Abstract
The expression and function of a glycoprotein Ib (GPIb) complex on human umbilical vein endothelial cells (HUVECs) is still a matter of controversy. We characterized HUVEC GPIb using viper venom proteins: alboaggregins A and B, echicetin, botrocetin, and echistatin. Echicetin is an antagonist, and alboaggregins act as agonists of the platelet GPIb complex. Botrocetin is a venom protein that alters von Willebrand factor (vWF) conformation and increases its binding affinity for the GPIb complex. Echistatin is a disintegrin that blocks vβ3. Echistatin, but not echicetin, inhibited the adhesion to vWF of Chinese hamster ovary (CHO) cells transfected with vβ3. We found the following: (1) Binding of monoclonal antibodies against GPIb to HUVECs was moderately increased after stimulation with cytokines and phorbol ester. Echicetin demonstrated an inhibitory effect. (2) Both echicetin and echistatin, an vβ3 antagonist, inhibited the adhesion of HUVECs to immobilized vWF in a dose-dependent manner. The inhibitory effect was additive when both proteins were used together. (3) Botrocetin potentiated the adhesion of HUVECs to vWF, and this effect was completely abolished by echicetin, but not by echistatin. (4) CHO cells expressing GPIbβ/IX adhered to vWF (in the presence of botrocetin) and to alboaggregins; GPIb was required for this reaction. Echicetin, but not echistatin, inhibited the adhesion of cells transfected with GPIbβ/IX to immobilized vWF. (5) HUVECs adhered strongly to immobilized vWF and alboaggregins with extensive spreading, which was inhibited by LJ1b1, a monoclonal antibody against GPIb. The purified vβ3 receptor did not interact with the alboaggregins, thereby excluding the contribution of vβ3 in inducing HUVEC spreading on alboaggregins. In conclusion, our data confirm the presence of a functional GPIb complex expressed on HUVECs in low density. This complex may mediate HUVEC adhesion and spreading on immobilized vWF and alboaggregins.
VON WILLEBRAND factor (vWF) secreted by endothelial cells is an important component of the extracellular matrix maintaining adhesion of these cells. It is well established that the interaction of vWF with human umbilical vein endothelial cells (HUVECs) is mediated by αvβ3 integrin, a constitutively expressed protein on the membranes of these cells.1,2 There have also been reports suggesting that vWF interaction with HUVECs could be partially mediated by an endothelial glycoprotein Ib (GPIb) complex; however, this is still controversial. Several investigators reported immunoreactive GPIb in HUVECs.3-5 Konkle et al6identified endothelial GPIbα in tonsil sections by an immunochemical method and subsequently isolated GPIbα cDNA clones from HUVECs. Furthermore, Beacham et al5 showed that the binding of vWF to HUVECs could be inhibited by monoclonal antibodies (MoAbs) directed against GPIb. Wu et al7 demonstrated that the full GPIb/IX/V complex is expressed on the HUVEC surface. It has been shown that before cell harvest, combined treatment with interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) upregulates the synthesis and surface expression of GPIb on HUVECs.5-8Thus, both αvβ31,2,9 and GPIb5 have been shown to play a role in mediating HUVEC adhesion to vWF. Moreover, Beacham et al10 also found an increased biosynthesis of GPIb in HUVECs subjected to shear stress, a condition that can be found in vivo in stenosed arteries. Other researchers have found that GPIb participated in HUVEC binding to sickle cell erythrocytes.11 Recently, Bombeli et al12 have shown that endothelial GPIbα participates in platelet bridging to endothelial cells.
It should be noted that the biosynthesis of a GPIb complex in HUVECs has been questioned in some recent studies. Perrault et al13 could not identify GPIb mRNA in HUVECs by Northern blot analysis and they could not confirm protein expression on the surface of these cells by immunologic techniques. Zieger et al14 compared clone cDNA of GPIbβ precursors from human erythroleukemia cell lines exhibiting megakaryocytic properties with the cDNA of putative GPIbβ precursors from endothelial cells. They proposed that the 45-kD protein identified as an endothelial GPIbβ by Kelly et al15 may actually belong to an entirely different class of proteins. They suggested that endothelial GPIbβ “results from the incomplete polyadenylation” of an upstream gene and read through transcription into the GIPb gene.14 In his recent review, Ware16 suggested that GPIb gene expression in endothelial cells was low and might require very sensitive methods to detect. Expression of GPIb in HUVECs may be caused by the leaky activity of a promoter typically expressed in megakaryocytic cell lines. According to Ware,16 there are no convincing data supporting the presence of a functional GPIb/IX/V complex anywhere other than on the surface of megakaryocytes and platelets.
In the current study, we used the following viper venom proteins: alboaggregin A, alboaggregin B (also called big alboaggregin with a molecular weight of 50 kD, and small alboaggregin with a molecular weight of 25 kD),17,18 echicetin,19-21botrocetin,22,23 and echistatin24 for identification and functional characterization of the endothelial GPIb complex. Alboaggregins and echicetin compete with vWF in its binding to the platelet GPIb complex. Botrocetin22,23 modifies vWF, increasing the binding affinity of vWF for the platelet GPIb complex. Echicetin19 acts as a potent inhibitor of platelet agglutination induced by bovine vWF or by human vWF and a cofactor, either ristocetin or botrocetin. Alboaggregins A and B, on the other hand, induce agglutination of formalin-fixed platelets in the absence of any cofactors.17,25-27 Alboaggregin A, acting on the GPIb complex, also induces calcium-dependent signals for platelet activation, leading to platelet aggregation and the release reaction.26,27 The platelet-activating ability of alboaggregin A could be blocked by echicetin and by MoAbs that are specific for the vWF binding site on the N-terminal of GPIbα, but not by antibodies that recognize other regions on the GPIb complex.26
Alboaggregins and echicetin are highly homologous to each other26 and to a number of other viper venom proteins, including factor IXa/Xa binding protein,28botrocetin,22,23 agkicetin,29flavocetins,30 tokaracetins,31 and yoshitobin.32 Most proteins in this group are heterodimers,33 except for alboaggregin A, which has a tetrameric structure. Each subunit of these proteins constitutes a domain structure known as a carbohydrate recognition domain (CRD). This structure was first identified as the minimum functional motif of Ca2+-dependent animal lectins.34,35 Echistatin is a disintegrin inhibiting αIIbβ3 and αvβ3.24 36
Our study using alboaggregins, echicetin, and botrocetin confirms the presence of a GPIb complex on endothelial cells and suggests that it may play a role, in concert with αvβ3 receptors, in the interaction of these cells with vWF leading to cell adhesion and spreading. We also found that selective stimulation of HUVEC GPIb by alboaggregins results in extensive cell spreading, suggesting cytoskeletal mobilization. In addition, we found that Chinese hamster ovary (CHO) αβ/IX cells and HUVECs share similar patterns of interaction with vWF and viper venom proteins, except that the spreading of transfected cells on immobilized ligands was minimal.
MATERIALS AND METHODS
Materials.
Lyophilized snake venoms of Trimeresurus albolabris andEchis carinatus were purchased from Latoxan (Rosans, France). Human vWF, fibronectin, and vitronectin were purchased from Calbiochem (San Diego, CA). TNF-α was purchased from Boehringer Mannheim (Indianapolis, IN), and IFN-γ was from GIBCO Life Technologies (Grand Island, NY). LJIb1, an anti-GPIbα MoAb that blocks vWF binding, was kindly provided by Dr Z.M. Ruggeri (Scripps Research Institute, La Jolla, CA). Other monoclonal GPIbα binding antibodies, SZ2 and WM23, and botrocetin were kindly provided by Dr M.C. Berndt (Baker Research Institute, Prahran, Victoria, Australia). A MoAb recognizing αvβ3 integrin, LM609, was purchased from Chemicon (Temecula, CA), and the anti-αvβ3/αIIbβ3 integrin antibody, 7E3, was kindly provided by Dr B.S. Coller (Mount Sinai Medical Center, New York, NY). C-18 reverse phase columns were purchased from Vydac (Hesperia, CA). Ion exchange Mono S and Mono Q HR 5/5 columns were from Pharmacia-LKB (Uppsala, Sweden). Other chemicals were purchased from Fisher Scientific (Pittsburgh, PA) and Sigma Chemical Co (St Louis, MO).
Purification of alboaggregins, echicetin, and echistatin.
Alboaggregin A and B were purified from the venom of Trimeresurus albolabris according to a previously described method.17,18 Echicetin and echistatin were purified from the same sample of Echis carinatus venom. The venom was dissolved in trifluoracetic acid and applied to a C18 column. Protein-containing fractions were eluted with an increasing acetonitrile gradient. Echistatin-containing fractions, eluted at 42% acetonitrile, were collected and reloaded on reverse-phase high-performance liquid chromatography (HPLC) using a shallow gradient. Echicetin-containing fractions were eluted at about 70% acetonitrile on reverse-phase HPLC and were then purified by means of cation-exchange chromatography.19 The purity of all proteins was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with the Phast Gel System (Pharmacia) using 20% homogeneous gels and by determination of the N-terminal sequence. Pooled pure fractions of these proteins were further tested for their functional activities. Alboaggregins A and B induced agglutination of formalin-fixed platelets with the same potency. Alboaggregin A aggregated washed platelets in a dose-dependent manner and this effect saturated at 20 nmol/L.27 At 200 nmol/L, echicetin completely inhibited the agglutination of formalin-fixed platelets by alboaggregins and by vWF in the presence of ristocetin. Echistatin, a disintegrin, inhibited ADP-induced platelet aggregation in platelet-rich plasma (PRP) with an IC50 of 130 nmol/L, as previously described.37 Eristostatin was obtained from Eristocophis macmahoni venom, as described by McLane et al.37 To test viper venom proteins, we isolated platelets by differential centrifugation as previously described.38 Formalin-fixed platelets were prepared using the method of Kirby.39
HUVEC culture.
HUVECs were isolated from freshly collected umbilical cords as described by Jaffe et al40 and cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO) containing 20% fetal calf serum, 2 mmol/L penicillin/streptomycin and L-glutamine, 10 μg/mL heparin, and 60 μg/mL endothelial cell growth supplement obtained from porcine hypothalamus extract. The cells were incubated at 37°C in the presence of 5% CO2. HUVECs were passaged at a 1:3 ratio, and cultures from the third to sixth passages were used for flow cytometry or adhesion assays. Cell suspensions were obtained by washing the cultures twice with cation-free Hank’s Balanced Salt Solution (HBSS) and then detaching the cells with HBSS containing 5 mmol/L EDTA, 100 μmol/L leupeptin, and 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF).
Stimulation of HUVECs with cytokines or phorbol myristate acetate (PMA) treatment.
HUVECs were treated with combined IFN-γ and TNF-α, as originally described by Rajagopalan et al.41 Briefly, 10 ng/mL (final concentration) of IFN-γ was added to HUVECs at about 80% confluence and 72 hours before cell detachment. TNF-α (100 U/mL) was added to the culture medium 16 hours before cell detachment. The culture medium was changed daily. Treatment with IFN-γ caused a characteristic elongation of the endothelial cells. The addition of TNF-α resulted in an upregulation of intercellular adhesion molecule-1 (ICAM-1), which was confirmed by flow cytometry of the cell suspension using an anti–ICAM-1 MoAb (kindly provided by Dr D. Mosser, Temple University, Philadelphia, PA).
PMA treatment has been reported to upregulate GPIb in cancer cells.42 We therefore used a similar method to upregulate the endothelial GPIb complex. In brief, 20 ng/mL of PMA was added to the cell culture medium 24 hours before cell detachment. Resembling IFN-γ, PMA caused an elongation of individual cells. In addition, HUVECs exposed to PMA were packed more densely compared with nonstimulated cells.
Transfected CHO cells.
CHO cells transfected with αvβ3 receptors (VNRC3 cells)43 were kindly provided by Dr M.H. Ginsberg (Scripps Research Institute, La Jolla, CA). Nontransfected CHO-K1 cells were purchased from ATCC (Rockville, MD) and used as control cells. Both VNRC3 and K1 cells were maintained in DMEM containing 10% fetal bovine serum and supplemented with nonessential amino acids, L-glutamine, and penicillin/streptomycin. CHOαβ/IX cells (CHO cells that stably express the entire GPIb/IX complex) and CHOβ/IX (which express GPIbβ and GPIX) were maintained as previously described.44 Briefly, CHOαβ/IX cells were grown in α-minimal essential media (α-MEM; GIBCO) containing 10% fetal bovine serum and penicillin/streptomycin and supplemented with geneticin, methotrexate, and hygromycin. The medium for CHOβ/IX cells was supplemented only with geneticin and methotrexate. Confluent cells were detached from plates with trypsin-EDTA for passaging or with Versene (GIBCO) for flow cytometry and adhesion experiments to avoid proteolysis of GPIbα by trypsin. The cells were then resuspended in α-MEM containing 1% bovine serum albumin (BSA) for flow cytometry or cell adhesion assays. Both VNRC3 cells and CHOαβ/IX cells were routinely checked by flow cytometry for surface expression of the receptors. Specific MoAbs, LM609 and 7E3, were used to assess the expression of αvβ3. LJ1b1, SZ2, and WM23 were used to assess the GPIbα surface expression. The CHOαβIX cells were sorted occasionally to retain a cell population with a high degree of receptor expression.
Cell adhesion.
Ninety-six–well microplates were coated with various concentrations of proteins or antibodies in phosphate-buffered saline (PBS) and left at 4°C overnight. The plates were then rinsed twice with PBS and blocked with 3% BSA in PBS for at least 1 hour at 37°C. After rinsing with PBS, the plates were ready for application of the cells. HUVEC or CHO cell suspensions were prepared as described above, the concentration was adjusted to 2 × 105 cells/mL, and the suspensions were incubated in the absence or presence of inhibitors or antibodies for 15 minutes on ice before being applied (at 100 μL/well) to the plates. The cells were incubated in the microplates at 37°C in 5% CO2 for 2 hours. Unattached cells were gently washed away with PBS. The attached cells were then fixed for 30 minutes with 1% formaldehyde at room temperature and stained with methylene blue according to Oliver et al.45 The relative number of adherent cells was calculated by lysing the stained cells with 50% ethanol and 50% hydrochloric acid and then reading the absorbance at 630 nm on a microplate autoreader (Bio-Tek Instruments, Winooski, VT). The percentage of cells adhering to the plate was determined based on the linear relationship between the absorbance reading and the number of cells counted in a hemocytometer. The amount of nonspecific adhesion was determined by using wells precoated with BSA only. In cell adhesion assays in which an antibody was used as a substrate, mouse IgG was used as a control substrate. The stained adherent cells were photographed using an Olympus camera (Hitech Instruments Inc, Chester Pike, Edgmont, PA) and Kodak T-Max 100 film (Eastman Kodak, Rochester, NY). For fluorescence staining, the cells were applied to chamber glass slides that has been precoated with various agents, fixed with 1% formaldehyde, and dried with 100% ethanol. The slides were stained with sulforhodamine for detection of proteins and 4,6-diamidino-2-phenylindole (DAPI) for detection of DNA. Photographs were then taken under a fluorescence microscope.
Flow cytometry.
HUVECs and transfected CHO cells were prepared as described above and the concentration was adjusted to 5 × 106 cells/mL. Five hundred-microliter aliquots of each cell suspension were added to several sample tubes. In the case of platelets, the concentration was adjusted to 1 × 1010 platelets/mL, and 10 μL was used in a final volume of 50 μL for each sample. The cells were then preincubated with various proteins or antibodies for 15 minutes at room temperature. MoAbs, prepared in PBS containing 1% BSA, were then added to the cells for 1 hour on ice with occasional agitation. The cells were then spun down, washed three times with PBS containing 1% BSA, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG for 1 hour on ice. After washing three times with PBS, each cell sample was resuspended in 250 μL PBS and then fixed by the addition of 150 μL of 3% formaldehyde. The samples were then analyzed on a Coulter Epics Elite flow cytometer (Miami, FL). Debris and dead cells were excluded by forward- and side-scatter gating. Incubating the cells with FITC-labeled secondary antibody alone assessed nonspecific binding.
Interaction of purified αvβ3 receptor with disintegrins and alboaggregin B.
αvβ3 integrin was purified from VNRC3 cells as described by Marcinkiewicz et al.46 In brief, cells were lysed by octylglucoside. The cell lysates were then applied onto a GRGDSPK-agarose column and receptor-containing fractions were eluted with EDTA. Echistatin, eristostatin, or alboaggregin B were immobilized on a 96-well enzyme-linked immunosorbent assay (ELISA) plate in 0.05 mol/L bicarbonate buffer, pH 9.3, and by overnight incubation at 4°C. Echistatin was used as a positive control. For a negative control, we used eristostatin, a disintegrin that does not interact with αvβ3.35 The wells were blocked with 5% nonfat milk in PBS containing 0.05% Tween. Purified αvβ3 (0.7 μg/per well) was added to the wells and the plate was incubated for 30 minutes at 37°C. Binding of a rabbit polyclonal anti-αvβ3 antibody (Chemicon, Temecula, CA) to the bound receptor was assessed using a biotinylated goat antirabbit anti-body according to the Vecta Stain ABC HRP kit (#PK-4001; Vector Laboratories, Burlingame, CA).
RESULTS
GPIb expression on the resting and activated HUVECs.
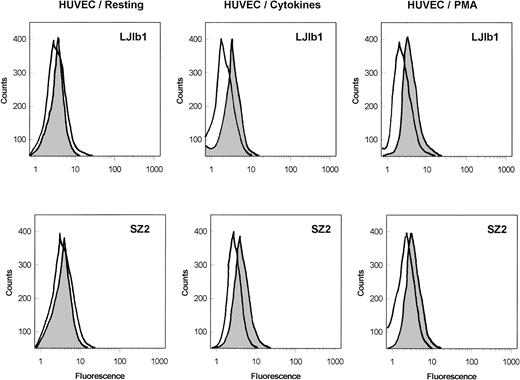
As shown in Fig 1, the binding of the monoclonal anti-GPIbα antibodies, LJ1b1 and SZ2, to nonstimulated HUVECs was very low; however, it was significantly increased after stimulation of HUVECs with IFN-γ and TNF-α or with PMA. Echicetin at concentrations greater than 100 nmol/L inhibited binding of these antibodies to stimulated HUVECs (not shown). In both stimulated and nonstimulated HUVECs, the binding of SZ2 to the cells was consistently less pronounced than the binding of LJb1. Binding of antibody WM23 and of nonimmune IgG to HUVEC was not significant (not shown). Both LJIb1 and SZ2 bound to washed platelets and to CHOαβIX cells, but not to CHOβIX cells (data not shown), confirming the specificity of these antibodies.
Binding of MoAbs against GPIb, LJ1b1, and SZ2 to the suspensions of HUVECs. Resting HUVECs: After HUVEC detachment, 500 μL aliquots of 5 × 106 HUVECs/mL were incubated with 2 μg/mL LJ1b1 or SZ2 for 1 hour on ice (shaded peak). As a control, an aliquot of HUVECs was treated only with FITC-conjugated goat-antimouse antibody (open peak). Flow cytometry was conducted after washing with PBS as described in Materials and Methods. HUVECs stimulated with cytokines: HUVECs were treated with IFN-γ and TNF- before cell detachment as described by Konkle et al.6 After HUVEC detachment, the experimental procedure was the same as that described for the resting HUVECs. HUVECs stimulated with PMA: The binding of LJ1b1 and SZ2 to HUVECs was increased by pretreating HUVECs in culture with 20 ng/mL PMA for 24 hours. A maximal increase in binding was observed under these conditions, although the increase was more pronounced for LJ1b1 than for SZ2.
Binding of MoAbs against GPIb, LJ1b1, and SZ2 to the suspensions of HUVECs. Resting HUVECs: After HUVEC detachment, 500 μL aliquots of 5 × 106 HUVECs/mL were incubated with 2 μg/mL LJ1b1 or SZ2 for 1 hour on ice (shaded peak). As a control, an aliquot of HUVECs was treated only with FITC-conjugated goat-antimouse antibody (open peak). Flow cytometry was conducted after washing with PBS as described in Materials and Methods. HUVECs stimulated with cytokines: HUVECs were treated with IFN-γ and TNF- before cell detachment as described by Konkle et al.6 After HUVEC detachment, the experimental procedure was the same as that described for the resting HUVECs. HUVECs stimulated with PMA: The binding of LJ1b1 and SZ2 to HUVECs was increased by pretreating HUVECs in culture with 20 ng/mL PMA for 24 hours. A maximal increase in binding was observed under these conditions, although the increase was more pronounced for LJ1b1 than for SZ2.
The effect of viper venom proteins on the adhesion of HUVECs to immobilized vWF.
Nonstimulated HUVECs adhered extensively to immobilized vWF and this adhesion was enhanced by the presence of botrocetin during incubation. However, cytokine-stimulated HUVECs adhered less extensively to immobilized vWF than did resting HUVECs (not shown). This was probably due to downregulation of αvβ3.47 The experiments to be described below were conducted with resting HUVECs in the presence or absence of botrocetin.
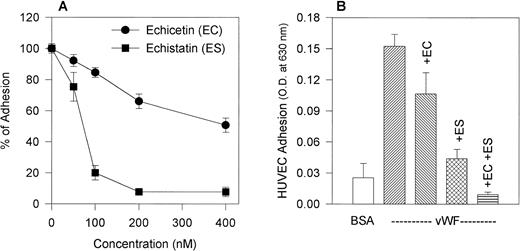
We compared the effect of the disintegrin echistatin, an αvβ3 inhibitor, and echicetin, a GPIb inhibitor, on the adhesion of HUVECs to immobilized vWF (Fig 2A). Both proteins inhibited adhesion in a dose-dependent manner with echistatin producing near complete inhibition at 200 nmol/L. Echicetin, on the other hand, inhibited only about 35% of the adhesion at the same concentration. A representative experiment shows that echistatin at 50 nmol/L and echicetin at 200 nmol/L partially inhibited HUVEC adhesion to vWF and that the effect of both inhibitors was additive (Fig 2B).
(A) Effect of echicetin and echistatin on HUVEC adhesion to immobilized vWF. HUVECs were preincubated with progressively increasing concentrations of echicetin and echistatin (an vβ3 antagonist) for 15 minutes on ice before being applied to a 96-well microplate previously coated with vWF as described in Materials and Methods. OD readings of the lysed cells indicated that about 45% of resting HUVECs adhered to immobilized vWF, and this value was used as 100% adhesion. (•) Echicetin; (▪) echistatin. The data represent the mean and SD of percentage of cell adhesion from four individual experiments. (B) Additive inhibition of echicetin and echistatin on HUVEC adhesion to immobilized vWF. HUVECs were preincubated with either 250 nmol/L echicetin (EC; lane 3), 50 nmol/L echistatin (ES; lane 4), or both 250 nmol/L echicetin and 50 nmol/L echistatin (EC + ES; lane 5) and were then applied onto a vWF-coated plate. For a negative control (lane 1), BSA-coated wells were used. Lane 2 represents HUVEC adhesion to vWF in the absence of inhibitors.
(A) Effect of echicetin and echistatin on HUVEC adhesion to immobilized vWF. HUVECs were preincubated with progressively increasing concentrations of echicetin and echistatin (an vβ3 antagonist) for 15 minutes on ice before being applied to a 96-well microplate previously coated with vWF as described in Materials and Methods. OD readings of the lysed cells indicated that about 45% of resting HUVECs adhered to immobilized vWF, and this value was used as 100% adhesion. (•) Echicetin; (▪) echistatin. The data represent the mean and SD of percentage of cell adhesion from four individual experiments. (B) Additive inhibition of echicetin and echistatin on HUVEC adhesion to immobilized vWF. HUVECs were preincubated with either 250 nmol/L echicetin (EC; lane 3), 50 nmol/L echistatin (ES; lane 4), or both 250 nmol/L echicetin and 50 nmol/L echistatin (EC + ES; lane 5) and were then applied onto a vWF-coated plate. For a negative control (lane 1), BSA-coated wells were used. Lane 2 represents HUVEC adhesion to vWF in the absence of inhibitors.
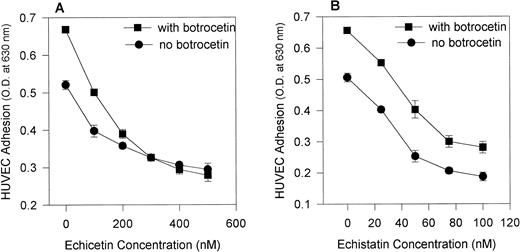
The addition of botrocetin, a venom protein that alters vWF conformation (making it more accessible to GPIb) increased the adhesion of HUVECs to vWF. Figure 3 shows that echicetin (Fig 3A) abolished botrocetin-induced cell adhesion in a dose-dependent manner, whereas echistatin (Fig 3B) did not. At a concentration of 300 nmol/L, echicetin completely inhibited botrocetin-enhanced adhesion of HUVECs to vWF. At concentrations ranging from 20 to 100 nmol/L, echistatin inhibited, with an identical pattern, the adhesion of HUVECs both to unmodulated vWF and to vWF modulated by botrocetin. Botrocetin-enhanced cell adhesion was not altered even in the presence of 100 nmol/L echistatin. These experiments exclude a contribution by αvβ3 to the botrocetin-enhanced adhesion of HUVECs to vWF. This observation was also confirmed with the use of MoAbs against GPIbα and αvβ3. Table 1 shows that MoAbs directed against αvβ3 inhibited to the same extent HUVEC adhesion to vWF and to botrocetin-treated vWF, whereas MoAbs against GPIbα inhibited more strongly HUVEC adhesion to botrocetin-treated vWF.
Effect of echicetin and echistatin on botrocetin-enhanced HUVEC adhesion to immobilized vWF. A 96-well microplate was coated with vWF, incubated overnight at 4°C, and then blocked with 3% BSA. HUVECs, after detachment, were applied to the microplate in the absence (•) and presence (▪) of 10 μg/mL botrocetin during the 2-hour incubation period. Various concentrations of echicetin (A) and echistatin (B) were added to the HUVECs before their application to the plate. In (B), the difference between samples examined in the presence and absence of botrocetin was significant at P < .05 for all concentrations of echistatin studied. In (A) (for echicetin), this statistically significant difference was not observed except for the control sample.
Effect of echicetin and echistatin on botrocetin-enhanced HUVEC adhesion to immobilized vWF. A 96-well microplate was coated with vWF, incubated overnight at 4°C, and then blocked with 3% BSA. HUVECs, after detachment, were applied to the microplate in the absence (•) and presence (▪) of 10 μg/mL botrocetin during the 2-hour incubation period. Various concentrations of echicetin (A) and echistatin (B) were added to the HUVECs before their application to the plate. In (B), the difference between samples examined in the presence and absence of botrocetin was significant at P < .05 for all concentrations of echistatin studied. In (A) (for echicetin), this statistically significant difference was not observed except for the control sample.
Inhibition of HUVEC Adhesion to Immobilized vWF in the Presence and Absence of Botrocetin by MoAbs Against GPIb (LJIb1 and SZ2) and Against vβ3 (LM609 and 7E3)
| MoAb . | Inhibition of HUVEC Adhesion (%) . | |
|---|---|---|
| vWF (control) . | vWF (+botrocetin) . | |
| LJIb1 | 21 ± 3.8 | 32 ± 3.2 |
| SZ2 | 16 ± 4.2 | 34 ± 4.4 |
| LM609 | 56 ± 4.3 | 55 ± 3.5 |
| 7E3 | 61 ± 2.5 | 63 ± 2.1 |
| MoAb . | Inhibition of HUVEC Adhesion (%) . | |
|---|---|---|
| vWF (control) . | vWF (+botrocetin) . | |
| LJIb1 | 21 ± 3.8 | 32 ± 3.2 |
| SZ2 | 16 ± 4.2 | 34 ± 4.4 |
| LM609 | 56 ± 4.3 | 55 ± 3.5 |
| 7E3 | 61 ± 2.5 | 63 ± 2.1 |
HUVECs were preincubated for 15 minutes on ice with antibodies against either GPIbα or with antibodies against αvβ3 at a concentration of 20 μg/mL, followed by the addition of botrocetin (10 μg/mL, final concentration). The cells were then applied to vWF-coated microplates and incubated in the presence of the antibodies for 2 hours at 37°C with 5% CO2. The results are shown as means of the percentage of inhibition of cell adhesion and standard deviations. These data represent results from three individual experiments. The difference between the inhibitory effects of LJIb1 and SZ2 on samples with and without botrocetin is at P < .05.
The experiments described above indicate that, besides αvβ3, an RGD-dependent receptor, GPIb, an RGD-independent receptor, mediates HUVEC adhesion to vWF in the substratum.
vWF and viper venom protein interactions with HUVECs and CHO cells transfected with GPIbαβ/IX or αvβ3.
In further experiments, we compared the adhesion of HUVECs, CHOαβIX cells, and VNRC3 cells to various substrates. HUVECs adhered comparably to immobilized vWF, alboaggregins, and echicetin. The adhesion of HUVECs to vWF did not require botrocetin (Fig 4A). CHOαβ/IX cells adhered to immobilized vWF but only in the presence of botrocetin. Adhesion of these cells to alboaggregins and echicetin did not require botrocetin (Fig 4B). Control (nontransfected) CHO cells and CHOβ/IX cells did not adhere to any of these substrates, indicating that GPIbα is critical for the interaction of CHOαβ/IX cells with these substrates. This finding is consistent with the previous observation that GPIbα was required for the agglutination of CHOαβ/IX cells in the presence of vWF.46
(A) HUVEC adhesion to immobilized ligands. A 96-well microplate was coated with various ligands of 1% BSA (lane 1), 15 μg/mL vWF (lane 2), 50 μg/mL echicetin (lane 3), 50 μg/mL alboaggregin A (lane 4), and 50 μg/mL alboaggregin B (lane 5) overnight at 4°C. After blocking with 3% BSA, 100-μL aliquots of 2 × 105 HUVECs/mL were added to each well. After 2 hours of incubation at 37°C in the presence of 5% CO2, the attached cells were fixed and stained with methylene blue. The cells were then lysed and an OD reading was taken. The data represent the mean and SD of OD readings from five individual experiments. (B) Adhesion of transfected CHO cells to immobilized ligands. A 96-well microplate was coated with various ligands (BSA [lane 1], vWF [lane 2], echicetin [lane 3], alboaggregin A [lane 4], and alboaggregin B [lane 5]) and blocked with BSA. Aliquots (100 μL) of CHOβIX cells at 2 × 105 cells/mL were added to each well. Wells coated with vWF received cells that had been treated with 10 μg/mL botrocetin. Cells were incubated on the plate for 2 hours at 37°C in the presence of 5% CO2. CHOβIX cells and control (untransfected) CHO cells showed baseline adhesion to immobilized vWF that was comparable to the adhesion level of CHOβIX cells to BSA (lane 1).
(A) HUVEC adhesion to immobilized ligands. A 96-well microplate was coated with various ligands of 1% BSA (lane 1), 15 μg/mL vWF (lane 2), 50 μg/mL echicetin (lane 3), 50 μg/mL alboaggregin A (lane 4), and 50 μg/mL alboaggregin B (lane 5) overnight at 4°C. After blocking with 3% BSA, 100-μL aliquots of 2 × 105 HUVECs/mL were added to each well. After 2 hours of incubation at 37°C in the presence of 5% CO2, the attached cells were fixed and stained with methylene blue. The cells were then lysed and an OD reading was taken. The data represent the mean and SD of OD readings from five individual experiments. (B) Adhesion of transfected CHO cells to immobilized ligands. A 96-well microplate was coated with various ligands (BSA [lane 1], vWF [lane 2], echicetin [lane 3], alboaggregin A [lane 4], and alboaggregin B [lane 5]) and blocked with BSA. Aliquots (100 μL) of CHOβIX cells at 2 × 105 cells/mL were added to each well. Wells coated with vWF received cells that had been treated with 10 μg/mL botrocetin. Cells were incubated on the plate for 2 hours at 37°C in the presence of 5% CO2. CHOβIX cells and control (untransfected) CHO cells showed baseline adhesion to immobilized vWF that was comparable to the adhesion level of CHOβIX cells to BSA (lane 1).
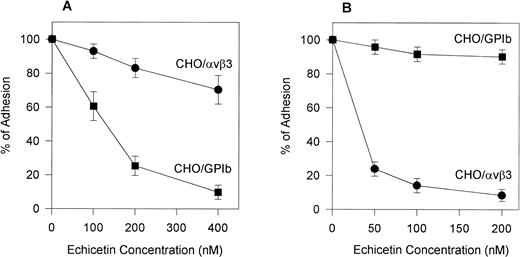
To confirm the specificity of echistatin and echicetin, we tested their inhibitory effect on CHOαβ/IX cells and VNRC3 cells adhering to vWF. Echicetin (Fig 5A) had a profound inhibitory effect on the adhesion of CHOαβ/IX cells to vWF but little effect on the adhesion of VNRC3 cells to immobilized vWF. At a concentration of 50 nmol/L, echistatin (Fig 5B) inhibited the adhesion of VNRC3 cells to vWF by approximately 80%. This percentage of inhibition was much higher than that seen (<50%) when echistatin was used as an inhibitor of HUVEC adhesion to vWF. Echistatin had no significant effect on the adhesion of CHOαβIX cells to vWF (Fig 5B).
Effect of echicetin (A) and echistatin (B) on CHO cells transfected with either vβ3 or GPIb complex. A 96-well microplate was coated with vWF. Confluent CHO cells transfected with either vβ3 (•) or GPIbβIX (▪) were detached with Versene (GIBCO) for 30 minutes at 37°C. After washing with PBS, the cells were resuspended in PBS containing 1 mmol/L CaCl2 and MgCl2. The concentration of the cell suspension was adjusted to 2 × 105 cells/mL. Varying concentrations of echicetin and echistatin were added to 100 μL aliquots of the cell suspension 15 minutes before 2 hours of incubation on the plate. Unbound cells were gently washed away with PBS. The adhesion procedure was followed according to the legend of Fig 2.
Effect of echicetin (A) and echistatin (B) on CHO cells transfected with either vβ3 or GPIb complex. A 96-well microplate was coated with vWF. Confluent CHO cells transfected with either vβ3 (•) or GPIbβIX (▪) were detached with Versene (GIBCO) for 30 minutes at 37°C. After washing with PBS, the cells were resuspended in PBS containing 1 mmol/L CaCl2 and MgCl2. The concentration of the cell suspension was adjusted to 2 × 105 cells/mL. Varying concentrations of echicetin and echistatin were added to 100 μL aliquots of the cell suspension 15 minutes before 2 hours of incubation on the plate. Unbound cells were gently washed away with PBS. The adhesion procedure was followed according to the legend of Fig 2.
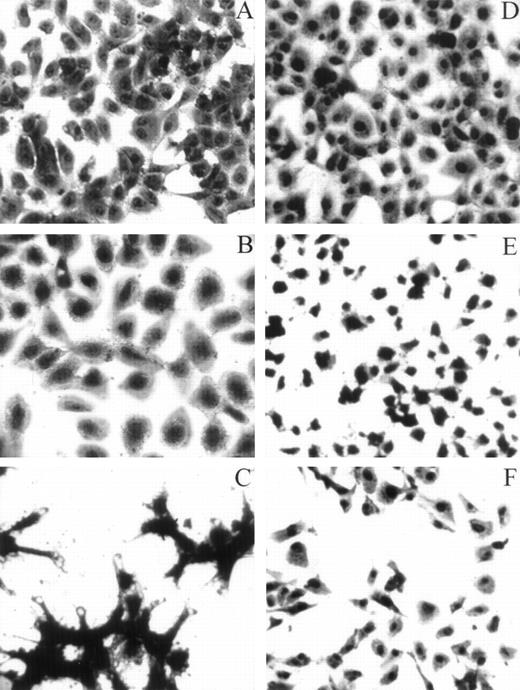
HUVECs adherent to various substrata examined by light microscopy. For the adhesion assay, refer to the legend for Fig 4A. Whenever inhibitors were used, HUVECs were preincubated with either LJIb1 or echicetin for 15 minutes on ice before being applied, together with the inhibitors, onto the precoated plate. This figure shows the morphology of HUVECs adherent to different ligands observed under light microscopy. HUVECs adherent to vWF (A), alboaggregin A (B), and alboaggregin B (D) showed extensive cell spreading, whereas cells attached to echicetin (C) showed cell clumping without prominent cell spreading. The presence of 50 μg/mL LJIb1 (E) or 500 nmol/L echicetin (F) demonstrated inhibition of cell adhesion and cell spreading on alboaggregin B.
HUVECs adherent to various substrata examined by light microscopy. For the adhesion assay, refer to the legend for Fig 4A. Whenever inhibitors were used, HUVECs were preincubated with either LJIb1 or echicetin for 15 minutes on ice before being applied, together with the inhibitors, onto the precoated plate. This figure shows the morphology of HUVECs adherent to different ligands observed under light microscopy. HUVECs adherent to vWF (A), alboaggregin A (B), and alboaggregin B (D) showed extensive cell spreading, whereas cells attached to echicetin (C) showed cell clumping without prominent cell spreading. The presence of 50 μg/mL LJIb1 (E) or 500 nmol/L echicetin (F) demonstrated inhibition of cell adhesion and cell spreading on alboaggregin B.
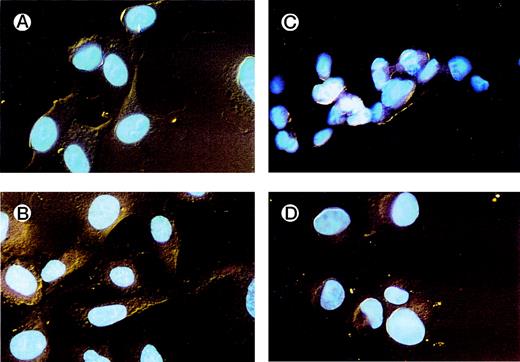
HUVECs adherent to various substrata examined by fluorescence microscopy. HUVECs were allowed to adhere to different ligands on chamber slides. After fixation with formaldehyde and staining with sulforhodamine and DAPI, the slides were examined by fluorescence microscopy. Details of the nuclei and the cytoplasm were better visualized using this dual stain. HUVECs adherent to vWF (A) and alboaggregin B (B) demonstrated extensive cell spreading, whereas HUVECs adherent to echicetin (C) and echistatin (D) showed minimal cell spreading.
HUVECs adherent to various substrata examined by fluorescence microscopy. HUVECs were allowed to adhere to different ligands on chamber slides. After fixation with formaldehyde and staining with sulforhodamine and DAPI, the slides were examined by fluorescence microscopy. Details of the nuclei and the cytoplasm were better visualized using this dual stain. HUVECs adherent to vWF (A) and alboaggregin B (B) demonstrated extensive cell spreading, whereas HUVECs adherent to echicetin (C) and echistatin (D) showed minimal cell spreading.
Effect of GPIb-binding proteins on HUVEC spreading.
HUVECs adhered comparably to immobilized vWF, echicetin, and alboaggregins A and B as assessed by measuring the optical density of lysed cells (Fig 4A). HUVECs adhered and spread extensively on both immobilized vWF and alboaggregins as visualized by methylene blue staining (Fig 6). Cells adhering to echicetin clumped with little, if any, spreading. There was minimal adhesion to immobilized BSA or mouse IgG (not shown). In the presence of 50 μg/mL of LJIb1, the adhesion of HUVECs to alboaggregin A was decreased by 55% as determined by OD reading, and spreading was also significantly inhibited. Figure 7 shows adherent HUVECs stained with sulforhodamine and DAPI. This double fluorescence staining visualizes in greater detail the cytoplasmic alterations of adhering cells. There were well-defined surface protrusions on HUVECs adherent to vWF and alboaggregins, but no such protrusions were visible on cells adherent to echicetin and echistatin.
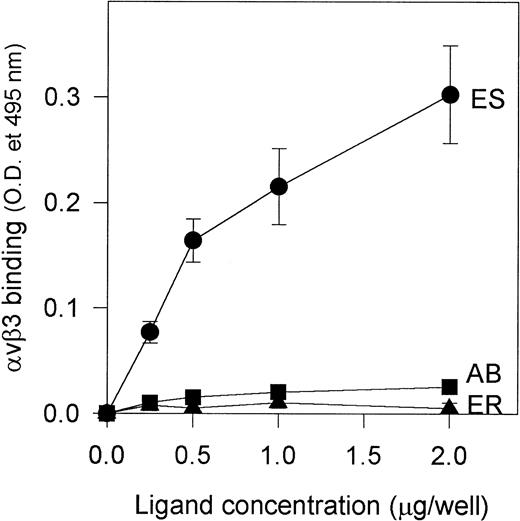
Because alboaggregins induced such dramatic HUVEC cell spreading and αvβ3 is the most abundant receptor on HUVECs mediating cell attachment and cell shape change, we studied the interaction of purified αvβ3 receptor with alboaggregins, echistatin, and eristostatin (as a negative control) to evaluate the direct involvement of αvβ3 in this adhesive phenomenon. Purified αvβ3 bound to immobilized echistatin in a dose-dependent manner but not to alboaggregin B (a similar finding with alboaggregin A; data not shown) or eristostatin (Fig 8). This experiment strongly suggests that alboaggregins, by interacting with the endothelial GPIb complex, might induce the cytoskeletal reorganization that is necessary for cell spreading. It is noteworthy that there was no significant spreading of CHOαβIX cells on immobilized vWF, alboaggregins, or echicetin at least after 2 hours of incubation, suggesting that the cellular signaling apparatus that is necessary for GPIb-mediated cell activation in HUVECs is not available.
Binding of purified vβ3 to immobilized alboaggregin B, echistatin, and eristostatin. A 96-well microplate was coated with various concentrations of alboaggregin B, eristostatin, and echistatin. The plate was then blocked with PBS containing 0.05% Tween (TPBS) and 5% nonfat dry milk for at least 1 hour. Seven hundred nanograms of purified vβ3 receptors was then added to each well and the plate was then incubated for 30 minutes at 37°C. Extensive washing with TPBS followed. Polyclonal rabbit IgG against vβ3 was used as primary antibody. After 1 hour of incubation at 37°C and subsequent washing, a biotinylated goat antirabbit IgG was used as secondary antibody. (•) vβ3 binding to immobilized echistatin; (▴) binding to immobilized eristostatin; (▪) binding to immobilized alboaggregin.
Binding of purified vβ3 to immobilized alboaggregin B, echistatin, and eristostatin. A 96-well microplate was coated with various concentrations of alboaggregin B, eristostatin, and echistatin. The plate was then blocked with PBS containing 0.05% Tween (TPBS) and 5% nonfat dry milk for at least 1 hour. Seven hundred nanograms of purified vβ3 receptors was then added to each well and the plate was then incubated for 30 minutes at 37°C. Extensive washing with TPBS followed. Polyclonal rabbit IgG against vβ3 was used as primary antibody. After 1 hour of incubation at 37°C and subsequent washing, a biotinylated goat antirabbit IgG was used as secondary antibody. (•) vβ3 binding to immobilized echistatin; (▴) binding to immobilized eristostatin; (▪) binding to immobilized alboaggregin.
DISCUSSION
The present study indicates that a GPIb complex is expressed on the surface of HUVECs in low quantities but in sufficient quantities to mediate the interaction of these cells with vWF. The viper venom proteins, alboaggregins, and echicetin proved to be useful in characterizing the endothelial GPIb complex because, in contrast to vWF, they interact with platelet GPIb/IX without the need for modulators. Ristocetin, or the viper venom protein botrocetin, are required for the interaction of vWF with the platelet GPIb complex in vitro. Alboaggregins and echicetin, on the other hand, bind platelet GPIb/IX directly without the assistance of any cofactor. Our data indicate that the same is true for CHOαβ/IX cells. These cells required botrocetin for adhesion to immobilized vWF, but they adhered extensively to immobilized alboaggregins and echicetin without the presence of any cofactor (Fig 4B).
The following evidence indicates that a GPIb/IX complex is expressed on the surface of HUVECs and mediates, together with αvβ3, HUVEC interaction with vWF. (1) HUVECs bound two MoAbs, LJ1b1 and SZ2, that recognize distinct epitopes in the GPIbα N-terminus. (2) The adhesion of HUVECs to immobilized vWF was inhibited by echistatin, an αvβ3 antagonist, and by echicetin, a GPIb antagonist, in a dose-dependent manner. Furthermore, the inhibitory effect was additive. (3) The adhesion of HUVECs to vWF was enhanced by botrocetin, which forms a complex with vWF and makes it more accessible to GPIb. This enhanced adhesion was inhibited by echicetin but not by echistatin. Botrocetin-enhanced adhesion was also inhibited by MoAbs against GPIb but not by MoAbs against αvβ3. (4) HUVECs adhered to immobilized alboaggregins and subsequently spread. Cell spreading was inhibited by the GPIbα MoAb LJ1b1. Unlike vWF, alboaggregin A does not contain an αvβ3 binding site. Nevertheless, alboaggregin A exhibits the same activity as vWF does in the presence of its cofactor botrocetin.
Our study is consistent with the results of Beacham et al,8 Konkle et al,6 and Wu et al.7 It is at variance with Perrault et al,13who could not detect GPIb/IX on their preparations of HUVECs and reported that this putative endothelial complex was irrelevant for cell attachment to vWF. The reasons for these differences are not clear but may relate to differences in cell passage number, cell preparation, or culture techniques. Interindividual variations may also exist in the level of GPIbα expression on HUVECs, an issue that has not been examined systematically.
The platelet GPIb complex has been extensively investigated and its structure and function have mostly been identified.48-50There are several differences between the platelet and the endothelial GPIb complexes. Wu et al,7 using radiolabeled MoAb L1Jb1, found that endothelial cells and platelets each express 330,000 and 35,000 copies of a GPIb/IX complex, respectively. However, the percentage of this MoAb bound at saturation to endothelial cells was low. They also found that a number of MoAbs reacting with platelet GPIb/IX did not recognize the endothelial counterpart of this complex, and we confirmed this observation in flow cytometry experiments using the various MoAbs, LJIb1, SZ2, and WM23, of which only LJIb1 showed pronounced binding.
The differences between platelet and endothelial GPIb may relate to differences in posttranslational modifications in platelets and HUVECs, respectively.48-50 WM23 recognizes an epitope in the GPIbα macroglycopeptide, a region that undergoes extensive O-glycosylation. Because protein glycosylation is a cell-type–specific process, the epitopes for this antibody may vary with the cell type studied. This may be a reason why WM23 does not recognize GPIbα on endothelial cells. Similarly, SZ2 binds to a region of GPIbα that undergoes posttranslational sulfation of tyrosine and the differences in binding may relate to differences in protein sulfation in platelets and HUVECs.49,50 Despite these differences in endothelial and platelet complexes, there are some common mechanisms in their interaction with vWF. Botrocetin enhanced vWF binding to both platelet and endothelial GPIb. It is well established that the vWF binding site on platelet GPIb is located on GPIbα.48-53Our studies with CHOαβ/IX cells show that GPIbα is also required for the adhesion of these cells to immobilized alboaggregins and echicetin. The LJ1b1 binding site is also located on GPIbα. We conclude that endothelial GPIbα interacts specifically with alboaggregins, echicetin, and vWF. It is noteworthy that echicetin acts as an antagonist of both platelet and endothelial cell GPIb complexes, whereas alboaggregins act as agonists. In platelets, echicetin inhibits vWF-induced agglutination, whereas alboaggregins induce platelet agglutination and aggregation. In endothelial cells, echicetin inhibited cell adhesion to vWF and HUVECs adherent to echicetin did not spread. In contrast, HUVECs adherent to alboaggregins spread extensively. Thus, the ability of GPIb-binding proteins to promote HUVEC spreading correlates with their ability to activate platelets. Results of our study on the inhibitory effect of echicetin and echistatin on the adhesion of CHOαβ/IX and VNRC3 cells to vWF were in general agreement with the contention that both the GPIb binding site (the A1 domain) and the αvβ3 binding site (the RGD sequence in the C1 domain) of vWF participate in the interaction between vWF and endothelial cells.
Dong et al54 could not detect any significant intracellular signaling after the interaction of vWF with CHOαβ/IX cells. In our hands, CHOαβ/IX cells did not spread on immobilized vWF or alboaggregins during the 2-hour incubation period. In contrast, HUVEC adherence and spreading on alboaggregins was very rapid, suggesting that the endothelial GPIb complex may confer a signal resulting in reorganization of cytoskeletal structures in endothelial cells.
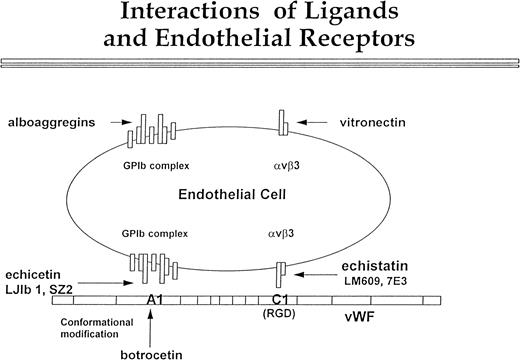
In conclusion, our data confirm the presence of GPIb on the surface of the endothelial cells (Fig 9). Although this complex is expressed in low density, it is definitely functional. We propose that it interacts with the A1 domain of vWF, and this interaction proceeds in parallel with αvβ3 binding to the C1 domain of vWF. Echicetin and MoAbs LJIb1 and SZ2 inhibit GPIb interaction with the A1 domain of vWF, and botrocetin potentiates this interaction. Echistatin and MoAbs LM609 and 7E3 inhibit interaction between αvβ3 and the C domain of vWF. Figure 9 also shows the possibility that endothelial GPIb and αvβ3 may interact independently of each other with alboaggregins and vitronectin. Nevertheless, the cooperation of both αvβ3 and GPIb receptors in endothelial cell interaction with vWF and other matrix components may significantly contribute to efficient hemostasis and tissue repair.
Interactions of ligands with endothelial cell receptors. This figure shows a putative scheme of the interaction between vβ3 and GPIb complex with vWF, vitronectin, and alboaggregins. The GPIb complex is composed of seven molecules. One molecule of GPV is flanked symmetrically by two molecules of GPIX, GPIb, and GPIbβ. vWF interaction with vβ3, mediated by the C1 domain, is inhibited by echistatin and MoAbs LJ1b and SZ2. Botrocetin alters the conformation of the A1 domain of vWF so that it can interact with GPIb complex. This interaction can be blocked by echicetin and by anti-GPIb MoAbs LJIb and SZ2. The GPIb complex and vβ3 cooperate in binding vWF, but each of these two receptors may independently bind specific ligands. Alboaggregins bind to the GPIb complex, and vitronectin binds to vβ3. Each of these interactions leads to cell spreading and cytoskeletal mobilization.
Interactions of ligands with endothelial cell receptors. This figure shows a putative scheme of the interaction between vβ3 and GPIb complex with vWF, vitronectin, and alboaggregins. The GPIb complex is composed of seven molecules. One molecule of GPV is flanked symmetrically by two molecules of GPIX, GPIb, and GPIbβ. vWF interaction with vβ3, mediated by the C1 domain, is inhibited by echistatin and MoAbs LJ1b and SZ2. Botrocetin alters the conformation of the A1 domain of vWF so that it can interact with GPIb complex. This interaction can be blocked by echicetin and by anti-GPIb MoAbs LJIb and SZ2. The GPIb complex and vβ3 cooperate in binding vWF, but each of these two receptors may independently bind specific ligands. Alboaggregins bind to the GPIb complex, and vitronectin binds to vβ3. Each of these interactions leads to cell spreading and cytoskeletal mobilization.
ACKNOWLEDGMENT
The authors thank Dr Michael C. Berndt for carefully reading the manuscript, Dr Dorothy Beacham and Dr Cezary Marcinkiewicz for helpful discussions, John Gibas for performing flow cytometry, and Dr Yuqing Wang and Mariola Marcinkiewicz for their help in preparing cultured cells.
Supported in part by National Institutes of Health Grant No. HL 45486 (to S.N.); a Training in Thrombosis and Hemostasis Grant No. T3 HL00777 (to L.T.); a grant from the American Heart Association Grant-in-Aid, Southeastern Pennsylvania affiliate (to S.N.); and an Established Investigator grant from the American Heart Association (96002750; to J.A.L.). The manuscript represents part of the dissertation (of L.T.) submitted to Temple University as partial fullfillment for the requirement of the degree of Doctor of Philosophy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stefan Niewiarowski, PhD, Department of Physiology, Temple University School of Medicine, 3400 N Broad St, OMS 200, Philadelphia, PA 19140; e-mail, stni@astro.ocis.temple.edu.

![Fig. 4. (A) HUVEC adhesion to immobilized ligands. A 96-well microplate was coated with various ligands of 1% BSA (lane 1), 15 μg/mL vWF (lane 2), 50 μg/mL echicetin (lane 3), 50 μg/mL alboaggregin A (lane 4), and 50 μg/mL alboaggregin B (lane 5) overnight at 4°C. After blocking with 3% BSA, 100-μL aliquots of 2 × 105 HUVECs/mL were added to each well. After 2 hours of incubation at 37°C in the presence of 5% CO2, the attached cells were fixed and stained with methylene blue. The cells were then lysed and an OD reading was taken. The data represent the mean and SD of OD readings from five individual experiments. (B) Adhesion of transfected CHO cells to immobilized ligands. A 96-well microplate was coated with various ligands (BSA [lane 1], vWF [lane 2], echicetin [lane 3], alboaggregin A [lane 4], and alboaggregin B [lane 5]) and blocked with BSA. Aliquots (100 μL) of CHOβIX cells at 2 × 105 cells/mL were added to each well. Wells coated with vWF received cells that had been treated with 10 μg/mL botrocetin. Cells were incubated on the plate for 2 hours at 37°C in the presence of 5% CO2. CHOβIX cells and control (untransfected) CHO cells showed baseline adhesion to immobilized vWF that was comparable to the adhesion level of CHOβIX cells to BSA (lane 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/8/10.1182_blood.v93.8.2605/5/m_blod40818004x.jpeg?Expires=1763626374&Signature=FkqkNib8XduqzoQTA3vhM363F6la~~HXuNHSSX4SXyqXbAbLd7FpS6PwmXExRgp2AIaG75It4B1Gx~N~xo~ojWMON9An5HrieAwkNfM0Fxmm1KRH9XivngdHEtd8jjnEgM2DfienOe0IOWawgZ66PlT9OkAK51zwtnpjm6U404Lj-NYOP~AlSsQNe7zUj7v2K4-OR~6XPVQvRiqqvKnJjf9pEbTzw~fazw46XeMubwrfBuREXT4my4LnsuKzdD8PPWnvbjSt4CUWgDRXbP~-VdW7dpWj373oSmNY4yqxKzz65snqlqkH0T3eW~VC8JBcuWeaKu6ili2begCis9j35w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal