Abstract
The spleen has two main functions. The first is to provide a proper microenvironment to lymphoid and myeloid cells, whereas the second involves clearance of abnormal erythrocytes. Ad4BP/SF-1, a product of the mammalian FTZ-F1 gene (mFTZ-F1), was originally identified as a steroidogenic, tissue-specific transcription factor. Immunohistochemical examination of the mammalian spleens confirmed the expression of Ad4BP/SF-1 in endothelial cells of the splenic venous sinuses and pulp vein. In mFtz-F1 gene–disrupted (KO) mice, several structural abnormalities were detected in the spleen, including underdevelopment and nonuniform distribution of erythrocytes. Examination of the spleen of KO fetuses showed failure of development of certain tubular structures during embryogenesis. These structures are normally assembled by Ad4BP/SF-1 immunoreactive cells, and most likely form the vascular system during later stages of development. Other structural abnormalities in the spleen of the KO mice included defects in the tissue distribution of type-IV collagen, laminin, c-kit, and vimentin. These morphologic defects in the vascular system were associated with a decrease in the proportion of hematopoietic cells, although differentiation of these cells was not affected significantly. A high number of abnormal red blood cells containing Howell-Jolly bodies were noted in the KO mice, indicating impaired clearance by the splenic vascular system. We also detected the presence of an mRNA-encoding cholesterol side-chain cleavage P450 in the spleen, resembling the findings in steroidogenic tissues such as the gonads and adrenal cortex. The mRNA transcript was not involved in splenic structural defects as it was detected in the spleens of both normal and KO mice, indicating that the regulatory mechanism of theP450 gene in the spleen is different from that in steroidogenic tissues. Our results indicate that a lack of the mFtz-F1 gene in mice is associated with structural and functional abnormalities of the splenic vascular system.
Ad4BP/SF-1, A MEMBER of the nuclear receptor family, was originally identified as a steroidogenic, tissue-specific transcription factor implicated in the expression of steroidogenic CYP genes encoding cytochrome P450s.1,2 Expression of Ad4BP/SF-1 correlates with the distribution of P450s in steroidogenic tissues such as gonads and adrenal cortex.3-5 In addition, Ad4BP/SF-1 immunoreactive cells are also present in nonsteroidogenic cells such as the pituitary gonadotrophs and ventromedial hypothalamic neurons.6-8These two cell types are involved in the establishment and maintenance of reproductive function by displaying a coordinated function with the gonads.9
Recent studies of genes downstream of Ad4BP/SF-1 have shown that several genes are under the control of Ad4BP/SF-1. These include the 3β-hydroxysteroid dehydrogenase gene,10 the steroidogenic acute regulatory protein gene,11,12 the prolactin receptor gene,13 the adrenocorticotropin receptor gene,14 the oxytocin gene,15 the Müllerian inhibitory substance gene,16 the gonadotropin gene,17-20 and the Leydig insulin-like gene.21 These genes are closely related to functions specific for the corresponding tissues. In addition to the functions identified in the studies mentioned, gene disruption studies of the mammalian Ftz-F1 (mFtz-F1)-encoding Ad4BP/SF-1 indicated an additional role for the transcription factor by showing that both gonads and the adrenal gland are absent in mFtz-F1gene–disrupted mice.8,22 23 Therefore, it is assumed that Ad4BP/SF-1 also plays an important role in the differentiation and development of steroidogenic tissues, although the precise mechanism remains to be clarified.
In addition to the above functionally or developmentally related tissues, recent studies using reverse transcription-polymerase chain reaction (RT-PCR) or in situ hybridization also detected themFtz-F1 gene transcript in the nonsteroidogenic spleen.24,25 The spleen of adult animals consists of two major parts, the red pulp and white pulp. The former contains a large number of erythrocytes, whereas the latter contains tightly packed lymphatic cells.26 In the white pulp, lymphopoiesis occurs from immature lymphoid cells analogous to lymph nodes, whereas in the red pulp, abnormal erythrocytes are thought to be eliminated when they pass through the meshwork structure of the splenic vasculature. In addition, the spleen transiently exhibits erythropoietic activity similar to the liver during fetal life.
The spleen has a unique vascular system composed of venous sinuses as well as a network of arteries and veins. Blood enters through splenic arteries that branch into trabecular arteries. These run into the white pulps making further branches as the central arteries. Some of fine branches from the central arteries run out of the white pulps and terminate in the surrounding marginal zone or in the red pulp. After flowing into the splenic cord, blood is collected in the venous sinus and thereafter runs directly into the pulp vein. Finally, the pulp veins coalesce forming the trabecular veins. Because the lymphopoietic and erythropoietic cells originate from their stem cells, which are not intrinsic splenic cells, they are transferred through the vascular system to settle into the spleen. Therefore, with respect to hematopoiesis in the spleen, an inherent function of the tissue is to provide a proper microenvironment for the settlement and differentiation of different blood cells.
In the present study, we examined the expression of Ad4BP/SF-1 in the spleen of normal and mFtz-F1 gene–disrupted mice. Our results showed that the gene product, Ad4BP/SF-1, is implicated in differentiation of splenic architecture and functions.
MATERIALS AND METHODS
Immunoblot analysis.
Spleens were isolated from the E16.5 mouse fetuses, newborn and adult mice, and then lysed with 50 mmol/L Tris-HCl (pH 7.5) containing 2% sodium dodecyl sulfate (SDS). This was followed by sonication to disrupt viscous cellular DNA. Total cellular proteins (25 μg) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Samples of total tissue lysates prepared from the adrenal glands of the adult mice were used as control. The procedure for immunoblotting using a rabbit antiserum to bovine Ad4BP/SF-1 was described previously.5 27 Electrochemiluminescence (ECL) Western blot reagents (Amersham, Arlington Height, IL) were used for the detection.
Immunohistochemical analysis.
For immunohistochemical analysis, the spleens were fixed with 4% paraformaldehyde-0.1 mol/L potassium phosphate (pH 7.0), dehydrated, and embedded in paraffin. To remove blood cells, the spleens of the adult animals were perfused, then fixed by using an arterial and venous pressure-loading perfusion fixation method that was originally developed for preparation of the specimen for scanning electron microscopy, as described previously.28,29 The human spleen was washed with phosphate-buffered saline (PBS) to remove red blood cells before fixation. Sections were prepared for immunohistochemical staining using the antiserum to Ad4BP/SF-1.5,30 For detection of laminin, type-IV collagen, c-kit, and vimentin, frozen sections of newborn mice spleens were fixed with acetone/methanol (1:1) at −20°C. A rat monoclonal antibody to mouse laminin and a rabbit polyclonal antibody to mouse type-IV collagen were purchased from Chemicon International Inc (Temecula, CA). An anti–c-kit monoclonal antibody (ACK2) was kindly provided by Dr Nishikawa (Kyoto University, Kyoto, Japan).31 Mouse monoclonal antibody to bovine vimentin was purchased from Boehringer Mannheim (Mannheim, Germany). Antibody-antigen complexes were detected by the streptavidin-biotin-peroxidase or -alkaline phosphatase method using the Histofine kit (Nichirei Co, Tokyo, Japan).
Characterization of myeloid and lymphoid cells.
Single-cell suspensions were prepared from spleens of newborn mice. For this purpose, splenocytes were stained with fluorescein dye-labeled monoclonal antibodies for 30 minutes on ice in PBS containing 2% fetal calf serum and 0.05% sodium azide. The cells were washed with PBS and analyzed with FACScan cytometer using the Lysis II program (Becton Dickinson, Mountain View, CA). The monoclonal antibodies used for cell staining were phycoerythrin (PE)-conjugated anti-CD4 (Pharmingen, San Diego, CA), fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (Pharmingen), FITC-conjugated anti-CD3 (Pharmingen), PE-conjugated anti-B220 (GIBCO-BRL, Gaithersburg, MD), and biotin–anti-Mac1 in conjunction with streptavidin-Red670 (GIBCO-BRL).
The number of erythrocytes containing Howell-Jolly bodies was determined by the method described by Higashikuni et al.32In brief, blood samples obtained from newborn mice were placed on a glass slide coated with acridine orange. Howell-Jolly bodies were detected under a fluorescence microscope. At least 1,000 erythrocytes were examined in each specimen.
RT-PCR.
Complementary DNAs (cDNAs) were obtained by reverse-transcription using total RNAs (1 μg) prepared from the spleens of newborn and adult mice. One-twentieth of the cDNAs was subjected to amplification of the Ad4BP/SF-1, Cyp11A, and β-actin transcripts using specific primers. The primers for Ad4BP/SF-1 were 5′-GACCAGATGACACTGCTGC-3′ and 5′-TCCTTGGCCTGCATGCTCA-3′,4 those for Cyp11A were 5′-GCACACAACTTGAAGGTACAGGAG-3′ and 5′-CAGCCAAAGCCCAAGTACCGGAAG-3′,33 and those for β-actin were 5′-GCTGTATTCCCCTCCATCGTG-3′ and 5′-CGGTTGGCCTTAGGGTTCAGG-3′. The expected lengths of the amplified products by the above sets of primers are 400 bp for the Ad4BP/SF-1, 343 bp for the Cyp11A, and 265 bp for β-actin.34
RESULTS
Microscopic and macroscopic features of the spleen of anmFtz-F1 gene–disrupted mouse.
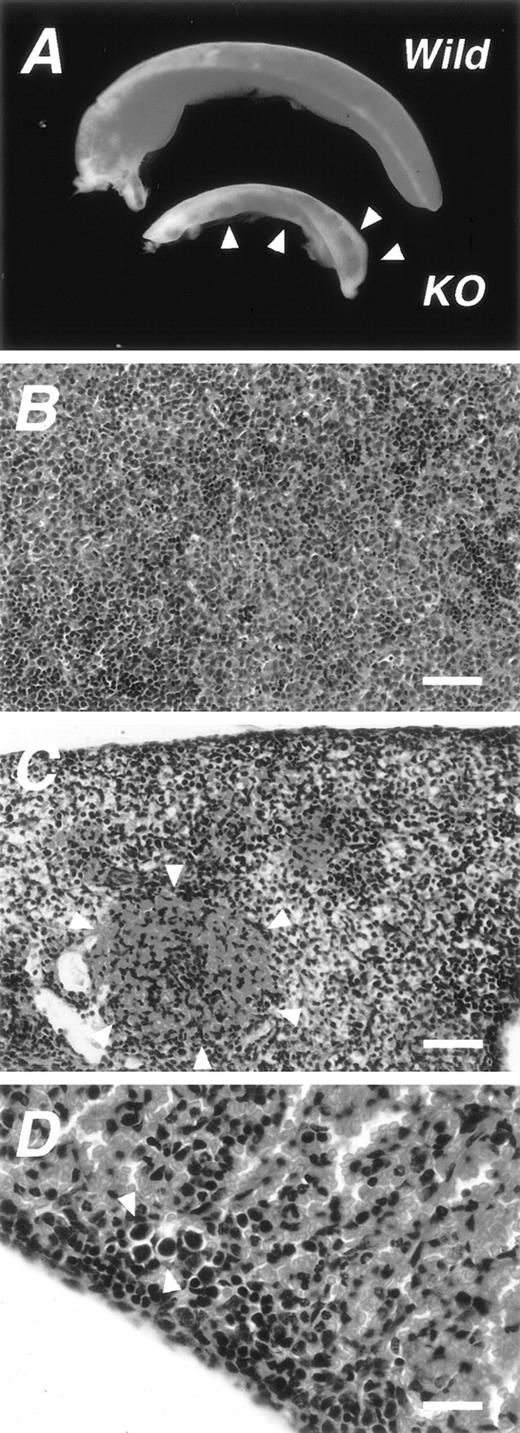
The results of studies by Ninomiya et al24 and Ramayya et al,25 in which the Ad4BP/SF-1 transcript was detected in the spleen, prompted us to examine the tissue of the mFtz-F1gene–disrupted (KO) mice. As described elsewhere,8,22,23the KO mouse dies within 1 week after birth, probably because of very low levels of circulating adrenocorticoids due to a developmental defect of the adrenal gland.34 Accordingly, we examined spleen tissues obtained from the KO fetuses and newborn mice. Interestingly, the spleen of the KO mouse was abnormal macroscopically and microscopically (Fig 1A). The size of the spleen was significantly smaller than the normal spleen. Wheras the spleen of the wild-type litter mates was uniformly red in color, the spleen of the KO mouse was characterized by the presence of scattered red spots that varied in size, distribution, and intensity from one animal to another. Histological examination of the spleen of the wild-type showed densely packed myeloid and lymphoid cells at various stages of maturation (Fig 1B), together with features of erythropoiesis throughout the whole tissue. In contrast, the spleen of the KO mouse contained sparsely packed areas among densely packed areas. These areas correspond to the white and red areas, respectively, shown macroscopically in Fig 1A. The densely packed red area contained mainly erythrocytes as indicated by arrowheads in Fig 1C. Furthermore, we also detected several megakaryocytes in the spleen of the KO mouse (Fig 1D).
Macroscopic and microscopic features of the spleen ofmFtz-F1 gene–disrupted mice. (A) Comparison of macroscopic features of wild-type spleens (Wild) and mFtz-F1gene–disrupted (KO) newborn mice. Arrowheads show red spots characteristic of the KO spleen. Photomicrographs of splenic tissues of the wild-type (B) and KO (C and D) mice stained with hematoxylin and eosin. Note the erythrocyte-rich region (encircled by arrowheads in C), probably corresponding to the red regions in (A). (D) Photomicrograph showing megakaryocytes in the spleen of KO mouse (arrowheads). Scale bars: 100 μm in B and C; 25 μm in D.
Macroscopic and microscopic features of the spleen ofmFtz-F1 gene–disrupted mice. (A) Comparison of macroscopic features of wild-type spleens (Wild) and mFtz-F1gene–disrupted (KO) newborn mice. Arrowheads show red spots characteristic of the KO spleen. Photomicrographs of splenic tissues of the wild-type (B) and KO (C and D) mice stained with hematoxylin and eosin. Note the erythrocyte-rich region (encircled by arrowheads in C), probably corresponding to the red regions in (A). (D) Photomicrograph showing megakaryocytes in the spleen of KO mouse (arrowheads). Scale bars: 100 μm in B and C; 25 μm in D.
Expression of Ad4BP/SF-1 in the adult spleen.
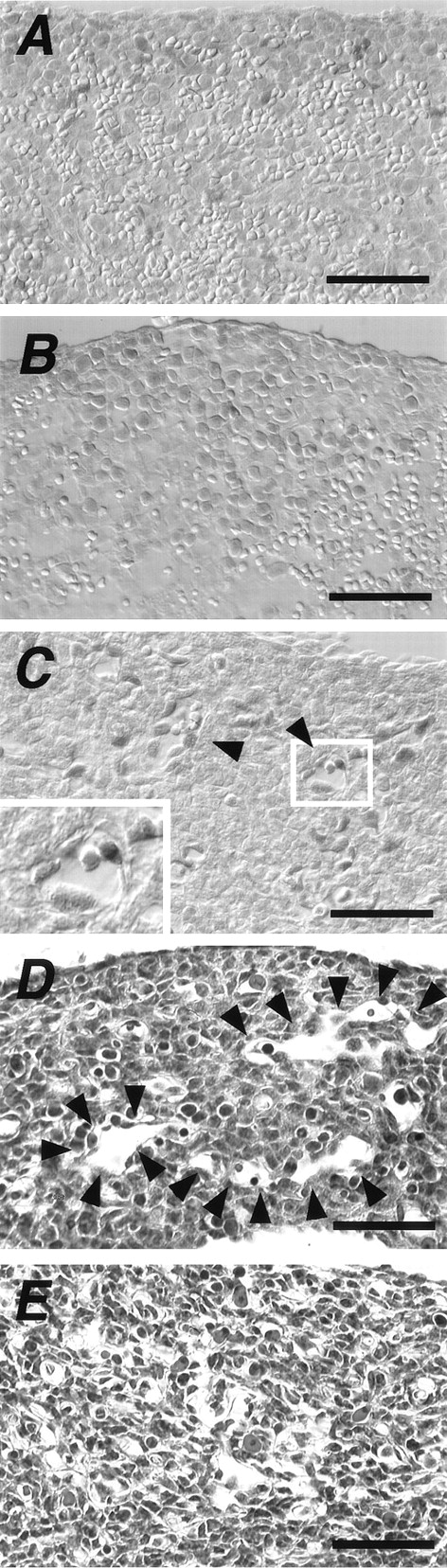
Expression of Ad4BP/SF-1 was detected in total tissue lysates of spleen tissues of adult and fetal (E16.5) mice using immunoblot analysis (Fig2A). As expected, the signal was not detected in the spleen of the KO mouse. The significantly low level of Ad4BP/SF-1 expression in the spleen of this mouse, relative to that in the adrenal gland, was probably due to a limited expression in certain cell types or otherwise represented at a uniformly low level throughout the entire tissue. In the next step, we examined the distribution of Ad4BP/SF-1 immunoreactive cells by immunohistochemistry. In the spleen of adult mice, Ad4BP/SF-1–immunoreactive cells were observed in the red pulp (indicated by “r” in Fig 2B) but not in the white pulp (indicated by “w” in Fig 2B). Although some immunoreactive cells showed an irregular distribution pattern, others were arranged in distinct patterns (Fig 2B). Because the sinus structure in the red pulp varies among animal species, we also investigated the spleen of the adult rat. Interestingly, several immunoreactive cells in the rat were arranged in a regular pattern probably along the sinuses (Fig 2C). Our results were, however, inconclusive with regard to the exact cell type that was immunoreactive for Ad4BP/SF-1 due to the large number of blood cells. Therefore, further investigation was performed using perfused spleens. Even after perfusion, immunoreactive cells remained at the luminal side of the pulp vein (indicated by a black asterisk in Fig 2D) and venous sinuses (indicated by red asterisks in Fig 2D). Examination at a higher magnification clearly indicated that these cells were attached to the basement membrane of the venous sinuses (arrowheads in Fig 2E). Alignment of immunoreactive cells along the venous sinus was also seen in the marginal sinus surrounding the white pulp (indicated by arrowheads in Fig 2F). A similar staining pattern was noted in the perfused spleens of adult rats (Fig 2G) and washed spleens of humans (Fig 2H). As in the mouse, Ad4BP/SF-1 immunoreactive cells were found to lay inside the sinus in both rats and humans. Furthermore, the pulp vein of the rat spleen was found to contain immunoreactive cells in its luminal side (data not shown).
Expression of Ad4BP/SF-1 in the spleen. Expression of Ad4BP/SF-1 was investigated using immunoblot (A) and immunohistochemical analysis (B, C, D, E, F, G, and H) using the antiserum to Ad4BP/SF-1. Total tissue lysates (25 μg) were prepared from spleen tissues of adult (Adult), E16.5 fetus (E16.5), and wild [B0 (Nor)] and KO [B0 (KO)] newborn mice. Adrenal glands (Adrenal) of adult mice were used as the positive control for detection of Ad4BP/SF-1. Molecular weight markers are indicated on the left. Arrows indicate signals for Ad4BP/SF-1. Immunohistochemistry was performed using mouse (B) and rat (C) splenic tissues, and perfused mouse (D, E, and F) and rat (G) spleen tissues. A tissue sample of human spleen was washed with PBS to remove erythrocytes and immunostained (H). Note that almost all positive nuclei are localized in the red pulp (r) but not in the white pulp (w) (B and C). Red and black asterisks in D, F, G, and H indicate venous sinuses and pulp vein, respectively. Arrowheads in E indicate the basement membrane of the venous sinus. Arrowheads in F indicate Ad4BP/SF-1 immunoreactive nuclei surrounding the white pulp. Ca, central artery. Scale bars: 50 μm in B, C, D, F, and H; 120 μm in E and G.
Expression of Ad4BP/SF-1 in the spleen. Expression of Ad4BP/SF-1 was investigated using immunoblot (A) and immunohistochemical analysis (B, C, D, E, F, G, and H) using the antiserum to Ad4BP/SF-1. Total tissue lysates (25 μg) were prepared from spleen tissues of adult (Adult), E16.5 fetus (E16.5), and wild [B0 (Nor)] and KO [B0 (KO)] newborn mice. Adrenal glands (Adrenal) of adult mice were used as the positive control for detection of Ad4BP/SF-1. Molecular weight markers are indicated on the left. Arrows indicate signals for Ad4BP/SF-1. Immunohistochemistry was performed using mouse (B) and rat (C) splenic tissues, and perfused mouse (D, E, and F) and rat (G) spleen tissues. A tissue sample of human spleen was washed with PBS to remove erythrocytes and immunostained (H). Note that almost all positive nuclei are localized in the red pulp (r) but not in the white pulp (w) (B and C). Red and black asterisks in D, F, G, and H indicate venous sinuses and pulp vein, respectively. Arrowheads in E indicate the basement membrane of the venous sinus. Arrowheads in F indicate Ad4BP/SF-1 immunoreactive nuclei surrounding the white pulp. Ca, central artery. Scale bars: 50 μm in B, C, D, F, and H; 120 μm in E and G.
Expression of Ad4BP/SF-1 in the fetal spleen.
Immunohistochemical analysis was also performed using spleen tissues of wild and KO newborn mice. Ad4BP/SF-1–immunoreactive cells were detected in the wild mouse but not in the KO mouse (Fig3A and B). As in the case of adult spleen, a large number of erythrocytes packed the entire spleen of the newborn control mice, making it difficult to determine the exact cell type that was immunoreactive to Ad4BP/SF-1. Therefore, we examined the spleen at an early developmental stage before the onset of erythropoiesis. Expression of Ad4BP/SF-1 appeared in the spleen of wild-type mice at day 14.5 of gestation (Fig 3C). Interestingly, some of the immunoreactive cells formed tubular structures in concert with other immunoreactive cells (indicated by arrowheads in Fig 3C). Although it is difficult to establish that these structures develop into the splenic vascular system, some of the tubular structures were observed to contain erythrocytes, suggesting that they are embryonic components of the vascular system. The tubular structures, which were sectioned longitudinally during tissue processing, were also occasionally seen in the wild-type spleen (indicated by arrowheads in Fig 3D). In contrast, the spleens of KO mice of the same littermate were less likely to contain similar well-assembled tubular structures.
Expression of Ad4BP/SF-1 in fetal and newborn spleens and structural changes observed in the early stages of splenic development. Immunohistochemical analysis was performed using splenic tissues from wild-type (A) and KO (B) newborn mice and spleen of an E 14.5 mouse fetus. Arrowheads in (C) indicate tubular structure composed of Ad4BP/SF-1 immunoreactive cells. Inset: higher magnification of the tubular structure. Spleens of E16.5 fetuses of wild-type (D) and KO (E) mice were histologically examined with hematoxylin and eosin. Arrowheads in D indicate longitudinal sections of the tubular structures. Scale bars: 100 μm.
Expression of Ad4BP/SF-1 in fetal and newborn spleens and structural changes observed in the early stages of splenic development. Immunohistochemical analysis was performed using splenic tissues from wild-type (A) and KO (B) newborn mice and spleen of an E 14.5 mouse fetus. Arrowheads in (C) indicate tubular structure composed of Ad4BP/SF-1 immunoreactive cells. Inset: higher magnification of the tubular structure. Spleens of E16.5 fetuses of wild-type (D) and KO (E) mice were histologically examined with hematoxylin and eosin. Arrowheads in D indicate longitudinal sections of the tubular structures. Scale bars: 100 μm.
Alteration of expression profiles of marker proteins.
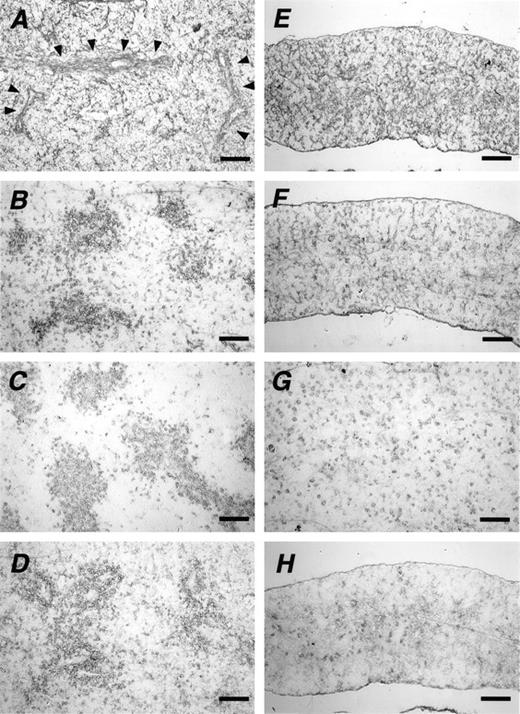
In the next step, we compared the expression and distribution of extracellular matrix proteins in spleen tissues of newborn wild mice with those in KO mice by using silver-stained sections. Fibrous structures were similarly stained in the spleens of both strains of mice without any obvious differences (data not shown). Similar staining patterns were also detected with the antibody to type-IV collagen. However, staining along certain structures, notably trabeculae, was clearly observed in the wild-type (arrowheads in Fig4A) but not in the KO mouse (Fig 4E). More importantly, striking differences were detected between the two spleens when tissue samples were stained for another extracellular matrix protein, laminin (Fig 4B and F). In the spleen of the newborn wild-type mouse, laminin was often assembled around vessel-like structures. In contrast, in spite of the persistent presence of laminin, such a unique pattern was never seen in the spleens of newborn KO mice. Staining patterns similar to that of laminin were also observed in c-kit– (Fig 4C and G) and vimentin- (Fig 4D and H) positive cells. Immunoreactive cells for these antigens were also assembled around the vessel-like structures in the spleen of the wild-type mouse. In contrast, the spleen of the newborn KO mouse was characterized by the absence of such assembly and fewer c-kit– and vimentin-positive cells.
Altered distribution of marker proteins. Frozen sections of spleens of newborn wild-type (A, B, C, and D) and KO mice (E, F, G, and H) stained with antibodies to type-IV collagen (A and E), laminin (B and F), c-kit (C and G), and vimentin (D and H). Arrowheads in (A) indicate a fibrous structure. Scale bars: 100 μm.
Altered distribution of marker proteins. Frozen sections of spleens of newborn wild-type (A, B, C, and D) and KO mice (E, F, G, and H) stained with antibodies to type-IV collagen (A and E), laminin (B and F), c-kit (C and G), and vimentin (D and H). Arrowheads in (A) indicate a fibrous structure. Scale bars: 100 μm.
Splenic hypofunction in KO mice.
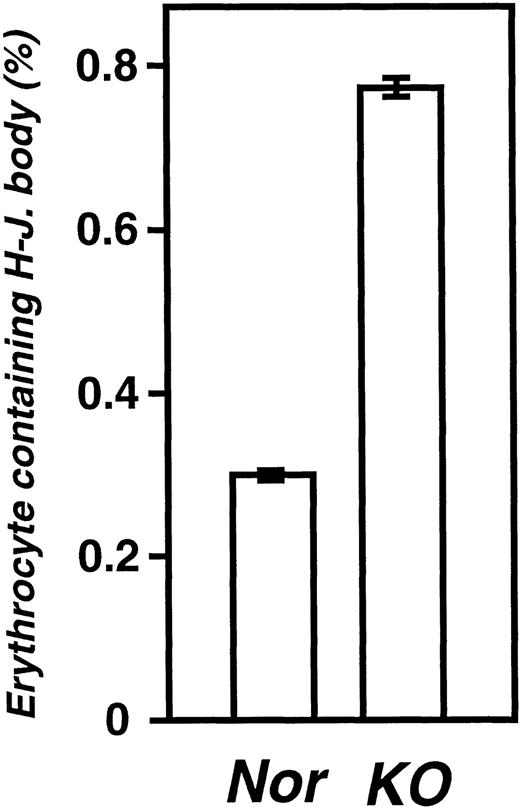
Splenic venous sinuses are involved in the clearance of abnormal erythrocytes. Accordingly, we counted the number of erythrocytes that contained Howell-Jolly bodies as a measure of splenic function, using blood smears prepared from adult and newborn mice (wild and KO mice at postnatal day 0). A few Howell-Jolly body–containing erythrocytes were present in healthy adult mice as well as newborn mice of the wild-type (Fig 5). In newborn KO mice, there was a 2.6-fold increase in the number of abnormal erythrocytes (Fig 5).
Percentage of erythrocytes containing Howell-Jolly bodies. Blood samples were prepared from wild (Nor) and KO newborn mice at postnatal day 0. Erythrocytes containing Howell-Jolly bodies were counted as described in Materials and Methods. Data represent the mean ± SEM of 10 wild-type and 6 KO mice.
Percentage of erythrocytes containing Howell-Jolly bodies. Blood samples were prepared from wild (Nor) and KO newborn mice at postnatal day 0. Erythrocytes containing Howell-Jolly bodies were counted as described in Materials and Methods. Data represent the mean ± SEM of 10 wild-type and 6 KO mice.
To investigate if the structural changes described above affected the differentiation of blood cells, we compared the percentages of different cellular components in the spleens of two newborn wild-type mice to those in a similar number of KO mice. Hypocellularity of both lymphocytes and erythrocytes was evident in the spleen of the KO mouse (<20% of those in wild-type mice) compared with those of the wild-type (Table 1). These findings correlated well with the small size of the organ and irregular distribution of erythrocytes in the KO mouse. In newborn KO mice, the numbers of mature CD3+ T cells, B220+ B cells, and Mac-1+ macrophages in the spleens were greatly decreased, but the proportion of T and B cells did not change as compared with that in the wild-type. The major cell population in the spleen of newborn mice was CD3−B220−Mac1− mononuclear cells in both strains. Furthermore, the presence of megakaryocytes was confirmed by histological examination of spleens of both KO and wild-type mice (Fig 1D).
Cellular Components of the Newborn Mouse Spleens
| . | No. of Nucleated Cells/Spleen . | % of Nucleated Cells . | No. of Erythrocytes/ Spleen . | |||||
|---|---|---|---|---|---|---|---|---|
| CD3+ . | CD4+ . | CD8+ . | Mac-1+ . | B220+ . | CD3− Mac-1− B220− . | |||
| Nor-1 | 1.94 × 106 | 1.0 | 0.3 | 0.1 | 19.1 | 14.7 | 65.2 | 2.56 × 106 |
| Nor-2 | 1.78 × 106 | 1.2 | 0.3 | 0.3 | 17.7 | 24.9 | 56.2 | 2.72 × 106 |
| KO-1 | 0.46 × 106 | 2.6 | 1.2 | 0.6 | 13.9 | 13.8 | 69.7 | 0.58 × 106 |
| KO-2 | 0.34 × 106 | 0.9 | 0.3 | 0.4 | 14.2 | 14.5 | 70.4 | 0.54 × 106 |
| . | No. of Nucleated Cells/Spleen . | % of Nucleated Cells . | No. of Erythrocytes/ Spleen . | |||||
|---|---|---|---|---|---|---|---|---|
| CD3+ . | CD4+ . | CD8+ . | Mac-1+ . | B220+ . | CD3− Mac-1− B220− . | |||
| Nor-1 | 1.94 × 106 | 1.0 | 0.3 | 0.1 | 19.1 | 14.7 | 65.2 | 2.56 × 106 |
| Nor-2 | 1.78 × 106 | 1.2 | 0.3 | 0.3 | 17.7 | 24.9 | 56.2 | 2.72 × 106 |
| KO-1 | 0.46 × 106 | 2.6 | 1.2 | 0.6 | 13.9 | 13.8 | 69.7 | 0.58 × 106 |
| KO-2 | 0.34 × 106 | 0.9 | 0.3 | 0.4 | 14.2 | 14.5 | 70.4 | 0.54 × 106 |
Cells prepared from the spleens of the wild-type (Nor-1 and Nor-2) and the mFtz-F1 gene–disrupted mice (KO-1 and KO-2) were used for detection of cell surface markers, CD3, CD4, CD8, Mac-1, and B220, as described in Materials and Methods. Numbers of the nucleated cells and erythrocytes were counted under the microscope.
Expression of steroidogenic Cyp genes in the spleen.
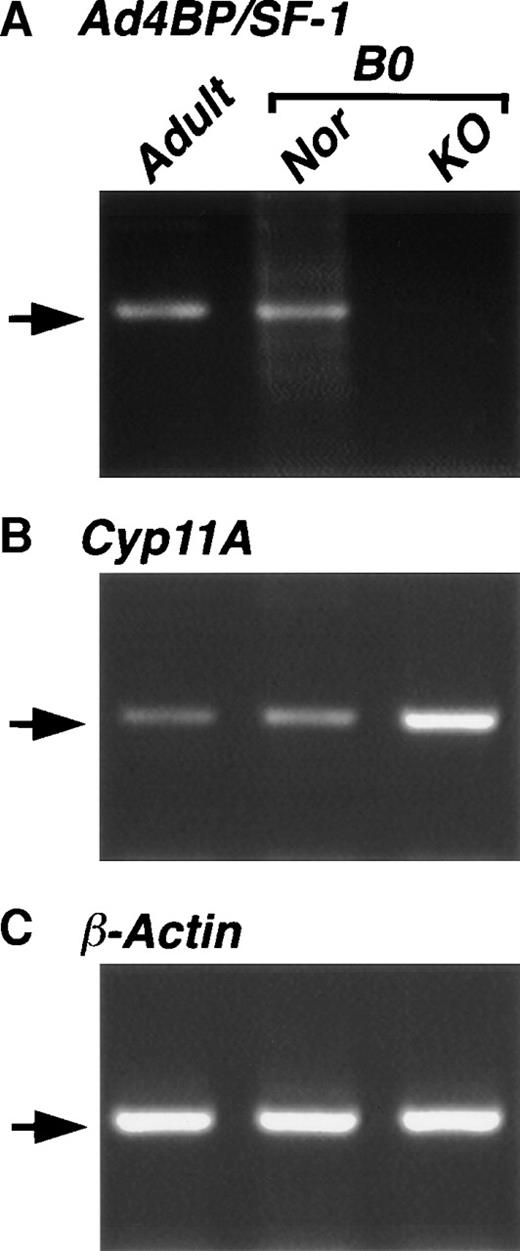
Because the regulation of steroidogenic Cyp gene is mediated by Ad4BP/SF-1 in steroidogenic tissues, we investigated the expression ofCyp genes in the spleen by RT-PCR. When total RNA prepared from the spleen of adult mice was used, the mRNA transcripts of both themFtz-F1 gene and Cyp11A gene, which encodes P450SCC, were detected (Fig 6), whereas transcripts of other steroidogenic Cyp genes, Cyp11B1, Cyp11B2, Cyp11B3, and Cyp17, were undetectable (data not shown). We also employed RT-PCR using cDNA of the wild-type and KO spleens. ThemFtz-F1 gene transcript was detected in the spleen of wild-type but not KO mice. In contrast, the Cyp11A gene transcript was detected in both strains, showing a similar observation with the Cyp11A expression in the placenta.23
Expression of Cyp11A in the spleen. Total RNAs were prepared from the spleens of wild-type adult (Adult), and wild-type (Nor) and mFtz-F1 gene–disrupted (KO) new born (B0) mice. cDNAs reverse-transcribed from the RNAs were used for PCR with specific primers for Ad4BP/SF-1 (A), Cyp11A (B), and β-actin (C) as described in Materials and Methods. Arrows indicate the expected length of PCR products.
Expression of Cyp11A in the spleen. Total RNAs were prepared from the spleens of wild-type adult (Adult), and wild-type (Nor) and mFtz-F1 gene–disrupted (KO) new born (B0) mice. cDNAs reverse-transcribed from the RNAs were used for PCR with specific primers for Ad4BP/SF-1 (A), Cyp11A (B), and β-actin (C) as described in Materials and Methods. Arrows indicate the expected length of PCR products.
DISCUSSION
The spleen has a unique structure composed of extrinsic cells derived mainly from the bone marrow and intrinsic cells. The latter cells constitute the framework of the spleen and include blood vessels, venous sinuses, and trabeculae, whereas both myeloid and lymphoid cells differentiate from bone marrow cells. The white pulp consists of T and B lymphocytes that can potentially produce antibodies in response to antigenic stimulation, similar to the lymph nodes. The red pulp, on the other hand, is packed with erythrocytes and leukocytes, both of which are in the process of transport from the arteries to the veins. Blood cells released from the arterial terminals into the splenic cord pass through slitlike structures in the sinus wall and enter into the luminal side. Examination by electron microscopy26,35-37 and scanning electron microscopy29 has shown that the major component of the sinus wall consists of endothelial cells arranged side-by-side, forming a tubular structure. Based on these morphologic studies, a remarkable feature of the endothelial cell is the presence of nuclei that protrude into the luminal side of the sinus.
Recent studies using RT-PCR and in situ hybridization24 25have confirmed the expression of Ad4BP/SF-1 in the spleen. However, the exact cell type that expresses Ad4BP/SF-1 could not be identified. A major finding of the present study was the localization of splenic cells that express Ad4BP/SF-1. In the first step, routine immunohistochemical analysis showed the presence of a large number of blood cells that interfered with the identification of immunoreactive cells. Consequently, we used perfused spleens for immunodetection. Our results showed that immunoreactivity was localized to endothelial lining cells of venous sinuses and pulp veins. In a series of preliminary experiments, we also investigated the expression of Ad4BP/SF-1 in other tissues that are functionally related to the spleen. To this effect, immunohistochemical staining of the thymus, lymph node, fetal liver, and bone marrow showed that none of these tissues contained immunoreactive cells for Ad4BP/SF-1 (data not shown).
Gross examination of the spleen showed a clear underdevelopment in themFtz-F1 gene–disrupted mice. Furthermore, histological examination showed the presence of fewer erythrocytes that appeared in clusters irregularly distributed in the spleen, reflecting a state of hypoerythropoiesis. Thus, in the KO mouse, erythropoiesis is likely to occur in limited regions of the spleen, in sharp contrast to the wild-type mice, where it occurs throughout the whole organ. These abnormal features were detected as early as day 14.5 of gestation. During normal fetal development, the expression of Ad4BP/SF-1 appeared in cells that formed the tubular framework, which probably form the splenic vascular system, including the venous sinuses and pulp vein. In contrast, such tubular structures could not be detected in the spleen of KO mice, probably leading to a poor organization of the vascular system. This conclusion is supported by our finding of the altered profile of the expression of type-IV collagen and more strikingly by that of laminin. Because these proteins are components of the extracellular matrix, our results suggest that disruption of themFtz-F1 gene not only results in an immature vascular system but also altered architecture of the spleen in the KO mouse.
Interestingly, our results showed a similar distribution of c-kit, a receptor tyrosine kinase, and vimentin, an intermediate filament, in normal and KO mice. These proteins are known to be expressed in the immature hematopoietic cells, and their expression is dependent on the cell lineage.38,39 Our results showed that in the wild-type spleen, immunoreactive cells for these proteins were arranged around structures that probably represented immature blood vessels. In contrast, such peculiar assembly was not detected in the KO spleen. Hematopoietic cells originate from the bone marrow and then settle in other hematopoietic tissues, including the spleen. The presence of structural and/or functional defects in the embryonic splenic vascular system is likely to interfere with the splenic blood flow, thereby affecting the extension and settlement of hematopoietic cells in the spleen. In this regard, our results showed a clear overlap in the distribution of laminin with that of c-kit and vimentin. Laminin is known as a ligand molecular for members of the integrin family and is expressed on the surface of several cell lineages, including the hematopoietic cells.40 Therefore, altered expression of laminin is probably associated with inefficient distribution of the c-kit– and vimentin-expressing cells in the KO spleen. It is also possible that the altered microenvironment in the KO spleen does not support a proper differentiation of hematopoietic cells. To investigate this possibility, we compared the cellular components of the spleen in KO mice with those of the wild-type by using several peripheral cell markers. Our results showed that full maturation was persistent in all hematopoietic lines tested and no obvious difference was detected, indicating clearly that differentiation of the hematopoietic cells is not impaired in KO mice.
Our results showed a higher proportion of erythrocytes containing Howell-Jolly bodies in the mFtz-F1 gene–disrupted mice than in control mice. A similar finding was also reported in the asplenic mouse41 and Hox11 gene–disrupted mouse, both of which showed failure of splenic development.42 These results are also in agreement with the appearance in the blood of erythrocytes containing Howell-Jolly bodies following splenectomy in humans.43
Ad4BP/SF-1 was originally identified as a specific steroidogenic transcription factor expressed in the adrenal cortex and gonads. In these tissues, the steroidogenic Cyp genes are downstream of Ad4BP/SF-1. Although the spleen is not classified as a steroidogenic tissue, we examined the expression of Cyp genes in the present study. Interestingly, we detected the Cyp11A gene transcript–encoding cholesterol side-chain cleavage P450 in the spleen. However, the same mRNA was also present in KO mice, strongly suggesting that the Cyp11A gene is not under the control of Ad4BP/SF-1 in the spleen. A similar Ad4BP/SF-1–independent regulation has been reported for the steroidogenic Cyp11A gene in the placenta,23 the primitive gut and dermal mesenchyme,44 and for the Cyp19 gene–encoding aromatase P450 in the hypothalamus.8 Although the mechanism remains to be clarified, the Cyp genes can be transcribed under a distinct mechanism present in steroidogenic tissues where Ad4BP/SF-1 is the crucial transcription factor for Cyp genes.
Ad4BP/SF-1 is thought to play a crucial role in the establishment of hypothalamic-pituitary-gonadal and -adrenal axes, in addition to its role in steroid hormone biosynthesis through the regulation of steroidogenic P450 gene transcription. In the present study, we identified a new function for Ad4BP/SF-1, ie, the construction of basic splenic architecture based on its expression in endothelial cells lining splenic venous sinuses and pulp vein. However, the exact function of Ad4BP/SF-1 in splenic cells has not yet been explained. Therefore, the mechanisms that lead to impaired splenogenesis in KO mice could not be clearly identified. This is mainly due to a lack of information on the exact gene regulated by Ad4BP/SF-1 in the spleen. Identification of the downstream genes will help explain the mechanisms involved in the development of splenic architecture, including the vascular system.
ACKNOWLEDGMENT
The authors thank Drs N. Yanai (Tohoku University), K. Sasaki (Kawasaki Medical School), and S. Sutou (Itoham Central Research Institute) for their critical discussions. The antibody for c-kit was generously provided by Dr Nishikawa (Kyoto University).
Supported by a Grant-in-Aid for Scientific Research from Ministry of Education, Science, Sports, and Culture of Japan, and grants from Naito Foundation, Sumitomo Foundation, Suzuken Memorial Foundation, and Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ken-ichirou Morohashi, Prof, PhD, Division of Cell Differentiation, Department of Developmental Biology, National Institute for Basic Biology, Myodaiji-cho, Okazaki 444-8585, Japan; e-mail:moro@nibb.ac.jp.

![Fig. 2. Expression of Ad4BP/SF-1 in the spleen. Expression of Ad4BP/SF-1 was investigated using immunoblot (A) and immunohistochemical analysis (B, C, D, E, F, G, and H) using the antiserum to Ad4BP/SF-1. Total tissue lysates (25 μg) were prepared from spleen tissues of adult (Adult), E16.5 fetus (E16.5), and wild [B0 (Nor)] and KO [B0 (KO)] newborn mice. Adrenal glands (Adrenal) of adult mice were used as the positive control for detection of Ad4BP/SF-1. Molecular weight markers are indicated on the left. Arrows indicate signals for Ad4BP/SF-1. Immunohistochemistry was performed using mouse (B) and rat (C) splenic tissues, and perfused mouse (D, E, and F) and rat (G) spleen tissues. A tissue sample of human spleen was washed with PBS to remove erythrocytes and immunostained (H). Note that almost all positive nuclei are localized in the red pulp (r) but not in the white pulp (w) (B and C). Red and black asterisks in D, F, G, and H indicate venous sinuses and pulp vein, respectively. Arrowheads in E indicate the basement membrane of the venous sinus. Arrowheads in F indicate Ad4BP/SF-1 immunoreactive nuclei surrounding the white pulp. Ca, central artery. Scale bars: 50 μm in B, C, D, F, and H; 120 μm in E and G.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1586/4/m_blod40528002w.jpeg?Expires=1769091975&Signature=dePCfCvOoPOp9lDOxHNNT0HONOPiAaa8AY9zpsqhyHaOyuW0MwIWFgSYgek~S56xz3Gap8amCTwClUxEybFeZix6uGh299fnEuBTdd7fHVbr~n9KcYUiWQm6ntFUgbnCPNzQ6ExBhRmn2kP9F2byKb~IfLkGxCQZtuSteEtl2gwXg9Z4nOrKt53fy8nHSIMUk0I8-Z0ewEl3IsyLcWE5kbqj7FvQEhqGe6O-scpthPSM06roPGUt-GDR4xa2-GqFQ7IcXysiQQqNEe5OIUNQCMwfpm3QTK~XRjK2wboe6AHI9nTOIn4RCVkhT395wXTV4DmVeMH4bd-i2whYG4zITw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal