Abstract
The human platelet alloantigen 1 system (HPA-1) is determined by a polymorphism at position 33 in the N-terminus of human glycoprotein IIIa (GPIIIa). This naturally occurring substitution creates a conformation in the HPA-1a allelic form that can be antigenic when presented to an individual expressing the HPA-1b form. Anti–HPA-1a antibodies generated by this immune response can lead to the destruction of platelets, as seen in the clinical disorders, neonatal alloimmune thrombocytopenia (NAIT) and posttransfusion purpura (PTP). To understand better the structural requirements for recognition by these pathogenic antibodies, we investigated the N-terminal 66 amino acids from the HPA-1a form of human GPIIIa and the analogous amino acids from the nonimmunogenic murine homolog. Our objectives were to define further the boundaries of the HPA-1a epitope(s) in the N-terminus of human GPIIIa, to isolate the murine 5’ nucleotide sequence and compare the deduced murine N-terminal sequence to that of human, and to mutate the murine sequence systematically to include an HPA-1a epitope(s). Murine amino acids that differed from human were changed by site-directed mutagenesis to the analogous residues in the HPA-1a form of human GPIIIa, starting and radiating from murine position 33 (site of human polymorphism). This systematic approach allowed us to pinpoint amino acids critical to a conformation recognized by anti–HPA-1a antibodies. Our results show that an HPA-1a epitope can be created within the N-terminus of murine GPIIIa and raise the possibility that murine models of HPA-1a sensitization can be developed.
HUMAN PLATELET alloantigen system 1 (HPA-1) involves a polymorphism in glycoprotein IIIa (GPIIIa), a subunit of the fibrinogen receptor. In this human biallelic system, the HPA-1a allele encodes a leucine at position 33 of GPIIIa and the allele for HPA-1b encodes a proline.1 In Caucasians from North America and Europe, the gene frequency is estimated to be 83% for HPA-1a and 17% for HPA-1b.2
Two bleeding disorders, neonatal alloimmune thrombocytopenia (NAIT) and posttransfusion purpura (PTP), can be attributed to an immune response of HPA-1b homozygous individuals to HPA-1a platelets.3 In each disorder, antibodies are made against HPA-1a platelets, leading to thrombocytopenia. Alloantibodies are made by HPA-1b homozygous mothers against HPA-1a fetal platelets in NAIT. The thrombocytopenia in NAIT is a leading cause of prenatal and perinatal intracranial hemorrhage. PTP results from the transfusion of HPA-1a–positive platelets to previously sensitized HPA-1b homozygous individuals.
Anti–HPA-1a antisera from patients with these disorders recognize a three-dimensional structure in the HPA-1a form of human GPIIIa.4 This conformation is dependent on the leucine at position 33.1 Disulfide bridges between cysteines (Cys) in the protein are also important; alteration of the disulfide bonds by reducing agents results in loss of HPA-1a antigenicity.5The N-terminal region of GPIIIa contains seven Cys and the disulfide bridges between Cys residues have been assigned.6 The proposed Cys-pairing creates a cloverleaf-like structure with three loops (see review by Newman7). This cloverleaf structure is thought to be pulled into apposition with the highly Cys-rich core of GPIIIa by a disulfide bond between Cys5 and Cys435.6
A 66–amino acid segment of the N-terminus of human GPIIIa binds anti–HPA-1a antisera from some individuals with NAIT or PTP. This N-terminal segment includes the entire cloverleaf structure. Bowditch et al8 first demonstrated the binding of this domain by anti–HPA-1a antibodies and our laboratory confirmed his finding by the production of soluble HPA-1 antigens.9 In the intact human GPIIIa, it has been proposed that the Cys5-Cys435 bridge between the N-terminal domain and Cys-rich core may be necessary for optimal binding of anti–HPA-1a antibodies.10 Alternatively, two types of anti–HPA-1a antibodies have been proposed, one that binds solely the N-terminus and another that binds the N-terminus bound to the Cys-rich core by the Cys5-Cys435bridge.11 12
To combat the specific antibodies that are problematic in NAIT and PTP, a better understanding of their structural requirements for binding human GPIIIa is needed. In this investigation, we focused on the requirements for recognition of the 66–amino acid segment of the N-terminus of human GPIIIa by anti–HPA-1a antibodies. By deletion analysis, we further truncated the N-terminal and C-terminal ends of the 66-residue segment and found shorter fragments that could still maintain a high degree of immunoreactivity. For comparison, we also isolated the nucleotide sequence coding for the first 66 residues of murine GPIIIa. The deduced amino acid sequence from mouse is highly homologous to human GPIIIa. However, polyclonal human anti–HPA-1a antibodies do not recognize the murine protein. To determine whether we might identify residues that are critical for binding anti–HPA-1a antibodies, residues within the murine GPIIIa N-terminus were systematically changed to the human amino acid counterpart. We describe herein the creation of an anti–HPA-1a binding domain within the context of the nonimmunoreactive mouse protein. This accomplishment is the first step towards the possible generation of a murine model of the HPA-1 system.
MATERIALS AND METHODS
Construction of Deletion Mutations of the Human GPIIIa N-Terminus
Deletions in the N-terminal 66–amino acid segment of human GPIIIa were made by polymerase chain reaction (PCR) techniques. N-terminal and C-terminal deletions were made to the segment by amplifying coding sequence sequentially shorter at either the 5′ and 3′ end. pGEXPlA1 (HPA-1a), described previously,9 was used as the template in the PCR reactions.
N-terminal deletions.
Using various 5′ oligonucleotides that annealed to sequences located between the initiating methionine codon and the site of the human polymorphism, the position of the N-terminal truncation was generated. The 3′ amplification primer annealed to the originalEcoRI cloning site. These primers contained restriction endonuclease sites for directional cloning. After digestion with the appropriate enzymes, the amplified fragments were cloned in frame into pPROEX-1 expression system (Life Technologies, Gaithersburg, MD) and subsequently moved into a pGST/HIS T2 prokaryotic expression system (Pharmacia Biotech, Piscataway, NJ). This vector is essentially pGEX4T2 modified to include a 6xHis tag at the 3′ end of the fusion segment. In our deletion constructs, the fusion proteins generated possess only a glutathione S-transferase (GST) fusion segment, because our inserts provide a stop codon before the 6xHis coding sequence. These N-terminal deletions include pGEXh3a9-66, pGEXh3a17-66, and pGEXh3a23-66.
C-terminal deletions.
Similarly, 3′ truncations were generated by PCR. In these constructions, various 3′ primers annealed to sequences downstream of the polymorphism. The 5′ oligonucleotide primer annealed to a vector sequence 5′ to the codon for the initiating methionine of GPIIIa. Conveniently engineered restriction sites, one previously made at the initiating methionine and the other in the 3′ primer, were used to clone the PCR fragments into pGST/HIS T2. The C-terminal truncations are pGEXh3a1-34, pGEXh3a1-40, and pGEXh3a1-50.
The sequence of each deletion mutation was verified by cycle sequencing (dsDNA Cycle Sequencing System; Life Technologies).
Isolation and Sequencing of the 5′ End of Murine GPIIIa cDNA (BALB/c strain)
The 5′ end of the cDNA sequence (nucleotides 1 to 309) of murine GPIIIa was completed by rapid amplification of cDNA ends, or the RACE procedure.13 Total RNA was isolated from the spleen of BALB/c mice using a standard protocol13; poly(A)+ RNA was purified using mini-oligo (dT) cellulose spin columns (5 Prime-3 Prime, Boulder, CO). cDNA was generated from the mRNA using Moloney murine leukemia virus reverse transcriptase and the reverse primer (5′-GGTAATCCTCCACCTGCCGC-3′), which anneals to the 5′ end of the partial sequence previously described for murine GPIIIa.14 After the 5′ cDNA was synthesized, a poly (A)+ tail was added to the 3′ end with terminal transferase. The murine GPIIIa sequence was then amplified by PCR using the above gene-specific primer and an oligo (dT)-containing primer. Another gene-specific, nested primer and the oligo (dT) primer were used to reamplify the sequence. The resulting PCR fragment was cloned into the cloning vector, pCR2.1 (Invitrogen, Carlsbad, CA), and sequenced using a cycle-sequencing system (Life Technologies). Subsequently, the murine sequence was cloned in frame into pGEX4T2 and named pGEXm3a1-66.
Isolation and Sequencing Fragments of the Murine GPIIIa Gene From Genetically Diverse Strains of Mice
Genomic clones, containing the exon that codes for position 33 of mouse GPIIIa, from 10 genetically diverse strains of mice were also isolated and sequenced. DNA from mice with dissimilar ancestries was obtained, including that from strains A/J, BALB/cBy, C3H/He, C57BL/6J, C58/J, CBA/J, DBA/2J, SEC/1Gn, SM/J, and NZW (Jackson Laboratory, Bar Harbor, ME). In the region of interest, the genomic structure of the mouse GPIIIa gene was the same as that of the human GPIIIa gene.15 As in humans, a murine intron, approximately 9 kb in size (analogous to intron 1 of human), precedes an exon that begins coding at amino acid 30 (analogous to exon 2 of human). Since the coding sequence for residue 33 was located very close to the 5′ end of the exon and the upstream intronic sequence was unknown, long PCR techniques were required to examine the residue coded for position 33. A forward primer that annealed to the end of the upstream exon (analogous to exon 1 in humans) and a reverse primer to the end of the exon in question (exon 2 in humans) were used to amplify the genomic sequence using a kit (Expand Long Template PCR System; Boehringer Mannheim, Indianapolis, IN). The resulting PCR fragments included a 9-kb intron and an exon encoding amino acids 30 to 84. The region encoding amino acids 30 to 84 from each fragment was sequenced.
Construction of Substitution Mutations in the Murine GPIIIa N-Terminus
Several techniques were used to generate the substitutions of human amino acids into the murine GPIIIa N-terminus.
Substituting amino acids at position 33 and 32.
Initially, the mutations at positions 32 and 33 in combination were constructed by PCR. Two overlapping PCR fragments with convenient 5′ and 3′ restriction sites were generated. The forward oligonucleotide primer annealed to the initiating methionine codon and included a unique 5′ BspHI restriction site. The reverse primers contained nucleotide substitutions that mutated the codons for positions 32 and 33 and, in addition, they silently mutated positions 34 and 35 to provide a unique BamHI restriction site. An overlapping 3′ PCR fragment was generated using a forward primer that recreated the BamHI site and a reverse primer that included a unique NotI restriction site. After digesting the 5′ fragment with BspHI and BamHI and the 3′ fragment with BamHI and NotI, the two fragments were ligated together and inserted in-frame into pPROEX1 (Life Technologies). The insert with both positions 32 and 33 mutated was subsequently cloned into pGEX4T2 and named pGEXm3a32,33.
Adding additional substitutions.
Using a site-directed mutagenesis kit (Clontech, Palo Alto, CA) and the pGEXm3a32,33 template, nucleotide substitutions were introduced individually into the codons for residues 22, 30, and 39. The resulting plasmids were (1) pGEXm3a22,32,33, (2) pGEXm3a30,32,33, and (3) pGEXm3a32,33,39. Further combinations of mutations were constructed either by a megaprimer technique16 17 or by exchanging restriction fragments with mutations. The first technique used a megaprimer made by PCR using a mutagenic oligonucleotide for position 22, the 3′ NotI primer, and the pGEXm3a30,32,33 template to generate pGEXm3a22,30,32,33. Further combinations were created by exchanging BamHI restriction fragments from pGEXm3a22,32,33, pGEXm3a30,32,33, and pGEXm3a22,30,32,33 with that from pGEXm3a32,33,39 which resulted in the addition of a mutation at position 39 to each construct. This exchange produced (1) pGEXm3a22,32,33,39, (2) pGEXm3a30,32,33,39, and (3) pGEXm3a22,30,32,33,39.
The sequence of each substitution mutation was verified by cycle sequencing.
Expression and Purification of Recombinant Proteins
GST fusion proteins.
The fusion proteins were expressed in Escherichia coli DH5α and purified under the standard, nondenaturing protocol given for pGEX expression systems (GST Gene Fusion System manual; Pharmacia Biotech, Piscataway, NJ). In the midst of these experiments, we found that the amount of contaminating GST protein decreased and the amount of specific fusion protein increased, when the bacterial cultures were grown at 30°C. To insure the proper folding of the proteins, the purified proteins were incubated in a mixture of oxidized and reduced glutathione as previously described.9 For clarity, the control proteins originally9 known as GST-PlA1and GST-PlA2 were renamed h3a 1-66 HPA-1a and HPA-1b in this study. These control proteins are expressed from the earliest GST expression vector, pGEX-1 (Amrad, Kew, Australia).
Immunoassays
The HPA-1a antisera tested were obtained from NAIT mothers or patients with PTP. The specificity of each antiserum was verified with a solid-phase enzyme-linked immunosorbent assay (ELISA) using isolated GPIIb/IIIa complexes derived from platelets of HPA-1a and 1b homozygotes.18 Previously described, anti–HPA-1a antibodies, affinity-purified against the N-terminal human GPIIIa (amino acids 1 to 66) fusion protein, were also used in some of the immunoassays,9 as well as the monoclonal antibody, SZ21 (Immunotech, Westbrook, ME).
ELISA.
Control and mutated proteins were assayed for reactivity to anti–HPA-1a antisera using an ELISA. Samples were tested in triplicate. Microtiter wells were coated with 200 ng purified fusion protein in 200 μL of 64 mmol/L carbonate buffer, pH 9.5 overnight at 4°C. After washing the wells with PBST, pH 7.2 (7.72 mmol/L Na2HPO4, 2 mmol/L NaH2PO4-H2O, 0.15 mol/L NaCl, and 0.05% Tween 20), each was blocked with 2% bovine serum albumin (BSA) in PBST and washed again with PBST. Antisera were diluted 1:40 and incubated with the immobilized antigen for 2 hours at 37°C. After washing, a 1:25,000 dilution of Protein A/G-horseradish peroxidase (HRP) conjugate (Pierce, Rockville, IL) was added to the well and the peroxidase was detected with tetramethylbenzidine (TMB) peroxidase substrate (Kirkegaard & Perry Labs, Gaithersburg, MD). Microtiter plates were read at optical density (OD) 450.
To adjust for possible differences in the amount of proteins loaded in the wells, a parallel plate (samples in duplicate) was prepared and assayed for GST-containing proteins using 5 μg/mL anti-GST antibody (preadsorbed polyclonal; Pharmacia, Piscataway, NJ) as the primary antibody. The signal was detected as above using Protein A/G-HRP and TMB substrate. The average of the results of each triplicate tested with anti–HPA-1a antibodies was divided by the average of the duplicate samples reacted with anti-GST antibodies.
Competitive inhibition in an ELISA was also used to test the ability of the mutated murine proteins to compete with the human GPIIIa N-terminal protein for binding anti–HPA-1a antibodies. In these experiments, microtiter wells were coated overnight at 4°C with the human fusion protein, h3a 1-66 HPA-1a. Dilutions of anti–HPA-1a antisera giving approximately 50% saturation were determined by ELISA; dilutions ranged from 1:50 to 1:2,500. Sera were preincubated with increasing amounts of mutated murine recombinant protein for 30 to 60 minutes at room temperature. The pretreated antisera were then added to the microtiter wells and allowed to react for 60 minutes at 37°C with the human protein bound to the well. Immunoreactivity was detected with Protein A/G-HRP conjugate and developed with TMB.
Immunoblots.
The reactivity of the clinical antibodies to the deletion and substitution mutations was assayed by immunoblots. Samples (5 to 10 μg) were electrophoresed on 10% to 15% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto nitrocellulose as previously described.9 Membranes were then blocked for 1 hour with 10% dried milk, washed with TBST (150 mmol/L NaCl, 0.05% Tween 20,10 mmol/L Tris-HCl, pH 8.0), and incubated with 5.0 μg/mL affinity-purified anti–HPA-1a antibody or 1:10 dilution of antisera. After washing with TBST, the blots were incubated with a 1:10,000 to 1:30,000 dilution of the Protein A/G-HRP conjugate. The immunoreactive proteins were detected by enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL) or SuperSignal (Pierce).
SZ21 blots were performed as described by Weiss et al.19Briefly, 20 μg of each protein sample was electrophoresed under nonreducing conditions and blotted onto nitrocellulose. The membranes were blocked with 0.3% Tween and 10% dried milk and incubated with 0.1 μg/mL of the SZ21 monoclonal antibody.
RESULTS
Truncations of the Human GPIIIa N-Terminus
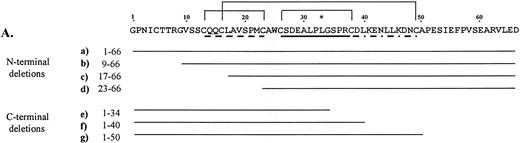
Using recombinant proteins, we have further defined the binding domain of an HPA-1a epitope by systematically dissecting the immunoreactive region of the N-terminal 66-residue segment of human GPIIIa (Fig1A). Three N-terminal and three C-terminal truncations of the 66-residue segment were constructed and tested for anti–HPA-1a recognition. By ELISA (Fig 1B), we showed a progressive reduction in immunoreactivity from fusion proteins with sequential deletions from either direction. Removal of residues 1 to 8 from the human GPIIIa N-terminus reduces the reactivity approximately 30% relative to the intact segment. This deleted segment includes Cys5 which is believed to be joined via a disulfide bond to Cys435 in the intact protein. When residues 1 to 17 are deleted, an approximate 53% decrease from the intact segment is observed. This missing segment extends into the first loop of the proposed cloverleaf structure and includes Cys16 that pairs with Cys49 to generate the third loop of the structure. Loss of six additional amino acids within the first loop in the deletion mutation m3a1-22 results in a 75% reduction in signal from the control. Truncations from the C-terminal direction show that removal of residues 51 to 66, not contained within the cloverleaf, decreases reactivity approximately 30% from control. Deletions of residues 41 to 66 and 35 to 66, which interrupt the second and third loops, drop the signal 73% and 86% from control, respectively.
Deletion analysis of the 66–amino acid segment from the N-terminus of human GPIIIa that is recognized by anti–HPA-1a antibodies. (A) Amino acid sequence of the immunoreactive 66–amino acid segment is shown above. *Site of the human polymorphism. Proposed loops of the cloverleaf structure are indicated, loop 1 (dashed line), loop 2 (solid line), and loop 3 (dashed and dotted line) with the proposed disulfide bonds between Cys13-Cys23, Cys26-Cys38, and Cys16-Cys49 shown with solid lines above the sequence. The deletion mutations and their sequences are shown below. (B) Results of an ELISA are graphically displayed. Letters (a-g) refer to deletion mutations illustrated in A. In this assay, microtiter wells were coated in triplicate with the deletion mutations (expressed as GST fusion proteins). An anti–HPA-1a antiserum was tested for its ability to bind the various human mutations. Antibodies bound were detected using Protein A/G conjugated with HRP, and TMB (tetramethylbenzidine) was used as the colorometric substrate. The average of the absorption at 450 nm of the triplicate was divided by the average of duplicate wells reacted with anti-GST. (C) Immunoblot (top box) of the deletion mutations incubated with the same anti–HPA-1a antiserum tested in ELISA, and corresponding Coomassie blue–stained SDS-polyacrylamide gel electrophoresis (PAGE) (bottom box) of deletion mutations. Molecular weight of the intact fusion protein, h3a 1-66, is indicated on the left of the blot and gel.
Deletion analysis of the 66–amino acid segment from the N-terminus of human GPIIIa that is recognized by anti–HPA-1a antibodies. (A) Amino acid sequence of the immunoreactive 66–amino acid segment is shown above. *Site of the human polymorphism. Proposed loops of the cloverleaf structure are indicated, loop 1 (dashed line), loop 2 (solid line), and loop 3 (dashed and dotted line) with the proposed disulfide bonds between Cys13-Cys23, Cys26-Cys38, and Cys16-Cys49 shown with solid lines above the sequence. The deletion mutations and their sequences are shown below. (B) Results of an ELISA are graphically displayed. Letters (a-g) refer to deletion mutations illustrated in A. In this assay, microtiter wells were coated in triplicate with the deletion mutations (expressed as GST fusion proteins). An anti–HPA-1a antiserum was tested for its ability to bind the various human mutations. Antibodies bound were detected using Protein A/G conjugated with HRP, and TMB (tetramethylbenzidine) was used as the colorometric substrate. The average of the absorption at 450 nm of the triplicate was divided by the average of duplicate wells reacted with anti-GST. (C) Immunoblot (top box) of the deletion mutations incubated with the same anti–HPA-1a antiserum tested in ELISA, and corresponding Coomassie blue–stained SDS-polyacrylamide gel electrophoresis (PAGE) (bottom box) of deletion mutations. Molecular weight of the intact fusion protein, h3a 1-66, is indicated on the left of the blot and gel.
An immunoblot using the same antiserum as in the ELISA showed slightly different results (Fig 1C). The stepwise decrease in signal was seen; however, the reactivity of deletion mutation h3a9-66 (lane b) was not equivalent in signal to h3a1-50 (lane g), nor was the signal from mutation h3a23-66 (lane d) equal to that of h3a1-40 (lane f), unlike the ELISA. These differences may be due to different sensitivities of the techniques. The combined data show that the anti–HPA-1a antibodies recognize the intact 66–amino acid segment better than any truncation. Both assays show that a high degree of immunoreactivity is maintained when the N-terminus is shortened to amino acid 9 or the C-terminus reduced to amino acid 50. They also indicate a sequential decrease in immunoreactivity as the deletions increase in size.
Murine N-Terminal GPIIIa Sequence
To compare the amino acid sequence of the immunoreactive human GPIIIa N-terminus to the nonimmunoreactive murine GPIIIa N-terminus, the 5′ end of the murine (BALB/c) GPIIIa cDNA (Genbank accession no.U83167) was isolated and sequenced. The nucleotide sequence of the segment that codes for amino acids 1 to 66 is 83% identical to the analogous sequence in human. The degree of identity is similar to the 86% DNA homology determined for the remainder of the cDNA by Cieutat et al14 (amino acid sequence begins at position 79, Genbank accession no. 386151). The high degree of nucleotide conservation translates into an overall amino acid identity of 89% between the full-length murine and human proteins.
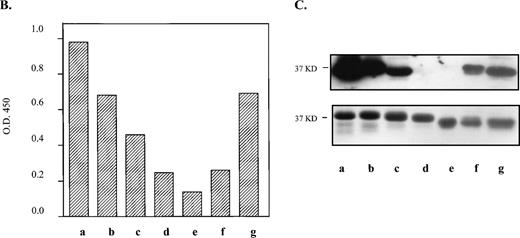
Within the 66 residue N-terminal segment that contains the alloepitope, we identified 12 amino acid differences between mouse and human (Fig2A). However, a recent cDNA sequence from osteoclasts in mouse strain C3H/HeN (Genbank accession no. AF026509) shows only 11 differences, with the residue at position 7 of the mature protein being a threonine as in human. Within the human sequence that retains strong anti–HPA-1a immunoreactivity (residues 9 to 50 shown underlined), only six amino acid differences between mouse and human occur. Neither a leucine (HPA-1a) nor a proline (HPA-1b) resides at the analogous site in the murine protein for the human polymorphism. Instead, the mouse gene codes for a glutamine at position 33. Due to their proximity to position 33, the differences in amino acid residues at positions 30 and 32 are also notable. At position 30, a conservative change from an alanine to a threonine occurs, whereas a more nonconservative difference takes place at position 32, where a proline is switched to a serine.
Substitution of murine GPIIIa residues with analogous human amino acids to generate an HPA-1a–like epitope. (A) Comparison of first 66 amino acids from the N-termini of human and murine GPIIIa. Amino acid differences are highlighted and italicized. *Site of human polymorphism for HPA-1a. Underlined sequence refers to the shortest human fragment that maintains the majority of immunoreactivity with clinical anti–HPA-1a antibodies. (B) By site-directed mutagenesis, several amino acids in the N-terminus of the murine GPIIIa were mutated to the human counterpart. The N-terminal regions containing the substitution mutations (left) were expressed as GST fusion proteins (37 kD), electrophoresed on 10% to 15% SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblot (top, right) of murine GPIIIa substitution mutations incubated with an affinity-purified anti–HPA-1a antibody is shown. The same set of proteins (bottom, right) stained with Coomassie blue. Smaller band on gel is contaminating GST protein.9
Substitution of murine GPIIIa residues with analogous human amino acids to generate an HPA-1a–like epitope. (A) Comparison of first 66 amino acids from the N-termini of human and murine GPIIIa. Amino acid differences are highlighted and italicized. *Site of human polymorphism for HPA-1a. Underlined sequence refers to the shortest human fragment that maintains the majority of immunoreactivity with clinical anti–HPA-1a antibodies. (B) By site-directed mutagenesis, several amino acids in the N-terminus of the murine GPIIIa were mutated to the human counterpart. The N-terminal regions containing the substitution mutations (left) were expressed as GST fusion proteins (37 kD), electrophoresed on 10% to 15% SDS-PAGE and transferred onto nitrocellulose membranes. Immunoblot (top, right) of murine GPIIIa substitution mutations incubated with an affinity-purified anti–HPA-1a antibody is shown. The same set of proteins (bottom, right) stained with Coomassie blue. Smaller band on gel is contaminating GST protein.9
To determine whether a naturally occurring mouse polymorphism that might generate an alloimmune reaction in certain mice upon exposure to platelets, we sequenced the appropriate region of the murine GPIIIa gene (coding for amino acids 30 to 84) from various mouse strains. Genomic DNA from various genetically diverse strains of mice was available through the Jackson Laboratory. Sequence of the exon that encodes position 33 of the murine GPIIIa gene from 10 genetically diverse strains of mice was compared. These data showed no evidence of a naturally occurring murine polymorphism at position 33 or nearby sites (amino acids 30 to 84, data not shown) when compared with each other, with our mouse (strain BALB/c) spleen cDNA sequence, and with the mouse (strain C3H/HeN) osteoclast cDNA sequence. A difference in a residue located at position 80 was seen between these 12 sequences and that of Cieutat et al.14
It is not clear whether this difference in Cieutat’s sequence is real or an artifact generated from one of the human oligonucleotide primers used in the study.
Reactivity of Anti–HPA-1a Antibodies and Antisera to Substitution Mutations in the Murine GPIIIa N-Terminus
We took advantage of the fact that GPIIIa from mouse was not reactive to anti–HPA-1a antibodies. By site-directed mutagenesis, human amino acids were substituted into the murine N-terminus at five of the six sites that differ within the 9 to 50 residue segment shown to maintain the majority of immunoreactivity to anti–HPA-1a antibodies. The reactivity of HPA-1a antibodies to the modified murine GPIIIa N-terminal fusion proteins was assayed by immunoblots using anti–HPA-1a antisera and an affinity-purified antibody.
These immunoblots revealed residues necessary for anti–HPA-1a antibody recognition. The blot shown in Fig 2B, which is reacted with an affinity-purified anti–HPA-1a antibody, is representative of the results seen with its corresponding antisera and an additional antisera (data not shown). Initial mutations at positions 32 and 33 in the murine protein were not sufficient for recognition by anti–HPA-1a antibodies. Addition of a third substitution at position 22 also did not show reactivity. However, a substitution at position 39, in combination with 32 and 33, exhibited slight to moderate reactivity. A substitution at position 30 was strongly reactive; however, the degree of recognition was not comparable to that of the human N-terminal 66–amino acid segment until a substitution at position 39 was added (Fig 2B, lane h).
Competition With the Human Recombinant Epitope
To determine how similar the epitope generated in the murine N-terminal GPIIIa sequence is to that of the human epitope, we tested the ability of the mutated murine proteins to compete with the human recombinant protein for antibody binding. After pretreating the anti–HPA-1a antisera with increasing concentrations of the modified murine GPIIIa N-termini, antisera were assayed by ELISA for their ability to bind the corresponding human 66–amino acid segment. The results of a competition ELISA using an antiserum are shown in Fig 3; similar results were observed with two additional anti–HPA-1a antisera (data not shown).
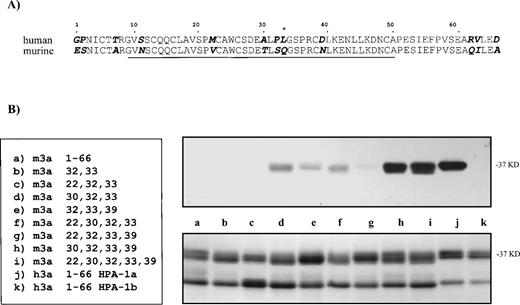
Competition of murine GPIIIa N-termini containing substitution mutations with the HPA-1a–positive human GPIIIa N-terminus. In an ELISA, murine substitution mutations were tested for their ability to compete with the human HPA-1a containing 66 amino acid segment. An antiserum was incubated with various murine GPIIIa N-termini containing substitution mutations before being assayed with the human N-terminal segment. Due to the numerous samples, the results were separated into three groups. Some samples are present in more than one group. In (A), substitution mutations (m3a32,33and m3a22,30,32) were tested for their ability to compete with the human GPIIIa N-terminal segment. The results in graph (B) compare the ability of the murine proteins with substitutions at position 30 to compete with the human segment; those in graph (C) compare the ability of the murine proteins with substitutions at position 39 to compete. The legend for the substitution mutations is given (bottom, right).
Competition of murine GPIIIa N-termini containing substitution mutations with the HPA-1a–positive human GPIIIa N-terminus. In an ELISA, murine substitution mutations were tested for their ability to compete with the human HPA-1a containing 66 amino acid segment. An antiserum was incubated with various murine GPIIIa N-termini containing substitution mutations before being assayed with the human N-terminal segment. Due to the numerous samples, the results were separated into three groups. Some samples are present in more than one group. In (A), substitution mutations (m3a32,33and m3a22,30,32) were tested for their ability to compete with the human GPIIIa N-terminal segment. The results in graph (B) compare the ability of the murine proteins with substitutions at position 30 to compete with the human segment; those in graph (C) compare the ability of the murine proteins with substitutions at position 39 to compete. The legend for the substitution mutations is given (bottom, right).
Figure 3A demonstrates that mutations m3a32,33 and m3a22,32,33 showed little reactivity with anti–HPA-1a antibodies. When compared with the unaltered mouse protein, those murine proteins with mutations at position 32 and position 33 (the site of the human polymorphism) inhibited slightly anti–HPA-1a binding to the human epitope in the GPIIIa N-terminus. An additional mutation at position 22 did not increase the degree of competition. At low doses, the degree of inhibition by the protein with mutations at positions 22, 32, and 33 was equal to the protein mutated at positions 32 and 33, and at higher concentrations showed no competition compared with mouse wild type.
In contrast, the substitutions at positions 30 and 39, in combination with those at positions 32 and 33, appear to be essential for antibody recognition. These results are shown in Fig 3B and C. Figure 3B analyzes all of the murine proteins that contain position 30 substitutions (m3a30,32,33, m3a22,30,32,33, m3a30,32,33,39, and m3a22,30,32,33,39). These competition experiments demonstrate that position 30 alone makes a large contribution to the antigenic determinant and greatly enhances the ability of the murine peptide to compete with the human N-terminal segment. These results are confirmed when all the modified murine proteins substituted at position 39 are compared (m3a32,33,39, m3a22,32,33,39, m3a30,32,33,39, and m3a22,30,32,33,39) in Fig 3C. Unlike the position 30 mutation, we found that a mutation at position 39 in combination with those at positions 32 and 33 in the murine protein could compete only moderately for antibody binding to the human epitope. As before, an additional mutation at position 30 markedly increased the degree of competition. The addition of a mutation at position 22 to any of the reactive constructs showed no increased effect.
The greatest competition against the human recombinant epitope occurred with murine proteins m3a22,30,32,33,39 and m3a30,32,33,39. Similar to the immunoblot data, these murine proteins were slightly less efficient in the competition ELISA than the human recombinant protein. This may indicate that additional residues such as position 11 or others that reside outside the 9– to 50–amino acid domain influence the conformation recognized by the antibodies. It is interesting that the lines in the graph of the most reactive murine proteins remain parallel to that of human at high concentrations with no hint of intersection. This suggests that the anti–HPA-1a antibodies are not binding with lower affinity to the murine site, but that at least some of the antibodies recognize a site or sites slightly different in the human than in the modified mouse.
Recognition of the Panel of Murine Substitution Mutations by the Monoclonal Antibody SZ21
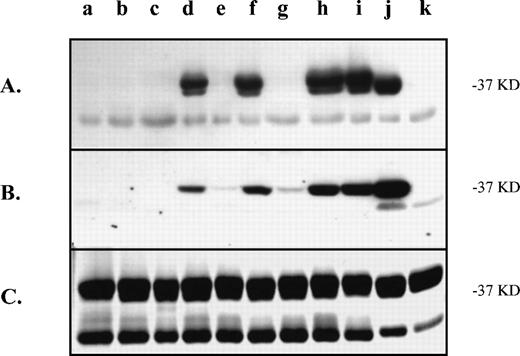
We also tested the murine monoclonal antibody SZ21 with the mutated murine proteins to determine whether it recognized the same amino acids as the clinical antibodies. This monoclonal antibody SZ21 can distinguish between the HPA-1a and HPA-1b forms of GPIIIa under certain conditions. We used the immunoblot conditions of Weiss et al19 to discriminate the HPA-1a form from the HPA-1b form of human GPIIIa. When compared with clinical antibodies, our immunoblot results show that the SZ21 monoclonal antibody has a similar pattern of recognition of the substitution mutations with one notable difference (Fig 4A). The substitution at position 39 is not important in the antigenic determinant of this antibody. Unlike the clinical antibodies (Fig 4B), substitution mutations m3a32,33,39 (lane e) and m3a22,32,33,39 (lane g) show no immunoreactivity with SZ21 (Fig 4A). Furthermore, the SZ21 signal (Fig 4A) from mutation m3a30,32,33 (lane d) is as intense as any of the reactive proteins, indicating that neither position 22 nor 39 is key to this epitope. This finding is consistent with published reports of the SZ21 binding site (residues 28 to 35 of human GPIIIa) for this antibody.10
Recognition of substitution mutations in the N-terminus of the murine GPIIIa by the monoclonal antibody, SZ21. The same modified murine proteins assayed in Fig 2 (samples a-k) were tested for immunoreactivity with the monoclonal antibody, SZ21. The immunoblot with SZ21 (A) can be compared with that with a clinical anti–HPA-1a antibody (B) to illustrate the difference in binding to the position 39-containing mutations. (C) Coomassie blue–stained gel of the proteins. Approximate molecular weight of the protein of interest is indicated on the right. The contaminating GST protein (lower band) is present on the stained gel.
Recognition of substitution mutations in the N-terminus of the murine GPIIIa by the monoclonal antibody, SZ21. The same modified murine proteins assayed in Fig 2 (samples a-k) were tested for immunoreactivity with the monoclonal antibody, SZ21. The immunoblot with SZ21 (A) can be compared with that with a clinical anti–HPA-1a antibody (B) to illustrate the difference in binding to the position 39-containing mutations. (C) Coomassie blue–stained gel of the proteins. Approximate molecular weight of the protein of interest is indicated on the right. The contaminating GST protein (lower band) is present on the stained gel.
DISCUSSION
The objective of this study was to identify critical elements in human GPIIIa that generate a conformation recognized by anti–HPA-1a antibodies. By revealing the binding requirements of the anti–HPA-1a antibodies, we may be able to regulate or neutralize those antibodies that are problematic in NAIT and PTP in the future. We have taken advantage of the fact that murine GPIIIa is similar to the human protein, yet not reactive to anti–HPA-1a antibodies. By comparing the two sequences, residues that are important in the development of the antigen conformation were identified. Having narrowed the immunoreactive region in the human GPIIIa N-terminus to 42 amino acids that include residues 9 to 50, we concentrated on the six differences between the mouse and human sequence that occur within this epitope-containing domain.
Using the cloverleaf structure proposed for the N-terminal region of human GPIIIa to map the six amino acid differences, we find that three are clustered in the second loop and three are spread throughout the remaining structure. The second loop (loop 2) includes the HPA-1 polymorphism (HPA-1a form = Leu33 and HPA-1b form = Pro33); this site is occupied by a glutamine (Q or Gln33) in mouse. Two neighboring residues in loop 2 that differ from the human sequence occur at positions 30 and 32. The other three differences occur at (1) position 11 within the sequence leading to the cloverleaf, (2) position 22 within the first loop, and (3) position 39 within the third loop. There are no differences in the cysteines in the N-terminal region (or the rest of the murine GPIIIa) from those of the human GPIIIa.
We discovered in our initial experiments that changing the glutamine to a leucine at position 33 in mouse (as in the HPA-1a form of the human polymorphism) and the adjacent serine to proline at position 32 was not sufficient for anti–HPA-1a recognition. These changes conferred weak antibody recognition, observed not by immunoblots, but by a competition ELISA. This indicated that other residues, in addition to these two positions, are pertinent to the conformation recognized by the antibodies. An additional substitution at position 22 did not improve the immunoreactivity of the structure, whereas additional substitution mutations at positions 30 and 39 proved to be critical for antibody recognition. Our results show that the conservative change from a threonine to an alanine at position 30 is more essential in the determinant than the switch from an asparagine to an aspartic acid at position 39. The combination of mutations at positions 30 and 39, in addition to the initial two mutations, produced an immunoreactive murine protein that could compete well with the human N-terminus for the binding of anti–HPA-1a antibodies.
The substitution mutation data in the mouse GPIIIa suggest that a core structure is formed that includes positions 30, (perhaps 32), 33, and 39. The structure is not limited to the four amino acids identified in the substitution study, but is confined to amino acids 9 to 50, as determined in the deletion analysis. These four residues reside in the second and third loops of the hypothetical cloverleaf structure. The majority of anti–HPA-1a antibodies that bind the N-terminal 66–amino acid segment recognize this core structure. A fifth substitution at position 22 located in the first loop was not critical for immunoreactivity. One explanation is that this residue is not important in the antigenic determinant. Another possibility is that the swap of the murine residue (valine) for the human residue (methionine) at position 22 makes little difference because the residues are similar and can interchange in this structure with no effect. Other residues in the first loop are important to the determinant, as shown by the N-terminal human deletion mutations. Further structural studies are necessary to understand fully the immunoreactive conformation of HPA-1a.
Amino acids outside the core structure also influence the immunoreactive conformation. The removal of amino acids 1 to 8 or residues 51 to 66 results in a 30% decrease in immunoreactivity by ELISA. Furthermore, the inability of the murine N-terminus with four substitutions to inhibit completely the human N-terminal segment in the competition ELISA, indicates that additional amino acids that differ between mouse and human (ie, positions 1, 2, 7, 11, 22, 62, 63, or 66) may contribute as well. These outer residues may serve an ancillary role to stabilize or complete the conformation so that additional antibodies can bind optimally. Alternatively, perhaps the core structure is a dominant epitope and the outer residues compose additional less antigenic determinants.
Our experiments focus on the conformation recognized by the anti–HPA-1a antibodies directed solely against the N-terminus of human GPIIIa. The question remains whether there are one or two classes of anti–HPA-1a antibodies. The work by Honda et al10 suggests that the Cys5-Cys435 bridge is necessary for the proper orientation of N-terminal segment within the context of intact human GPIIIa. However, the experiments of Valentin et al11 and Liu et al12 suggest two classes of anti–HPA-1a antibodies: type I recognizes the N-terminal region only and type II requires the N-terminus to be tethered to the core of GPIIIa.11 We have shown in our experiments with the monoclonal antibody SZ21 that differences in binding of anti–HPA-1a antibodies can be detected using a panel of substitution mutations. The panels of human deletion and murine substitution mutations may prove useful in deciphering differences in binding properties of various human antisera, and possibly in classifying the antisera. By organizing the antisera by their binding specificities, we may be able to discern whether their classification correlates with severity of the alloimmune disorder.
In these experiments, the HPA-1a epitope was studied in the context of a fusion protein using solid-phase ELISAs and immunoblots. Thus, the theoretical concern that our results may not reflect the true conformation of GPIIIa on the platelet surface must be considered. However, we have previously demonstrated that the human N-terminal GPIIIa fusion protein inhibits binding of anti–HPA-1a antibodies to the native protein on HPA-1a platelets in suspension.9Also, antibodies bound only to the HPA-1a form, but not to the HPA-1b form of the fusion protein. This suggests that the fusion proteins do contain a conformational structure relevant to the intact, membrane-bound protein. Further studies will be needed to show conclusively that the conformation in our fusion proteins is a good model of the native protein.
To determine the feasibility of developing murine models of the platelet alloimmune disorders, the generation of an HPA-1a binding site in mouse GPIIIa is critical. Such models would provide systems to study the nature of the alloimmune responses in these disorders, and also allow the testing of new and perhaps more specific therapeutic agents. The demonstration that an HPA-1a epitope can be created within the context of the murine GPIIIa is an encouraging step towards that goal.
ACKNOWLEDGMENT
We thank Ruth M. Graupera for subcloning the deletion mutations into the pGEX expression vector.
Supported by a National Blood Foundation grant, American Heart Established Investigator Grant No. 9740238N, and National Institutes of Health Grant No. HL-59955. G.N. was supported by a training fellowship from the University of Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Emily A. Barron-Casella, PhD, Division of Hematology/Department of Pediatrics, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Bldg, Room 1129, Baltimore, MD 21205; e-mail: ebcasell@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal