Abstract
Nonhuman primate models are useful to evaluate the safety and efficacy of new therapeutic modalities, including gene therapy, before the inititation of clinical trials in humans. With the aim of establishing safe and effective approaches to therapeutic gene transfer, we have been focusing on a small New World monkey, the common marmoset, as a target preclinical model. This animal is relatively inexpensive and easy to breed in limited space. First, we characterized marmoset blood and bone marrow progenitor cells (BMPCs) and showed that human cytokines were effective to maintain and stimulate in culture. We then examined their susceptibility to transduction by retroviral vectors. In a mixed culture system containing both marmoset stromal cells and retroviral producer cells, the transduction efficiency into BMPCs and peripheral blood progenitor cells (PBPCs) was 12% to 24%. A series of marmosets then underwent transplantation with autologous PBPCs transduced with a retroviral vector carrying the multidrug resistance 1 gene (MDR1) and were followed for the persistence of these cells in vivo. Proviral DNA was detectable by polymerase chain reaction (PCR) in peripheral blood granulocytes and lymphocytes in the recipients of gene transduced progenitors up to 400 days posttransplantation. To examine the function of the MDR1 gene in vivo, recipient maromsets were challenged with docetaxel, an MDR effluxed drug, yet the overall level of gene transfer attained in vivo (<1% in peripheral blood granulocytes) was not sufficient to prevent the neutropenia induced by docetaxel treatment. Using this model, we safely and easily performed a series of in vivo studies in our small animal center. Our results show that this small nonhuman primate, the common marmoset, is a useful model for the evaluation of gene transfer methods targeting hematopoietic stem cells.
THE DEVELOPMENT of new therapeutic modalities such as cytokine therapy and gene therapy has opened a new era in the treatment of intractable diseases such as genetic disorders, cancer, and acquired immunodeficiency syndrome (AIDS). Preclinical animal models are important for evaluating the safety and therapeutic efficacy of such modalities. Murine models have been commonly used for the study of cytokine and gene transfer technology, yet murine models may not reliably predict biology in larger animals and humans. Nonhuman primate models have emerged as desirable models from both a pathophysiologic and pharmacokinetic viewpoint. Particularly in the field of gene therapy, the study of nonhuman primates can provide important information on the suitability of gene transfer vector systems in vivo before human trials are undertaken. Gene transfer studies using large nonhuman primates have been reported by several institutions.1-4 However, a practical, economical primate model is needed for institutions without the space or resouces to support a large primate colony. Such a model would accelerate basic studies of gene therapy.
For this reason, we have recently focused on a small New World monkey, the common marmoset (Callithrix jacchus). This monkey has several advantages that make it suitable for use in preclinical studies. It is small (200 to 500 g), relatively inexpensive, easy to breed, and does not require special facilities or a well-trained breeder. Furthermore, marmosets are available from inbred colonies and their use lessens the risk of infections, which may be imported from foreign countries.5 Because their basic biology is relatively close to humans, marmosets will likely provide a powerful preclinical model for gene therapy.
Several studies on the physiology and immunology of marmosets have been reported.6-9 Based on the results of these studies, the marmoset has been used in toxicology studies of new reagents, including human interleukin-6 (IL-6).10 Although various human cytokines have been studied in large nonhuman primates,11 12 there have been very few such studies in New World monkeys. Moreover, relatively little is known about the characteristics of marmoset hematopoietic stem and progenitor cells or about the effects of new reagents on marmoset hematopoiesis. Before the results of newly developed cytokines or gene therapy vectors can be extrapolated from marmosets to humans, a systematic characterization of marmoset hematopoietic stem and progenitor cells is necessary. The physiologic differences between humans and marmosets must also be elucidated.
Recent advances in the field of gene therapy have facilitated the study of various intractable disorders including hematologic disorders (eg, adenosine deaminase deficiency, thalassemia, and leukemia/lymphoma), and a number of reports have demonstrated successful gene transfer into long-term reconstituting hematopoietic stem cells in both murine and canine models.13,14 In addition, several investigators have used nonhuman primate models for evaluating the safety and efficacy of retroviral vector systems.15-18 However, the overall levels of vector containing cells attainable in both human and nonhuman primates by transplantation of hematopoietic stem cells transduced by retroviral vectors remains low compared with that of mice.1To evaluate the in vivo efficacy of newly developed vectors for human use, it is important to first establish the optimal conditions for gene transduction into hematopoietic stem cells using nonhuman primates both in vitro and in vivo.
We first characterized marmoset bone marrow progenitor cells (BMPCs) using various human cytokines. We then determined the optimal conditions for transduction of marmoset BMPCs and peripheral blood progenitor cells (PBPCs) using a retroviral vector carrying a selectable marker gene, the multidrug resistance 1 gene (MDR1). We also showed the suitability of marmosets as a preclinical animal model for hematopoietic stem cell-based gene therapy by infusing autologous MDR1 gene-transduced PBPCs after conditioning radiation, followed by the systemic administration of an MDR-effluxed chemotherapeutic agent, docetaxel. Our results suggest that the marmoset is an appropriate model for the study of hematopoietic stem cell-based gene transfer methods suitable for laboratories where resources and space do not permit the use of larger nonhuman primate models.
MATERIALS AND METHODS
Animals and preparation of bone marrow mononuclear cells.
Common marmosets were bred in the Central Institute for Experimental Animals, Kawasaki, Japan. This primate center was established in 1952 and has bred common marmosets for over 20 years. All experiments were performed according to the guidelines of The Institute of Medical Science, The University of Tokyo. Bone marrow mononuclear cells (MNCs) were isolated from femoral bone marrow cells by Ficoll-Hypaque density gradient centrifugation. Cells were frozen using a programmed freezer and stored in liquid nitrogen until use. Human bone marrow cells were collected from healthy volunteers after obtaining informed consent and prepared and frozen as described above.
Granulocyte colony-stimulating factor (G-CSF) mobilization of peripheral blood progenitor cells.
Four marmosets were treated with human G-CSF (10 μg/kg/d) subcutaneously for 5 days. Peripheral blood (500 μL to 1 mL) was obtained from the femoral vein and the number of leukocytes, neutrophils, red blood cells, platelets, hematocrit level, and hemoglobin concentration were measured with an automatic blood cell analyzer (Sysmex K-1000; Toa-iyoudenshi, Kobe, Japan). MNCs were isolated and progenitor cell assays performed in the presence of human cytokines as described below.
Progenitor cell assays.
Erythropoietin (Epo) and G-CSF were provided by Chugai Pharmaceutical Co (Tokyo, Japan); granulocyte-macrophage CSF (GM-CSF) by Hoechst Japan (Tokyo, Japan); stem cell factor (SCF) and IL-3 by Amgen (Thousand Oaks, CA); thrombopoietin (Tpo) by Kirin Brewery Co (Maebashi, Japan); and IL-6 and IL-11 by Ajinomoto Co (Kawasaki, Japan) and Genetic Institute (Cambridge, MA), respectively.
Bone marrow and peripheral blood MNCs were cultured under standard methylcellulose culture conditions. Briefly, MNCs were plated in 35-mm petri dishes in 1 mL of α-minimal essential medium (α-MEM; GIBCO-BRL, Grand Island, NY) containing 1.2% methylcellulose (Wako Chemical, Osaka, Japan), 30% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% bovine serum albumin (BSA; Sigma, St Louis, MO), and 10−4 mol/L 2-mercaptoethanol (Wako Chemical) with human SCF (10 ng/mL), IL-3 (10 ng/mL), Epo (2 U/mL), and G-CSF (10 ng/mL). For serum-free cultures, MNCs were incubated with 1 mL of α-MEM containing 1.2% methylcellulose, 1% BSA, 10−4 mol/L 2-mercaptoethanol, 300 μg/mL iron-saturated human transferrin (Sigma), 160 μg/mL soybean lecithin (Sigma), 96 μg/mL cholesterol (Sigma), and 10−4mol/L sodium selenite (Sigma) in the presence of various human cytokines. To examine the colony-forming effects of each cytokine, cells were incubated at 37°C for 7 to 14 days in 5% CO2, in the presence of G-CSF or GM-CSF, and in 5% CO2, 5% O2, in the presence of Epo, IL-3, or SCF. Burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte (CFU-G), colony-forming unit-macrophage (CFU-M), colony-forming unit-granulocyte/macrophage (CFU-GM), and colony-forming unit-granulocyte/erythroid/megakaryocyte/macrophage (CFU-Mix) were enumerated following the morphologic criteria for human colonies. For colony-forming unit-megakaryocyte (CFU-MK) assays, bone marrow MNCs were cultured in a methylcellulose mixture (1.2% methylcellulose, 1% BSA, 30% human plasma, and 10−4 mol/L 2-mercaptoethanol). Human platelet-poor plasma was collected from a healthy volunteer after obtaining informed consent. Culture dishes were incubated at 37°C in 5% CO2 for 7 to 10 days. To confirm each colony component, several representative colonies were picked up and stained by standard May-Grünwald-Giemsa staining.
Gene transduction of bone marrow and peripheral blood progenitor cells.
An amphotropic retroviral vector, HaMDR1, carrying wild-type MDR1 driven by the Harvey murine sarcoma virus long terminal repeat19 20 was produced in the PA317 packaging cell 1ine. The producer cell line (PA317-HaMDR1) was maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO) with 10% FBS.
When the producer cells reached 80% confluence, the culture medium was renewed and the supernatant harvested after 24 hours. The viral supernatant was then filtered fresh through a 0.45 μm filter unit (Millipore, Bedford, MA) and used for transduction experiments. Target hematopoietic cells were cultured with or without allogeneic marmoset bone marrow stromal cells (prepared in advance) in fresh viral supernatant containing 4 μg/mL protamine sulfate (Sigma) and human cytokines (100 ng/mL SCF, 20 ng/mL IL-3, 80 ng/mL IL-6). The medium was renewed four times at 24-hour intervals. For gene transduction by cocultivation, bone marrow or peripheral blood MNCs were cultured directly on the retroviral producer cells or on a mixed population of retroviral producer cells and allogeneic marmoset bone marrow stromal cells at a ratio of 1:1 in DMEM supplemented with 10% FBS. The culture medium was supplemented with 4 μg/mL protamine sulfate, with or without human cytokines (SCF, IL-3, IL-6), and was changed every 48 hours. The cultures were maintained for 48 to 144 hours. The feeder layers were treated with 5 μg/mL mitomycin C (Sigma) over 2 hours before cocultivation with bone marrow or peripheral blood MNCs. The cells were then cultured in α-MEM with 10% FBS with or without cytokines for 48 hours after the cessation of infection, and then successively plated in methylcellulose culture medium as described above. Colony formation was assayed with or without 10 ng/mL vincristine (VCR; Sigma). The inhibitory effect of VCR on colony formation in untransduced bone marrow and peripheral blood MNCs was examined at increasing concentrations of VCR, with complete inhibition of colony formation at a concentration of 10 ng/mL of VCR. To confirm the correspondence between VCR resistance and the integrated MDR1 gene, representative VCR-resistant colonies were picked up and assayed by polymerase chain reaction (PCR) for the provirus as described below. The transduction efficiencies among various culture conditions were compared using a Student’s t-test.
Transplantation of peripheral blood progenitor cells.
Four 2-year-old marmosets (three males and one female), bred in the Central Institute for Experimental Animals, were used in the transplantation studies. The schedule for peripheral blood progenitor cell transplantation (PBPCT) is described in Fig 1. Autologous PBPCs were obtained from G-CSF–treated marmosets at 2 months and 1 month before PBPCT. Briefly, 10 μg/kg/d of G-CSF was subcutaneously administered to individual marmosets for 5 days. Peripheral blood was then collected from each marmoset once a day for 5 consecutive days after the final G-CSF administration. MNCs were isolated by Ficoll-Hypaque centrifugation, frozen using a programmed freezer, and stored in liquid nitrogen until use. Two thirds (nos. 2 and 3) or nine tenths (no. 4) of the peripheral blood MNCs obtained from three marmosets were transduced with the MDR1 gene by the cocultivation method. Briefly, cells were cultured on feeder cells consisting of the same number of retroviral producer and stromal cells in the presence of human cytokines (IL-3, IL-6, SCF) and 4 μg/mL protamine sulfate for 48 hours from day −2 to day 0. All four marmosets received two fractionated doses (2.5 Gy each) of total body irradiation from a 60Co source on days −1 and 0. The marmosets were then infused intravenously with the transduced and untransduced PBPCs via the femoral vein within 2 hours of the second irradiation. One control marmoset received only untransduced peripheral blood MNCs, while the other three marmosets received a mixture of transduced and untransduced peripheral blood MNCs. All marmosets were orally administered 1.5 × 104 U/body polymyxin B (Pfizer, Tokyo, Japan) and 0.5 mg/body fluconazole (Pfizer) daily from day −5 and a daily intramuscular injection of 5 mg/body ampicillin (Meiji-Seika, Tokyo, Japan) from day 0 until the peripheral neutrophil count exceeded 500/μL. Peripheral blood was collected in tubes containing EDTA every 2 or 3 days from day 0 to day 30, and thereafter, every 1 or 2 weeks. Complete blood cell counts were measured for each blood sample. The S+L− assay was performed on peripheral blood samples obtained on day 46 or day 58, to rule out the appearance of replication-competent retrovirus (RCR) using PG4 cells, as described elsewhere.21
Protocol for MDR1 transduction, transplantation of gene transduced or untransduced marmoset peripheral blood progenitor cells, and treatment with Docetaxel.
Protocol for MDR1 transduction, transplantation of gene transduced or untransduced marmoset peripheral blood progenitor cells, and treatment with Docetaxel.
PCR analysis of transduced gene in transplanted animals.
The presence of proviral HaMDR1 DNA was assayed by PCR analysis of genomic DNA extracted from peripheral blood or bone marrow cells in the transplanted marmosets. DNA was extracted from whole peripheral blood cells, granulocytes, or lymphocytes using a DNA extraction kit (Microtubogen; Invitrogen, San Diego, CA). A 353-bp DNA sequence specific for the HaMDR1 provirus was amplified using the sense-strand primer (F12) TGTTTCAGAATGGCAGAGTCA and the antisense-strand primer (R24) AAACAGAAAGGCGAGCAGAGA. The F12 primer is specific for the MDR1 region and the R24 primer is specific for the viral long terminal repeat region in the Ha vector. An equal amount of DNA from each sample was separately amplified using β-globin specific primers (sense-strand primer GAAGAGCCAAGGACAGGTAC, antisense-strand primer CATCAGGAGTGGACAGATCC) yielding a 485-bp fragment of the β-globin gene and was used as an internal control. PCR amplification was performed under the following conditions: initial denaturation at 94°C (5 minutes), followed by 35 cycles of 94°C denaturation (45 seconds), 60°C annealing (45 seconds), and 72°C extension (1 minute). The PCR products were electrophoresed on a 3% NuSieve (FMC, Rockland, ME) agarose gel and transferred to a nylon membrane (Hybond N; Amersham, Little Chalfont, UK). The membranes were then UV cross-linked. Hybridization was performed overnight at 65°C in Church’s phosphate buffer containing 1 mmol/L EDTA and 7% sodium dodecyl sulfate (SDS) using a 32P-labeled MDR1 cDNA probe. The amplified products were visualized by autoradiography. Using a Bio-Imaging Analyzer (FUJIX, Tokyo, Japan), the copy number of these samples was densitometrically compared with those of a standard curve generated by amplification of a series of dilutions of DNA from the packaging cell line (PA317-HaMDR1) containing a single copy of the provirus per cell.
Treatment with Docetaxel.
Docetaxel (Rhône-Poulenc Rorer, Vitry sur Seine, France) was dissolved in ethanol, followed by the addition of polysorbate 80 (Sigma), and its final dilution was prepared in a 5% glucose (Wako) solution (1:1:18 dH2O; vol/vol/vol). Three marmosets, including one control and two recipients of gene-transduced cells in whom the proviral DNA was detectable (nos. 1, 3, and 4), were intravenously administered 2 mg/kg Docetaxel. Complete blood cell counts were obtained every 2 or 3 days, and the animals received daily intramuscular injections with 5 mg/body ampicillin until the peripheral neutrophil count reached 500/μL. The Docetaxel treatment was repeated three times at 4-week intervals.
RESULTS
Responses of marmoset bone marrow progenitor cells to human cytokines.
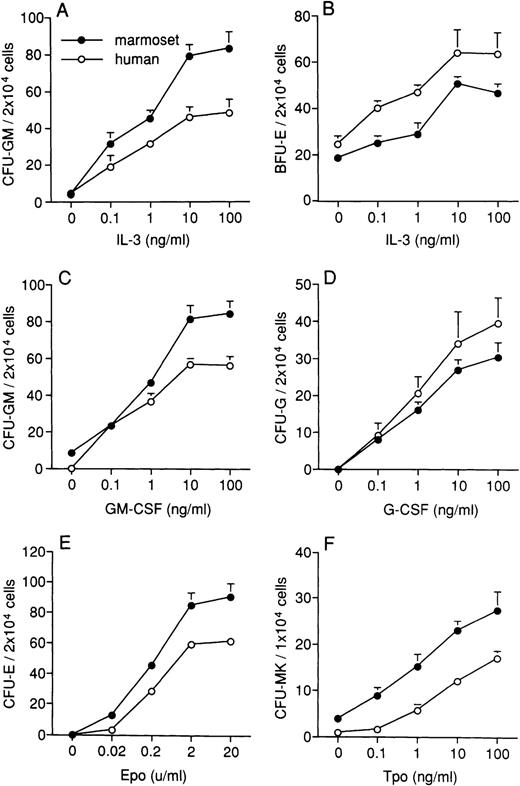
To investigate the cross-reactivity of human IL-3, GM-CSF, G-CSF, and Epo on marmoset BMPCs, we examined the effects of IL-3 on CFU-GM and BFU-E formation, GM-CSF on CFU-GM formation, G-CSF on CFU-G formation, and Epo on CFU-E formation in serum-free cultures. IL-3 and GM-CSF stimulated CFU-GM formation in a dose-dependent manner (Fig 2A and C). IL-3 also stimulated BFU-E formation (Fig 2B). The formation of CFU-G and CFU-E was stimulated in a dose-dependent manner by G-CSF and Epo, respectively (Fig 2D and E). The marmoset and human response patterns of BMPCs to human cytokines were similar. However, the frequency of CFU-GM formation in the presence of IL-3 and GM-CSF and CFU-E formation in the presence of Epo were both higher in marmoset than in human BMPCs. The frequency of BFU-E formation in the presence of IL-3 and Epo was higher in human than in marmoset BMPCs. The number of CFU-MK increased in a dose-dependent manner by treatment with Tpo (Fig 2F), and the frequency of CFU-MK formation in BMPCs was higher in marmosets than in humans.
Effects of human cytokines on colony formation of marmoset (•) and human (○) BMPCs. The effects of IL-3 on CFU-GM (A) and BFU-E (B) formation in the presence of 2 U/mL Epo, GM-CSF on CFU-GM formation (C), G-CSF on CFU-G formation (D), and Epo on CFU-E formation in the presence of 10 ng/mL IL-3 (E) were examined in serum-free cultures. The effects of Tpo were assessed using human platelet-poor plasma (F). The human cytokines effectively stimulated colony formation dose-dependently. The response patterns of BMPCs to human cytokines was similar in marmosets and humans. Data are expressed as the mean ± standard error of mean (SEM) from three separate triplicate experiments.
Effects of human cytokines on colony formation of marmoset (•) and human (○) BMPCs. The effects of IL-3 on CFU-GM (A) and BFU-E (B) formation in the presence of 2 U/mL Epo, GM-CSF on CFU-GM formation (C), G-CSF on CFU-G formation (D), and Epo on CFU-E formation in the presence of 10 ng/mL IL-3 (E) were examined in serum-free cultures. The effects of Tpo were assessed using human platelet-poor plasma (F). The human cytokines effectively stimulated colony formation dose-dependently. The response patterns of BMPCs to human cytokines was similar in marmosets and humans. Data are expressed as the mean ± standard error of mean (SEM) from three separate triplicate experiments.
We next examined the effects of human SCF in the presence of other cytokines to determine the best conditions for the proliferation of more primitive progenitor cells. SCF demonstrated significant activity on marmoset BMPCs, and with IL-3 or GM-CSF, increased CFU-GM formation by 2.0-fold and 2.2-fold compared with the value obtained in the absence of SCF, respectively (Table 1). Similarly, SCF enhanced colony formation of BFU-E and CFU-Mix with IL-3 and Epo. Culture in the presence of SCF plus Epo and SCF plus IL-3 and Epo increased BFU-E formation by 2.7-fold and 3.6-fold, respectively, compared with culture with Epo alone. The combination of SCF, IL-3, and Epo increased the formation of CFU-Mix relative to SCF plus IL-3 or SCF plus Epo (Table 1). SCF also enhanced the effects of both Tpo and Tpo plus IL-3 in forming CFU-MK (Table 1). IL-6 and IL-11 also enhanced the formation of CFU-MK, but to a lesser extent. The number of CFU-MK was 11.3 ± 1.6 (/1 × 104 cells) in the presence of IL-6 and 10.3 ± 1.5 (/1 × 104 cells) in the presence of IL-11, about half the level obtained with Tpo.
Effects of Human SCF on Colony Formation of Marmoset Bone Marrow Progenitor Cells
| Cytokine* . | CFU-GM . | BFU-E . | CFU-Mix . | Cytokine† . | CFU-MK . |
|---|---|---|---|---|---|
| Epo | 3.5 ± 1.4 | 18.6 ± 2.4 | 0.0 ± 0.0 | IL-3 | 8.6 ± 1.5 |
| IL-3 | 68.0 ± 7.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | Tpo | 22.9 ± 2.2 |
| SCF + Epo | 112.5 ± 6.0 | 51.0 ± 3.1 | 6.0 ± 1.0 | SCF + Tpo | 36.9 ± 2.8 |
| SCF + IL-3 | 135.5 ± 4.8 | 1.0 ± 0.6 | 4.8 ± 0.6 | SCF + IL-3 + Tpo | 47.9 ± 3.2 |
| SCF + IL-3 + Epo | 105.5 ± 3.3 | 67.5 ± 4.1 | 24.0 ± 1.6 | ||
| GM-CSF | 61.5 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| SCF + GM-CSF | 134.5 ± 2.9 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Cytokine* . | CFU-GM . | BFU-E . | CFU-Mix . | Cytokine† . | CFU-MK . |
|---|---|---|---|---|---|
| Epo | 3.5 ± 1.4 | 18.6 ± 2.4 | 0.0 ± 0.0 | IL-3 | 8.6 ± 1.5 |
| IL-3 | 68.0 ± 7.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | Tpo | 22.9 ± 2.2 |
| SCF + Epo | 112.5 ± 6.0 | 51.0 ± 3.1 | 6.0 ± 1.0 | SCF + Tpo | 36.9 ± 2.8 |
| SCF + IL-3 | 135.5 ± 4.8 | 1.0 ± 0.6 | 4.8 ± 0.6 | SCF + IL-3 + Tpo | 47.9 ± 3.2 |
| SCF + IL-3 + Epo | 105.5 ± 3.3 | 67.5 ± 4.1 | 24.0 ± 1.6 | ||
| GM-CSF | 61.5 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| SCF + GM-CSF | 134.5 ± 2.9 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Data are expressed as the mean ± SEM from three separate experiments performed in triplicate. Human SCF: 10 ng/mL, IL-3: 10 ng/mL, Epo: 2 U/mL, GM-CSF: 10 ng/mL, Tpo: 10 ng/mL.
Progenitor cell assays were performed in serum-free conditions. CFU-GM, BFU-E, CFU-Mix/2 × 104 marmoset bone marrow MNCs.
Progenitor cell assays were performed using human platelet poor plasma. CFU-MK/1 × 104 marmoset bone marrow MNCs.
G-CSF mobilization of peripheral blood progenitor cells.
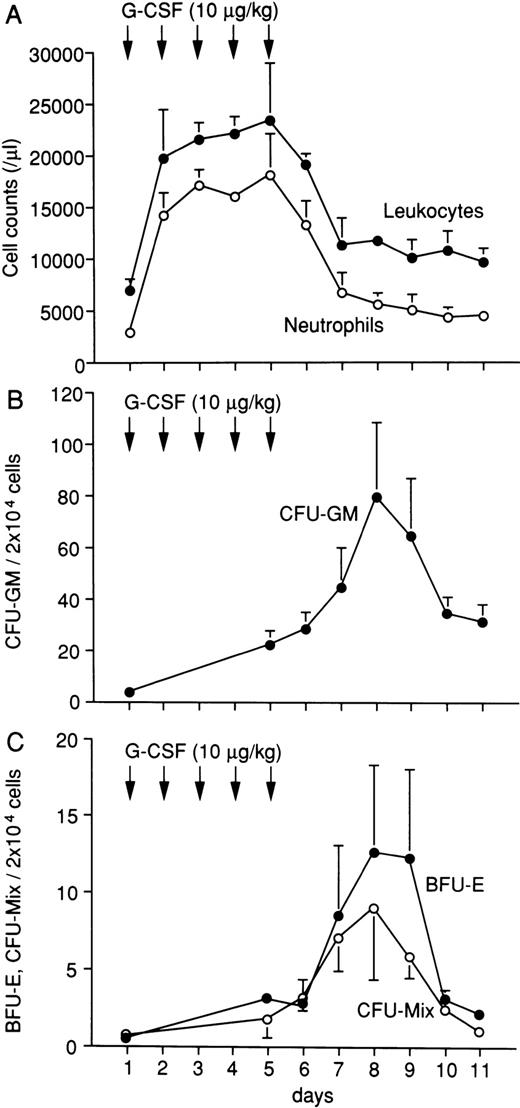
Both the total white blood cell and neutrophil counts increased immediately after the administration of G-CSF and remained high during treatment (Fig 3A). The numbers of CFU-GM, BFU-E, and CFU-Mix in peripheral blood were highest on day 8 and were approximately 21-fold, 25-fold, and 13-fold increased over their unstimulated values, respectively (Fig 3B and C). The PBPCs increased in number from days 6 to 8 and persisted until day 10. These results show that G-CSF has potent mobilizing activity at 10 μg/kg/d in marmosets, suggesting the availability of G-CSF–mobilized PBPCs for autologous hematopoietic progenitor cell transplantation.
Changes of leukocytes (•) and neutrophils (○) in G-CSF–treated marmosets (A). Changes of CFU-GM (•) (B), BFU-E (•), and CFU-Mix (○) (C) in peripheral blood of G-CSF–treated marmosets. First, 10 μg/kg/d of human G-CSF was administered subcutaneously for 5 days. The maximal numbers of CFU-GM, BFU-E, and CFU-Mix in peripheral blood were obtained on day 8 and were approximately 21-fold, 25-fold, and 13-fold, respectively, of the value obtained in the unstimulated state. The data shown were obtained from four animals and are expressed as the mean ± SEM.
Changes of leukocytes (•) and neutrophils (○) in G-CSF–treated marmosets (A). Changes of CFU-GM (•) (B), BFU-E (•), and CFU-Mix (○) (C) in peripheral blood of G-CSF–treated marmosets. First, 10 μg/kg/d of human G-CSF was administered subcutaneously for 5 days. The maximal numbers of CFU-GM, BFU-E, and CFU-Mix in peripheral blood were obtained on day 8 and were approximately 21-fold, 25-fold, and 13-fold, respectively, of the value obtained in the unstimulated state. The data shown were obtained from four animals and are expressed as the mean ± SEM.
Efficiency of transduction into bone marrow and peripheral blood progenitor cells.
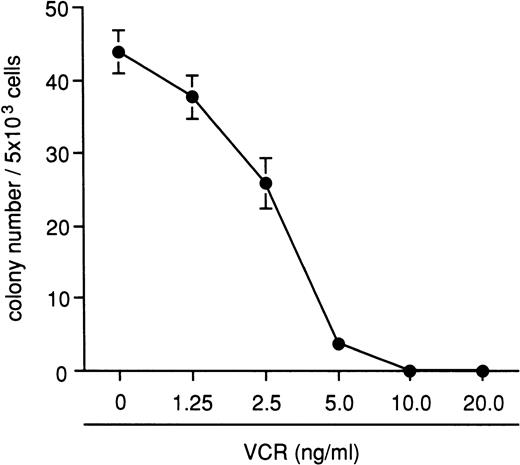
The inhibitory effects of VCR on bone marrow colony formation were examined using four different marmosets, and the results are shown in Fig 4. A total of 10 ng/mL of VCR completely inhibited the colony formation, and this concentration was chosen for all subsequent experiments. After demonstrating the presence of provirus by PCR analysis in all randomly sampled VCR-resistant colonies, the appearance of VCR-resistant colonies was used for estimating gene transduction efficiency. With the supernatant transduction method, 1.7% of CFU-GM colonies became VCR-resistant. The transduction efficiency increased when the cells were transduced with the viral supernatant in the presence of bone marrow stromal cells, although the difference was not statistically significant (Table 2). The frequency of VCR-resistant colonies obtained by cocultivation with the retroviral producer cells was low despite an extended culture period (Table 2). In an attempt to increase the transduction efficiency, we mixed marmoset bone marrow stromal cells with retroviral producer cells. The frequency of VCR-resistant colonies was significantly higher when target cells were cocultured with viral producer cells and bone marrow stromal cells for each transduction period. Under these conditions, human cytokines significantly enhanced the transduction efficiency at both 96 and 144 hours (Table 2).
Inhibitory effects of VCR on the colony formation from normal marmoset bone marrow MNCs. A total of 10 ng/mL of VCR completely inhibited colony formation. The data shown here were obtained from four different animal experiments and are expressed as the mean ± SEM.
Inhibitory effects of VCR on the colony formation from normal marmoset bone marrow MNCs. A total of 10 ng/mL of VCR completely inhibited colony formation. The data shown here were obtained from four different animal experiments and are expressed as the mean ± SEM.
Efficiency of Transduction Into Marmoset Hematopoietic Progenitor Cells
| Transduction Method . | Cell . | Stroma . | Cytokine* . | Transduction Period . | ||
|---|---|---|---|---|---|---|
| 48 h . | 96 h . | 144 h . | ||||
| Supernatant | BMPC | − | + | ND | 1.7 ± 0.6 | ND |
| + | + | ND | 5.6 ± 1.2 | ND | ||
| Cocultivation | BMPC | − | − | 1.0 ± 0.6 | 3.2 ± 1.0 | 5.9 ± 0.9 |
| − | + | 1.6 ± 0.2 | 5.5 ± 0.5 | 6.3 ± 1.2 | ||
| + | − | 11.6 ± 1.5† | 15.3 ± 1.9† | 16.5 ± 1.2† | ||
| + | + | 15.2 ± 1.5† | 23.2 ± 1.7†,‡ | 20.9 ± 0.7†,‡ | ||
| PBPC | + | + | 15.9 ± 4.0 | 14.8 ± 2.7 | ND | |
| Transduction Method . | Cell . | Stroma . | Cytokine* . | Transduction Period . | ||
|---|---|---|---|---|---|---|
| 48 h . | 96 h . | 144 h . | ||||
| Supernatant | BMPC | − | + | ND | 1.7 ± 0.6 | ND |
| + | + | ND | 5.6 ± 1.2 | ND | ||
| Cocultivation | BMPC | − | − | 1.0 ± 0.6 | 3.2 ± 1.0 | 5.9 ± 0.9 |
| − | + | 1.6 ± 0.2 | 5.5 ± 0.5 | 6.3 ± 1.2 | ||
| + | − | 11.6 ± 1.5† | 15.3 ± 1.9† | 16.5 ± 1.2† | ||
| + | + | 15.2 ± 1.5† | 23.2 ± 1.7†,‡ | 20.9 ± 0.7†,‡ | ||
| PBPC | + | + | 15.9 ± 4.0 | 14.8 ± 2.7 | ND | |
Transduction efficiency is represented as the percentage of VCR resistant colonies. Repetitive transduction in a coculture system of viral producer and marmoset bone marrow stromal cells facilitated efficient transduction efficiency. Data are expressed as the mean ± SEM from two separate experiments with the supernatant method and from three separate experiments with the cocultivation method.
Abbreviation: ND, not done.
Human SCF (100 ng/mL), IL-3 (20 ng/mL), IL-6 (80 ng/mL).
P < .01 when compared with the group without stromal cells, but with the same cytokine combination during the same transduction period.
P < .05 when compared with the group without cytokines, but with the same stromal cells condition during the same transduction period.
The coculture of target cells on retroviral producer cells and stromal cells was also effective for the transduction of PBPCs. The frequency of VCR-resistant colonies was approximately 15% regardless of the transduction period, either 48 or 96 hours (Table 2). These results indicate that cocultivation using mixed populations of retroviral producer cells and stromal cells enhances the efficiency of transduction of the MDR1 gene into marmoset BMPCs and PBPCs.
Transplantation of gene transduced or untransduced peripheral blood progenitor cells.
The total number of autologous peripheral blood MNCs and CFU-GM harvested and frozen ranged from 2.6 × 107 to 4.5 × 107 cells and from 2.0 × 104 to 1.4 × 105 cells, respectively. The actual numbers of peripheral blood MNCs and CFU-GM transplanted into the four marmosets are summarized in Table 3. One control animal was transplanted with untransduced cells only. Three marmosets were transplanted with both transduced and a fraction of untransduced cells to ensure engraftment. In vitro progenitor cell assays showed that 6.7%, 5.9%, and 13.7% of the CFU-GM in the transduced fractions from marmoset nos. 2, 3, and 4 became VCR-resistant after MDR1 gene transduction, respectively. The total numbers of CFU-GM were decreased during the culture and transduction procedure. The calculated percentage of transduced CFU-GM infused into each animal was 2.5%, 2.1%, and 8.9%, respectively, given the coadministration of the untransduced fraction of cells. Recovery of hematopoiesis was observed in all four animals. The peripheral neutrophil count reached 500/μL on days 25, 39, and 33 in recipients of gene-transduced cells, and on day 35 in the control marmoset. The platelet count reached 5 × 104/μL on days 28, 39, and 33 in the recipients of gene-transduced cells, and on day 28 in the control marmoset. No RCR was detected in serum obtained on day 46 or day 58 in any animal by the S+L− assay using PG4 cells. One marmoset (no. 2) who received gene transduced cells died suddenly on day 63, possibly of a mechanically induced ileus due to ingestion of vinyl fragments. Autopsy of this animal showed full recovery of bone marrow hematopoiesis.
Summary of Transplanted MNC, CFU-GM, and Hematologic Recovery
| No. . | MNC (×107/kg) . | CFU-GM (×104/kg) . | Days to Neutrophils >500/μL . | Days to Platelets >5 × 104/μL . | ||||
|---|---|---|---|---|---|---|---|---|
| Transduced . | Untransduced . | Total . | Transduced . | Untransduced . | Total . | |||
| 1 | — | 7.8 | 7.8 | — | 5.9 | 5.9 | 35 | 28 |
| 2 | 4.0 | 3.0 | 7.0 | 1.5 | 2.5 | 4.0 | 25 | 28 |
| (6.7%)3-150 | (2.5%)3-151 | |||||||
| 3 | 5.7 | 2.8 | 8.5 | 2.5 | 3.3 | 5.8 | 39 | 39 |
| (5.9%)3-150 | (2.1%)3-151 | |||||||
| 4 | 6.9 | 1.1 | 8.0 | 8.1 | 4.4 | 12.5 | 33 | 33 |
| (13.7%)3-150 | (8.9%)3-151 | |||||||
| No. . | MNC (×107/kg) . | CFU-GM (×104/kg) . | Days to Neutrophils >500/μL . | Days to Platelets >5 × 104/μL . | ||||
|---|---|---|---|---|---|---|---|---|
| Transduced . | Untransduced . | Total . | Transduced . | Untransduced . | Total . | |||
| 1 | — | 7.8 | 7.8 | — | 5.9 | 5.9 | 35 | 28 |
| 2 | 4.0 | 3.0 | 7.0 | 1.5 | 2.5 | 4.0 | 25 | 28 |
| (6.7%)3-150 | (2.5%)3-151 | |||||||
| 3 | 5.7 | 2.8 | 8.5 | 2.5 | 3.3 | 5.8 | 39 | 39 |
| (5.9%)3-150 | (2.1%)3-151 | |||||||
| 4 | 6.9 | 1.1 | 8.0 | 8.1 | 4.4 | 12.5 | 33 | 33 |
| (13.7%)3-150 | (8.9%)3-151 | |||||||
Animal No. 1 was transplanted only with untransduced PBPC as a control.
Transduction efficiency (percentage of VCR resistant colonies).
Transduction efficiency was calculated from the numbers of transduced and untransduced CFU-GM.
Detection of transduced gene in transplanted animals.
DNA from peripheral blood or bone marrow cells from recipients of MDR1 transduced PBPCs was extracted and examined for the presence of the proviral DNA by vector specific PCR followed by Southern blotting analysis. In marmoset no. 2, peripheral blood cells from day 28 posttransplantation showed the appearance of proviral DNA, which was also detected on day 53, just before the animal’s death (Fig 5A). The estimated percentage of MDR1 positive peripheral blood cells ranged from 0.2% to 0.4% by densitometric comparison with the standard DNA curve. The provirus was also detected in the blood cells of marmoset no. 3 on day 35 and on day 81 (Fig 5A). In this animal, the provirus was detected in DNA isolated from peripheral blood granulocytes and lymphocytes on day 142, and the provirus was also detected on day 412. The percentage of MDR1 positive granulocytes and lymphocytes ranged from 0.2% to 1.0%. Additionally, a bone marrow aspiration was performed on day 105, and the provirus was detected in both bone marrow MNCs and in pooled colonies (10 colonies) derived from the bone marrow MNCs (Fig 5A). In marmoset no. 4, the provirus was detected in granulocytes and lymphocytes on days 36, 74, 158, and 216 (Fig 5B). The estimated percentage of MDR1 positive granulocytes and lymphocytes ranged from 0.2% to 0.9%. These results indicate that the MDR1 gene-transduced PBPCs were capable of engraftment and prolonged contribution to hematopoiesis in vivo.
Detection of provirus DNA in peripheral blood cells or bone marrow cells of the transplanted marmosets. On the indicated days, DNA was obtained from each sample and analyzed for the presence of provirus DNA and β-globin DNA as an internal control by PCR, followed by Southern blot analysis. Peripheral blood cells from day 28 and day 53 of transplantation showed the presence of provirus DNA in marmoset no. 2. The provirus was detected in marmoset no. 3 in peripheral blood cells on day 35 and day 81. In this animal, the provirus was detected both in peripheral blood granulocytes and lymphocytes on day 142 (just before the first treatment with Docetaxel) and on day 245 (just after the third treatment with Docetaxel), and the provirus was also detected on day 412. A bone marrow biopsy was performed on this marmoset on day 105; the provirus was detected in bone marrow MNCs and in pooled colonies (10 colonies) (A). The provirus DNA in granulocytes and lymphocytes was also detected from day 36 to day 216 in marmoset no. 4. Day 74 was the day before the first treatment with Docetaxel, and day 158 was the day after the third treatment with Docetaxel (B). The standard provirus DNA sample was obtained from serial dilutions of DNA from the packaging cell line, PA317-HaMDR1, containing a single copy of the provirus per cell (C). The standard β-globin DNA sample was obtained from serial fivefold to 10-fold dilutions of DNA from normal PB (D).
Detection of provirus DNA in peripheral blood cells or bone marrow cells of the transplanted marmosets. On the indicated days, DNA was obtained from each sample and analyzed for the presence of provirus DNA and β-globin DNA as an internal control by PCR, followed by Southern blot analysis. Peripheral blood cells from day 28 and day 53 of transplantation showed the presence of provirus DNA in marmoset no. 2. The provirus was detected in marmoset no. 3 in peripheral blood cells on day 35 and day 81. In this animal, the provirus was detected both in peripheral blood granulocytes and lymphocytes on day 142 (just before the first treatment with Docetaxel) and on day 245 (just after the third treatment with Docetaxel), and the provirus was also detected on day 412. A bone marrow biopsy was performed on this marmoset on day 105; the provirus was detected in bone marrow MNCs and in pooled colonies (10 colonies) (A). The provirus DNA in granulocytes and lymphocytes was also detected from day 36 to day 216 in marmoset no. 4. Day 74 was the day before the first treatment with Docetaxel, and day 158 was the day after the third treatment with Docetaxel (B). The standard provirus DNA sample was obtained from serial dilutions of DNA from the packaging cell line, PA317-HaMDR1, containing a single copy of the provirus per cell (C). The standard β-globin DNA sample was obtained from serial fivefold to 10-fold dilutions of DNA from normal PB (D).
Changes of peripheral blood neutrophils and transduced cells after Docetaxel treatment.
Docetaxel was administered to three marmosets after the presence of proviral DNA was confirmed in granulocytes and lymphocytes by vector-specific PCR in the two recipients of MDR1 gene-transduced PBPCs. Docetaxel treatment began on day 168 in marmosets no. 1 (negative control) and no. 3 and on day 78 in marmoset no. 4. A reduction in the neutrophil count was observed in all marmosets after each cycle of Docetaxel treatment. The period required for the recovery of neutrophil count to baseline level after each Docetaxel treatment was not significantly different among the three marmosets (data not shown). The percentage of MDR1-positive neutrophils after three courses of Docetaxel in the two recipients of MDR1 gene-transduced PBPCs increased slightly relative to the pretreatment level (Fig 5A and B), but remained less than 1%.
DISCUSSION
Nonhuman primate models are invaluable as tools for the preclinical evaluation of both cytokine and gene therapy applications. However, studies using large nonhuman primates are difficult to perform and are currently being performed only in large primate centers. We have recently introduced a small primate, the common marmoset, as a model for hematologic research. These animals are relatively inexpensive compared with large primates such as the macaque or baboon, and the cost of marmosets in Japan is about one third to one half of that of macaques because of the large number of colonies available. Marmosets usually bear two to four offspring per year and have a 140 to 150 day gestation period. They become sexually active at the age of 18 months and require one half to one third the cage size required for macaques. Moreover, it is possible to breed two animals per cage. The handling of marmosets is relatively simple and requires no special training, another advantage of this model. However, little published information regarding the hematologic characteristics of marmosets exists. We therefore analyzed the hematologic characteristics of common marmosets to clarify the advantages and disadvantages of their use as a primate model for preclinical studies.
Transduction of hematopoietic cells by retroviral vectors requires cell division, and the majority of transduction methods achieve in vitro stimulation by culture in the presence of cytokines. Gene transfer efficiency has been reported to be enhanced by culture in multicytokine combinations such as SCF, IL-3, and IL-6.13,22,23 In this study, we first compared the responsiveness of marmoset BMPCs with human cytokines in serum-free conditions. Our results show that both marmoset and human BMPCs respond in a similar, dose-dependent manner to human IL-3, GM-CSF, G-CSF, and Epo. It is well known that both the human IL-3 and GM-CSF react in a species-specific manner.24,25 In the present study, however, we show that marmoset BMPCs respond appropriately to both human IL-3 and GM-CSF in vitro. Human SCF, also known to be highly species-specific,26 is also active on marmoset BMPCs, stimulating colony formation in the presence of other human cytokines. There have been several reports concerning the effects of human cytokines in Old World monkeys.10-12 The present findings strongly support the use of New World monkeys for such investigations, which in contrast to other large animal models such as canine or swine models,27,28 does not require the cloning of species-specific cytokines to stimulate hematopoietic cells for gene transduction. In addition, our in vivo results show that marmoset hematopoietic progenitor cells are effectively mobilized into the peripheral blood circulation by human G-CSF, and that these cells are capable of restoring hematopoiesis after irradiation as previously reported in humans.29
Although hematopoietic stem cells, including PBPCs, are considered to be important targets for gene therapy applications, the efficiency of transduction of hematopoietic stem cells in human and nonhuman primates remains low compared with that in mice.1 The evaluation of new strategies in a relevant animal model is therefore essential to the eventual goal of clinical gene therapy in humans. A previous report showed that the transduction of hematopoietic cells by retroviral vectors was generally more efficient in the presence of viral producer cells than viral supernatant alone.30 Furthermore, others have reported increased efficiency of transduction into hematopoietic stem cells in the presence of bone marrow stromal cells or extracellular matrix molecules.31-33 As both the supernatant and cocultivation methods were not effective in increasing the transduction efficiency into marmoset BMPCs and PBPCs, we designed a novel transduction system to exploit the advantages of both cocultivation and stromal cell support with improved transduction efficiency using this method for both BMPCs and PBPCs.
Based on these findings, we performed PBPCT in marmosets using MDR1 gene-transduced PBPCs. Hematologic recovery after PBPCT was observed in all animals. Our preliminary studies showed that irradiation with 7.5 Gy was uniformly lethal in marmosets. In animals irradiated with 5.5 Gy, hematologic recovery was delayed (neutrophil count <500/μL on day 50), yet all survived. These results suggested that 5.0 Gy of irradiation was a suitable dose for our studies, and hematologic reconstitution was accelerated by PBSCT. Provirus was detected in DNA from granulocytes and lymphocytes in marmosets nos. 3 and 4 up to day 412 and day 216, respectively. These results suggested that the MDR1 gene had been introduced into long-term reconstituting cells and proved that our novel transduction method resulted in successful genetic modification detectable in vivo. However, the hematologic recovery seen in these animals was slower than is typical for PBPCT in humans. We presume that the number of transplanted progenitor cells were few, or that primitive progenitor cells differentiated during the transduction procedure. Thus, further study is required to clarify the relationship between the number of transplanted PBPCs and hematologic recovery in marmosets.
Previous reports in murine models suggest that the MDR1 gene can be used as a selectable marker gene in vivo allowing enrichment for cells transduced with the MDR1 gene by treatment with chemotherapeutic agents such as paclitaxel.34-37 Additionally, inclusion of a second gene with the MDR1 gene using a bicistronic vector enhanced its expression after the transduced cells were treated with VCR.38 We administered Docetaxel to marmoset recipients of gene-transduced progenitors to examine whether in vivo selection of MDR1 transduced cells was feasible in our primate model. While paclitaxel-induced leukopenia can be completely prevented in murine recipients of MDR1 gene transduced progenitors,39,40 Docetaxel-induced neutropenia was not completely prevented in our animals. The percentage of MDR1-positive neutrophils was slightly increased in our marmosets after treatment with Docetaxel, but the percentage was still very low. These low levels were not considered to be high enough to prevent the neutropenia induced by Docetaxel treatment. Similar results have recently been reported in human cancer patients undergoing autologous transplantation with MDR1-transduced progenitor cells despite a relatively high transduction efficiency into CD34+ cells.33 41
Finally, RCR was not detected in any of the recipients of gene-transduced progenitors by the S+L−assay, nor did we detect any simian retrovirus in these animals (data not shown). Development of lymphoma by contaminating RCR in macaques transplanted with gene-transduced bone marrow cells has been reported previously17; therefore, studies to prove the safety of new retroviral vectors using primates are essential. Because the common marmoset is available at a lower cost than the macaque, the marmoset can also be used to detect RCR for newly developed retroviral vectors.
In summary, our results show that a small New World primate, the common marmoset, is a useful alternative to large primates for the evaluation of hematopoietic stem cell based gene transfer methodologies in vivo. This animal model can be used for nonhuman primate studies in institutions without special primate centers and will potentially contribute to the future development of successful human gene therapy.
Supported in part by grants from the Ministry of Health and Welfare, the Ministry of Education, Science and Culture, and the Science and Technology Agency, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenzaburo Tani, MD, PhD, 4-6-1 Shirokanedai Minato-ku, Tokyo 108, Japan; e-mail: taniken@ims.utokyo.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal