Abstract
Interleukin-12 (IL-12) inhibits angiogenesis in vivo by inducing interferon-γ (IFN-γ) and other downstream mediators. Here, we report that neutralization of natural killer (NK) cell function with antibodies to either asialo GM1 or NK 1.1 reversed IL-12 inhibition of basic fibroblast growth factor (bFGF)-induced angiogenesis in athymic mice. By immunohistochemistry, those sites where bFGF-induced neovascularization was inhibited by IL-12 displayed accumulation of NK cells and the presence of IP-10–positive cells. Based on expression of the cytolytic mediators perforin and granzyme B, the NK cells were locally activated. Experimental Burkitt lymphomas treated locally with IL-12 displayed tumor tissue necrosis, vascular damage, and NK-cell infiltration surrounding small vessels. After activation in vitro with IL-12, NK cells from nude mice became strongly cytotoxic for primary cultures of syngeneic aortic endothelial cells. Cytotoxicity was neutralized by antibodies to IFN-γ. These results document that NK cells are required mediators of angiogenesis inhibition by IL-12, and provide evidence that NK-cell cytotoxicity of endothelial cells is a potential mechanism by which IL-12 can suppress neovascularization.
INTERLEUKIN-12 (IL-12), a disulfide-linked heterodimer composed of two subunits, p35 and p40, stimulates T and natural killer (NK) cells to secrete interferon-gamma (IFN-γ) and augments T- and NK-cell proliferation and cytolytic activity.1-5 Through these functions, IL-12 plays a critical role in the regulation of early inflammatory responses and promotes the development of Th1-type T-cell responses that favor cell-mediated immunity.6,7 Preclinical testing has demonstrated that IL-12 can be effective against certain microbial and fungal agents, can serve as an immune adjuvant, and can exert antitumor activity against several experimental malignancies.8-13
Recently, IL-12 was reported to inhibit new vessel formation in an in vivo assay that detects murine corneal vascularization induced by basic fibroblast growth factor (bFGF), a potent inducer of angiogenesis.14,15 This property of IL-12 was also demonstrated in another in vivo angiogenesis assay that measures bFGF-induced neovascularization of subcutaneous Matrigel (Becton-Dickinson Labware, Bedford, MA) plugs in nude mice.16 Neutralizing antibodies to IFN-γ removed most of the IL-12 inhibitory effect on neovascularization in both mouse models, and experiments in vitro failed to demonstrate a direct effect of IL-12 on endothelial-cell growth.16 Furthermore, a neutralizing antibody to IP-10, a CXC chemokine induced by IFN-γ, substantially reduced the inhibition of angiogenesis induced by IL-12.16Previous studies had documented that IP-10 can act as an inhibitor of neovascularization induced by bFGF and other stimuli.17These findings indicated that IL-12 inhibits angiogenesis indirectly through the stimulation of IFN-γ and, at least in part, IP-10.
The mechanisms by which IP-10 inhibits neovascularization are presently unknown, and it is unclear whether the chemokine can act directly on endothelial cells.18 In one study, a recombinant IP-10 alkaline phosphatase fusion protein was found to bind to endothelial cells and other cells through heparan sulfate proteoglycan.19 This binding was specific and saturable, and was reversed by heparin, but signaling was not reported. Endothelial-cell proliferation in vitro was inhibited by IP-10, albeit at substantially higher concentrations than those required for an angiostatic effect in vivo.19 Recently, a signaling receptor for human IP-10, CXCR3, was identified on activated T cells, NK cells, and other cells.20 Using reverse-transcriptase polymerase chain reaction (RT-PCR), we found no evidence of CXCR3 expression in primary cultures of human umbilical cord–derived endothelial cells (unpublished results, November 1996 to January 1997). While others have also reported that human umbilical cord–derived endothelial cells do not express CXCR3,21 a recent study found evidence of CXCR3 expression in murine endothelial cells.22 Thus, it is currently unclear whether endothelial cells can be direct targets of IP-10 effects.
In the present study, we examined further the mechanisms that mediate angiogenesis inhibition by IL-12. Our results provide evidence that NK cells can play a critical role in the regulation of angiogenesis.
MATERIALS AND METHODS
Mice, reagents, cytokine, and antibodies.
Five- to 6-week-old female BALB/c nude mice (National Cancer Institute, Bethesda, MD, Frederick Cancer Research Center [FCRC]) maintained in pathogen-limited conditions were used throughout, except for one experiment that used 4- to 6-week-old female C57BL/6 nude mice (Taconic, Germantown, NY). Matrigel was purchased from Becton Dickinson Labware. Recombinant murine IL-12 was a gift of Genetics Institute, Inc (Cambridge, MA). Recombinant murine IFN-γ was a gift of Genentech, Inc (South San Francisco, CA). Rabbit antimurine IP-10 antiserum was a gift of Dr J.M. Farber (Laboratory of Clinical Investigation, National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIH], Bethesda, MD). Rabbit antiasialo GM1 antibody was purchased from WAKO (Dallas, TX). Murine antiasialo GM1 monoclonal antibody (clone SH34) was a gift of Dr R. Stout (James H. Quillen College of Medicine, Johnson City, TN).23 24 Murine antimouse NK1.1 (clone PK136) and rat antimouse B220 (clone RA3-6B2) monoclonal antibodies were purchased from PharMingen (San Diego, CA). Murine anti–alpha-smooth muscle actin monoclonal antibody (clone 1A4) and a rabbit antihuman von Willebrand factor (factor VIII–related antigen) polyclonal antibody were purchased from DAKO (Carpinteria, CA). Mouse antirat IFN-γ (clone DB-1, cross-reactive to mouse) monoclonal antibody was a gift of Dr D.S. Finbloom (Division of Cytokine Biology, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, MD). bFGF was purchased from R&D Systems (Minneapolis, MN).
Treatment with antiasialo GM1 antibody and in vivo Matrigel assay.
BALB/c nude mice were injected intraperitoneally (IP) with rabbit antiasialo GM1 antibody (1 mg/mouse; WAKO) and C57BL/6 nude mice were injected IP with purified monoclonal anti-NK1.1 antibody (clone PK136, 100 μg/mouse) 1 day before Matrigel injection, and the same treatment was repeated on day 3 after Matrigel injection. Antiasialo GM1 or anti NK1.1 antibodies were also mixed with Matrigel and injected subcutaneously. The Matrigel assay was performed as described previously.16 17 In brief, Matrigel (liquid at 4°C) was mixed with medium alone; with 150 ng/mL bFGF (R&D system); with bFGF (150 ng/mL) plus 100 ng/mL IL-12; with bFGF (150 ng/mL) plus 250 μg/mL antiasialo GM1 (BALB/c mice) or 50 μg/mL anti–NK 1.1 (C57BL/6 mice) antibodies; or with bFGF (150 ng/mL) plus IL-12 (100 ng/mL) plus antiasialo GM1 (BALB/c mice, 250 μg/mL) or anti–NK 1.1 (C57BL/6 mice, 50 μg/mL). A 0.5-mL quantity of the Matrigel mixture was injected subcutaneously into the midabdominal region of BALB/c or C57BL/6 nude mice. Five mice were injected with each mixture. At body temperature, Matrigel polymerizes to form a plug. After 7 days, the animals were killed, Matrigel plugs were removed together with the abstract epidermis and subepidermis, fixed in 10% neutral buffered formalin solution (Sigma Chemical Co, St Louis, MO), and then embedded in paraffin. All tissues were sectioned (5-μm thickness) and mounted onto slides for further staining.
Histologic sections from Matrigel plugs were stained with Masson’s trichrome or esterase by standard techniques. Quantitative analysis of angiogenesis in Matrigel plugs used a computerized semiautomated digital analyzer (Optomax, 40-10 System, Hollis, NH) essentially as described.17 In brief, the instrument is adjusted to measure the total area occupied by cells within a circular area measuring 1.26 × 105 μm2 of the Matrigel plug. For each plug, 12 to 15 separate fields (each field reflecting a circular area of 1.25 × 105 μm2) are evaluated; the total plug area measures 12 to 18 × 106μm2. The fields are randomly selected from each plug, and the operator is blind to the experimental design. The average area occupied by infiltrating cells/1.26 × 105μm2 Matrigel field is calculated. Results are expressed as the mean area occupied by cells per Matrigel field (±SD) of five replicate plugs from five distinct mice.25
Isolation of RNA and RT-PCR.
Total cellular RNA was isolated form Matrigel plugs by Trizol (GIBCO-BRL, Gaithersburg, MD). RNA 4 μg was reverse-transcribed using Superscript Preamplification System for First Strand cDNA Synthesis (GIBCO-BRL), according to the manufacturer’s instructions. The resultant cDNA was diluted with H2O to a final volume of 200 μL. For RT-PCR analysis, cDNA equivalents to 80 ng of total RNA were subjected to PCR amplification in a 50-μL reaction mixture containing 20 mmol/L Tris-HCl (pH 8.5), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 μmol/L of each dNTP, 2.5 U Taq DNA polymerase (GIBCO-BRL), and 0.2 μmol/L of each primer (Table1).
Primers, Annealing Temperature, Sizes, and Amplification Cycles for PCR Analysis
| Name . | Primer Pair . | Temperature (°C) . | Size (bp) . | Cycles . |
|---|---|---|---|---|
| m-Crg-2/IP-10 | ACCATGAACCCAAGTGCTGCCGTC | 64 | 311 | 32 |
| GCTTCACTCCAGTTAAGGAGCCCT | ||||
| m-IFN-γ | TGCGGCCTAGCTCTGAGACAATGA | 62 | 413 | 40 |
| TGAATGCTTGGCGCTGGACCTGTG | ||||
| m-Perforin | CTGAGAAGACCTATCAGGACC | 58 | 429 | 35 |
| ACCAAGTACTTCGACGTGACG | ||||
| m-Granzyme B | ACACGCTACAAGAGGTTGAGC | 55 | 295 | 35 |
| GCAGGATCCATGTTGCTTCTG | ||||
| m-GAPDH | GCCACCCAGAAGACTGTGGATGGC | 62 | 446 | 28 |
| CATGTAGGCCATGAGGTCCACCAC |
| Name . | Primer Pair . | Temperature (°C) . | Size (bp) . | Cycles . |
|---|---|---|---|---|
| m-Crg-2/IP-10 | ACCATGAACCCAAGTGCTGCCGTC | 64 | 311 | 32 |
| GCTTCACTCCAGTTAAGGAGCCCT | ||||
| m-IFN-γ | TGCGGCCTAGCTCTGAGACAATGA | 62 | 413 | 40 |
| TGAATGCTTGGCGCTGGACCTGTG | ||||
| m-Perforin | CTGAGAAGACCTATCAGGACC | 58 | 429 | 35 |
| ACCAAGTACTTCGACGTGACG | ||||
| m-Granzyme B | ACACGCTACAAGAGGTTGAGC | 55 | 295 | 35 |
| GCAGGATCCATGTTGCTTCTG | ||||
| m-GAPDH | GCCACCCAGAAGACTGTGGATGGC | 62 | 446 | 28 |
| CATGTAGGCCATGAGGTCCACCAC |
Immunohistochemistry.
Paraffin-embedded tissue sections were deparaffinized twice in xylene and twice in 100% ethanol. Tissue sections were further incubated with 3% hydrogen peroxide in methanol, and then rehydrated through graded ethanol washes (100%, 90%, 70%, and 50%) followed by Tris-buffered saline (TBS). Sections were either treated with 1× trypsin (Sigma) for 10 minutes, or immersed in 10 mmol/L citrate buffer and heated in a microwave pressure cooker (Nordic Ware, Minneapolis, MN) at maximum power (800 W) for 25 minutes. Sections were then blocked with 3% goat serum in TBS, followed by incubation (overnight at 4°C) with primary antibody (undiluted SH34 hybridoma culture supernatant for antiasialo GM1; 1:200 dilution of rabbit antimurine IP-10 antiserum; 1:50 dilution of purified rabbit anti–von Willebrand factor [factor VIII–related antigen]; 1:200 dilution of rat antimouse B220 monoclonal antibody; and 0.25 μg/mL mouse anti–alpha smooth muscle actin monoclonal antibody). After washing, biotinylated goat antirabbit, goat antirat, or horse antimouse secondary antibody (2 μmol/L/mL; Vector Labs, Burlingame, CA) was applied followed by VECTASTAIN ABC peroxidase complex (Elite ABC-kit; Vector Labs). The sections were developed using peroxidase 3,3′-diaminobenzidine (DAB) substrate and then counterstained with hematoxylin. Double staining for asialo GM1 and von Willebrand factor was performed by first staining with antiasialo GM1 monoclonal antibody as described earlier using DAB substrate (color brown) followed by staining with anti–von Willebrand factor as described earlier using Vector VIP substrate (color purple). No counterstaining was done after double staining. All sections were mounted, dehydrated, and examined by light microscopy.
Purification and culture of murine cells.
Mouse aortic endothelial cells were obtained from aorta of C57BL/6 nude mice using 1 mg/mL collagenase (Worthington, Biochemical Corp, Freehold, NJ). The cells were cultured in endothelial cells’ growth medium (GIBCO) supplemented with 15 μg/mL of endothelial cell growth supplement (ECGS) (Sigma). Cells from passages 4 or less were used for cytotoxicity assay. By immunocytochemical staining, greater than 90% of the cells were positive for the endothelial cells’ marker von Willebrand factor (factor VIII–related antigen).
Mouse splenocytes were prepared from spleens of littermate C57BL/6 nude mice. For NK-cell enrichment, the cells were cultured in RPMI 1640 at 5 × 106/mL in the presence of 500 IU/mL of IL-2 (Chiron, Emeryville, CA) for 6 days.26 At completion of cultures with IL-2, more than 95% of the cells were positive for asialo GM1 by flow cytometry. One day before cytotoxicity was tested, NK-cell–enriched splenocytes were washed free of IL-2 and incubated with medium alone, medium plus murine IFN-γ (200 ng/mL), murine IL-12 (10 ng/mL), or murine IL-12 plus antibody to rat IFN-γ (BD-1, 5 μg/mL) for 24 hours. Cells were collected, washed, and suspended at the desired concentrations for cytotoxicity assay.
Cytotoxicity assay.
Cytotoxicity assays were performed in 96-well flat-bottom plates (Becton Dickinson, Lincoln Park, NJ). Mouse aortic endothelial cells (5 × 103) were added to each well and incubated overnight. Subsequently, the cells were labeled with 2 μci of Na251CrO4 (New England Nuclear, Boston, MA) per well for 16 hours. After the labeled cells were washed, NK-cell–enriched splenocytes were added to each well at effector-to-target (E:T) ratios of 120:1 in a final volume of 100 μL/well. After a 4-hour incubation at 37°C in a humidified 5% CO2 atmosphere, the plates were centrifuged for 4 minutes at 100 × g. The supernatants, harvested using a Skatron-Titertek harvester (Skatron, Sterling, VA), were counted in an automated gamma counter (Pharmacia, Piscataway, NJ). Maximum release of 51Cr was obtained by lysis of the cells in 2% sodium dodecyl sulfate (SDS). Spontaneous 51Cr release was determined in wells containing labeled target cells incubated with medium alone. Spontaneous release was less than 15% of maximum release in all experiments. Each condition was tested in quadruplicate wells. Percent specific 51Cr release was calculated by using the following equation: Percent specific release: = ([Experimental Release − Spontaneous Release] ×100)/Maximum Release − Spontaneous Release.
Mouse tumor model.
BALB/c nude mice, 6 to 8 weeks of age, received 400 rad (1 rad = 0.01 Gy) total-body irradiation and 24 hours later were injected subcutaneously in the right abdominal quadrant with 107exponentially growing human Burkitt lymphoma cells (CA49 cell line)27 in 0.2 mL RPMI medium 1640. Tumor size was estimated (in square centimeters) twice weekly as the product of two-dimensional caliper measurements (longest perpendicular length and width). Test mice bearing Burkitt tumors (≥0.2 cm2 in size) were injected subcutaneously next to the tumor daily for 34 days with either murine IL-12 (200 ng/mL) or formulation buffer (saline containing 50 mg/mL human serum albumin and 5 mg/mL mannitol); the total injection volume was 100 μL. The care and use of mice was in accordance with NIH guidelines.
RESULTS
NK-cell infiltration at sites of IL-12–inhibited vascularization.
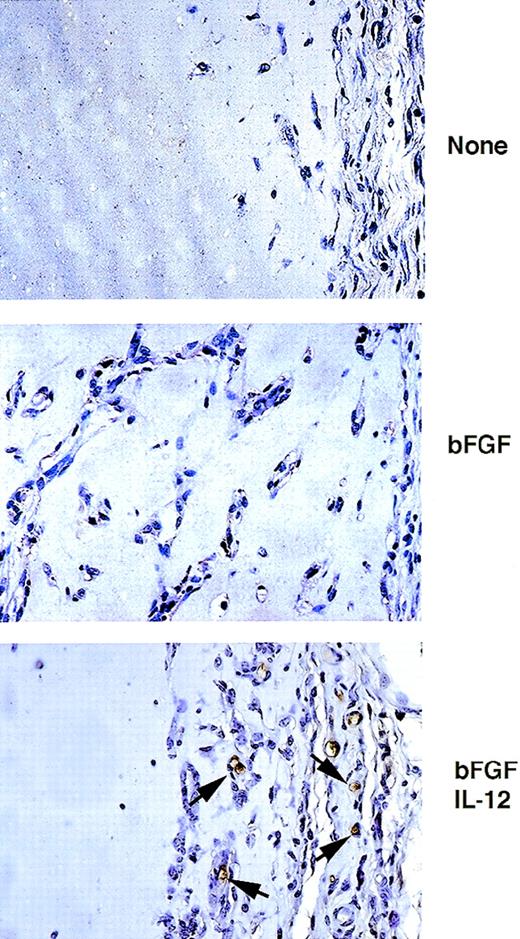
When Matrigel, a basement membrane extract that is liquid at 4°C but rapidly solidifies at body temperature, is inoculated with bFGF subcutaneously into athymic mice, an angiogenic response is induced within 5 to 7 days.17 Histologically, bFGF-impregnated Matrigel plugs, but not Matrigel-alone plugs, reveal the presence of capillary vessels containing red blood cells, in conjunction with tubular structures resembling primordial vessels, and many isolated cells. When IL-12 is present in the Matrigel plugs in conjunction with bFGF, the angiogenic response induced by bFGF is markedly reduced.16 Since IL-12 is known to directly activate T and NK cells and to indirectly promote their migration, through stimulation of IFN-γ and the IFN-γ–inducible chemokine IP-10,18 we looked for the presence of NK cells in Matrigel plugs containing IL-12. Plugs of Matrigel alone, Matrigel impregnated with bFGF (150 ng/mL) showing evidence of neovascularization, and Matrigel impregnated with bFGF (150 ng/mL) plus murine IL-12 (100 ng/mL) showing evidence of profound (>80%) inhibition of bFGF-induced neovascularization by IL-12, were stained with a monoclonal antibody to asialo GM1. In a representative experiment (Fig 1), asialo GM1-positive cells were identified in sections from Matrigel plugs impregnated with bFGF plus IL-12, but generally not from Matrigel plugs impregnated with bFGF or from Matrigel-alone plugs. Most of the strongly positive cells localized at the margins of the plug and at the interface between Matrigel and mouse tissues (epidermis and subepidermis). Since NK cells, and to a lesser extent monocytes, express asialo GM1,28 29 these results suggested that NK cells may localize in proximity and within IL-12–impregnated Matrigel plugs displaying histologic evidence for IL-12–inhibited neovascularization.
Immunohistochemical analysis of asialo GM1-positive cells infiltrating and surrounding Matrigel plugs. Paraffin-embedded sections from Matrigel plugs alone or Matrigel plugs impregnated with bFGF, or bFGF + IL-12 were stained with a monoclonal antimouse asialo GM1 antibody, counterstained with hematoxylin, and examined by light microscopy. Original magnification ×40.
Immunohistochemical analysis of asialo GM1-positive cells infiltrating and surrounding Matrigel plugs. Paraffin-embedded sections from Matrigel plugs alone or Matrigel plugs impregnated with bFGF, or bFGF + IL-12 were stained with a monoclonal antimouse asialo GM1 antibody, counterstained with hematoxylin, and examined by light microscopy. Original magnification ×40.
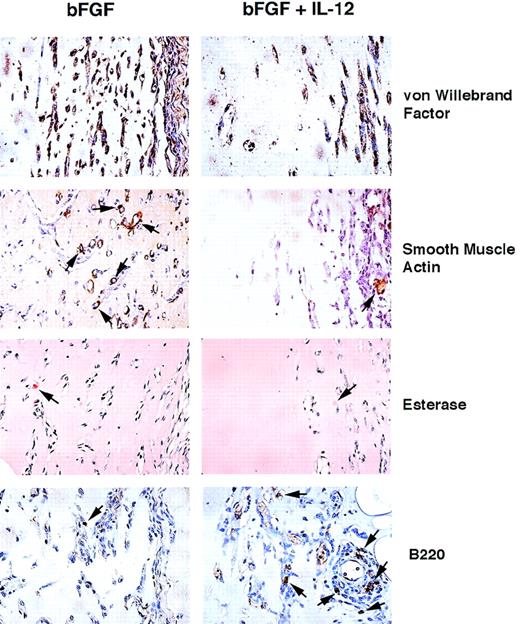
We wanted to define further the cellular composition of Matrigel plugs stimulated with bFGF alone or in conjunction with IL-12. Using an antibody against von Willebrand factor (factor VIII–related antigen) that identifies endothelial cells, bone marrow megakaryocytes, and platelets (due to their storage of von Willebrand factor), we found most of the nucleated cells to be immunopositive in both control and IL-12–induced plugs (Fig 2). This result provided evidence that most cells in bFGF-stimulated Matrigel plugs with or without IL-12 are of endothelial-cell origin. It should be noted that the total number of immunopositive cells infiltrating the bFGF plus IL-12–induced plugs was lower than in bFGF-induced plugs, reflecting inhibition of angiogenesis by the cytokine. Several cells in control (bFGF) and occasional cells in IL-12–induced plugs (bFGF plus IL-12) displayed staining for the alpha-smooth muscle isoform of actin that identifies smooth muscle cells of vessels and other parenchymas (Fig 2). In addition, rare cells in control and IL-12–induced plugs were esterase-positive, displaying a red staining that identifies cells of monocyte/macrophage lineage (Fig 2). Staining for B220, an antigen expressed on B cells and subsets of activated NK cells,30identified several positive cells at the margins of IL-12 plus bFGF-induced plugs and at the interface between the plug and the surrounding mouse tissues, but only rare B220-positive cells in control Matrigel plugs (bFGF, Fig 2). Because the pattern of B220 staining was similar to the pattern with antiasialo GM1 antibody, and because IL-12 is not known to directly stimulate B cells, we concluded that activated NK cells are likely to contribute most of the B220-positive cells in IL-12 plus bFGF-induced plugs.
Histochemical analysis of cells infiltrating Matrigel plugs. Paraffin-embedded sections from Matrigel plugs induced by either bFGF alone or in conjunction with IL-12 were stained with rabbit anti–von Willebrand factor, mouse anti–alpha smooth muscle actin monoclonal antibody, or rat antimouse B220 monoclonal antibody and counterstained with hematoxylin. Staining for esterase used conventional methods. Original magnification ×40.
Histochemical analysis of cells infiltrating Matrigel plugs. Paraffin-embedded sections from Matrigel plugs induced by either bFGF alone or in conjunction with IL-12 were stained with rabbit anti–von Willebrand factor, mouse anti–alpha smooth muscle actin monoclonal antibody, or rat antimouse B220 monoclonal antibody and counterstained with hematoxylin. Staining for esterase used conventional methods. Original magnification ×40.
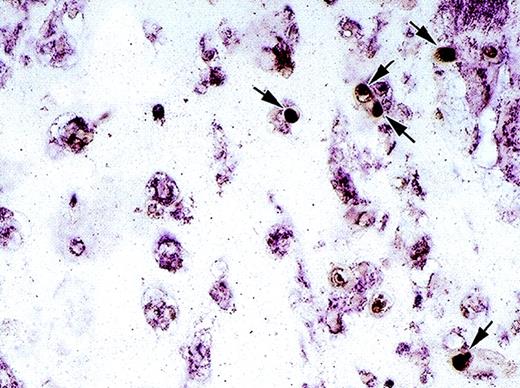
Spatial relationships between endothelial cells and NK cells infiltrating Matrigel plugs were examined. Using double staining for asialo GM1 (dark brown) and von Willebrand factor (purple, Fig3), NK cells were identified at the periphery of the plug in close proximity to endothelial cells present as isolated cells or less frequently in tubular structures. Together, these results provided evidence that IL-12 stimulates NK-cell migration to the margins of bFGF-induced Matrigel plugs, where endothelial cells are also recognized.
Cell identification and localization by double staining for asialo GM1 and von Willebrand factor. Immunohistochemical analysis of a paraffin-embedded section from a Matrigel plug stimulated with bFGF + IL-12 using mouse monoclonal antibody to asialo GM1 and a rabbit anti–von Willebrand factor antiserum. Asialo GM1-positive cells (dark brown) are indicated by arrows; von Willebrand factor positive cells are purple. Original magnification ×40.
Cell identification and localization by double staining for asialo GM1 and von Willebrand factor. Immunohistochemical analysis of a paraffin-embedded section from a Matrigel plug stimulated with bFGF + IL-12 using mouse monoclonal antibody to asialo GM1 and a rabbit anti–von Willebrand factor antiserum. Asialo GM1-positive cells (dark brown) are indicated by arrows; von Willebrand factor positive cells are purple. Original magnification ×40.
NK-cell requirement for angiogenesis inhibition by IL-12.
To dissect a potential role for NK cells as mediators of angiogenesis inhibition by IL-12, we used two reagents that can suppress NK-cell function: antiasialo GM1 antibody31,32 and anti-NK1.1 antibody.33 Groups of five athymic mice (BALB/c or C57BL/6) were first inoculated IP with antiasialo GM1 antibody (BALB/c, 1 mg/mouse) or anti-NK1.1 antibody (C57BL/6, 100 μg/mouse), and 24 hours later were injected subcutaneously (0.5 mL total injection volume) with Matrigel plus bFGF (150 ng/mL) plus antiasialo GM1 antibody (BALB/c, 250 μg/mL) or anti–NK 1.1 antibody (C57BL/6, 50 μg/mL), or with Matrigel plus bFGF (150 ng/mL) plus IL-12 (100 ng/mL) plus antiasialo GM1 antibody (BALB/c, 250 μg/mL) or anti–NK1.1 antibody (C57BL/6, 50 μg/mL). On day 3 after the injection of Matrigel, the mice were injected again IP with antiasialo GM1 antibody (BALB/c, 1 mg/mouse) or anti–NK1.1 antibody (C57BL/6, 100 μg/mouse). Groups of five control mice received subcutaneous injections (0.5 mL total injection volume) or Matrigel alone, Matrigel plus bFGF (150 ng/mL), or Matrigel plus bFGF (150 ng/mL) plus IL-12 (100 ng/mL). All plugs were removed on day 7, processed for histology, and angiogenesis quantified by a semiautomated digital analyzer set to measure areas occupied by cells on Matrigel sections. The results of four experiments using antiasialo GM1 antibodies and of one experiment using NK 1.1 antibody are listed in Table 2. In both sets of experiments, bFGF stimulated angiogenesis as documented by a 19- and 23-fold increase in the area occupied by cells on Matrigel sections, and IL-12 inhibited this bFGF-induced angiogenesis by 67.9% and 76.9%. Antiasialo GM1 and anti–NK 1.1 antibody treatment reversed by 92.6% and 76.9%, respectively, the inhibition of angiogenesis by IL-12, but had little effect on the stimulation of angiogenesis by bFGF. These results demonstrated that neutralization of NK-cell function reduces significantly the ability of IL-12 to act as an inhibitor of bFGF-induced angiogenesis in vivo, and strongly suggested that NK cells can mediate angiogenesis inhibition by IL-12.
Effects of Antiasialo GM1 and Anti–NK 1.1 Antibodies on IL-12 Regulation of Angiogenesis In Vivo
| Additions to Matrigel . | Surface Area Occupied by Cells (μm2/1.26 × 105 μm2) (±SD) . | Inhibition (%) . |
|---|---|---|
| BALB/c nude | ||
| None | 484 (±179) | — |
| bFGF | 9,329 (±1,476) | — |
| bFGF + IL-12 | 2,989 (±638) | 67.9 |
| bFGF + antiasialo GM1 | 10,269 (±2,302) | — |
| bFGF + IL-12 + antiasialo GM1 | 8,638 (±1,688) | 7.4 |
| B57BL/6 nude | ||
| None | 565 (±89) | — |
| bFGF | 13,472 (±1,118) | — |
| bFGF + IL-12 | 3,112 (±389) | 76.9 |
| bFGF + anti–NK 1.1 | 12,363 (±3,347) | — |
| bFGF + IL-12 + anti–NK 1.1 | 10,361 (±604) | 2.3 |
| Additions to Matrigel . | Surface Area Occupied by Cells (μm2/1.26 × 105 μm2) (±SD) . | Inhibition (%) . |
|---|---|---|
| BALB/c nude | ||
| None | 484 (±179) | — |
| bFGF | 9,329 (±1,476) | — |
| bFGF + IL-12 | 2,989 (±638) | 67.9 |
| bFGF + antiasialo GM1 | 10,269 (±2,302) | — |
| bFGF + IL-12 + antiasialo GM1 | 8,638 (±1,688) | 7.4 |
| B57BL/6 nude | ||
| None | 565 (±89) | — |
| bFGF | 13,472 (±1,118) | — |
| bFGF + IL-12 | 3,112 (±389) | 76.9 |
| bFGF + anti–NK 1.1 | 12,363 (±3,347) | — |
| bFGF + IL-12 + anti–NK 1.1 | 10,361 (±604) | 2.3 |
Mice (BALB/c or C57BL/6 nudes) were injected subcutaneously with Matrigel alone, Matrigel + bFGF, Matrigel + bFGF + IL-12, or Matrigel + bFGF + IL-12 + rabbit antiasialo GM1 (BALB/c mice) or anti–NK 1.1 (C57BL/6 mice) antibodies. The mice injected with Matrigel containing antiasialo GM1 or anti–NK 1.1 antibodies were also treated with antiasialo GM1 (1 mg/mouse) or anti–NK 1.1 (100 mg/mouse) antibodies IP, 1 day before as well as 3 days after Matrigel injection. The results reflect the mean (±SD) surface area occupied by cells (μm2 area/1.26 × 105 μm2area) from 12 to 15 fields in each Matrigel plug, 5 Matrigel plugs/group; 4 independent experiments with antiasialo GM1 and 1 experiment with anti–NK 1.1 were performed. Determinations of surface area were performed by a semiautomated digitized analyzer.
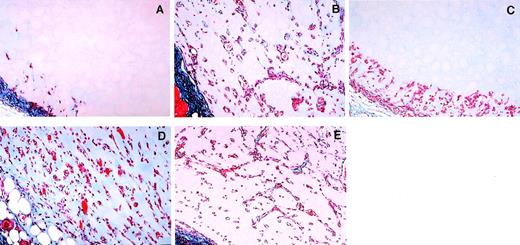
Histologic sections depicting representative Matrigel plugs derived from the five experimental groups are depicted in Fig 4. As shown, plugs with Matrigel alone (Fig4A) characteristically contained only few infiltrating cells at the margin of the plug, while bFGF-impregnated plugs (Fig 4B) contained abundant infiltrating cells, often organized to form tubular structures or capillaries containing red blood cells. Plugs containing bFGF and IL-12 (Fig 4C) usually displayed cells confined to the margin of the plug, but not reaching deeper in the plug. bFGF-impregnated plugs from mice treated systemically and locally with antibody to asialo GM1 (Fig 4D) were similar in morphology to bFGF-impregnated plugs from animals not treated with the antibody (Fig 4B). In addition, bFGF plus IL-12 impregnated plugs from mice treated with antibody to asialo GM1 (Fig4E) contained numerous cells, often organized in tubular structures and capillaries, and thus differed substantially from bFGF plus IL-12–treated plugs in mice not treated with the antibody (compare Fig4E and C), resembling instead plugs containing bFGF alone (compare Fig4E and B). Thus, the angiogenic process induced by bFGF appeared to be only modestly reduced by IL-12 when NK cells were functionally impaired.
Representative photomicrographs depicting the effects of antiasialo GM1 antibody on inhibition of bFGF-induced neovascularization by IL-12. Female BALB/c mice were injected subcutaneously with Matrigel alone (A), Matrigel impregnated with bFGF alone (B), bFGF + IL-12 (C), bFGF + antiasialo GM1 antibody (D), or bFGF + antiasialo GM1 antibody + IL-12 (E). Matrigel plugs were removed 7 days after injection and were processed for histology. The sections were stained with Masson’s trichrome. Original magnification ×20.
Representative photomicrographs depicting the effects of antiasialo GM1 antibody on inhibition of bFGF-induced neovascularization by IL-12. Female BALB/c mice were injected subcutaneously with Matrigel alone (A), Matrigel impregnated with bFGF alone (B), bFGF + IL-12 (C), bFGF + antiasialo GM1 antibody (D), or bFGF + antiasialo GM1 antibody + IL-12 (E). Matrigel plugs were removed 7 days after injection and were processed for histology. The sections were stained with Masson’s trichrome. Original magnification ×20.
Pathway of NK-cell recruitment and activation.
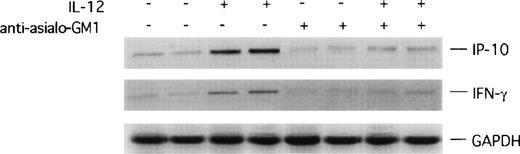
Since the data above demonstrated that IL-12 requires NK cells to act as an inhibitor of angiogenesis, and that NK cells are present at sites of inhibited neovascularization by IL-12, we examined potential mechanisms by which NK cells might mediate the angiostatic effect of IL-12. It is known that IL-12 is a potent inducer of IFN-γ4 and IP-10, an IFN-γ–inducible chemokine that acts as a potent chemotactic factor for activated T and NK cells.18,34 35 It was thus possible that in the nude mouse that is T-cell–immunodeficient, IFN-γ and IP-10 stimulation by IL-12 might account for the presence of activated NK cells at the sites of inhibited vascularization by IL-12. We therefore looked for evidence of IFN-γ and IP-10 expression in Matrigel plugs removed from nude mice displaying evidence of an angiostatic effect by IL-12. Using RT-PCR analysis applied to total RNA extracted from bFGF-induced Matrigel plugs, we found the PCR products of IFN-γ and IP-10 amplifications to be more abundant from Matrigel plugs impregnated with IL-12 than without it (representative results from duplicate plugs shown in Fig4). By contrast, in antiasialo GM1–treated animals, the amplification products of IFN-γ and IP-10 from plugs induced or not induced with IL-12 were similar in magnitude, and less abundant than the corresponding PCR products from Matrigel plugs impregnated with IL-12 in the absence of antibody (representative results shown in Fig5). These results suggested that IL-12 induces expression of the IFN-γ and IP-10 genes at the Matrigel plug site, and that this effect of IL-12 requires the presence of functional NK cells.
RT-PCR analysis of IP-10 and IFN-γ expression in Matrigel plugs. mRNA was prepared from duplicate sets of Matrigel plugs impregnated with bFGF alone, bFGF + IL-12, bFGF + antiasialo GM1 antibody, or bFGF + IL-12 + antiasialo GM1 antibody, reverse-transcribed, and subjected to PCR amplification using specific primers for murine IP-10 and IFN-γ. The amplified products were electrophoresed through a 1.5% agarose gel.
RT-PCR analysis of IP-10 and IFN-γ expression in Matrigel plugs. mRNA was prepared from duplicate sets of Matrigel plugs impregnated with bFGF alone, bFGF + IL-12, bFGF + antiasialo GM1 antibody, or bFGF + IL-12 + antiasialo GM1 antibody, reverse-transcribed, and subjected to PCR amplification using specific primers for murine IP-10 and IFN-γ. The amplified products were electrophoresed through a 1.5% agarose gel.
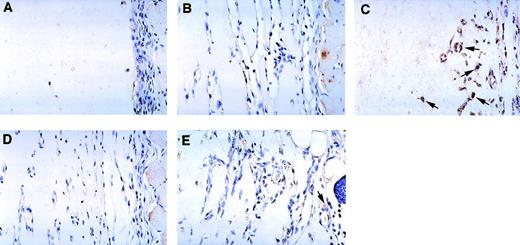
We wished to confirm these results and looked for the presence of IP-10 protein by immunohistochemistry (Fig 6, see page 1616). Using an antiserum to murine IP-10, essentially no immunopositive cells were detected in Matrigel plugs alone (Fig 6A), Matrigel plugs induced by bFGF (Fig 6B), or Matrigel plugs induced by bFGF in mice treated with anti–NK 1.1 antibody (Fig6D). By contrast, strong positive staining was detected in Matrigel plugs impregnated with bFGF and IL-12, mostly localized at the periphery of the plug (Fig 6C). Since most of the cells in these plugs were identified as endothelial cells due to their staining for von Willebrand factor (Fig 2), we concluded that endothelial cells infiltrating the plugs are a likely source of IP-10 in the presence of IL-12. After treatment with anti–NK 1.1 antibody, Matrigel plugs impregnated with bFGF and IL-12 displayed occasional IP-10 faintly immunopositive cells (Fig 6E). These results are consistent with those from RT-PCR analysis, and demonstrate the presence of IP-10 at those sites where angiogenesis is inhibited by IL-12.
Immunohistochemical detection of murine IP-10 in Matrigel plugs from C57BL/6 mice treated with or without anti–NK 1.1 monoclonal antibody treatment. Paraffin-embedded sections from Matrigel plugs alone (A); plugs impregnated with bFGF (B); plugs impregnated with bFGF + IL-12 (C); plugs impregnated with bFGF + monoclonal anti–NK 1.1 antibody (D); and plugs impregnated with bFGF + IL-12 + monoclonal anti–NK 1.1 antibody (E) were stained with a rabbit antimouse IP-10 antiserum and counterstained with hematoxylin. Some IP-10–positive cells (brown) are indicated by arrows. Original magnification ×40.
Immunohistochemical detection of murine IP-10 in Matrigel plugs from C57BL/6 mice treated with or without anti–NK 1.1 monoclonal antibody treatment. Paraffin-embedded sections from Matrigel plugs alone (A); plugs impregnated with bFGF (B); plugs impregnated with bFGF + IL-12 (C); plugs impregnated with bFGF + monoclonal anti–NK 1.1 antibody (D); and plugs impregnated with bFGF + IL-12 + monoclonal anti–NK 1.1 antibody (E) were stained with a rabbit antimouse IP-10 antiserum and counterstained with hematoxylin. Some IP-10–positive cells (brown) are indicated by arrows. Original magnification ×40.
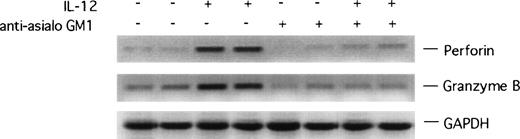
To examine whether IL-12–induced NK cells at the Matrigel plug site are activated, we looked for evidence of increased expression of perforin and granzyme B, molecules that are involved in NK-cell–mediated killing, and expression of which is increased with NK-cell activation.36 37 As shown in representative results (Fig 7), the RT-PCR products of perforin and granzyme B amplifications were more abundant in RNA samples from Matrigel plugs impregnated with IL-12 plus bFGF as opposed to plugs containing bFGF alone, suggesting that IL-12 promotes NK-cell activation in this system. As expected, Matrigel plugs from antiasialo GM1–treated animals did not display evidence of IL-12–induced expression of perforin and granzyme B genes (Fig 7).
RT-PCR analysis of murine perforin and granzyme B expression in Matrigel plugs. mRNA was prepared from Matrigel plugs impregnated with bFGF alone, bFGF + IL-12, bFGF + antiasialo GM1 antibody, or bFGF + IL-12 + antiasialo GM1 antibody, reverse-transcribed, and subjected to PCR amplification using specific primers for murine perforin and granzyme B. The amplified products were electrophoresed through a 1.5% agarose gel.
RT-PCR analysis of murine perforin and granzyme B expression in Matrigel plugs. mRNA was prepared from Matrigel plugs impregnated with bFGF alone, bFGF + IL-12, bFGF + antiasialo GM1 antibody, or bFGF + IL-12 + antiasialo GM1 antibody, reverse-transcribed, and subjected to PCR amplification using specific primers for murine perforin and granzyme B. The amplified products were electrophoresed through a 1.5% agarose gel.
NK-cell accumulation and vascular damage in experimental Burkitt tumors treated locally with IL-12.
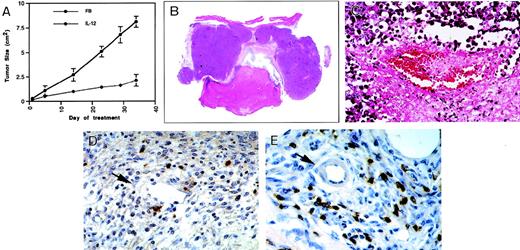
The results presented above demonstrated that NK cells are required for inhibition of angiogenesis by IL-12, and that NK cells expressing the activation markers perforin and granzyme B accumulate at sites where angiogenesis is inhibited by IL-12. We asked whether NK cells might contribute to angiogenesis inhibition by IL-12 more directly than by providing for IFN-γ and IP-10. Particularly, we looked for evidence that activated NK cells might kill endothelial cells and disrupt vascularization by this mechanism. To this end, we examined the effects of IL-12 on a preexisting and yet rapidly evolving tumor vascular bed, and selected a model in which human Burkitt tumors are reproducibly established subcutaneously in athymic mice.27,38 As expected,39 local inoculations of IL-12 (200 ng/d, 7 d/wk), but not buffer alone, reduced Burkitt tumor growth (Fig 8A, see page 1616). Burkitt tumors treated locally with IL-12 for 34 days displayed the characteristic central necrosis, extending to the epidermis (Fig 8B). Histologically, there was diffuse evidence of vascular injury, characterized by intraluminal thrombosis (Fig 8C) and intimal thickening in IL-12–treated, but not buffer-treated tumors. Immunocytochemical staining for activated NK cells using either anti-B220 monoclonal antibody (Fig 8D and E) or staining with antiasialo GM1 antibody (not shown) showed intense focal staining in IL-12–treated, but not buffer-treated Burkitt tumors. NK cells localized almost exclusively within the boundary area that separates live from necrotic tumor tissue. Here, NK cells were detected surrounding capillaries (Fig 8D) and small arterioles (Fig 8E). RT-PCR analysis demonstrated expression of perforin and granzyme genes in IL-12–treated, but not control-treated tumors, suggesting the presence of activated NK cells (not shown). These experiments demonstrate that, when applied to a tumor vascular bed, IL-12 can promote extensive vascular damage and tumor tissue necrosis. In addition, IL-12 can induce the accumulation of activated NK cells at those sites where tumor tissue necrosis usually progresses, particularly surrounding blood vessels.
Vascular effects and NK-cell recruitment in experimental Burkitt tumors treated locally with IL-12. (A) Effects of local diluent (FB) or IL-12 inoculation (200 ng/mouse/d, 7 d/wk) on the growth of Burkitt tumors established subcutaneously in nude mice. The results reflect mean (±SD) tumor sizes of 4 animals/group. (B) Gross morphology of a representative Burkitt tumor treated locally with IL-12 for 34 days (no magnification). (C) Microscopic morphology of a representative Burkitt tumor treated locally with IL-12 for 34 days depicting vascular pathology (original magnification ×40). (D, E) Immunohistochemical detection of NK cells by the B220 monoclonal antibody in representative Burkitt tumors treated locally with IL-12 depicting positive cell staining surrounding a capillary vessel with discontinuous endothelium (D, original magnification ×40), and positive cell staining surrounding a small arteriole (E, original magnification ×40).
Vascular effects and NK-cell recruitment in experimental Burkitt tumors treated locally with IL-12. (A) Effects of local diluent (FB) or IL-12 inoculation (200 ng/mouse/d, 7 d/wk) on the growth of Burkitt tumors established subcutaneously in nude mice. The results reflect mean (±SD) tumor sizes of 4 animals/group. (B) Gross morphology of a representative Burkitt tumor treated locally with IL-12 for 34 days (no magnification). (C) Microscopic morphology of a representative Burkitt tumor treated locally with IL-12 for 34 days depicting vascular pathology (original magnification ×40). (D, E) Immunohistochemical detection of NK cells by the B220 monoclonal antibody in representative Burkitt tumors treated locally with IL-12 depicting positive cell staining surrounding a capillary vessel with discontinuous endothelium (D, original magnification ×40), and positive cell staining surrounding a small arteriole (E, original magnification ×40).
NK-cell cytolysis of endothelial-cell targets.
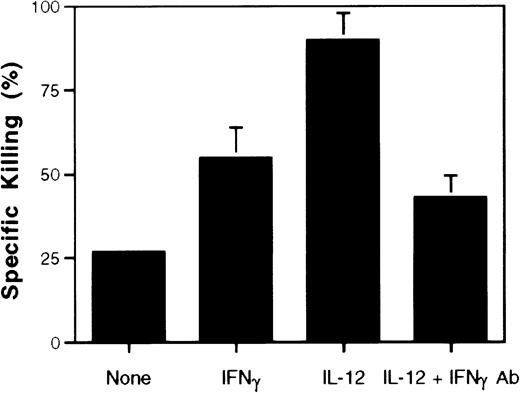
We wished to test directly whether activated NK cells could kill endothelial cells in an in vitro syngeneic system. For these experiments, we used NK-cell–enriched populations (95% positive for asialo GM1) derived from spleens of C57BL/6 nude mice as effector cells, and activated them by 24-hour culture with IL-12 (10 ng/mL). As targets, we used primary cultures of mouse aortic endothelial cells (>90% positive for von Willebrand factor) obtained from C57BL/6 nude littermates. In a representative 51Cr release experiment (Fig 9), IL-12–treated NK-cell–enriched splenocytes exhibited strong cytolytic activity against aortic endothelial cells (63% killing above background). IFN-γ treatment alone increased NK-cell killing by 28% above background. A neutralizing antibody to IFN-γ added in conjunction with IL-12 during preculture reduced significantly (47% reduction) endothelial-cell killing by NK cells stimulated with IL-12. These experiments demonstrate that activated NK cells can kill endothelial-cell targets.
Endothelial-cell killing by IL-12–activated NK cells. NK-cell–enriched splenocytes from C57BL/6 nude mice were first incubated for 24 hours in medium alone, medium supplemented with IL-12 (10 ng/mL), IFN-γ (100 ng/mL) or a combination of IL-12 (10 ng/mL) and an anti–IFN-γ antibody (5 μg/mL), and then tested for cytotoxicity against 51Cr-labeled aortic endothelial cells from littermate C57BL/6 nude mice at an E:T ratio of 120:1. Results are expressed as percent specific cytotoxicity, and reflect the mean (±SD) of four replicate cultures.
Endothelial-cell killing by IL-12–activated NK cells. NK-cell–enriched splenocytes from C57BL/6 nude mice were first incubated for 24 hours in medium alone, medium supplemented with IL-12 (10 ng/mL), IFN-γ (100 ng/mL) or a combination of IL-12 (10 ng/mL) and an anti–IFN-γ antibody (5 μg/mL), and then tested for cytotoxicity against 51Cr-labeled aortic endothelial cells from littermate C57BL/6 nude mice at an E:T ratio of 120:1. Results are expressed as percent specific cytotoxicity, and reflect the mean (±SD) of four replicate cultures.
DISCUSSION
These studies demonstrate that NK-cell function is required for IL-12 to act as an inhibitor of angiogenesis. Using Matrigel plugs impregnated with bFGF in an athymic mouse model, we found that neutralization of NK-cell activity with antibodies to asialo GM1 or NK 1.1 antigens reduced by 77% to 93% the level of angiogenesis inhibition induced by IL-12. NK cells were readily demonstrable in those sites where neovascularization was inhibited by IL-12, but not in controls, and there was evidence that these NK cells were activated because IFN-γ, as well as the lytic mediators perforin and granzyme B, were locally expressed. The chemokine IP-10 was also expressed at these sites, suggesting that it may serve to chemoattract NK cells at the plug site. When applied to a preexisting, and yet rapidly evolving, tumor vascular bed, IL-12 caused extensive vascular damage, tumor tissue necrosis, and accumulation of activated NK cells surrounding small tumor vessels. In vitro, IL-12–activated NK cells exerted effective cytotoxicity against syngeneic endothelial cells. Together, these results may explain how IL-12 can inhibit angiogenesis. In brief, we believe that IL-12 stimulates the production of IFN-γ by the rare NK cells present locally in the tissues, and IFN-γ produced by these NK cells in turn stimulates IP-10 secretion by neighboring endothelial cells, monocyte/macrophages, and other resident cells. IP-10 chemoattracts additional NK cells, and these cells contribute to the secretion of additional IFN-γ and, secondarily, IP-10. In addition to being locally recruited, the NK cells become activated by IFN-γ. Once activated, NK cells can become more strongly cytolytic and kill neighboring endothelial cells. As a result, neovascularization is impaired. It should be noted that the current results derive from experiments in athymic mice that are T-cell–immunodeficient. The relative contribution of NK cells in the context of euthymic mice was not examined here, and it is clear that CD8-positive T cells have been found to mediate most of the antitumor effects of IL-12.11-13
A number of reports have documented effective endothelial cells killing in vitro by activated human allogeneic NK cells.40 41 We have shown here that once activated in vitro by IL-12, murine NK cells become strongly cytotoxic for primary cultures of syngeneic endothelial cells. However, the extent to which NK-cell killing of endothelial cells contributes to IL-12 inhibition of bFGF-induced angiogenesis in vivo is presently unclear. The current experiments localized NK cells at sites where angiogenesis was inhibited by IL-12, and detected expression of the perforin and granzyme B genes, results that are consistent with the presence of activated cytolytic NK cells. However, we cannot exclude the involvement of soluble inhibitors of angiogenesis produced by activated NK cells.
In an athymic murine tumor model, treatment of established tumors with IP-10 over a period of 30 to 40 days produced extensive damage to the tumor vasculature, including capillary wall fragmentation, intimate thickening, and intravascular thrombosis.27 Similar extensive destruction of the established tumor vasculature was noted with prolonged local treatment with IL-12.39 Here, we found that tumors treated in this manner also displayed evidence of NK-cell recruitment and activation at the tumor site, particularly at the interface between viable and necrotic tumor tissue, surrounding small vessels. Since Burkitt cells are resistant to NK cytotoxicity,42 these results further support the notion that IL-12 can initiate an NK-cell–mediated process leading to extensive damage of established or developing vasculature.
Once activated with IL-2, NK cells can adhere to and lyse human endothelial cells cultured in vitro.40,43 The destruction of endothelial cells by IL-2–activated NK cells was proposed to contribute to the toxicity of high-dose IL-2 treatment, particularly to the capillary leakage syndrome.43,44 One might speculate that NK-cell killing of endothelial cells at a tumor site might directly contribute to the antitumor effects of IL-2 treatment by reducing tumor vascularization. However, when tested for their effects on bFGF-induced Matrigel neovascularization, IL-2 exhibited little or no effect.45 Unlike IL-12, IL-2 is not known to directly or indirectly promote NK-cell migration to a particular site. In the absence of sufficient accumulation of activated NK cells at a site of neovascularization, endothelial-cell killing may be insufficient to cause inhibition of neovascularization. It is of interest that IL-15, a cytokine functionally related to IL-2, stimulated angiogenesis.45 Stimulation by IL-15 was attributed to a direct effect of this cytokine on endothelial cells that express the α, β, and γ subunits of the IL-15 receptor and undergo rapid protein phosphorylation in response to IL-15.45
For many inhibitors of angiogenesis, including fumagillin, angiostatin, endostatin, platelet factor 4, and thrombospondin, the mechanisms of action are presently unknown, but for others mechanisms have been proposed.46 Apoptosis in endothelial cells engaged in neovascularization was believed to represent the mechanism by which antagonists of the integrin αvβ3 inhibit angiogenesis.47 Neutralization of a growth factor required by endothelial cells for growth was the reason why a monoclonal antibody to vascular endothelial growth factor could inhibit neovascularization.48,49 Intravascular thrombosis induced by a monoclonal antibody to a cell-surface domain of human tissue factor was the cause of reduced blood supply in an experimental tumor model.50 Inactivation of enzymes that degrade extracellular matrix proteins thereby favoring endothelial-cell spread may contribute to the inhibition of angiogenesis by inhibitors of metalloproteinase.51 52 However, NK-cell cytotoxicity of angiogenic endothelial cells have not been proposed previously as effective mechanisms for regulation of tissue vascularization.
IL-12 has shown promise as an anticancer agent in a number of experimental tumor models. The anticancer properties of IL-12 have generally been attributed to its ability to boost host immunity, particularly the activity of cytotoxic T and NK cells.11-13The observation that IL-12 is also an effective inhibitor of neovascularization raised the possibility that this mechanism of action might account for some of the antitumor activities of the cytokine, even in the context of T-cell immunodeficiency.11 13 The findings, described in this report, that NK-cell activation, chemotaxis, and ultimately lysis of endothelial cells engaged in neovascularization is a mechanism by which IL-12 may act as an inhibitor of angiogenesis, confirm the central role of T and NK cells as mediators of the biologic activities of IL-12 through their secretion of IFN-γ. They further stress the important role of the interplay between host immunity and tumor-cell biology.
ACKNOWLEDGMENT
The authors thank Drs J.A. Farber, A. Rosenberg, and R. Yarchoan for their suggestions and critical review of the manuscript.
Supported in part by an appointment to the Postgraduate Research Participation Program at the Center for Biologics Evaluation and Research administered by Oak Ridge Institute for Science and Education through an Interagency Agreement between the Department of Energy and the Food and Drug Administration.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions on its use.
REFERENCES
Author notes
Address reprint requests to Lei Yao, PhD, Division of Hematologic Products, Center for Biologics Evaluation and Research, FDA, Building 29A, Room 2D06, 8800 Rockville Pike, Bethesda, MD 20892; e-mail:YAO@A1.CBER.FDA.GOV.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal