratories, Rahway, NJ; diluted from a 10 mmol/L stock in ethanol prepared as previously described5) and processed for 3-4,5-dimethylthiazolyl-2,2,5-diphenyl tetrazolium bromide (MTT), colony growth, flow cytometric, and electron microscopic analysis.
MTT assay.
In a 96-well flat-bottom plate (Nunc, Naperville, IL) approximately 10,000 cells/150 μL of cell suspension was used to seed each well. After 2 days of lovastatin treatment (0 to 150 μmol/L), 50 μL of a 5 mg/mL solution in phosphate-buffered saline of the MTT tetrazolium substrate (ICN, Toronto, Ontario, Canada) was added and incubated for 6 hours at 37°C. The resulting violet formazan precipitate was solubilized by the addition of 100 μL of a 0.01 mol/L HCl/10% sodium dodecyl sulfate (SDS; Sigma) solution overnight at 37°C.18 The plates were then analyzed on an SLT Labinstruments 340 ATTC enzyme-linked immunosorbent assay (ELISA) plate reader (SLT Labinstruments, Crailsheim, Germany) at 450 nm to determine the optical density of the samples.
Trypan blue exclusion assay.
A total of 5 × 105 leukemic cells in 1 mL of media were seeded in a 24-well plate (Falcon; Fisher Scientific, Mississauga, Ontario, Canada) and exposed to either solvent control or 1, 10, or 20 μmol/L lovastatin for up to 4 days in triplicate. Lovastatin was replenished after 2 days of treatment. Cell counts were evaluated using a 1:1 dilution of cell suspension in trypan blue (GIBCO-BRL, Mississauga, Ontario, Canada). Viable and nonviable cells were counted using a hemocytometer as trypan blue excluded or stained cells, respectively, as previously described.19
Colony growth assay.
Mononuclear cell cultures from normal bone marrow were either seeded at 106 cells/mL of cell suspension in a 24-well plate and treated for 2 days or plated directly in methylcult (Stem Cell Technologies, Vancouver, British Columbia, Canada) with 0 to 150 μmol/L lovastatin following the manufacturer’s instructions. Incubation in methylcult was for 14 days and aggregates of more than 50 cells were scored as colonies. AML primary cultures were seeded at 5 × 104 cells/150 μL of cell suspension to each well in a 96-well plate and treated for 2 days with 0 to 150 μmol/L lovastatin. The clonogenic assay used here has been well described.20 Briefly, approximately 2 × 104 of the lovastatin-treated cells were plated in 0.1 mL of growth medium containing 10% fetal calf serum, 10% 5637 conditioned medium, and 0.8% methylcellulose (GIBCO-BRL) in triplicate in a 96-well plate. Incubation was for 7 days and aggregates of more than 50 cells were scored as colonies.
Flow cytometry and electron microscopy.
Cell-cycle parameters were determined by flow cytometry using propidium iodide labeling of single cells. The method used was described previously.10,21 Single-cell suspensions were labeled with 50 μg/mL propidium iodide (Sigma), and approximately 106cells in 100 μL were analyzed by flow cytometry. Ten thousand cells were evaluated and the percentage of cells in pre-G1 phase was determined using the Modfit LT program (Verity Software House, Topsham, ME). Reduced glutathione (GSH) was measured using monobromobimane and mitochondrial membrane potential (MMP) was measured using the cyanine dye DiIC1(5).22 A combined labeling method using triple laser excitation was used as previously described22 to examine the associations between cellular GSH and MMP. Ultrathin sections of cultured cell pellets were cut and prepared for electron microscopy as previously described.10 21 Cultured cell pellets were fixed in phosphate-buffered 2% glutaraldehyde and 1% osmium tetroxide, dehydrated through acetone, and embedded in epon araldite.
RESULTS
Increased sensitivity of AML-derived cell lines to lovastatin.
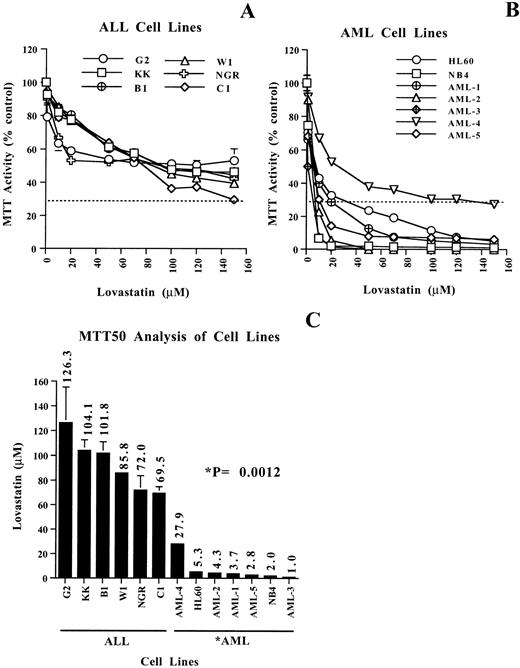
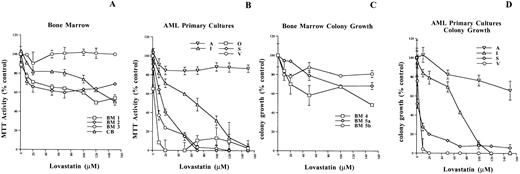
The potential growth-inhibitory and cytotoxic effects of targeting HMG-CoA reductase function using lovastatin in leukemia-derived cell lines was first evaluated using the MTT assay. The MTT assay is a measure of mitochondrial dehydrogenase activity in viable cells; this assay has been used extensively to evaluate the cytotoxic effects of chemotherapeutics on tumor cells in vitro.18 The 6 ALL and the 7 AML cell lines used in this study were treated with various concentrations of lovastatin ranging from 1 to 150 μmol/L for 2 days (Fig 1). In our previous work, this timepoint and the lovastatin concentrations given above clearly delineated the apoptotic sensitive neuroblastoma cells.10Whereas the ALL cell lines tested consistently displayed a gradual decline in MTT activity with increasing concentrations of lovastatin, the AML cell lines tested were significantly more sensitive to lovastatin as determined by MTT activity (compare Fig 1A and Fig 1B). An analysis of the concentration of lovastatin to induce a decrease in MTT activity by 50% (MTT50) clearly highlighted the different responses to lovastatin in these cell lines. The MTT50 of the ALL cell lines was in the range of 70 to 125 μmol/L lovastatin, whereas in the AML cell lines, the range was 1 to 28 μmol/L, a significant difference of P = .0012 (Fig 1C). In 6 of 7 AML cell lines, the MTT50 values obtained were less than 5.3 μmol/L lovastatin, indicating a greater than 10-fold increased sensitivity to this agent in comparison to the ALL cell lines. Clear demarcation between the ALL and the AML apoptotic response groups was distinguished by the latter showing a decrease of MTT activity to less than 30% (MTT30) under these experimental conditions (Fig 1A and B).
Evaluating the cytotoxic effects of lovastatin on leukemic cell lines using the MTT assay. (A and B) MTT enzyme activity, after exposure to 0 to 150 μmol/L lovastatin for 2 days, of 6 representative ALL and 7 AML cell lines, respectively. The dotted lines are at the level of MTT30. Results shown are the average of two independent experiments performed in quadruplicate, where the error bars represent the standard deviation of the mean. The values obtained were normalized to the solvent controls set at 100 for clarity of presentation. (C) Histogram showing the concentration of lovastatin required to achieve a decrease of MTT activity by 50% (MTT50) in the ALL and AML cell lines examined. MTT50 values for the ALL and AML cell lines were determined by regression analysis by the method of Chou-Talalay as previously described.49 The significant difference (P = .0012) in the MTT50 values between the ALL and AML cell lines was determined by the Wilcoxon test.
Evaluating the cytotoxic effects of lovastatin on leukemic cell lines using the MTT assay. (A and B) MTT enzyme activity, after exposure to 0 to 150 μmol/L lovastatin for 2 days, of 6 representative ALL and 7 AML cell lines, respectively. The dotted lines are at the level of MTT30. Results shown are the average of two independent experiments performed in quadruplicate, where the error bars represent the standard deviation of the mean. The values obtained were normalized to the solvent controls set at 100 for clarity of presentation. (C) Histogram showing the concentration of lovastatin required to achieve a decrease of MTT activity by 50% (MTT50) in the ALL and AML cell lines examined. MTT50 values for the ALL and AML cell lines were determined by regression analysis by the method of Chou-Talalay as previously described.49 The significant difference (P = .0012) in the MTT50 values between the ALL and AML cell lines was determined by the Wilcoxon test.
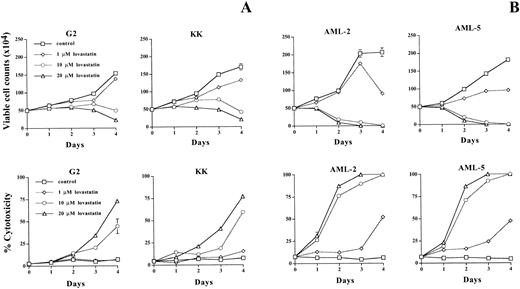
To determine if the decrease in MTT activity was due to lovastatin-induced cytotoxicity, we measured the effect of this agent on cell viability using trypan blue exclusion. Viable cells can exclude the dye trypan blue, whereas nonviable cells lose this ability, retain dye, and stain blue.19 Treatment of the ALL cell lines G2 and KK with either solvent control or 1, 10, or 20 μmol/L lovastatin displayed decreases in viable cell counts and an increase in cell cytotoxicity after prolonged exposure (3 to 4 days) to 10 and 20 μmol/L lovastatin (Fig 2A). In the AML cell lines, AML-2 and AML-5, these concentration of lovastatin had dramatic effects on cell viability with extensive cytotoxicity. The effect of this agent on cell counts and viability was more extensive and occurred more rapidly than within the ALL cell lines tested (Fig2B). Importantly, effects on cell viability were evident in the AML cell lines at 1 μmol/L lovastatin, at which, after 4 days of treatment, approximately 50% of the cells were nonviable (Fig 2B). Therefore, the AML cell lines were significantly more sensitive to lovastatin-induced cytotoxicity than the ALL cell lines.
The effect of lovastatin on leukemic cell viability determined by trypan blue exclusion. Five hundred thousand cells from two ALL cell lines KK and G2 (A) and two AML cell lines AML-2 and AML-5 (B) were seeded in triplicate and exposed to solvent control or 1, 10, or 20 μmol/L lovastatin for 4 days. The number of viable cells that can exclude the dye trypan blue and the percentage of nonviable cells that stained blue were evaluated daily by microscopy using a hemocytometer. Results shown are the average of the triplicate counts at each timepoint, where the error bars represent the standard deviation of the mean. This experiment was repeated with similar results.
The effect of lovastatin on leukemic cell viability determined by trypan blue exclusion. Five hundred thousand cells from two ALL cell lines KK and G2 (A) and two AML cell lines AML-2 and AML-5 (B) were seeded in triplicate and exposed to solvent control or 1, 10, or 20 μmol/L lovastatin for 4 days. The number of viable cells that can exclude the dye trypan blue and the percentage of nonviable cells that stained blue were evaluated daily by microscopy using a hemocytometer. Results shown are the average of the triplicate counts at each timepoint, where the error bars represent the standard deviation of the mean. This experiment was repeated with similar results.
Lovastatin-induced apoptosis of AML cell lines.
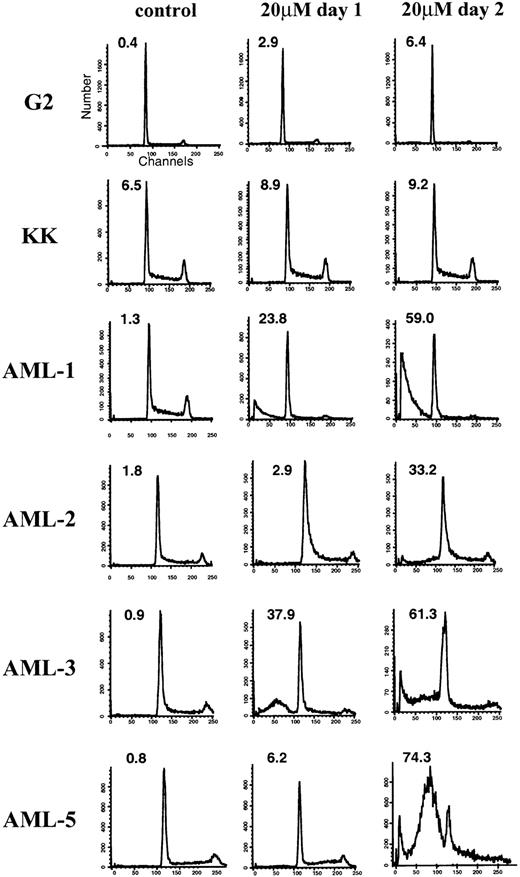
To better understand the mechanism of lovastatin-induced cytotoxicity, we further analyzed cells during the death process. To determine whether the decrease in MTT activity and the increase in cytotoxicity observed by trypan blue staining after exposure to lovastatin was due to an apoptotic response, flow cytometric, ultrastructural, and biochemical alterations characteristic of this response were evaluated. Cells undergoing apoptosis typically show a pre-G1 peak due to nuclear fragmentation.23 24 Flow cytometric analysis indicated that the G2 and KK ALL cell lines lacked significant evidence of apoptosis upon 20 μmol/L lovastatin exposure for 2 days (Fig 3). By contrast, under identical experimental conditions, the AML cell lines showed a significant apoptotic response highlighted by the presence of a prominent pre-G1 peak after lovastatin exposure (Fig 3).
Representative flow cytometric analysis of ALL (G2 and KK) and AML (AML-1, -2, -3, and -5) cell lines after exposure to lovastatin. The percentage of cells in the pre-G1 (apoptotic) fraction is shown in the upper left quadrant of the individual histograms. Cells were exposed to solvent control (left column), 20 μmol/L lovastatin treatment for 1 day (center column), or 20 μmol/L lovastatin treatment for 2 days (right column).
Representative flow cytometric analysis of ALL (G2 and KK) and AML (AML-1, -2, -3, and -5) cell lines after exposure to lovastatin. The percentage of cells in the pre-G1 (apoptotic) fraction is shown in the upper left quadrant of the individual histograms. Cells were exposed to solvent control (left column), 20 μmol/L lovastatin treatment for 1 day (center column), or 20 μmol/L lovastatin treatment for 2 days (right column).
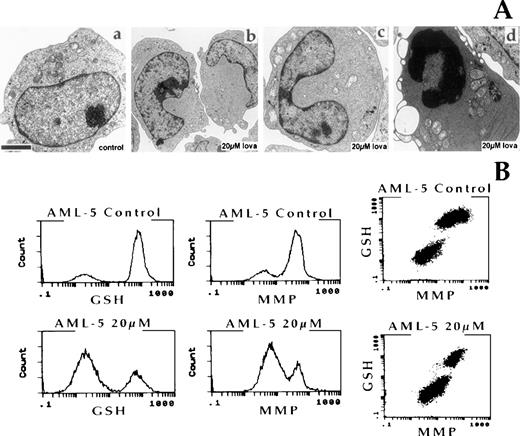
Ultrastructural features of apoptotic cell death include chromatin and cytoplasmic condensation, followed by nuclear and cellular fragmentation.25 Electron microscopic analysis of the effect of 20 μmol/L lovastatin for 2 days on AML blasts included an examination of the AML-5 cell line. Lovastatin-treated AML-5 cells displayed dramatic morphological changes to their nuclei, with a bilobed-indented nucleus present in cells before apoptosis. However, the majority of cells showed characteristic ultrastructural features of apoptosis, including nuclear and cytoplasmic condensation (Fig 4A).
(A) Ultrastructural features of the AML-5 cell line after 2 days of exposure to solvent control or 20 μmol/L lovastatin. (a) Control AML-5 cells; (b and c) lovastatin treatment-induced nuclear morphology changes in AML-5; (d) characteristic features of apoptosis were observed in the majority of lovastatin-treated AML-5 cells. The bar represents 1 μm. (B) Multilaser flow cytometric analysis of reduced GSH and MMP. AML-5 cells were exposed to solvent control or 20 μmol/L lovastatin for 2 days and analyzed. Dual-parameter dot plots of GSH and MMP for each sample are also presented.
(A) Ultrastructural features of the AML-5 cell line after 2 days of exposure to solvent control or 20 μmol/L lovastatin. (a) Control AML-5 cells; (b and c) lovastatin treatment-induced nuclear morphology changes in AML-5; (d) characteristic features of apoptosis were observed in the majority of lovastatin-treated AML-5 cells. The bar represents 1 μm. (B) Multilaser flow cytometric analysis of reduced GSH and MMP. AML-5 cells were exposed to solvent control or 20 μmol/L lovastatin for 2 days and analyzed. Dual-parameter dot plots of GSH and MMP for each sample are also presented.
In addition to the ultrastructural changes, several metabolic alterations also form part of the common phase of the apoptotic process.22,26-28 Apoptosis is typically accompanied by a depletion of intracellular reduced GSH, which lowers the capacity of cells to buffer against endogenous oxidants.26,28 In all cell types and in response to all inducers of apoptosis studied thus far, cells display a collapse of their MMP that precedes the nuclear signs of apoptosis.27 In this study, we used multilaser flow cytometry methods22 to analyze the cellular content of GSH and measurements of MMP coincident with lovastatin treatment. Analysis was limited to viable cells that retained surface membrane integrity identified by their ability to exclude the membrane impermeable fluorescent DNA stain propidium iodide. AML-5 cells exposed to 20 μmol/L lovastatin for 2 days displayed significant reductions in both GSH levels and MMP on exposure to lovastatin (Fig 4B). The dual-parameter dot plots of GSH content versus MMP clearly demonstrated that, in the responsive blast cells, lovastatin-induced GSH depletion occurred in the same population of cells showing a decrease in MMP (Fig4B). Similar results were demonstrated in the AML-2 and AML-3 cell lines; however, no changes in GSH or MMP levels were observed in the KK ALL cell line treated with lovastatin under the same experimental conditions (data not shown). Thus, lovastatin induced the flow cytometric, ultrastructural, and metabolic alterations characteristic of apoptosis in AML cell lines.
Lovastatin targets primary AML cultures.
After noting the dramatic response of lovastatin on cell viability in the AML cell lines, we next evaluated its effect on normal bone marrow as well as primary leukemic cells from a variety of AML patients. The responsiveness of this primary patient material to lovastatin-induced cytotoxicity was first evaluated by MTT analysis. Sensitivity to lovastatin-induced cytotoxicity was evaluated as nonresponsive, weak, and sensitive using the MTT50 and MTT30 values obtained after 2 days of exposure. Based on the responsiveness of the cell lines to lovastatin-induced cytotoxicity, the primary patient samples showing both MTT50 and MTT30 values greater than 100 μmol/L were considered nonresponsive and samples with an MTT50 less than 100 μmol/L and an MTT30 greater than 100 μmol/L lovastatin showed a weak response, whereas primary patient samples with both MTT50 and MTT30 values less than 100 μmol/L were considered sensitive. The effects of HMG-CoA reductase inhibitors, including lovastatin, have been evaluated extensively both in vitro and in vivo and have shown negligible effects on normal bone marrow progenitors and hematopoiesis.8 29Indeed, MTT analysis of three bone marrow samples and one from cord blood demonstrated that normal hematopoietic cells do not undergo significant cytotoxicity and were nonresponsive to lovastatin (Fig 5A). However, the MTT analysis of 22 AML patient samples (Table 1) indicated that 2 were nonresponsive, 7 showed a weak response, and 13 were sensitive to lovastatin-induced cytotoxicity (Fig 5B and Table 1). Age, gender, French-American-British (FAB) subtype, or total white blood cell and blast counts in peripheral blood at presentation were not predictive of lovastatin responsiveness in these AML primary cell cultures (Table 1).
The effect of lovastatin on primary cell cultures. (A and B) MTT enzyme activity after exposure to 0 to 150 μmol/L lovastatin for 2 days in bone marrow and cord blood samples as well as a representative sampling of the 22 AML primary cultures analyzed (see Table 1), respectively. The results are the average of six replicates of a single experiment, where the error bars represent the standard deviation of the mean. The MTT activity values were normalized to the solvent controls for each sample and set at 100 for clarity of presentation. (C) Colony growth potential of bone marrow progenitor cells after exposure to 0 to 150 μmol/L lovastatin for 2 days in vitro (BM 4 and BM 5a) or added to the methylcellulose at time of plating (BM 5b) and incubation for 14 days; colonies were scored where the values represent the mean of duplicate readings and error bars as the standard deviation of the mean. (D) Colony growth potential of various AML primary cultures after exposure to 0 to 150 μmol/L lovastatin for 2 days; cells were then plated in methylcellulose and incubated for 7 days. Blast colonies of greater than 50 cells were scored and the values represent the mean of triplicate readings, where the error bars are the standard deviation of the mean.
The effect of lovastatin on primary cell cultures. (A and B) MTT enzyme activity after exposure to 0 to 150 μmol/L lovastatin for 2 days in bone marrow and cord blood samples as well as a representative sampling of the 22 AML primary cultures analyzed (see Table 1), respectively. The results are the average of six replicates of a single experiment, where the error bars represent the standard deviation of the mean. The MTT activity values were normalized to the solvent controls for each sample and set at 100 for clarity of presentation. (C) Colony growth potential of bone marrow progenitor cells after exposure to 0 to 150 μmol/L lovastatin for 2 days in vitro (BM 4 and BM 5a) or added to the methylcellulose at time of plating (BM 5b) and incubation for 14 days; colonies were scored where the values represent the mean of duplicate readings and error bars as the standard deviation of the mean. (D) Colony growth potential of various AML primary cultures after exposure to 0 to 150 μmol/L lovastatin for 2 days; cells were then plated in methylcellulose and incubated for 7 days. Blast colonies of greater than 50 cells were scored and the values represent the mean of triplicate readings, where the error bars are the standard deviation of the mean.
Sensitivity of AML Primary Cultures to Lovastatin-Induced Cytotoxicity
| Patient . | UPN . | Source of Cells . | Age (yr)/ Sex . | WBC Count (×109/L) . | Blast Count (×109/L) . | FAB . | MTT 50 μmol/L Lovastatin . | MTT 30 μmol/L Lovastatin . |
|---|---|---|---|---|---|---|---|---|
| Nonresponsive | ||||||||
| A | 1054 | Cryo | 48/M | 71.3 | 10.0 | M4 | >150 | >150 |
| B | 1270 | Fresh | 60/F | 9.3 | 6.1 | M4 | 148 | >150 |
| Weak | ||||||||
| C | 683 | Cryo | 48/M | 97.0 | 34.9 | M4 | 26 | 106 |
| D | 1350 | Fresh | 73/M | 11.7 | 6.7 | M1 | 63 | 105 |
| E | 733 | Cryo | 65/M | 95.0 | 14.3 | M4 | 105 | 145 |
| F | 881 | Fresh | 59/F | 234.0 | 63.2 | M4 | 69 | 150 |
| G | 870 | Cryo | 38/F | 42.5 | 36.5 | M3 | 61 | >150 |
| H | 1475 | Fresh | 45/F | 6.7 | ND | M2 | 38 | >150 |
| I | 1333 | Fresh | 19/M | 99.6 | 44.9 | M2 | 69 | 103 |
| Sensitive | ||||||||
| J | 473 | Fresh | 36/F | 25.7 | 5.1 | M2 | 15 | 44 |
| K | 355 | Cryo | 70/F | 44.0 | 37.0 | M2 | 14 | 26 |
| L | 1234 | Fresh | 70/F | 37.0 | 5.0 | M4 | 32 | 62 |
| M | 1083 | Fresh | 81/M | 20.4 | 17.0 | M1 | 39 | 64 |
| N | 1352 | Fresh | 65/F | 8.1 | 4.9 | M2 | 9 | 90 |
| O | 841 | Cryo | 32/M | 143.5 | 140.6 | M4 | 3 | 6 |
| P | 328 | Cryo | 16/F | 301.0 | 273.9 | M4 | 20 | 56 |
| Q | 1373 | Fresh | 75/F | 146.0 | 93.7 | M4 | 8 | 20 |
| R | 805 | Cryo | 71/F | 51.6 | 35.1 | M5 | 22 | 84 |
| S | 1344 | Fresh | 45/F | 41.7 | 37.7 | M2 | 16 | 33 |
| T | 1119 | Cryo | 71/M | 208.5 | 204.3 | M1 | 8 | 27 |
| U | 1316 | Fresh | 36/M | 5.6 | 2.2 | M5a | 6 | 50 |
| V | 913 | Fresh | 48/F | 12.2 | 7.4 | M2 | 7 | 15 |
| Patient . | UPN . | Source of Cells . | Age (yr)/ Sex . | WBC Count (×109/L) . | Blast Count (×109/L) . | FAB . | MTT 50 μmol/L Lovastatin . | MTT 30 μmol/L Lovastatin . |
|---|---|---|---|---|---|---|---|---|
| Nonresponsive | ||||||||
| A | 1054 | Cryo | 48/M | 71.3 | 10.0 | M4 | >150 | >150 |
| B | 1270 | Fresh | 60/F | 9.3 | 6.1 | M4 | 148 | >150 |
| Weak | ||||||||
| C | 683 | Cryo | 48/M | 97.0 | 34.9 | M4 | 26 | 106 |
| D | 1350 | Fresh | 73/M | 11.7 | 6.7 | M1 | 63 | 105 |
| E | 733 | Cryo | 65/M | 95.0 | 14.3 | M4 | 105 | 145 |
| F | 881 | Fresh | 59/F | 234.0 | 63.2 | M4 | 69 | 150 |
| G | 870 | Cryo | 38/F | 42.5 | 36.5 | M3 | 61 | >150 |
| H | 1475 | Fresh | 45/F | 6.7 | ND | M2 | 38 | >150 |
| I | 1333 | Fresh | 19/M | 99.6 | 44.9 | M2 | 69 | 103 |
| Sensitive | ||||||||
| J | 473 | Fresh | 36/F | 25.7 | 5.1 | M2 | 15 | 44 |
| K | 355 | Cryo | 70/F | 44.0 | 37.0 | M2 | 14 | 26 |
| L | 1234 | Fresh | 70/F | 37.0 | 5.0 | M4 | 32 | 62 |
| M | 1083 | Fresh | 81/M | 20.4 | 17.0 | M1 | 39 | 64 |
| N | 1352 | Fresh | 65/F | 8.1 | 4.9 | M2 | 9 | 90 |
| O | 841 | Cryo | 32/M | 143.5 | 140.6 | M4 | 3 | 6 |
| P | 328 | Cryo | 16/F | 301.0 | 273.9 | M4 | 20 | 56 |
| Q | 1373 | Fresh | 75/F | 146.0 | 93.7 | M4 | 8 | 20 |
| R | 805 | Cryo | 71/F | 51.6 | 35.1 | M5 | 22 | 84 |
| S | 1344 | Fresh | 45/F | 41.7 | 37.7 | M2 | 16 | 33 |
| T | 1119 | Cryo | 71/M | 208.5 | 204.3 | M1 | 8 | 27 |
| U | 1316 | Fresh | 36/M | 5.6 | 2.2 | M5a | 6 | 50 |
| V | 913 | Fresh | 48/F | 12.2 | 7.4 | M2 | 7 | 15 |
Age, WBC, and blast counts in peripheral blood at registration.
Abbreviations: UPN, unique patient number; Cryo, cryopreserved; WBC, white blood cell; ND, not detected.
The ability of leukemic cells to grow and form colonies in a semisolid media such as methylcellulose is an indicator of their growth and malignant potential.17,29 Using this approach, previous studies have demonstrated that HMG-CoA reductase inhibitors, including lovastatin, can significantly affect the colony-forming ability of AMLs with minimal effects on normal bone marrow progenitor cells29 30; however, this effect was attributed to growth arrest of the leukemic blasts. In line with these previous reports, lovastatin had minimal effects on the colony-forming ability of normal bone marrow progenitor cells when treated for 2 days in vitro (Fig 5C, BM 4 and BM 5a) or when plated directly (Fig 5C, BM 5b) in the methylcellulose with 0 to 150 μmol/L lovastatin. A representative sampling of the AML primary cultures was also treated for 2 days with the same concentrations of lovastatin used in the MTT analysis and subsequently plated in methylcellulose. Of the 12 samples tested, the 4 that demonstrated a plating efficiency of greater than 10% are presented (Fig 5D). The colony growth potential of these AML primary cultures paralleled their responsiveness to lovastatin-induced cytotoxicity as determined by MTT analysis. For example, the nonresponsive AML primary sample A showed a marginal decrease in colony-forming ability and the intermediate responsive patient I displayed a partial response, whereas the sensitive primary cultures S and V dramatically reduced their colony-forming potential (compare Fig5B to 5D). Thus, in the majority of AML patient samples tested, a significant lovastatin-induced cytotoxic response was observed.
Analysis of the apoptotic response of lovastatin in AML primary samples.
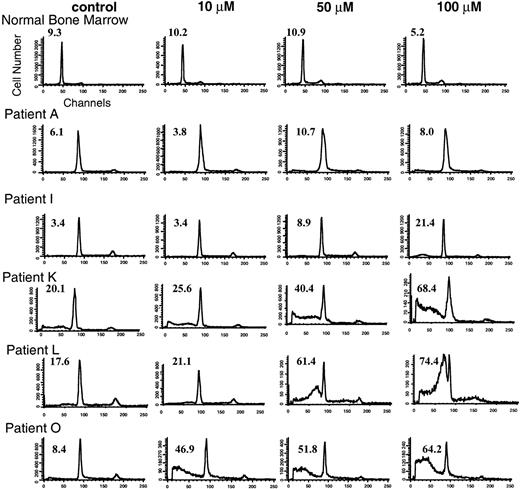
Similar to its effect on the cell lines, the potential apoptotic response in the primary samples after lovastatin exposure was investigated by analyzing characteristic flow cytometric, ultrastructural, and biochemical changes. The primary cultures were exposed to ethanol control or to low (10 μmol/L), intermediate (50 μmol/L), or high (100 μmol/L) concentrations of lovastatin for 2 days and then analyzed for the appearance of a pre-G1 peak indicative of cells undergoing nuclear fragmentation.23 24 Lovastatin did not induce significant apoptosis in normal bone marrow cells and the leukemic cells of two patients (A and B). Flow cytometric analysis of one of the bone marrow samples and patient A are representative and are shown in Fig 6. By comparison, the weak responding patient samples as determined by MTT analysis displayed weak apoptotic responses only at the highest concentration of lovastatin used (patients C through I), with patient I shown in Fig 6. Finally, patient samples J through V showed a dramatic induction of apoptosis visualized by the presence of an abundant pre-G1 peak (representative patient samples K, L, and O are shown in Fig 6).
Flow cytometric analysis of normal bone marrow and AML primary cultures exposed to solvent control or to low (10 μmol/L), intermediate (50 μmol/L), and high (100 μmol/L) concentrations of lovastatin for 2 days. Representative primary cultures from each of the three lovastatin response groups are shown, including a nonresponsive sample A, an intermediate responsive sample I, and sensitive samples K, L, and O. The percentage of cells in the pre-G1 (apoptotic) fraction of the cell cycle is shown in the upper left corner of the individual histograms.
Flow cytometric analysis of normal bone marrow and AML primary cultures exposed to solvent control or to low (10 μmol/L), intermediate (50 μmol/L), and high (100 μmol/L) concentrations of lovastatin for 2 days. Representative primary cultures from each of the three lovastatin response groups are shown, including a nonresponsive sample A, an intermediate responsive sample I, and sensitive samples K, L, and O. The percentage of cells in the pre-G1 (apoptotic) fraction of the cell cycle is shown in the upper left corner of the individual histograms.
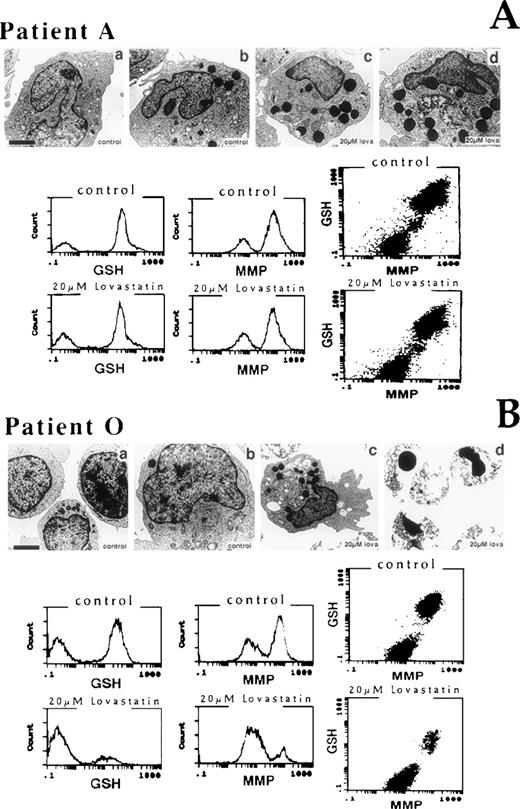
Electron microscopic and biochemical analyses of the effect of 20 μmol/L lovastatin for 2 days on the AML primaries included an examination of a nonresponsive (patient A, Fig 7A) and a sensitive (patient O, Fig 7B) primary culture. In the AML primary samples, the most striking effect of lovastatin in both the nonresponsive and the sensitive primary cultures examined was the presence of numerous, large lysosomal or granular structures within the cytoplasm of these cells. In the ethanol-treated control cells, only a small proportion of cells possessed these structures. Ultrastructural features of apoptosis were not evident in the nonresponsive sample but constituted a prominent feature of the sensitive primary sample examined. Primary cells from this nonresponsive patient (patient A) exposed to 20 μmol/L lovastatin for 2 days showed no significant changes to GSH or MMP measurements compared with ethanol-treated controls. By contrast, primary cells from the sensitive patient (patient O) displayed dramatic reduction in both GSH levels and MMP on exposure to lovastatin. As shown in the AML-5 cell line, the dual-parameter dot plots of GSH content versus MMP clearly demonstrated that, in the sensitive blast cells, lovastatin-induced GSH depletion occurred in the same population of cells showing a decrease in MMP. Thus, as demonstrated in the cell lines examined, lovastatin-induced cytotoxicity possessed the flow cytometric, ultrastructural, and metabolic alterations typical of apoptosis in sensitive AML blasts.
Ultrastructural and biochemical features of AML leukemic blast cells after 2 days of exposure to solvent control or lovastatin. (A) A nonresponsive primary sample (patient A) and (B) a sensitive primary sample (patient O) were evaluated. (A) (a and b) Control blast cells; (c and d) 20 μmol/L lovastatin-treated blast cells from patient A displayed a number of large lysosomal granules. (B) (a and b) Control blast cells; (c) 20 μmol/L lovastatin-treated blast cells from patient O also displayed a number of large lysosomal granules in the few surviving cells; (d) 20 μmol/L lovastatin induced a prominent apoptotic response in patient O. The bar represents 1 μm except in (B) (b), where it represents 3 μm. Multilaser flow cytometric analysis of reduced GSH and MMP in the AML primary cultures A and O exposed to 20 μmol/L lovastatin for 2 days and analyzed. Dual-parameter dot plots of GSH and MMP for each sample are also presented.
Ultrastructural and biochemical features of AML leukemic blast cells after 2 days of exposure to solvent control or lovastatin. (A) A nonresponsive primary sample (patient A) and (B) a sensitive primary sample (patient O) were evaluated. (A) (a and b) Control blast cells; (c and d) 20 μmol/L lovastatin-treated blast cells from patient A displayed a number of large lysosomal granules. (B) (a and b) Control blast cells; (c) 20 μmol/L lovastatin-treated blast cells from patient O also displayed a number of large lysosomal granules in the few surviving cells; (d) 20 μmol/L lovastatin induced a prominent apoptotic response in patient O. The bar represents 1 μm except in (B) (b), where it represents 3 μm. Multilaser flow cytometric analysis of reduced GSH and MMP in the AML primary cultures A and O exposed to 20 μmol/L lovastatin for 2 days and analyzed. Dual-parameter dot plots of GSH and MMP for each sample are also presented.
DISCUSSION
Present therapeutic regimens for the treatment of patients with AML are toxic and often ineffective.31 Despite initial favorable responses to a variety of chemotherapeutics, most patients relapse, develop drug resistance, and quickly succumb to their disease.14,31-33 Clearly, novel therapeutic approaches are urgently needed. In this study, we demonstrated that the targeting of HMG-CoA reductase, the rate-limiting enzyme of de novo cholesterol synthesis,1 represents a potential novel therapeutic approach in the treatment and control of AML. Inhibition of enzyme function with lovastatin induced a significant apoptotic response in the majority of the AML samples tested. Apoptosis is a well-defined mechanism of programmed cell death that is distinguished by characteristic morphological and metabolic features.25-27Lovastatin treatment of sensitive AML cells demonstrated these hallmarks of apoptosis, including nuclear and cytoplasmic condensation and fragmentation, depletion of GSH, and a decrease in MMP. Serum levels of approximately 4 μmol/L are achievable with oral administration of lovastatin.8 This concentration in vitro significantly affected cell viability of the majority of AML blast cells tested but had little effect on ALL blasts or normal bone marrow cells, indicating specificity in response to this agent.
We have now identified two retinoic acid-responsive tumors, neuroblastoma10 and AML, that demonstrate increased sensitivity to lovastatin-induced apoptosis. The mechanism by which lovastatin triggers apoptosis and the determinants of tumor cell sensitivity and specificity to this response remains unknown. Lovastatin’s ability to suppress proliferation is thought to be mediated by its ability to block receptor signaling, particularly from the insulin-like growth factor-I and the platelet-derived growth factor receptors.34-37 Because mitogenic receptors such as insulin-like growth factor-1 are also mediators of cell survival,38,39 lovastatin-induced apoptosis may result from abrogation of cell survival signals. The dependency of cells for specific survival factors is tissue and developmental stage restricted.40 This phenomenon may account for the tumor specificity of lovastatin-induced apoptosis observed in our studies. Further work is ongoing to delineate the mechanism of action of lovastatin-induced apoptosis. Whether the retinoic acid responsiveness documented in the lovastatin-sensitive tumor types is a phenotypic marker or mechanistically linked to the mevalonate pathway remains to be elucidated.
Understanding the mechanism of lovastatin-induced apoptosis in the sensitive tumor types will likely require an evaluation of the potential roles of the various endproducts of the mevalonate pathway. Because lovastatin inhibits HMG-CoA reductase, critical mevalonate metabolites such as farnesyl and geranylgeranyl isoprenoids, dolichol, ubiquinone, and cholesterol are reduced,1,41 possibly contributing to lovastatin-induced apoptosis. The addition of these endproducts or other inhibitors further along this pathway that may modulate the apoptotic response of lovastatin may delineate its mechanism of action. For example, the mevalonate metabolite dolichol, involved in N-linked glycosylation,1 plays a role in the translocation of cell survival factor receptors to the cell membrane.34,35 In fact, treatment with lovastatin can result in the diminished trafficking of a number of receptors to the cell surface.34,35 Furthermore, essential regulatory proteins such as ras are dependent on isoprenylation for their proper localization to cellular membranes and their function.1,41Ras has been demonstrated to be a key effector of mitogenic and cell survival stimuli,42 with constitutive activation of ras through mutation as a common feature of many human cancers.43 As such, targeting of ras isoprenylation through farnesyl transferase inhibitors has been evaluated as a potential therapeutic target.41,44 The growth arrest properties of lovastatin have been shown to be independent of ras mutational status.45 Similarly, the extent of ras mutations are approximately equal in AML and ALL samples46,47; yet, our work shows that AML, but not ALL, is sensitive to lovastatin-induced apoptosis. However, because the role of ras in mediating cell survival signals is an independent signaling pathway to its mitogenic effects,43 48 a formal evaluation of ras family of signaling molecules and their downstream targets in the apoptotic response triggered by lovastatin is required.
Our work suggests that lovastatin has potential as an immediate, novel therapeutic approach in the treatment of AML. First, lovastatin can induce a specific apoptotic response in AML blast cells within its therapeutic range. Second, it has a proven record in the clinic as a safe and effective drug.3,4,8 The well-documented potent growth arrest property of HMG-CoA reductase inhibitors5 has led to their evaluation as potential chemotherapeutics in human cancers.8 In a phase I clinical trial of lovastatin, this inhibitor displayed minimal adverse side effects at high doses; however, lovastatin had little effect in reducing tumor-load in these patients.8 The therapeutic potential of lovastatin on the apoptotic sensitive neuroblastoma10 and AML cells identified in our studies were not evaluated in this phase I trial. HMG-CoA reductase inhibitors have been used extensively in the treatment of hypercholesterolemia and as a result have well-defined pharmacokinetics.3,4,8,9 There is no evidence of acquired resistance to HMG-CoA reductase inhibitors with respect to their ability to lower plasma cholesterol levels, even with extended use.3,4 9 The tumor-specific apoptosis induction as well as the biological properties of the HMG-CoA reductase inhibitors suggests that they are potentially ideal therapeutic agents in AML. The efficacy of lovastatin alone or in combination with other therapeutics in the treatment of AML can be evaluated in clinical trials.
ACKNOWLEDGMENT
The authors are grateful to Dr S. Minkin for the statistical analysis;to Dr J.E. Dick and the Penn lab for critically reviewing this manuscript; to Dr J. Squire, A. Pandita, and J. Bayani for helpful discussions; to J. Kao, N. Jamal, J. Sheldon, and J. Hwang for technical assistance; and to Drs H. Messner and J.E. Dick for providing the bone marrow and cord blood cells used in this study, respectively. Our thanks is extended to Merck and Frost (Montreal, Quebec, Canada) for generously supplying the lovastatin used in this study.
Supported by funds from The Medical Research Council of Canada (L.Z.P.) as well as a joint industry grant from MRC/Apotex Inc (L.Z.P.). Fellowship support from OCI/Amgen (J.D.) is gratefully acknowledged.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Linda Z. Penn, PhD, Department of Molecular and Cellular Biology, Ontario Cancer Institute, the Princess Margaret Hospital, 610 University Ave, Toronto, Ontario, Canada, M5G 2M9.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal