We have assessed tumor contamination of peripheral blood progenitor cells (PBPC) in 203 high-risk breast cancer patients who were prospectively randomized to mobilization with stem cell factor (SCF) plus granulocyte colony-stimulating factor (G-CSF) versus G-CSF alone. The patients then received high-dose cyclophosphamide, cisplatin, and carmustine (BCNU) with PBPC support. One bone marrow aspirate obtained before treatment, one whole blood specimen obtained before cytokine infusion, and one to five leukapheresis products were tested for the presence of tumor cells by an alkaline phosphatase immunocytochemical technique with a targeted sensitivity of 1.7 tumor cells per 106 hematopoietic cells. Tumor cells were detected in the bone marrow, peripheral blood, and/or PBPC of 21 patients (10%). In 14 patients, bone marrow specimens were tumor-positive; in seven patients, premobilization whole blood specimens were tumor-positive, and in eight patients, leukapheresis products were tumor-positive. In five patients, repetitive or multiple specimens were tumor-positive, and in three cases, marrow, peripheral blood, and PBPC products were all tumor-positive. Nine of the patients in whom tumor cells were found in marrow or peripheral blood were clinical stage II to III and 12 were clinical stage IV. Nine of the tumor-positive patients were in the SCF + G-CSF arm and 12 were in the G-CSF arm. Tumor cells were detected in leukapheresis products of eight patients: three in the G-CSF + SCF arm and five in the G-CSF arm. We conclude that detectable tumor-cell contamination of bone marrow, peripheral blood, and/or PBPC occurred in approximately 10% of patients in this trial and was observed in stage II to III patients, as well as in stage IV patients. No significant difference could be found in the rate of PBPC tumor-cell contamination between patients who received SCF + G-CSF compared with those who received G-CSF alone. Neither mobilization regimen was found to increase the rate of tumor-cell contamination when control premobilization blood samples were compared with leukapheresis products.
THE PROGNOSIS OF LATE-STAGE breast carcinoma treated with standard-dose therapy is poor, with only 41% of stage III and 10% of stage IV patients surviving for 5 years.1 These poor results have led to the increasing use of high-dose chemotherapy followed by autologous hematopoietic progenitor cell support with encouraging preliminary results.2-4 With the widespread availability of cytokines to mobilize hematopoietic progenitor cells from the marrow, peripheral blood has become an increasingly important source of hematopoietic stem-cell support. Peripheral blood progenitor cells (PBPC) collected following cytokine administration appear to produce more rapid hematopoietic recovery than autologous bone marrow, with comparable long-term hematopoietic reconstitution.5-8 The cytokines commercially available for PBPC mobilization include granulocyte colony-stimulating factor (G-CSF; Amgen Inc, Thousand Oaks, CA) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Recently, recombinant methionyl human stem cell factor (SCF) has been investigated as a potential means to enhance the mobilization of PBPCs. The combination of SCF with G-CSF leads to an increase in the number of CD34+ cells that can be collected from the peripheral blood.9-13
A potential complication of autologous hematopoietic progenitor cell infusion, regardless of its source, is the possibility of contamination of progenitor cell preparations with tumor cells. Numerous studies have indicated that tumor cells may be present in both bone marrow and PBPC preparations of patients with carcinoma of the breast8,14-22 with one suggesting that PBPC collections are less contaminated with tumor cells than corresponding marrow harvests.20 Since no large studies of homogenous patient populations have been reported, the true incidence of tumor cells in bone marrow and PBPC products of carefully staged and uniformly treated patients is not known. Whether specific cytokine combinations influence the extent of tumor contamination in the leukapheresis product is also unknown.
The primary aim of the present study was to compare tumor-cell contamination of the leukapheresis products from patients mobilized with SCF + G-CSF versus G-CSF alone. To assist in the interpretation of the data, baseline bone marrow samples obtained before cytokine administration were also examined. Furthermore, since it was clinically impractical to obtain a baseline leukapheresis sample, peripheral blood samples were evaluated before the initiation of cytokine therapy and on each day of leukapheresis for comparative purposes. The study included breast cancer patients who were participating in a large clinical trial that involved prospective randomization to PBPC mobilization with either SCF + G-CSF or G-CSF alone.
MATERIALS AND METHODS
Tumor-cell quantification method.
In a previous study, we found that the number of tumor cells detected in cytocentrifuge preparations of hematologic specimens stained by the alkaline phosphatase–antialkaline phosphatase (APAAP) technique is proportional to the number of tumor cells present in the test cell suspension and to the number of slides examined.20 That study used an antibody mixture that exhibited low-level cross-reactivity with nonmalignant hematopoietic cells, particularly in cases treated with high-dose chemotherapy.
To improve staining specificity and to compare the effectiveness of two different monoclonal antibodies for tumor-cell detection, the current study used antibodies against both cytokeratin (AE1/AE3 cocktail; Signet Corp, Dedham, MA) and MUC-1 (Bre-3; generously donated by Dr Roberto Ceriani, Cancer Research Institute of Contra Costa, Walnut Creek, CA). The sensitivity of the detection assay using the two different antibodies was documented by adding CAMA breast cancer cells to leukapheresis products at concentrations ranging from 0.5 to 16 tumor cells per million hematopoietic cells. The spiked samples were then centrifuged and stained using the APAAP immunocytochemical staining technique23 and tumor cells counted. The APAAP staining technique used is particularly applicable to hematologic specimens, since endogenous leukocyte alkaline phosphatase is completely blocked by levamisole and nonspecific background staining is absent. The number of tumor cells detected was plotted against tumor-cell concentration and the slope of the regression line for the combined results was used to estimate tumor-cell concentrations in trial samples.
Patients and samples.
Two hundred three patients from several institutions (Appendix 1) with stage II disease involving 10 or more axillary lymph nodes, or stage III or stage IV disease that was stable or responding to induction therapy were prospectively randomized to PBPC mobilization with SCF + G-CSF (Filgrastim, Amgen, Thousand Oaks, CA) or G-CSF alone as shown in Table 1. All patients who entered the clinical study underwent baseline bone marrow biopsy, which was assessed by conventional histologic examination. Twelve of these baseline marrows contained breast carcinoma and in all of these tumor-positive specimens less than 10% of the marrow was involved by tumor.
Patient Allocation
| Stage . | G-CSF + SCF . | G-CSF Only . | Total . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| II/III | 46 | 48.9 | 48 | 51.1 | 94 |
| IV | 54 | 49.5 | 55 | 50.5 | 109 |
| Total | 100 | 49.3 | 103 | 50.7 | 203 |
| Stage . | G-CSF + SCF . | G-CSF Only . | Total . | ||
|---|---|---|---|---|---|
| No. . | % . | No. . | % . | ||
| II/III | 46 | 48.9 | 48 | 51.1 | 94 |
| IV | 54 | 49.5 | 55 | 50.5 | 109 |
| Total | 100 | 49.3 | 103 | 50.7 | 203 |
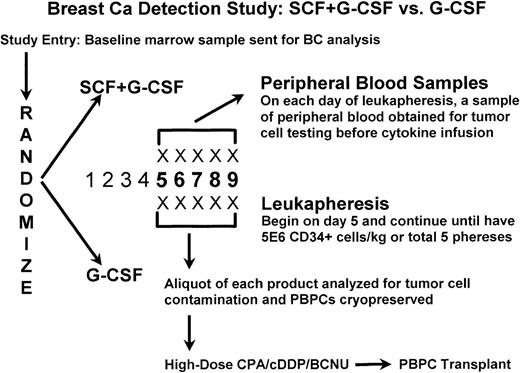
The details of the treatment protocol are illustrated in Fig1. All patients entering the clinical study were entered into the immunocytochemical tumor detection protocol described below. Cytokines were administered daily and leukapheresis initiated on day 5. Cytokine administration and leukapheresis continued daily until a target CD34+ cell yield of 5 × 106 cells/kg had been reached or a total of five leukaphereses performed (ie, a maximum of 9 days of cytokine administration). A sample (1 to 2 mL) of each leukapheresis product was obtained from all patients on each of the 1 to 5 days of leukapheresis, as appropriate. In addition, baseline heparinized samples of bone marrow (5 to 10 mL) and peripheral blood (20 mL) were obtained 5 days before the initiation of cytokine treatment. Heparinized peripheral blood samples (10 mL) were also obtained on each day of leukapheresis prior to cytokine infusion and to the leukapheresis procedure. All hematopoietic cell samples, other than those obtained from University of Colorado patients, were shipped at 4°C by overnight courier to the University of Colorado Stem Cell Engineering Laboratory.
Treatment schema. BC, breast cancer; CPA, cyclophosphamide; cDDP, cisplatin; BCNU, carmustine.
Treatment schema. BC, breast cancer; CPA, cyclophosphamide; cDDP, cisplatin; BCNU, carmustine.
Specimen preparation and evaluation.
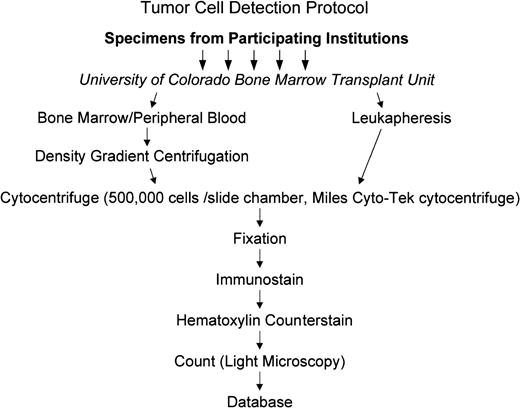
Specimens received in the Stem Cell Engineering Laboratory were centrifuged and stained as shown in Fig 2. Mononuclear fractions of the bone marrow and peripheral blood samples were isolated using Ficoll gradient centrifugation, washed with phosphate-buffered saline (PBS) containing 10% bovine serum, and adjusted to a concentration of 5.0 × 106 cells/mL. Two hundred microliters of the cell suspension was added to cytocentrifuge chambers and the cells centrifuged at 500 rpm for 5 minutes onto silane-coated slides.
Routing of specimens and tumor detection protocol. Specimens were shipped to the central testing laboratory in Colorado. Mononuclear cells were isolated from marrow and peripheral blood while leukapheresis products were tested directly as shown in the figure and described in the text.
Routing of specimens and tumor detection protocol. Specimens were shipped to the central testing laboratory in Colorado. Mononuclear cells were isolated from marrow and peripheral blood while leukapheresis products were tested directly as shown in the figure and described in the text.
Twenty slides per sample were stained by the APAAP technique, 10 with anti–MUC-1 and 10 with anti-cytokeratin. When insufficient cells were present in a sample to permit preparation of 20 slides, fewer slides were evaluated. Immunostained slides were counterstained with hematoxylin and examined microscopically in a blinded fashion at low magnification (10× objective) using a conventional light microscope equipped with a mechanical stage. Immunoreactive cells identified at scanning magnification were scrutinized at high magnification to confirm the presence of malignant histologic features. These features included large cell size, high nuclear/cytoplasmic ratio, active appearing granular nuclear chromatin, prominent nucleoli, and clustering of tumor cells. All cell counts were recorded and manually entered into an Oracle database (Redwood Shores, CA). Data were analyzed using StatExact from Cytel Software Corp (Cambridge, MA).
RESULTS
Tumor-cell quantification method.
Rates of detection of tumor cells in leukapheresis products containing various known tumor-cell concentrations are shown in Fig3. In multiple replicate experiments using either the anti–MUC-1 or anti-cytokeratin antibodies, regression of the number of CAMA tumor cells detected on number added to leukapheresis product gives similar linear results with slopes of 0.464 (r2 = .997) for anti–MUC-1 and 0.395 (r2 = .941) for anti-cytokeratin. The slope for combined results was 0.418 (r2 = .971), suggesting uniform loss of approximately 60% of tumor cells across all dilutions.
Plot of known CAMA cell concentrations against number of CAMA cells detected. Each point represents the mean of two to three replicate experiments. At tumor-cell concentrations ≤2/106 hematopoietic cells, a total of 78 to 164 slides were evaluated; at concentrations ≥4/106, 11 to 21 slides were evaluated.
Plot of known CAMA cell concentrations against number of CAMA cells detected. Each point represents the mean of two to three replicate experiments. At tumor-cell concentrations ≤2/106 hematopoietic cells, a total of 78 to 164 slides were evaluated; at concentrations ≥4/106, 11 to 21 slides were evaluated.
The sensitivity of the tumor-cell detection assay is also dependent on number of slides examined. The slopes for proportion of positive slides regressed on tumor-cell prevalence at prevalences less than 8 per 106 are 0.083 for anti–MUC-1 and 0.107 for anti-cytokeratin. These slopes (B) multiplied by dilution numbers give the probability of detecting a single positive slide for a given dilution. For multiple slides, the probability (P) of detecting at least one positive slide among y slides when the average number (x) of tumor cells are present among 106 hematopoietic cells is given by the equation P = 1 − (1 − Bx)y.20 By setting B = 0.08 (the more conservative slope), it is possible to estimate the sensitivity of the detection assay, ie, tumor-cell frequency that can be detected with a high degree of confidence (P > .95), by examining a specific number of slides. Evaluation of 20 slides, the targeted number in this study, results in a sensitivity of 1.7 tumor cells per million hematopoietic cells. When insufficient cells are present in a sample to permit preparation of 20 slides, evaluation of one to 19 slides still provides sensitivities that range from 1.8 to 11.9 tumor cells per million mononuclear cells. This is still well within the range of sensitivity for immunocytochemical testing reported for the detection of minimal residual epithelial tumor in hematopoietic organs (0.5 to 40 tumor cells per million hematopoietic cells).
Sample collection/adequacy.
A total of 1,730 specimens from 203 patients were evaluated. Slides were judged to be adequate for quantification if an even, nearly continuous monolayer of cells was deposited on the glass slides at the base of the cytocentrifuge funnel and if cells were well preserved. Only specimens in which no slide contained an evaluable monolayer were considered inadequate for quantification. No tumor cells were identified in any of the samples considered inadequate for quantification.
The number of adequate specimens obtained from patients in the present trial is shown in Table 2. Of the 203 patients included in this analysis, baseline bone marrow samples were received from 168 (83%). Sufficient cells to allow preparation of 1 to 20 slides were obtained from 95% of the baseline marrow specimens. One hundred eighty-one (89%) of the 203 patients contributed baseline peripheral blood samples and nearly 92% of the samples yielded 1 to 20 slides. However, in only 35% of these specimens were sufficient cells recovered to reach the target sensitivity of 1.7 tumor cells per 106 hematopoietic cells. Peripheral blood samples were received on 680 of the 721 aggregate days of apheresis. Sufficient cells to reach a target sensitivity of 1.7 tumor cells per 106 hematopoietic cells were available for 75% of these peripheral blood specimens. Finally, samples from 701 (97%) of the 721 leukaphereses were received. Sufficient cells to allow preparation of one to 20 slides were obtained from 98% of these samples and in 89%, detection sensitivity was 1.7 tumor cells per 106hematopoietic cells.
Specimen Adequacy
| Specimen Type . | Optimal* (20 slides) . | Adequate† (1-19 slides) . | Inadequate . | Total . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Bone marrow | 130 | 77 | 29 | 17 | 9 | 5 | 168 |
| Baseline peripheral blood | 63 | 35 | 103 | 57 | 15 | 8 | 181 |
| Treatment blood | 509 | 75 | 144 | 21 | 27 | 4 | 680 |
| Leukapheresis | 627 | 89 | 59 | 8 | 15 | 2 | 701 |
| Total | 1,329 | 77 | 335 | 19 | 66 | 4 | 1,730 |
| Specimen Type . | Optimal* (20 slides) . | Adequate† (1-19 slides) . | Inadequate . | Total . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Bone marrow | 130 | 77 | 29 | 17 | 9 | 5 | 168 |
| Baseline peripheral blood | 63 | 35 | 103 | 57 | 15 | 8 | 181 |
| Treatment blood | 509 | 75 | 144 | 21 | 27 | 4 | 680 |
| Leukapheresis | 627 | 89 | 59 | 8 | 15 | 2 | 701 |
| Total | 1,329 | 77 | 335 | 19 | 66 | 4 | 1,730 |
Sensitivity = 1.4 tumor cells/106 hematopoietic cells.
Sensitivity = 1.5 to 11.3 tumor cells/106 hematopoietic cells.
Incidence of tumor contamination.
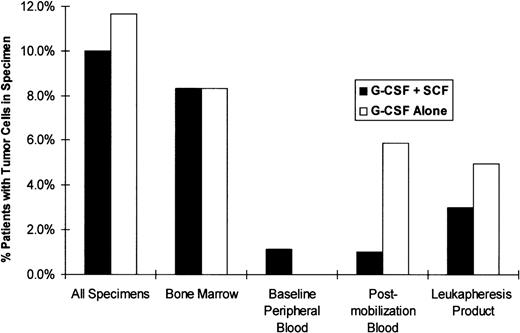
Tumor cells were readily distinguishable from background mononuclear cells by brightly staining cell membranes and cytoplasm (Fig4). Tumor cells were detected in specimens from 21 of the 203 patients (10.3%) enrolled in the study: 10 (10.0%) patients in the G-CSF + SCF arm and 12 (11.7%) patients in the G-CSF alone arm (Fig 5). The most heavily tumor-cell–contaminated specimens were pretreatment bone marrow specimens, 8.3% of which contained tumor cells regardless of treatment arm. Baseline peripheral blood samples rarely contained tumor cells and only one tumor-positive specimen was encountered in this group during this study. Lower rates of tumor-cell contamination were detected in premobilization peripheral blood (3.5%) and in leukapheresis products (4.5%) than in marrow specimens (8.3%). The two treatment groups were similar with respect to proportion of patients with tumor contamination in leukapheresis products (3.0% of the SCF + G-CSF patients and 5.0% of the G-CSF only patients). There was also no evidence of mobilization of tumor cells to any significant extent for bone marrow to peripheral blood with either cytokine regimen. The incidence of tumor contamination per leukapheresis sample was similar for the two groups: 1.9% (7 of 375 samples) for patients who received G-CSF alone and 1.0% (three of 311 samples) for the SCF + G-CSF patients, with an overall incidence of 1.5% (10 of 686 samples). Although more patients were found to have tumor in peripheral blood or leukapheresis specimens in the G-CSF only arm than in the G-CSF + SCF arm (8 of 103 patientsv 3 of 100 patients), this difference was not statistically significant (Fisher’s exact two-sided P = .72).
Photomicrograph shows staining patterns of tumor cells detected in peripheral blood. (A) Cell detected with the Bre-3 anti–MUC-1 antibody; (B) tumor cell detected with AE1/AE3 anti-cytokeratin cocktail. There is cell membrane staining in the anti–MUC-1 preparation and coarse granular or fibrillary cytoplasmic staining of tumor cells with anti-cytokeratin, consistent with the distribution of cytokeratin intermediate filaments.
Photomicrograph shows staining patterns of tumor cells detected in peripheral blood. (A) Cell detected with the Bre-3 anti–MUC-1 antibody; (B) tumor cell detected with AE1/AE3 anti-cytokeratin cocktail. There is cell membrane staining in the anti–MUC-1 preparation and coarse granular or fibrillary cytoplasmic staining of tumor cells with anti-cytokeratin, consistent with the distribution of cytokeratin intermediate filaments.
Numbers of tumor cells calculated to be present in tumor-positive cases are indicated in Table 3. Larger numbers of tumor cells were found in bone marrow specimens than in peripheral blood. In three cases, more than 100 tumor cells per million hematopoietic cells were found in the marrow, while the maximum tumor-cell count in the peripheral blood specimens of all types was only 21 tumor cells per million blood cells and in leukapheresis product was 48 per million blood cells. In all cases where more than nine tumor cells per million hematopoietic cells were detected, there was concordance between cytokeratin and Bre-3 results.
Analysis of Positive Specimens
| . | Bone Marrow . | Baseline Blood . | Postmobilization Blood . | Leukapheresis . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | |||||||||
| Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | |
| N | 5 | 5 | 6 | 6 | 0 | 1 | 0 | 0 | 1 | 0 | 6 | 5 | 2 | 1 | 7 | 4 |
| Min | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 | 0 | 1 | 1 | 3 | 1 | 1 | 1 |
| Max | 638 | 582 | 2,366 | 969 | 0 | 1 | 0 | 0 | 5 | 0 | 21 | 9 | 4 | 1 | 48 | 26 |
| Mean | 160 | 147 | 397 | 165 | 0 | 1 | 0 | 0 | 5 | 0 | 6 | 6 | 3 | 1 | 17 | 11 |
| Median | 4 | 8 | 4 | 5 | 0 | 1 | 0 | 0 | 5 | 0 | 1 | 6 | 3 | 1 | 10 | 8 |
| . | Bone Marrow . | Baseline Blood . | Postmobilization Blood . | Leukapheresis . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | |||||||||
| Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | Bre33-150 . | Ker3-151 . | |
| N | 5 | 5 | 6 | 6 | 0 | 1 | 0 | 0 | 1 | 0 | 6 | 5 | 2 | 1 | 7 | 4 |
| Min | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 | 0 | 1 | 1 | 3 | 1 | 1 | 1 |
| Max | 638 | 582 | 2,366 | 969 | 0 | 1 | 0 | 0 | 5 | 0 | 21 | 9 | 4 | 1 | 48 | 26 |
| Mean | 160 | 147 | 397 | 165 | 0 | 1 | 0 | 0 | 5 | 0 | 6 | 6 | 3 | 1 | 17 | 11 |
| Median | 4 | 8 | 4 | 5 | 0 | 1 | 0 | 0 | 5 | 0 | 1 | 6 | 3 | 1 | 10 | 8 |
Abbreviations: Min, minimum; Max, maximum; Ker, cytokeratin.
Indicates number of tumor cells detected in specimens stained with anti–MUC-1 antibody, Bre-3.
Indicates number of tumor cells detected in specimens stained with anti-cytokeratin antibodies, AE1/AE3.
Results by stage of disease.
The treatment groups were similar with regard to stage of disease and tumor contamination of the bone marrow, as determined by histology or immunocytochemistry. As expected, bone marrow immunocytochemistry testing was more sensitive than routine histology; this was particularly evident for stage II/III patients. Bone marrow immunocytochemistry, but not bone marrow histology, was also a significant predictive factor for tumor contamination in the postcytokine peripheral blood or leukapheresis samples (P < .02, Fisher’s exact test). It was expected that tumor cells would more frequently be found in peripheral blood and leukapheresis from stage IV patients than in patients from stage II/III patients. While this proved to be the case (Table 4), the difference in tumor contamination rates between stage II/III and stage IV patients was small and tumor cells were found in specimens from several patients with stage II/III disease. Nine of the patients in which tumor cells were found in the marrow or peripheral blood were stage II or III and 12 were clinical stage IV.
Tumor Detection by Tumor Stage
| Specimen . | Stage II/III . | Stage IV . | ||||||
|---|---|---|---|---|---|---|---|---|
| G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | |||||
| % Patients . | No. Tested . | % Patients . | No. Tested . | % Patients . | No. Tested . | % Patients . | No. Tested . | |
| Bone marrow | 8 | 39 | 5 | 40 | 9 | 45 | 11 | 44 |
| Baseline peripheral blood | 0 | 39 | 0 | 44 | 2 | 50 | 0 | 48 |
| Postmobilization blood | 2 | 45 | 2 | 49 | 0 | 53 | 9 | 53 |
| Leukapheresis product | 7 | 46 | 2 | 48 | 0 | 53 | 8 | 53 |
| Any specimen | 11 | 46 | 8 | 48 | 9 | 54 | 15 | 55 |
| Specimen . | Stage II/III . | Stage IV . | ||||||
|---|---|---|---|---|---|---|---|---|
| G-CSF + SCF . | G-CSF Only . | G-CSF + SCF . | G-CSF Only . | |||||
| % Patients . | No. Tested . | % Patients . | No. Tested . | % Patients . | No. Tested . | % Patients . | No. Tested . | |
| Bone marrow | 8 | 39 | 5 | 40 | 9 | 45 | 11 | 44 |
| Baseline peripheral blood | 0 | 39 | 0 | 44 | 2 | 50 | 0 | 48 |
| Postmobilization blood | 2 | 45 | 2 | 49 | 0 | 53 | 9 | 53 |
| Leukapheresis product | 7 | 46 | 2 | 48 | 0 | 53 | 8 | 53 |
| Any specimen | 11 | 46 | 8 | 48 | 9 | 54 | 15 | 55 |
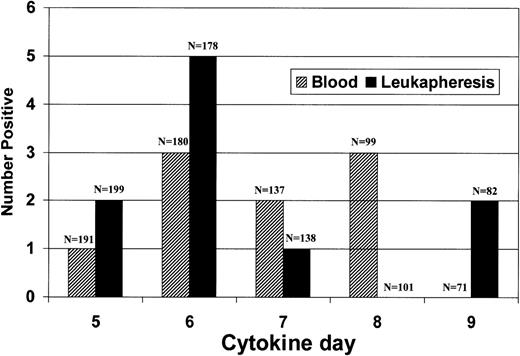
Relationship to day of apheresis.
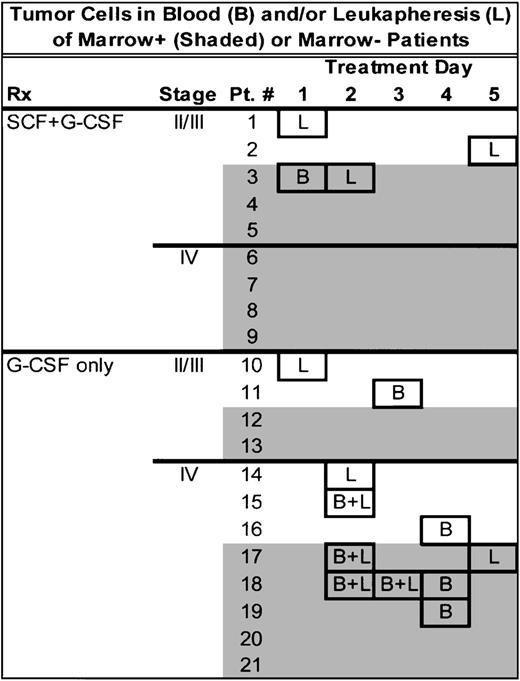
The relationship between the timing of cytokine infusion and tumor cell detection is shown in Figs 6 and 7. Tumor cells were detected on all days following the initiation of cytokine infusion and no relationship could be found between the timing of the cytokine infusion and the presence of tumor cells in peripheral blood or leukapheresis product. We found that, of the 11 patients found to have tumor cells in blood samples or leukapheresis products, both blood samples and leukapheresis products were tumor-positive on the same day in three patients. That the testing procedures probably detected a real biologic phenomenon is indicated by the fact that in five of the patients in whom tumor cells were found, multiple specimens were tumor-positive, one of which was from a clinical stage III patient.
Diagrammatic chart of the timing of each positive specimen. Shading indicates cases in which baseline bone marrow was positive. Boxes indicate tumor-positive specimen. L, tumor-positive leukapheresis; B, tumor-positive, premobilization peripheral blood sample; B + L, both leukapheresis and premobilization peripheral blood specimen positive.
Diagrammatic chart of the timing of each positive specimen. Shading indicates cases in which baseline bone marrow was positive. Boxes indicate tumor-positive specimen. L, tumor-positive leukapheresis; B, tumor-positive, premobilization peripheral blood sample; B + L, both leukapheresis and premobilization peripheral blood specimen positive.
Relation of tumor detection to day after beginning of cytokine infusion.
DISCUSSION
The primary aim of this study was to compare the incidence of tumor contamination in the leukapheresis product of patients mobilized with SCF + G-CSF or with G-CSF alone. The two treatment groups were evenly balanced with respect to stage of disease and baseline tumor contamination of the bone marrow and peripheral blood. The two groups also proved to be similar with respect to proportion of patients with tumor contamination in any of their leukapheresis samples (3.0% of the SCF + G-CSF patients and 5.0% of the patients who received G-CSF alone). There was no evidence of mobilization of tumor from bone marrow to peripheral blood with either cytokine regimen.
The assay used for tumor-cell detection was highly specific since the identity of each malignant cell could be verified by evaluation of the microscopic details of stained cells. In a previous study, we have shown that the sensitivity of this assay is directly proportional to the number of cytocentrifuge slides examined.20 At 20 slides, the sensitivity (95% confidence level) of the current assay is 1.7 tumor cells per million hematopoietic cells. This is similar to the reported sensitivities of previous studies employing immunocytochemical methods, which range from 0.5 to 2.5 breast cancer cells per million hematopoietic cells.19,21,24 25
Although the sensitivity of the tumor detection assay used in this study was similar to that previously reported, the proportion of patients with tumor-cell contamination of leukapheresis products in this study was lower than previously reported.17-20 This difference is primarily attributable to patient selection. In a previous trial conducted by the University of Colorado Bone Marrow Transplant Unit,20 17% of 155 patients had immunocytochemical evidence of breast cancer in their PBPC fractions. The vast majority of patients (141 of 155) had stage IV disease, while only 14 patients had stage II/III disease. Of the stage IV patients, 68 of 141 (48%) were enrolled on phase II studies, while 73 of 141 (52%) were enrolled on phase I protocols. Enrollment onto a phase I protocol required extensive prior therapy and/or extensive bulk disease at study entry. There were no limitations on the extent of bone marrow metastases and approximately two thirds of the patients on the phase I protocols had extensive bone and bone marrow metastases.26The present study enrolled a balanced number of stage II/III and stage IV patients and only 12 patients who entered the trial had light microscopic evidence of minimal bone marrow tumor contamination at baseline. Thus, the lower frequency of breast cancer cell detection in the PBPC fractions of the present trial appears appropriate for the study population.
The relatively low tumor burden in the stage IV patients in this study might also explain the unexpected lack of difference between tumor contamination rates of stage II/III and stage IV patients. Of the stage II/III patients, 4.3% had tumor-positive leukapheresis samples, while just 3.8% of the stage IV patients had tumor-positive leukapheresis samples. Recently, Passos-Coelho et al have also reported significant rates of tumor-cell contamination in baseline marrows (11%) and leukapheresis samples (8%) from stage III and IV breast cancer patients with no light microscopic evidence of bone marrow involvement before PBPC mobilization with G-CSF.27
To determine whether the cytokine infusion and leukapheresis procedure affected the incidence of tumor cells in the peripheral blood, a peripheral blood sample was obtained by venipuncture on the same day as leukapheresis before cytokine infusion. Of the 11 patients with tumor cells in blood samples or leukapheresis products, three had tumor cells in the corresponding peripheral blood and leukapheresis samples (four samples total). However, a stronger relationship between leukapheresis and peripheral blood results may be partially obscured due to missing data, since in four cases where a particular leukapheresis sample was tumor-positive, corresponding peripheral blood sample was inadequate for determination of tumor contamination. Similar proportions of patients in each arm of the trial had tumor cells in their peripheral blood samples taken on days of leukapheresis (1.0% of the SCF + G-CSF patients and 5.9% of the patients who received G-CSF alone). Although there was a trend towards greater tumor contamination in the peripheral blood samples of patients who received G-CSF alone (2.2% for the G-CSF alone patients, 0.3% for the SCF + G-CSF patients), the numbers of tumor-contaminated samples in either arm were too small to draw clinically relevant conclusions.
Only one positive case (0.6%) was identified for the baseline (pre-cytokine) peripheral blood samples, but only 35% of samples contained sufficient numbers of cells to obtain the level of sensitivity (1.7 tumor cells/106 hematopoietic cells) that was routinely reached for leukapheresis samples. The baseline peripheral blood samples were thus considered of limited value in providing reference levels for subsequent comparisons.
Tumor cells were detected in the leukapheresis product on 4 of 5 days following the initiation of cytokine administration and no relationship could be found between the day of cytokine injection and the presence of tumor cells in the leukapheresis products. Since the number of tumor-positive cases observed for each day was small, the results should be interpreted cautiously. However, it is of interest that in three patients, tumor cells were detected on 2 or more different days, suggesting that a subset of tumors is prone to seed the peripheral blood with relatively high levels of tumor cells. In most patients, only a single specimen was tumor-positive, suggesting that seeding of the peripheral blood in most patients is intermittent and sporadic. In this group, reducing the number of leukaphereses may lessen the chance of tumor-cell contamination of leukapheresis product.
The duration of follow-up in this study is currently too short to evaluate the relationship between tumor-cell contamination and clinical outcome. Whether the infusion of tumor in PBPC autografts will have an impact on clinical outcome remains to be determined. Results of a study conducted by Rill et al suggest this possibility.28 The investigators marked the bone marrow cells of patients with neuroblastoma and leukemia, using retroviral gene-mediated transfer of a neomycin-resistant gene. The majority of patients who relapsed had phenotypic and genotypic evidence of the marker gene in the malignant cells. Vredenburgh et al recently reported that the presence of occult bone marrow micrometastasis is a poor prognostic factor in patients with high-risk breast cancer undergoing high-dose chemotherapy with hematopoietic support.29 Moreover, infusion of bone marrow containing residual tumor detected by polymerase chain reaction (PCR) was associated with a significantly higher relapse rate in non-Hodgkin’s lymphoma patients and breast cancer patients than infusion of hematopoietic grafts that were normal by PCR analysis.30
In conclusion, this study of 203 patients with high-risk breast cancer demonstrated no difference in the proportion of patients with tumor cells in their leukapheresis product between patients mobilized with a combination of SCF and G-CSF and those who received G-CSF alone. The study also showed that the overall incidence of tumor-cell contamination of leukapheresis products in this patient population is low (4%), with no evidence of mobilization of tumor cells from bone marrow to peripheral blood by either cytokine regimen. Finally, when tumor cells were detected, they were often present on multiple days of leukapheresis and no clear relationship could be found between the day of the cytokine infusion and the presence of tumor cells in the leukapheresis product.
ACKNOWLEDGMENT
The authors thank Kim Hartsough and the Cytology Laboratory of the University of Colorado Health Sciences Center for expert technical assistance.
Principal Investigators and Clinical Coordinators and Participating Institutions
| Principal Investigators . | Clinical Coordinators . | Institutions . |
|---|---|---|
| Elizabeth J. Shpall | Sharon Taffs, Peggy Russell | University of Colorado Health Sciences Center, Denver, CO |
| John Glaspy | Linda Norton | UCLA School of Medicine, Los Angeles, CA |
| C.F. LeMaistre | Sheryl Oliversen | South Texas Cancer Institute, Dallas, TX |
| Stephanie Williams | Becky Malloy | University of Chicago Medical Center, Chicago, IL |
| Andrew Peccora | Arianne VanVliet | Northern New Jersey Cancer Institute, Hackensack, NJ |
| Thomas Shea | Betty Hinshaw | Lineburger Cancer Center, Chapel Hill, NC |
| Catherine Wheeler | Lisa Gaynes | Beth Israel Hospital, Boston, MA |
| Robert Negrin | Kate Tierney | Stanford Medical Center, Stanford, CA |
| Randy Brown | Carol Rush | Washington University School of Medicine, St Louis, MO |
| Saul Yanovich | Angela Brame | Medical College of Virginia, Richmond, VA |
| Kenneth Mangan | Lisa Matta | Temple University Cancer Center, Philadelphia, PA |
| Joe Rosenblatt | Sharon Heister | University of Rochester, Rochester, NY |
| Jeffrey Shogan | John W. Mucenski | Allegheny General Hospital, Pittsburgh, PA |
| Subhash Gulati | Suzanne (Simon) Donovon | New York Hospital, New York, NY |
| Principal Investigators . | Clinical Coordinators . | Institutions . |
|---|---|---|
| Elizabeth J. Shpall | Sharon Taffs, Peggy Russell | University of Colorado Health Sciences Center, Denver, CO |
| John Glaspy | Linda Norton | UCLA School of Medicine, Los Angeles, CA |
| C.F. LeMaistre | Sheryl Oliversen | South Texas Cancer Institute, Dallas, TX |
| Stephanie Williams | Becky Malloy | University of Chicago Medical Center, Chicago, IL |
| Andrew Peccora | Arianne VanVliet | Northern New Jersey Cancer Institute, Hackensack, NJ |
| Thomas Shea | Betty Hinshaw | Lineburger Cancer Center, Chapel Hill, NC |
| Catherine Wheeler | Lisa Gaynes | Beth Israel Hospital, Boston, MA |
| Robert Negrin | Kate Tierney | Stanford Medical Center, Stanford, CA |
| Randy Brown | Carol Rush | Washington University School of Medicine, St Louis, MO |
| Saul Yanovich | Angela Brame | Medical College of Virginia, Richmond, VA |
| Kenneth Mangan | Lisa Matta | Temple University Cancer Center, Philadelphia, PA |
| Joe Rosenblatt | Sharon Heister | University of Rochester, Rochester, NY |
| Jeffrey Shogan | John W. Mucenski | Allegheny General Hospital, Pittsburgh, PA |
| Subhash Gulati | Suzanne (Simon) Donovon | New York Hospital, New York, NY |
Supported by National Cancer Institute Grant No. RO1-CA615082 and by a research grant from Amgen Corp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Wilbur A. Franklin, MD, Department of Pathology, Box B-216, University of Colorado Health Sciences Center, 4200 E 9th Ave, Denver, CO 80262; e-mail: wilbur.franklin@uchsc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal