Abstract
Fibronectin matrix assembly is a cell-dependent process mediated by cell surface binding sites for the 70-kD N-terminal portion of fibronectin. We have shown that Rho-dependent cytoskeleton reorganization induced by lysophosphatidic acid (LPA) or the microtubule-disrupting agent nocodazole increases fibronectin binding (Zhang et al, Mol Biol Cell 8:1415, 1997). Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid implicated in mitogenesis and cytoskeletal remodelling. Both LPA and S1P are present in increased amounts in serum as compared with plasma as a result of platelet activation. Addition of S1P to human osteosarcoma MG63 cells or human foreskin fibroblasts increased cell-mediated binding and assembly of fibronectin. MG63 cells expressed the Edg-2 and Edg-4 G-protein–coupled receptors for bioactive lipids, whereas foreskin fibroblasts expressed Edg-2, Edg-3, and Edg-4. The stimulatory effect of S1P on the binding of fibronectin or the N-terminal 70-kD fragment of fibronectin was dynamic and due to increases in both the number and affinity of binding sites. The stimulation of 70-kD fragment binding by nanomolar S1P, like stimulation of binding by LPA or nocodazole, was blocked by inactivation of Rho with C3 exotoxin but not by pertussis toxin-mediated inactivation of Gi. These results indicate a common signal pathway leading to control of cellular fibronectin matrix assembly by bioactive lipids generated during blood coagulation.
SPHINGOSINE 1-PHOSPHATE (S1P), a bioactive phospholipid, is present in plasma and released from platelets after activation.1,2 S1P is the product of phosphorylation of sphingosine by sphingosine kinase3 and is subsequently cleaved by S1P lyase to a fatty aldehyde and ethanolamine phosphate4 or dephosphorylated by a sphingosine phosphatase.5 S1P, in addition to being an intermediary catabolite, is a bioactive lipid with interesting functions, including stimulation of mitogenesis of fibroblasts,6-8 inhibition of tumor cell motility,9 platelet activation,10 and neurite retraction.11
Initial studies focused on S1P as an intracellular second messenger mediating the proliferative effects of platelet-derived growth factor (PDGF) and evoking release of Ca2+ from internal stores by an inositol-phosphate (IP3)-independent mechanism.12-14 Recent studies show that some signaling responses to S1P are through cell surface G-protein–coupled receptors (GPCR), of which several candidates have been cloned.15,16A similar dependence on G proteins has been observed for response to lysophophatidic acid (LPA),17 a lysoglycerophospholipid that is structurally similar to S1P. Several GPCRs for LPA have also recently been cloned.15,18-20 The receptors for S1P and LPA are called endothelial cell differentiation genes (Edg) in recognition of the initial identification of Edg-1 in endothelial cells.21
Fibronectin matrix assembly is a cell-dependent process mediated by cell surface binding sites for the 70-kD N-terminal portion of fibronectin. LPA stimulates binding and assembly of fibronectin by a variety of fibroblastic cells.22,23 Enhanced binding of the 70-kD N-terminal fragment of fibronectin induced by LPA treatment correlates with changes in cell shape and actin-containing cytoskeleton.23-25 Actin stress fiber formation and cell contraction are mediated by the small Ras-type GTPase, Rho, and represent a rapid, labile way that cells can modulate fibronectin matrix assembly.24
Like LPA, S1P causes stress fiber formation and cell contraction.11,26 Both LPA and S1P are present in serum as a result of platelet activation.1 27 Therefore, we tested whether S1P stimulates cell surface binding and assembly of fibronectin. S1P (0.1 nmol/L to 10 μmol/L) stimulated 70-kD fragment binding to MG63 osteosarcoma cells and foreskin fibroblasts. The Edg receptors common to MG63 osteosarcoma cells and foreskin fibroblasts were Edg-2 and Edg-4. The stimulatory activity of S1P was inhibited by C3 exotoxin-mediated inactivation of Rho. These results indicate that a common signal pathway initiated by bioactive lipids generated during blood coagulation controls assembly of fibronectin by cells.
MATERIALS AND METHODS
Materials.
LPA was from Avanti Polar Lipids (Birmingham, AL). S1P was obtained from LC Laboratories (Woburn, MA). Sphingosylphosphorylcholine, sphingomyelin, and fatty acid-free bovine albumin were from Sigma Chemical Co (St Louis, MO). Stock solutions of 2 mmol/L S1P, sphingosylphosphorylcholine, or sphingomyelin were made in methanol. Recombinant C3 exotransferase28 was prepared by Dr Tracee Panetti of our laboratory. Pertussis toxin was purchased from List Biological Laboratories Inc (Campbell, CA). Recombinant factor XIII was a gift from Dr Paul Bishop (Zymogenetics, Seattle, WA). Human plasma fibronectin and the 70-kD N-terminal, gelatin-binding fragment of fibronectin generated by cathepsin D were isolated, iodinated, and reisolated as previously described.29,30 The labeled proteins were stored in portions at −70°C in TBS containing 0.1% (wt/vol) fatty acid-free bovine albumin. Purity of labeled proteins was assessed by gel electrophoresis with and without reduction followed by autoradiography. Fibronectin was also labeled with fluorescein isothiocyanate (FITC).30
Cells.
The MG63 human osteosarcoma cell line was obtained from the American Type Culture Collection (Rockville, MD). Human foreskin fibroblasts were a strain derived by Dr Lynn Allen-Hoffmann (University of Wisconsin-Madison, Madison, WI). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (for MG63 cells) or 10% (for foreskin fibroblasts) fetal bovine serum (FBS).
Pertussis toxin and C3 exotransferase treatment.
Cell monolayers were incubated in complete media containing pertussis toxin (100 μg/L) or C3 exotransferase (50 mg/L) for 24 hours before fibronectin or 70-kD fragment binding assay. The effects of pretreatment of activation of MAP kinase was assayed by immunoblotting with antibodies to dual phosphorylated ERK1, 2 from Promega (Madison, WI).
Binding and cross-linking assays.
Confluent cell layers were usually studied 3 days after sparse seeding in DMEM plus FBS. For binding studies, the confluent cell layers were washed with TBS and incubated with 125I-labeled fibronectin or 70-kD fragment of fibronectin in DMEM containing 0.2% (wt/vol) fatty acid-free bovine albumin in the absence or presence of different stimulators for the indicated amount of time. After washing out the unbound ligand, the cell layers were collected with 1% sodium dodecyl sulfate (SDS) for counting of the amounts of total cell layer-associated radioactivity.23 Nonspecific binding in the presence of 500 mg/L of unlabeled fibronectin or 20 mg/L of unlabeled 70-kD fragment was determined and subtracted from total binding to calculate specific binding. Cross-linking with recombinant factor XIIIa (10 mg/L) was performed after washing out the unbound ligand and assessed on reducing SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by phosphor-imaging as previously described.31
Microscopy studies.
Confluent cell layers were washed with TBS and incubated with FITC-labeled fibronectin in DMEM containing 0.2% (wt/vol) fatty acid-free bovine albumin in the absence or presence of different stimulators for the indicated amount of time. After washing out the unbound ligand, the cell layers were processed for fluorescent microscopy as previously described.23
RNA isolation, reverse transcription, and polymerase chain reaction (RT-PCR).
RNA was isolated and RT-PCR were performed using the RNAgents Total RNA Isolation and Access RT-PCR kit systems (Promega) as described.32 Primers were selected based on the sequences for human cDNAs: Edg-1, 5′ACGTCAACTATGATATCATCGTCCG3′ and 5′CATTTTCAGCATTGTGATATAGCGC3′, expected size 391 bp21; Edg-2, 5′AATCGAGAGGCACATTACGG3′ and 5′TGTGGACAGCACACGTCTAG3′, expected size 432 bp18; Edg-3, 5′CGATCGTTTTATCAATGGTCGC3′ and 5′GCGTCGTAAGGCCTCATTTTG3′, expected size 631 bp33; and Edg-4, 5′CATCATGCTTCCCGACAACG3′ and 5′GGGCTTACCAAGGATACGCAG3′, expected size 352 bp (Genebank entry no. AF011466).
RESULTS
S1P stimulates binding of fibronectin or the N-terminal 70-kD fragment to cells: Comparison to LPA and nocodazole.
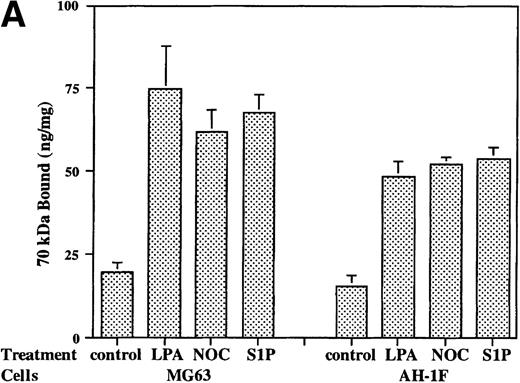
LPA and the microtubule-disrupting agent nocodazole are potent stimulators of fibronectin matrix assembly.22-24 The addition of S1P (200 nmol/L) to cell layers of strain of human foreskin fibroblasts or MG63 osteosarcoma cells increased the initial binding to cells of the N-terminal 70-kD matrix assembly domain of fibronectin (Fig 1A) to levels stimulated by LPA (200 nmol/L) or nocodazole (10 μmol/L). This effect was specific for S1P, inasmuch as the same dose of sphingosylphosphorylcholine or sphingomyelin, two related sphingolipids,34 did not increase the binding of 70-kD fragment to MG63 cells (data not shown).
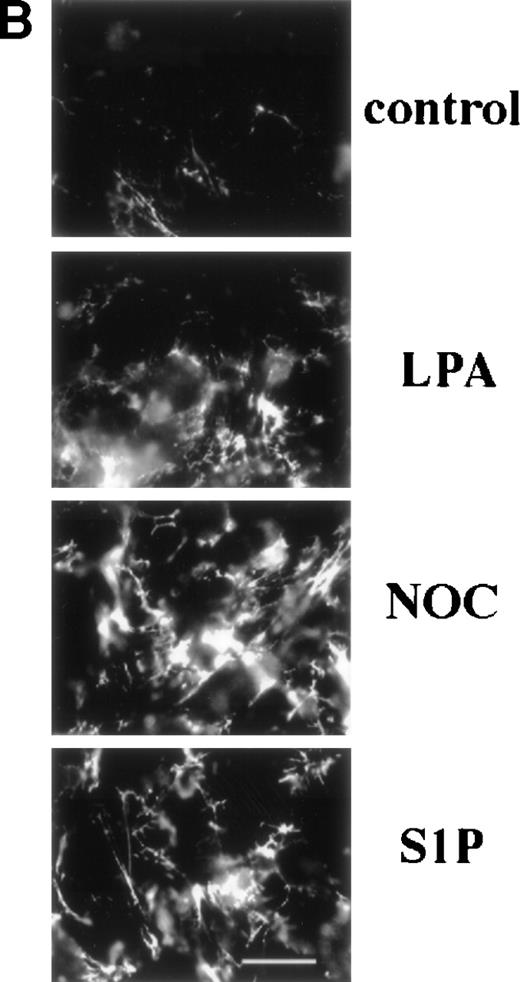
Binding of 70-kD N-terminal fragment of fibronectin (A) and FITC-fibronectin (B) to confluent cells. (A) Binding of125I-70-kD fragment of fibronectin to MG53 cells (MG63) or human foreskin fibroblasts (AH-1F) was determined in the absence (control) or presence of 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 200 nmol/L S1P (S1P) as described in Materials and Methods. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the mean ± SD (n = 3). (B) Deposition of FITC-fibronectin by confluent MG53 cells was determined after incubation for 1 hour in the absence (control) or presence of 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 200 nmol/L S1P (S1P). Bar = 35 μm.
Binding of 70-kD N-terminal fragment of fibronectin (A) and FITC-fibronectin (B) to confluent cells. (A) Binding of125I-70-kD fragment of fibronectin to MG53 cells (MG63) or human foreskin fibroblasts (AH-1F) was determined in the absence (control) or presence of 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 200 nmol/L S1P (S1P) as described in Materials and Methods. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the mean ± SD (n = 3). (B) Deposition of FITC-fibronectin by confluent MG53 cells was determined after incubation for 1 hour in the absence (control) or presence of 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 200 nmol/L S1P (S1P). Bar = 35 μm.
Fluorescence microscopy of MG63 cells incubated with FITC-labeled fibronectin showed that S1P enhanced deposition of fibronectin into extracellular matrix (Fig 1B). When MG63 cells were treated with S1P, LPA, or nocodazole, fluorescent fibers were more numerous and coarser.
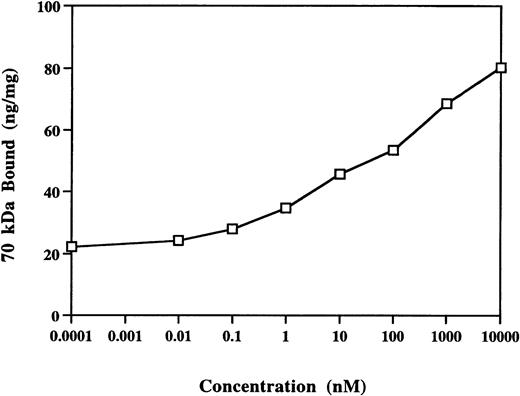
Starting at 0.1 nmol/L, S1P’s effect on 70-kD fragment binding to MG63 cells was dose-dependent over 6 orders of magnitude (Fig 2). Unlike the results with LPA,23 S1P caused increasing stimulation of 70-kD fragment binding at concentrations as high as 10 μmol/L, and a plateau was not achieved.
Dose response to S1P of binding of 70-kD N-terminal fragment of fibronectin to confluent MG63 cells. Binding of125I-70-kD fragment of fibronectin was determined in the presence of increasing concentrations of S1P. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the average of duplicates that varied by less than 10%.
Dose response to S1P of binding of 70-kD N-terminal fragment of fibronectin to confluent MG63 cells. Binding of125I-70-kD fragment of fibronectin was determined in the presence of increasing concentrations of S1P. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the average of duplicates that varied by less than 10%.
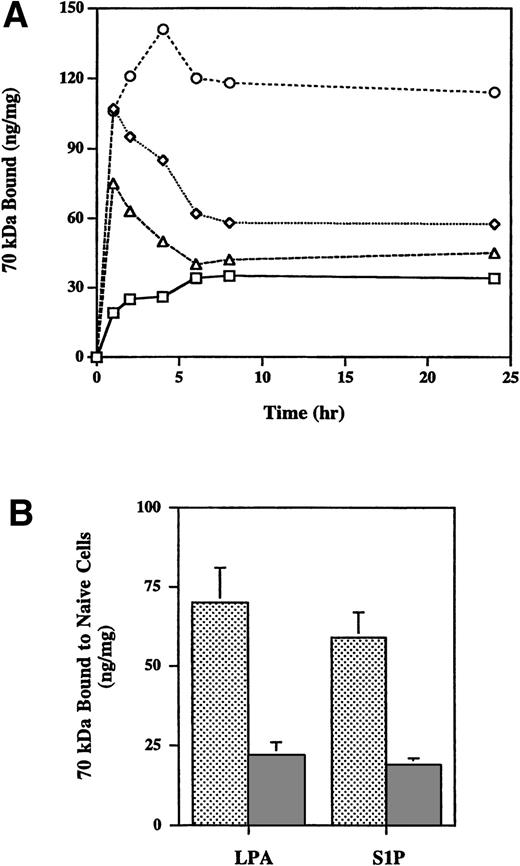
Long-term incubation of LPA with cells has been shown to result in loss of its activity to stimulate fibronectin binding to cells, whereas the effect of microtubule-disrupting agent nocodazole is sustained.23 24 When 100 nmol/L S1P was included in long-term binding assays, it lost the stimulatory activity by 6 hours (Fig 3A). S1P- or LPA-containing medium conditioned by MG63 cells for 20 hours both lost the ability to enhance binding of the 70-kD fragment to fresh cells, whereas S1P- or LPA-containing medium incubated at 37°C but not incubated with cells retained the ability to enhance binding when subsequently added to fresh cells (Fig 3B). In experiments not shown, S1P-containing medium conditioned by cells for 2 to 6 hours showed gradual loss of enhancing activity when added to fresh cells. These results indicate that both LPA and S1P are degraded or cleared from the medium by cell-based mechanisms.
Stability of S1P. (A) Time course of effects of LPA, nocodazole, and S1P on binding of 70-kD fragment to MG63 cells. Binding of 125I-70-kD fragment of fibronectin in the absence (□) or presence of 200 nmol/L LPA (◊), 10 μmol/L nocodazole (○), or 100 nmol/L S1P (▵) was determined at different time points. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the average of duplicates that varied by less than 10%. (B) 200 nmol/L LPA (LPA) or S1P (S1P) was preincubated with MG63 cells for 20 hours at 37°C (▩) or left at 37°C for 20 hours (▩) and was transferred to naive cells for a 45-minute 125I-70-kD fragment binding assay. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the mean ± SD (n = 3).
Stability of S1P. (A) Time course of effects of LPA, nocodazole, and S1P on binding of 70-kD fragment to MG63 cells. Binding of 125I-70-kD fragment of fibronectin in the absence (□) or presence of 200 nmol/L LPA (◊), 10 μmol/L nocodazole (○), or 100 nmol/L S1P (▵) was determined at different time points. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the average of duplicates that varied by less than 10%. (B) 200 nmol/L LPA (LPA) or S1P (S1P) was preincubated with MG63 cells for 20 hours at 37°C (▩) or left at 37°C for 20 hours (▩) and was transferred to naive cells for a 45-minute 125I-70-kD fragment binding assay. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per milligram of cellular protein. Data represent the mean ± SD (n = 3).
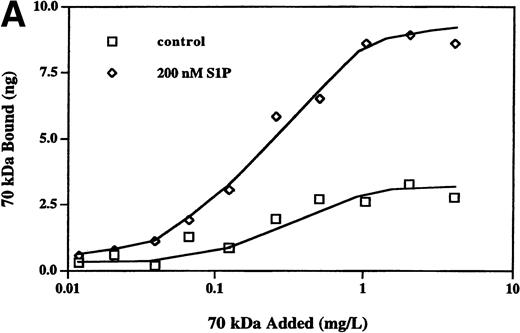
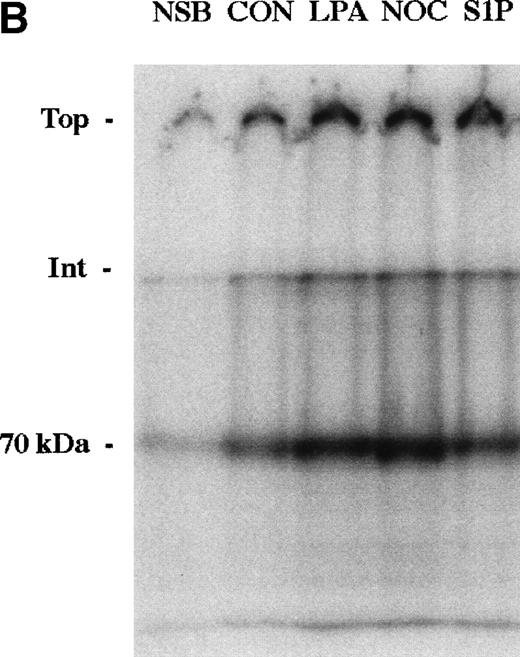
The enhancement of 70-kD fragment binding to MG63 cells by LPA or nocodazole is due to changes in both the kd and the cell surface binding sites for the N-terminal matrix assembly domain.23,24 Binding isotherms for binding of the 70-kD fragment to MG63 cells in the presence or absence of 200 nmol/L S1P were similarly determined (Fig 4A). Scatchard plotting of the result indicated that for S1P-treated MG63 cells, the kd was 3.1 nmol/L versus 6.4 nmol/L for control cells, and there were 218,000 binding sites per S1P-treated cell versus 92,000 sites for control cells. To compare S1P further with other modulators of fibronectin matrix assembly, we cross-linked bound125I-70-kD fragment with factor XIIIa. Cross-linking of the cell surface-bound 70-kD fragment to MG63 cells treated with S1P was to molecules of large apparent molecular mass of 3 MD and >>3 MD (Fig 4B), as is also the case with LPA or nocodazole treatment.24
Binding isotherm (A) and cross-linking (B) of125I-70-kD fragment to cells. (A) Increasing amounts of125I-70-kD fragment were incubated with MG63 cells in the absence (control) or presence of 200 nmol/L S1P (S1P) for 45 minutes. To achieve concentrations less than 0.5 mg/L, ligand with a specific activity of 1,000 cpm/ng was added. To achieve concentrations greater than 0.5 mg/L, unlabeled 70-kD fragment was added to125I-70-kD fragment to yield mixtures with progressively lower specific activity. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per well. Data represent the average of duplicate values. (B) Confluent MG63 cells were incubated for 60 minutes with125I-70-kD fragment (0.5 mg/L) in medium containing unlabeled 70-kD fragment (20 mg/L; NSB), control with nothing additional (CON), 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 100 nmol/L S1P (S1P). Cell layers were then washed and incubated for an addition of 5 minutes with factor XIIIa (10 mg/L). Cell lysates were analyzed by reducing SDS-PAGE and phosphorimaging. Top, top of the stacking gel; Int, interface of the 3% stacking and 8% separating polyacrylamide gels; and 70-kD, un–cross-linked 125I-70-kD fragment. Quantitation of bands at the top, the interface, and the position of the un–cross-linked probe yielded the following ratios of density of the three bands in treated cultures compared with control: LPA/CON: 2.0, 2.2, and 1.9; NOC/CON: 2.1, 2.5, and 2.3; and S1P/CON: 2.1, 1.9, and 1.7.
Binding isotherm (A) and cross-linking (B) of125I-70-kD fragment to cells. (A) Increasing amounts of125I-70-kD fragment were incubated with MG63 cells in the absence (control) or presence of 200 nmol/L S1P (S1P) for 45 minutes. To achieve concentrations less than 0.5 mg/L, ligand with a specific activity of 1,000 cpm/ng was added. To achieve concentrations greater than 0.5 mg/L, unlabeled 70-kD fragment was added to125I-70-kD fragment to yield mixtures with progressively lower specific activity. Specific binding was calculated by subtraction of nonspecific binding (in the presence of 500 mg/L unlabeled fibronectin) from total binding and expressed as nanograms of 70-kD fragment bound per well. Data represent the average of duplicate values. (B) Confluent MG63 cells were incubated for 60 minutes with125I-70-kD fragment (0.5 mg/L) in medium containing unlabeled 70-kD fragment (20 mg/L; NSB), control with nothing additional (CON), 200 nmol/L LPA (LPA), 10 μmol/L nocodazole (NOC), or 100 nmol/L S1P (S1P). Cell layers were then washed and incubated for an addition of 5 minutes with factor XIIIa (10 mg/L). Cell lysates were analyzed by reducing SDS-PAGE and phosphorimaging. Top, top of the stacking gel; Int, interface of the 3% stacking and 8% separating polyacrylamide gels; and 70-kD, un–cross-linked 125I-70-kD fragment. Quantitation of bands at the top, the interface, and the position of the un–cross-linked probe yielded the following ratios of density of the three bands in treated cultures compared with control: LPA/CON: 2.0, 2.2, and 1.9; NOC/CON: 2.1, 2.5, and 2.3; and S1P/CON: 2.1, 1.9, and 1.7.
Signaling mechanism: Involvement of Rho in enhancement of fibronectin assembly.
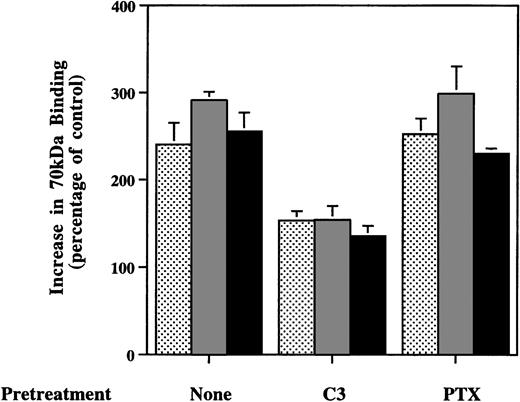
Exogenous S1P has been shown to influence cellular functions through GPCRs on the cell surface.1,7,10,11,16,35,36 RT-PCR indicated that MG-63 cells express Edg-2 and Edg-4 and that foreskin fibroblasts express Edg-2, Edg-3, and Edg-4 (data not shown). S1P’s effects on mitogenesis,7 MAP kinase activation,37 intracellular Ca2+mobilization,36 and activation ofIK(Ach) in atrial myocytes35 are abolished by pertussis toxin. In contrast, the effects of S1P on neurite retraction11 and cell aggregation16depend on Rho. Pretreatment of MG63 cells with Clostridium botulinum C3 exoenzyme, which ADP-ribosylates and thereby inactivates Rho,28 caused loss of enhancement of 70-kD fragment binding to cells in response to 200 nmol/L S1P (Fig 5). Enhanced deposition of FITC-fibronectin into extracellular matrix in response to S1P was also lost after treatment with C3 exo-enzyme (data not shown). S1P’s stimulation of 70-kD fragment binding was not affected by pretreatment of MG63 cells with pertussis toxin (Fig 5), even though the activation of MAP kinase in MG63 cells by S1P was totally abolished by the pertussis toxin pretreatment (data not shown). These results indicate that a nanomolar dose of S1P stimulates initial fibronectin binding to cells through a Rho-dependent signaling pathway.
Effects of C3 exotransferase or pertussis toxin on enhanced binding of 70-kD N-terminal fragment of fibronectin to MG63 cells by LPA, nocodazole, or S1P. Confluent MG63 cells were pretreated with complete medium (None), 50 mg/L C3 transferase (C3), or 100 μg/L pertussis toxin (PTX) for 24 hours and then incubated for 60 minutes with 125I-70-kD fragment in medium with 200 nmol/L LPA (▩), 10 μmol/L nocodazole ( ), or 100 nmol/L S1P (▪). Data are presented as specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
), or 100 nmol/L S1P (▪). Data are presented as specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
Effects of C3 exotransferase or pertussis toxin on enhanced binding of 70-kD N-terminal fragment of fibronectin to MG63 cells by LPA, nocodazole, or S1P. Confluent MG63 cells were pretreated with complete medium (None), 50 mg/L C3 transferase (C3), or 100 μg/L pertussis toxin (PTX) for 24 hours and then incubated for 60 minutes with 125I-70-kD fragment in medium with 200 nmol/L LPA (▩), 10 μmol/L nocodazole ( ), or 100 nmol/L S1P (▪). Data are presented as specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
), or 100 nmol/L S1P (▪). Data are presented as specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
Differentiation of the effects of S1P from the stimulatory effects of LPA and microtubule-disrupting agent.
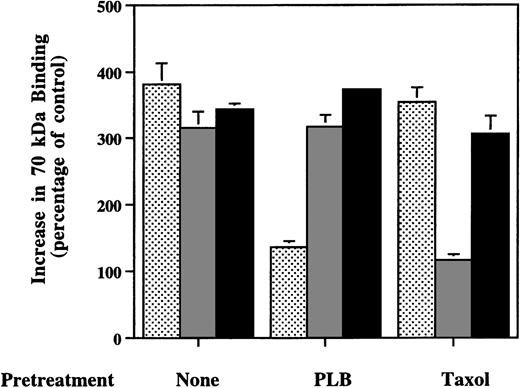
To check whether S1P’s effect on fibronectin binding to cells is secondary to production of LPA, phospholipase B, which cleaves the remaining fatty acid chain from the glycerol backbone and destroys LPA,23 was included in the binding mix with the stimulator. Phospholipase B (1,000 U/L) totally eliminated the stimulation of 70-kD fragment binding to MG63 cells by LPA, but did not affect the response to S1P (Fig 6). Furthermore, when the cells were preincubated for 2 hours with 20 μmol/L taxol, a microtubule-stabilizing agent,38 enhancement of 70-kD fragment binding by nocodazole was blocked, whereas MG63 cells pretreated with taxol responded to S1P just as well as the cells not treated with taxol (Fig 6). These results indicate that the stimulatory activity on fibronectin binding by S1P is neither secondary to extracellular LPA production nor due to the disruption of microtubules. When S1P and LPA at concentrations of 1, 10, or 100 mmol/L were combined, the enhancement of 70-kD fragment binding was 20% to 60% greater than if each lipid alone at the same concentration was incubated with cells (data not shown). This result indicates that the effects of the two lipids on cells are additive.
Effects of phospholipase B or taxol on enhanced binding of 70-kD N-terminal fragment of fibronectin to MG63 cells by LPA, nocodazole, or S1P. Confluent MG63 cells were pretreated with complete medium (None), 1,000 U/L phospholipase B (PLB), or 20 μmol/L taxol (Taxol) for 2 hours and were then incubated for 60 minutes with125I-70-kD fragment in medium with 200 nmol/L LPA (▩), 10 μmol/L nocodazole ( ), or 100 nmol/L S1P (▪). Data are presented as the specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
), or 100 nmol/L S1P (▪). Data are presented as the specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
Effects of phospholipase B or taxol on enhanced binding of 70-kD N-terminal fragment of fibronectin to MG63 cells by LPA, nocodazole, or S1P. Confluent MG63 cells were pretreated with complete medium (None), 1,000 U/L phospholipase B (PLB), or 20 μmol/L taxol (Taxol) for 2 hours and were then incubated for 60 minutes with125I-70-kD fragment in medium with 200 nmol/L LPA (▩), 10 μmol/L nocodazole ( ), or 100 nmol/L S1P (▪). Data are presented as the specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
), or 100 nmol/L S1P (▪). Data are presented as the specific binding in the presence of additive divided by specific binding in the absence of any additives as a percentage of control. Data represent the mean ± SD (n = 4).
DISCUSSION
Organization of soluble fibronectin into extracellular matrix is a tightly regulated process, mediated by initial reversible binding by the 70-kD N-terminal region of fibronectin to specific cell surface binding sites, followed by insolubilization into fibrils.39The adhesive information present after insolubilization of fibronectin is postulated to play a central role in various physiological and pathophysiologic processes, including embryogenesis, wound healing, inflammation, and degenerative disease processes such as atherosclerosis and fibrosis.40 41
S1P is abundantly stored in platelets and rapidly released into the extracellular environment upon stimulation with physiologic agonists,1,2 whereas LPA is quickly generated upon platelet activation.27 S1P is a normal constituent of human plasma as well, and the mean S1P levels in plasma and serum are 190 and 480 nmol/L, respectively.1 LPA is generated by activated platelets during blood clotting process, at least partially through secretion of phospholipase A2 from platelet granules that then acts on shedded microvesicles.42 Whole blood serum is estimated to contain 1 to 5 μmol/L LPA, whereas no LPA was detected in plasma prepared by methods that minimized platelet activation.27LPA has been identified as the major enhancer of fibronectin matrix assembly in 0.1% to 2% serum based on the ability of phospholipase B to destroy activity.23 Another component, viz, S1P, derived from platelet activation could play an additional role at higher serum concentrations. We found that S1P in the 100 to 500 nmol/L concentration range present in plasma and serum enhances fibronectin binding considerably and that LPA and S1P added together at a concentration of 100 nmol/L enhance binding more than each lipid by itself. Thus, S1P and LPA may function additively and/or redundantly during early wound healing. Redundancy may be important because of the fact that the pathways for generation of S1P and LPA are different: platelet secretion in the case of S1P and action of secretory phospholipase on microvesicles in the case of LPA. In addition, the pathways for destruction of S1P and LPA may be different. Finally, S1P, for which there is a higher plasma/serum concentration ratio than LPA, has the potential to act tonically on fibronectin assembly in tissues in which there is high permeability to plasma proteins.
S1P’s effect on 70-kD fragment binding to MG63 cells was dose-dependent over 6 orders of magnitude (Fig 2). Unlike the results with LPA,23 S1P caused increasing stimulation of 70-kD fragment binding at concentrations as high as 10 μmol/L, and a plateau was not achieved. The complex dose response is compatible with the suggestions that S1P’s effect on binding of 70-kD fragment to MG63 cells may involve both a receptor-mediated pathway at lower doses and second messenger functions at higher doses.34
S1P regulates intracellular second messengers and membrane channels through activation of specific receptors present in many different cell types. A family of seven transmembrane spanning domain receptor encoded by endothelial differentiation genes (Edg) have recently been recognized as receptors for S1P and LPA. Of this family, Edg-2 and Edg-4 are expressed by MG63 cells and Edg-2, Edg-3, and Edg-4 are expressed by foreskin fibroblasts. Edg-2 isolated from both mouse20,43 and human18 has been shown to be stimulated by LPA. S1P has been described as a ligand for Edg-315 as well as for Edg-1.16 LPA has been described as a ligand for Edg-4.44 We have developed evidence from transfection of MG63 cells and HT1080 fibrosarcoma cells that both LPA and S1P are able to signal through Edg-2 to cause increased stress fiber formation and shape change (Peyruchaud et al, unpublished observation). Thus, Edg-2, which is shared by MG63 cells and foreskin fibroblasts, likely plays a major role in the effect of S1P on matrix assembly with an additional unknown contribution from Edg-4 and perhaps still to be described Edg family receptors.
S1P-induced neurite retraction,11,45 cell aggregation,16 and 3T3 cell shape change have been shown to be mediated by Rho based on sensitivity to C3 exotransferase.26 S1P-stimulated fibronectin binding was blocked by the exotransferase, as in the case with LPA or nocodazole.24 These results indicate that a common Rho-dependent cytoplasmic mechanism similar to that described for other effects of S1P on cells regulates the display of cell surface sites for assembly of fibronectin. The stimulatory activity of S1P or LPA on fibronectin binding was not sensitive to pertussis toxin, indicating lack of the participation of Gi in this signaling. The G12/G13 family of heterotrimeric G protein has been shown to activate Rho specifically.46 Thus, a G12 or G13 complex likely links the S1P receptor(s) to the Rho-dependent machinery.
ACKNOWLEDGMENT
The authors thank Julie Nowlen and Renee Schultz for excellent technical support.
Supported by National Institutes of Health Grant No. HL21644. O.P. was a postdoctoral fellow of the Sanofi Association for Thrombosis Research.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Deane F. Mosher, MD, Departments of Medicine and Biomolecular Chemistry, University of Wisconsin-Madison, 1300 University Ave, Madison, WI 53706; e-mail:dfmosher@facstaff.wisc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal