In study NHL-BFM 90, we investigated whether the serum lactate dehydrogenase (LDH) concentration and early response are useful markers for stratification of therapy for childhood B-cell neoplasms in addition to stage, if the outcome of patients with abdominal stage III and LDH ≥500 U/L can be improved by high-dose (HD) methotrexate (MTX) at 5 g/m2 instead of intermediate-dose (ID) MTX at 500 mg/m2 in the preceding study 86; whether 2 therapy courses are enough for patients with complete resection; and whether combined systemic and intraventricular chemotherapy is efficacious for central nervous system-positive (CNS+) patients. After a cytoreductive prephase, treatment was stratified into 3 risk groups: patients in R1 (completely resected) received 2 5-day courses (ID-MTX, dexamethasone, oxazaphorins, etoposide, cytarabine, doxorubicin, and intrathecal therapy), patients in R2 (extra-abdominal primary only or abdominal tumor and LDH <500 U/L) received 4 courses containing HD-MTX, and patients in R3 (abdominal primary and LDH ≥500 U/L or bone marrow/CNS/multilocal bone disease) received 6 courses. Incomplete responders after 2 courses received an intensification containing HD-cytarabine/etoposide. Patients with no or necrotic tumor thereafter received 3 more courses; 6 patients with viable tumor received autologous bone marrow transplantation. From April 1990 through March 1995, 413 evaluable patients were enrolled (R1, 17%; R2, 40%; and R3, 43%). The 6-year event-free survival (pEFS) was 89% ± 2% for all and 100%, 96% ±2%, and 78% ± 3% in R1, R2, and R3, respectively. The pEFS of patients with abdominal stage III and LDH ≥500 U/L was 81% ± 4% as compared with 43% ± 10% in study 86. Of 26 CNS+ patients, 5 died early, but only 3 relapsed.
THE INTRODUCTION OF short, intensive therapy courses primarily based on cyclophosphamide, methotrexate (MTX), and intrathecal (i.th.) therapy resulted in improved survival rates of children suffering from mature B-cell neoplasms.1-8 Conclusions from 3 previous trials of the Berlin-Frankfurt-Münster (BFM) group were that 3 courses of chemotherapy are enough for patients with completely resected localized tumors and that 6 courses are as good as 8 for patients with advanced stage disease.3 High-dose (HD) MTX therapy (5 g/m2) instead of intermediate-dose (ID) MTX (0.5 g/m2) improved the outcome of patients with acute B-cell leukemia (B-ALL).5 Central nervous system-negative (CNS−) patients do not need CNS irradiation for prevention of CNS relapses.5,6 An intraventricularly applied chemotherapy is likely to be beneficial for patients with CNS disease.5 Patients with incomplete tumor regression after 2 or 3 therapy courses had an increased risk of failure, particularly if they had histologically active residual tumors. There was no evidence of a curative role of surgery or radiotherapy.5,6 9
However, these intensive treatment strategies carry acute and late risks. But, after failure of first-line therapy, the probability of survival is poor.10 Therefore, further improvement of therapy requires optimized tailoring of treatment intensity to the patient’s risk of failure. In our previous trials, treatment intensity was stratified according to the St. Jude staging system.11Descriptions of tumor burden as an important prognostic factor12-14 are in line with the hypothesis of Goldie and Coldman15 that the proportion of multiple resistant cells correlates with tumor mass. Yet, the parameters of tumor mass, eg, serum level of lactic dehydrogenase (LDH),12 were not used for stratification of therapy because they correlate with stage.16 However, stage III of the St. Jude’s staging system includes patients with largely varying tumor mass. In our previous trial, NHL-BFM 86, patients with stage III and pretherapeutic serum LDH concentrations ≥500 U/L had a significantly worse outcome than those with lower LDH values (6-year event-free survival [pEFS], 43% ± 10% [n = 23] v 85% ± 6% [n = 35]). Therefore, in study NHL-BFM 90, LDH was used as an additional parameter for stratification of therapy intensity.
In study NHL-BFM 90, the goals for the treatment group B-NHL/B-ALL were to investigate whether a system for stratification of treatment intensity based on resectability, LDH, and stage is appropriate; whether the outcome of patients with abdominal stage III and LDH ≥500 U/L can be improved by intensified chemotherapy; whether survival of patients with incomplete initial response can be improved by stepwise intensification of therapy, including autologous blood stem cell transplantation; whether for patients with completely resected localized tumors therapy can further be reduced from 3 to 2 5-day courses; whether for patients with low tumor mass (LDH <500 U/L) therapy can be reduced from 6 to 4 courses when HD-MTX is used instead of ID-MTX; and whether combined intravenously and intraventricularly applied chemotherapy without radiotherapy is efficacious treatment for patients with CNS disease.
We report here on the treatment strategy and results of children and adolescents suffering from mature B-cell neoplasms enrolled in the study NHL BFM-90 from Austria, Germany, and part of Switzerland during a period of 5 years. Results are compared with those of trial NHL-BFM 86 as a historical control.
PATIENTS AND METHODS
Patients.
Children and adolescents up to 18 years of age newly diagnosed with any kind of non-Hodgkin’s lymphoma (NHL) or B-ALL were eligible for trial NHL-BFM 90. Exclusion criteria were the following: acquired immunodeficiency syndrome (AIDS)-related NHL, severe immunodeficiency, posttransplantation lymphoma, and pre-existing disease prohibiting chemotherapy. From April 1990 through March 1995, 682 eligible patients were enrolled from 90 clinics in Austria, Germany, and Switzerland after informed consent of their guardians had been obtained. The study population was subdivided according to NHL subtype into 3 groups with different therapy strategies: therapy group non-B (n = 162, patients with lymphoblastic lymphoma [precursor-B-cell or T-cell] or peripheral T-cell lymphoma), therapy group B-NHL/B-ALL (n = 431, patients with mature B-cell-NHL [B-NHL] or B-ALL), and therapy group ALCL (n = 89, patients with anaplastic large-cell lymphoma [ALCL]). We report here on the treatment and results of therapy group B-NHL/B-ALL. Of the 431 patients of group B-NHL/B-ALL, 18 patients (4%) were excluded from the evaluation for the following reasons: no therapy applied due to an individual decision (1 patient, alive after complete resection), premature withdrawal (1 patient, alive), treatment according to therapy strategy non-B (2 patients, 2 relapses), treatment according to therapy strategy ALCL (1 patient, free of disease), treatment according to protocol of previous trial NHL-BFM 86 (1 patient, free of disease), treatment according to a pilot protocol (6 patients, 1 relapse), allogeneic bone marrow transplantation (BMT; 1 patient, free of disease), and no intraventricular chemotherapy despite CNS disease (5 patients [1 received cranial irradiation], 2 CNS relapses). Finally, 413 patients with B-cell neoplasms were evaluable for response.
Diagnosis.
Cases were classified according to the updated Kiel-Classification for Non-Hodgkin’s Lymphomas.17 The corresponding terms of the Revised European American Lymphoma (REAL) Classification18are given in Table 3. Immunophenotyping of fresh cell suspensions was performed as previously described.19 In 341 patients, the diagnosis of B-NHL was based on histopathology and immunohistochemistry. From 233 (68%) of these 341 patients, the histopathological material was reviewed centrally by a reference laboratory of the study. Sixteen cases were classified as Burkitt’s lymphoma due to L3 morphology according to French-American-British (FAB) criteria20 of cells from malignant effusions. The presence of surface Igs (sIg) was proven in 11 of these 16 patients. Immunophenotyping was incomplete in 2 cases due to inappropriate material, whereas from 3 patients no material was available for central immunopheotyping. Fifty-six patients were diagnosed with B-ALL due to the presence of 25% or more FAB L3 blasts in the bone marrow (BM). The expression of sIg was shown by immunophenotyping in 41 of these 56 patients. Immunophenotyping was incomplete in 9 cases due to inappropriate material, whereas from 5 patients no material was available for central immunopheotyping. In 1 case, the FAB-L3 blasts did not express sIg.
Staging.
The St. Jude staging system11 was used. Staging studies included physical examination, peripheral blood and BM aspiration smears, cerebrospinal fluid (CSF) analyses, ultrasonography, x-ray, computed tomography (CT) or magnetic resonance imaging (MRI), and skeletal scintigraphy. In patients with an otherwise proven diagnosis of NHL, bone involvement was diagnosed if imaging studies showed bone lesions. Initial CNS disease was diagnosed if at least 1 of the following was present: morphologically identifiable lymphoma cells in the CSF on cytospin-preparations (regardless of the number), cerebral infiltrates on cranial CT or MRI, or cranial nerve palsy that was not caused by an extradural or extracranial mass. Testicular involvement was diagnosed clinically as the presence of painless enlargement of 1 or both testicles, provided that the diagnosis of NHL was otherwise established. The total serum LDH activity was measured as a routine laboratory test using standard methods.21 The normal limits of serum LDH values varied between the participating clinics. The comparability of measured enzyme activities measured in different laboratories is monitored by an external quality control system.
Stratification of treatment intensity.
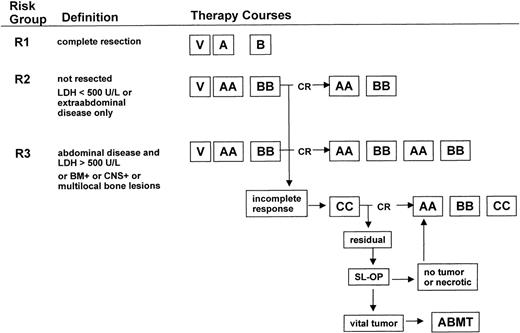
Therapy was stratified into 3 risk groups (Fig 1) according to the following definitions. Risk group 1 was defined as patients with initial complete resection of the lymphoma manifestation. Risk group 2 was defined as patients with no or incomplete resection of lymphoma manifestations, for which 1 of the following criteria is met: only extra-abdominal sites or abdominal site and LDH less than 500 U/L, measured before beginning chemotherapy. Risk group 3 was defined as patients with no or incomplete resection of abdominal lymphoma and LDH ≥500 U/L, all patients with BM involvement or/and CNS disease, or/and multifocal bone involvement.
Treatment strategy. Patients were stratified into 3 risk groups: R1, R2, and R3. The composition of therapy courses is given in Table 1. V, cytoreductive prophase; CR, complete response; SL-OP, second-look operation; ABMT, autologous BMT or blood stem cell transplantation.
Treatment strategy. Patients were stratified into 3 risk groups: R1, R2, and R3. The composition of therapy courses is given in Table 1. V, cytoreductive prophase; CR, complete response; SL-OP, second-look operation; ABMT, autologous BMT or blood stem cell transplantation.
Chemotherapy.
The therapy courses are given in Table 1. All patients received a 5-day cytoreductive prephase. Hydration of 3,000 to 4,500 mL/m2/d, alkalization with NaHCO3 intravenously (IV), and allopurinol were administered to prevent acute cell lysis syndrome. By July 1, 1993, an amendment of the cytoreductive prephase was introduced because of the incidence of early toxic death. Cyclophosphamide doses were reduced to 2, and prednisone was replaced by dexamethasone at 5 mg/m2/d on days 1 and 2 and at 10 mg/m2/d on days 3, 4, and 5. After the 5-day prephase, the first course of chemotherapy should be initiated the next day depending on the condition of the patient. The median delay of the initiation of the first course of chemotherapy was 0 days (range, 0 to 52 days). Patients in risk group R1 received 2 therapy courses A, B. Patients in risk group R2 received 4 courses AA-BB-AA-BB. Patients in risk group R3 received 6 courses AA-BB-AA-BB-AA-BB. Conditions for starting the second and third course of therapy were as follows: platelets greater than 50,000/μL and neutrophils greater than 200/μL after the nadir of postchemotherapeutic cytopenia was passed; for subsequent courses 4, 5, and 6, neutrophils should be greater than 500/μL. The minimal interval between the first day of 2 successive courses should be at least 2 weeks.
Therapy Courses
| . | Drug . | Dose . | Day . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |||
| Prephase V* | Prednisone (orally/IV) | 30 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Course A | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Ifosfamide (IV 1 h) | 800 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h† | 500 mg/m2 | x | |||||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Cytarabine IV 1 h | 150 mg/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | ||||
| Course B | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h† | 500 mg/m2 | x | |||||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | ||||
| Course AA1-155 | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Ifosfamide (IV 1 h) | 800 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h1-154 | 5 g/m2 | x | |||||
| Methotrexate‡ IT | 6 mg | x | x | ||||
| Cytarabine‡ IT | 15 mg | x | x | ||||
| Prednisolone‡ IT | 5 mg | x | x | ||||
| Vincristine IV | 1.5 mg/m2 # | x | |||||
| Cytarabine IV 1 h | 150 mg/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | ||||
| Course BB1-155 | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h1-154 | 5 g/m2 | x | |||||
| Methotrexate‡ IT | 6 mg | x | x | ||||
| Cytarabine‡ IT | 15 mg | x | x | ||||
| Prednisolone‡ IT | 5 mg | x | x | ||||
| Vincristine IV | 1.5 mg/m2 # | x | |||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | ||||
| Course CC1-155 | Dexamethasone (orally/IV) | 20 mg/m2 | x | x | x | x | x |
| Vindesine IV | 3 mg/m2 1-160 | x | |||||
| Cytarabine (IV 3 h) | 2 g/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 150 mg/m2 | x | x | x | |||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| . | Drug . | Dose . | Day . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |||
| Prephase V* | Prednisone (orally/IV) | 30 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Course A | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Ifosfamide (IV 1 h) | 800 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h† | 500 mg/m2 | x | |||||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Cytarabine IV 1 h | 150 mg/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | ||||
| Course B | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h† | 500 mg/m2 | x | |||||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | ||||
| Course AA1-155 | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Ifosfamide (IV 1 h) | 800 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h1-154 | 5 g/m2 | x | |||||
| Methotrexate‡ IT | 6 mg | x | x | ||||
| Cytarabine‡ IT | 15 mg | x | x | ||||
| Prednisolone‡ IT | 5 mg | x | x | ||||
| Vincristine IV | 1.5 mg/m2 # | x | |||||
| Cytarabine IV 1 h | 150 mg/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 100 mg/m2 | x | x | ||||
| Course BB1-155 | Dexamethasone (orally/IV) | 10 mg/m2 | x | x | x | x | x |
| Cyclophosphamide (IV 1 h) | 200 mg/m2 | x | x | x | x | x | |
| Methotrexate IV 24 h1-154 | 5 g/m2 | x | |||||
| Methotrexate‡ IT | 6 mg | x | x | ||||
| Cytarabine‡ IT | 15 mg | x | x | ||||
| Prednisolone‡ IT | 5 mg | x | x | ||||
| Vincristine IV | 1.5 mg/m2 # | x | |||||
| Doxorubicin IV 1 h | 25 mg/m2 | x | x | ||||
| Course CC1-155 | Dexamethasone (orally/IV) | 20 mg/m2 | x | x | x | x | x |
| Vindesine IV | 3 mg/m2 1-160 | x | |||||
| Cytarabine (IV 3 h) | 2 g/m2 | x − x1-153 | x − x1-153 | ||||
| Etoposide IV 1 h | 150 mg/m2 | x | x | x | |||
| Methotrexate‡ IT | 12 mg | x | |||||
| Cytarabine‡ IT | 30 mg | x | |||||
| Prednisolone‡ IT | 10 mg | x | |||||
Amendment July 1993: cyclophosphamide day 1, 2 only, dexamethasone instead of prednisone, see text.
Ten percent of dose within 0.5 hours, 90% of dose IV over 23.5 hours, leucovorin rescue at 12 mg/m2 IV at 48 and 54 hours.
Doses were adjusted for children less than 3 years of age. In courses A, B, AA, and BB, IT therapy was administered 2 hours after the beginning of MTX IV infusion.
Twelve hours apart.
For CNS-pos. patients, chemotherapy was applied intraventricularly as described in the chemotherapy section.
Ten percent of dose within 0.5 hours, 90% IV over 23.5 hours, leucovorin rescue (racemic folinic acid) IV at 30 mg/m2 at 42 hours and 15 mg/m2 at 48 and 54 hours. See text for adjustment of leucovorin dose in case of impaired MTX excretion.
#Maximum dose, 2 mg.
Maximum dose, 5 mg.
In the therapy courses AA and BB, which contain MTX at 5 g/m2, the MTX serum concentration were measured at 24, 36, 42, and 48 hours from the start of the MTX IV infusion. The serum levels of MTX should be ≤150 μmol/L at 24 hours from start of MTX infusion, less than 3 μmol/L at 36 hours, ≤1 μmol/L at 42 hours, and ≤0.4 μmol/L at 48 hours. Leucovorin rescue (racemic folinic acid) was administered IV at 30 mg/m2 at 42 hours and at 15 mg/m2 at 48 and 54 hours after the start of MTX infusion. If the MTX serum concentrations was higher than expected at 42 or 48 hours, measurements of the MTX serum levels and administration of leucovorin rescue were continued every 6 hours until the serum MTX concentration decreased to less than 0.25 μmol/L. The dose of leucovorin was adjusted as follows: MTX serum level of greater than 1 to 2 μmol/L, leucovorin at 30 mg/m2; MTX level of greater than 2 to 3 μmol/L, leucovorin at 45 mg/m2; MTX level of greater than 3 to 4 μmol/L, leucovorin at 60 mg/m2; and MTX level of greater than 4 to 5 μmol/L, leucovorin at 75 mg/m2. If the MTX level exceeded 5 μmol/L, the leucovorin dose was calculated according to the formula leucovorin (in milligrams) = MTX serum concentrations (in micromoles per liter) × body weight (in kilograms) and was administered as an IV infusion to avoid hypercalcemia.
In patients with overt CNS disease, a device for intraventricular application of chemotherapy was implanted before the second course of therapy. MTX at 3 mg and 2.5 mg prednisolone were administered on days 1, 2, 3, and 4 and 30 mg cytarabine was administered on day 5 of courses AA and BB. In courses CC, 3 mg MTX and 2.5 mg prednisolone were administered on days 3, 4, 5, and 6; 30 mg cytarabine was administered on day 7 (dose reduction for patients <3 years).
Patients in risk groups R2 and R3 who had a residual tumor and/or persistent blasts in the BM and/or in the CSF after 2 therapy courses received therapy course CC (Table 1). Patients were re-evaluated after course CC. In case of a persistent mass, a second-look operation was performed. If no viable lymphoma tissue was found, therapy was continued with 3 more courses AA-BB-CC. If viable lymphoma tissue was found, then megadose chemotherapy with autologous BM or blood stem cell rescue was performed. The high-dose chemotherapy consisted of busulfan, etoposide, and cyclophosphamide (Table 2). In CNS+ patients, thiotepa was administered instead of cyclophosphamide.
Autologous Stem Cell Transplantation
| Drug . | Dose . | Day . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −8 . | −7 . | −6 . | −5 . | −4 . | −3 . | −2 . | −1 . | 0 . | ||
| Busulfan (orally) | 120 mg/m2 * | x | x | x | x | |||||
| Etoposide (IV 4 h) | 300 mg/m2 | x | x | x | ||||||
| Cyclophosphamide† (IV 1 h) | 1500 mg/m2 | x | x | x | ||||||
| Stem cell transfusion | x | |||||||||
| Drug . | Dose . | Day . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −8 . | −7 . | −6 . | −5 . | −4 . | −3 . | −2 . | −1 . | 0 . | ||
| Busulfan (orally) | 120 mg/m2 * | x | x | x | x | |||||
| Etoposide (IV 4 h) | 300 mg/m2 | x | x | x | ||||||
| Cyclophosphamide† (IV 1 h) | 1500 mg/m2 | x | x | x | ||||||
| Stem cell transfusion | x | |||||||||
In 4 divided doses per day.
In CNS+ patients, thiotepa at 300 mg/m2/d was administered instead of cyclophosphamide. In this case, transfusion of stem cells was performed 48 hours after the third dose of thiotepa.
Local therapy modalities.
In case of localized disease, complete surgical resection was recommended if it could be easily and safely accomplished without the risk of functional impairments. In advanced stage disease, restricting initial surgery to the minimum required for gaining appropriate diagnostic tissue was recommended. Second-look surgery should follow the same guidelines. Local radiotherapy was not performed. In male subjects with testicular involvement, orchiectomy and radiotherapy was not foreseen.
Response criteria.
The treatment success was determined by the rate of EFS of the patients. Events were death due to any cause, tumor progression, and second malignancy. Tumor response was evaluated after each course of therapy. Follow-up studies were performed at 4- to 6-week intervals during the first 1.5 years. In patients with BM or/and CNS involvement, control punctures of BM or/and CSF were performed only until the BM or the CNS, respectively, was cleared from blasts. Progression was defined as a recurrence of lymphoma proven by biopsy or regrowth of an incompletely resolved tumor. The diagnosis of isolated progression in the BM was based on 25% or more blasts in the BM. The diagnosis of isolated progression in the CNS was based on the appearance of blasts in the CSF.
Statistical analysis.
Analyses of EFS were performed using the Kaplan and Meier method22 with differences compared by the log-rank test.23 EFS was calculated from the date of diagnosis to the first event (death from any cause, tumor progress, or second malignancy) or to the date of last follow-up. Patients lost to follow-up (LFU) were censored at the time of their withdrawal. Rank order comparisons of the prognostic relevance of different parameters were examined by stepwise Cox regression analysis.24Differences in the distribution of individual parameters among patients subsets were analyzed using the χ2 test or Fisher’s Exact Test. The statistical analysis was performed using the SAS statistical program (SAS-PC, Version 6.12; SAS Institute Inc, Cary, NC). Follow-up data were actualized as of October 1, 1998.
RESULTS
Patient characteristics.
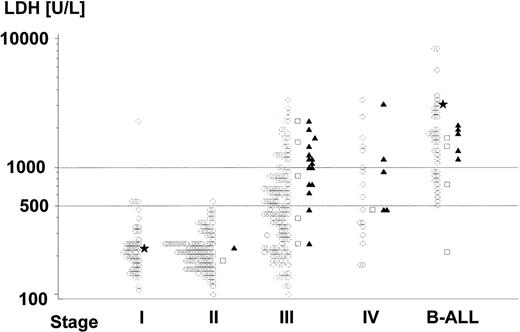
Of the 413 evaluable patients, 92 were girls and 321 were boys. The median age was 9.0 years (range, 1.2 to 17.9 years). The diagnoses of the patients are given in Table 3. The distribution of stages were stage I, II, III, IV, B-ALL in 12%, 28%, 41%, 6%, and 13% of patients, respectively. The median pretreatment LDH level was 365 U/L (range, 80 to 17,000 U/L). Figure 2 shows the distribution of serum LDH values according to stage. Table 4lists the distribution of patients according to risk groups and stages: 17% of patients were assigned to risk group R1, 40% to risk group R2, and 43% to risk group R3. Patients with stages I and II were subdivided between risk groups R1 and R2 according to complete versus incomplete tumor resection. Patients with stage III were subdivided 1:73:95 between risk groups R1:R2:R3 according to LDH and localization of the primary tumor. Six stage III patients with pretreatment LDH ≥500 U/L were assigned to risk group R2 due to exclusively extra-abdominal disease. Of the 24 patients with stage IV disease, 12 had BM involvement, 9 had CNS disease, and 3 had both BM and CNS disease. Fourteen of the 56 B-ALL patients had CNS involvement at diagnosis. Of the 26 patients with CNS disease, 18 had CSF blasts (1 had also an intraparenchymal mass). The median number of CSF cells of these patients was 9/μL (range, 1 to 1,000/μL). Three patients were considered CNS+ due to an intraparenchymal mass and cranial nerve palsy, and 5 patients had a cranial nerve palsy only. Four of the 26 CNS+ patients had an epidural mass. Another 5 patients had epidural tumors without CSF blasts or intraparenchymal lesions, and they were considered CNS−. Three boys had unilateral wheras 1 had bilateral testicular disease. The diagnosis of testicular involvement was established by biopsy in 2 cases and clinically plus by ultrasound in 2 cases. Twenty patients with impaired renal functions due to kidney infiltration or/and acute cell lysis syndrome needed hemodialysis.
Diagnosis and Site of Progression
| Diagnosis . | No. of Patients . | Patients Suffering From Progres- sion . | Site of Progression (any involvement)3-150 . | ||||
|---|---|---|---|---|---|---|---|
| Local . | BM . | CNS . | Testes . | New Site . | |||
| Burkitt type | 266 | 17 | 12 | 3 | 1 | 1 | 1 |
| B-ALL | 56 | 6 | 3 | 2 | 1 | 0 | 1 |
| Diffuse large B-cell lymphoma | |||||||
| Centroblastic | 42 | 2 | 2 | ||||
| Immunoblastic | 6 | 0 | |||||
| Mediastinal (thymic) B-cell lymphoma | 8 | 1 | 1 | ||||
| NHL, not further classified: | |||||||
| B-cell | 30 | 1 | 1 | ||||
| Immunophenotype NA3-151 | 5 | ||||||
| Total | 413 | 27 | |||||
| Diagnosis . | No. of Patients . | Patients Suffering From Progres- sion . | Site of Progression (any involvement)3-150 . | ||||
|---|---|---|---|---|---|---|---|
| Local . | BM . | CNS . | Testes . | New Site . | |||
| Burkitt type | 266 | 17 | 12 | 3 | 1 | 1 | 1 |
| B-ALL | 56 | 6 | 3 | 2 | 1 | 0 | 1 |
| Diffuse large B-cell lymphoma | |||||||
| Centroblastic | 42 | 2 | 2 | ||||
| Immunoblastic | 6 | 0 | |||||
| Mediastinal (thymic) B-cell lymphoma | 8 | 1 | 1 | ||||
| NHL, not further classified: | |||||||
| B-cell | 30 | 1 | 1 | ||||
| Immunophenotype NA3-151 | 5 | ||||||
| Total | 413 | 27 | |||||
Multiple sites can be involved in 1 patient.
Abdominal primary and lymphoid origin confirmed by CD45 positivity, cytokeratin negativity.
Distribution of pretreatment serum LDH concentrations (logarithmic scale) according to stage of disease. (○) Patients surviving event-free; (□) toxic death; (▴) patients suffering from progress; (*) patients who developed second malignancy. From 16 patyients (2, 7, 2, 1, and 4 of stage I, II, III, IV, and B-ALL, respectively) the LDH values are lacking. One of these patients (stage IV) relapsed, and 2 B-ALL patients died of acute cell lysis syndrome. One patient (stage III) with LDH less than 100 U/L and 3 patients (B-ALL) with LDH greater than 10,000 are not depicted; 1 of them (B-ALL) died of acute cell lysis syndrome.
Distribution of pretreatment serum LDH concentrations (logarithmic scale) according to stage of disease. (○) Patients surviving event-free; (□) toxic death; (▴) patients suffering from progress; (*) patients who developed second malignancy. From 16 patyients (2, 7, 2, 1, and 4 of stage I, II, III, IV, and B-ALL, respectively) the LDH values are lacking. One of these patients (stage IV) relapsed, and 2 B-ALL patients died of acute cell lysis syndrome. One patient (stage III) with LDH less than 100 U/L and 3 patients (B-ALL) with LDH greater than 10,000 are not depicted; 1 of them (B-ALL) died of acute cell lysis syndrome.
Risk Groups and Stages
| Risk Group . | Patients . | Stage . | ||||||
|---|---|---|---|---|---|---|---|---|
| I . | II . | III . | IV CNS− . | IV CNS+ . | B-ALL CNS− . | B-ALL CNS+ . | ||
| R1 | 71 (17%) | 27 | 43 | 1 | 0 | 0 | 0 | 0 |
| R2 | 167 (40%) | 22 | 72 | 73 | 0 | 0 | 0 | 0 |
| R3 | 175 (43%) | 0 | 0 | 95 | 12 | 12 | 42 | 14 |
| All | 413 | 49 | 115 | 169 | 12 | 12 | 42 | 14 |
| Risk Group . | Patients . | Stage . | ||||||
|---|---|---|---|---|---|---|---|---|
| I . | II . | III . | IV CNS− . | IV CNS+ . | B-ALL CNS− . | B-ALL CNS+ . | ||
| R1 | 71 (17%) | 27 | 43 | 1 | 0 | 0 | 0 | 0 |
| R2 | 167 (40%) | 22 | 72 | 73 | 0 | 0 | 0 | 0 |
| R3 | 175 (43%) | 0 | 0 | 95 | 12 | 12 | 42 | 14 |
| All | 413 | 49 | 115 | 169 | 12 | 12 | 42 | 14 |
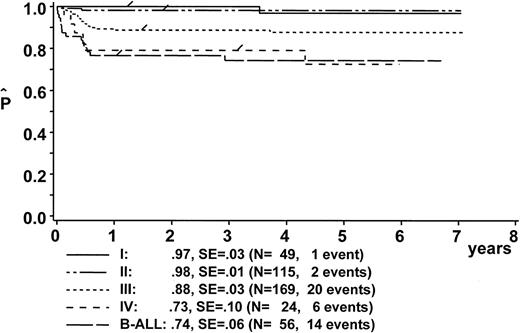
EFS.
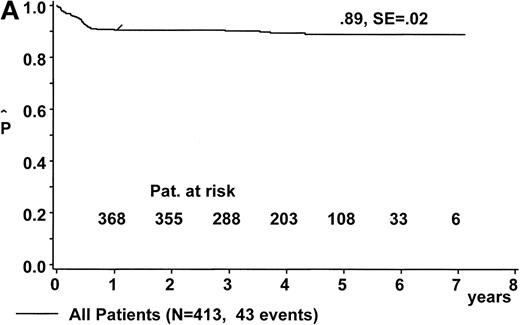
At a median follow-up of 4.2 years (range, 1.0 to 7.1 years), the estimate for pEFS is 88% ± 2% for the 431 eligible patients and 89% ± 2% for the 413 patients evaluable for response (Fig 3A). Twenty-one patients were lost to follow-up after a median event-free follow-up duration of 1.73 years (range, 0.2 to 5.46 years).
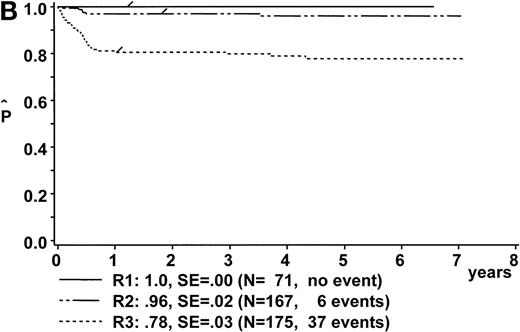
Kaplan-Meier estimate of EFS (A) for the whole group of evaluable patients and (B) according to risk groups R1, R2, and R3. SE, standard error.
Kaplan-Meier estimate of EFS (A) for the whole group of evaluable patients and (B) according to risk groups R1, R2, and R3. SE, standard error.
The following analysis of treatment results includes only the 413 evaluable patients. The pEFS at 6 years was 100%, 96% ± 2%, and 78% ± 3% for patients in risk groups R1, R2, and R3, respectively (Fig 3B). Eight patients in risk group R1 were treated according to branch R2, and 12 patients of risk group R2 were treated according to branch R3 due to individual decisions of the physicians in charge. The pEFS at 6 years according to the treatment applied was 100%, 96% ± 2%, and 79% ± 3% for patients treated according to branches R1, R2, and R3, respectively.
Events.
Forty-three patients suffered an adverse event (Table 5). Three children in risk group R3 died early of acute cell lysis syndrome. Eleven children (3 in risk group R2 and 8 in risk group R3) died of toxic death. Twenty-seven children suffered from progression (2 in risk group R2 and 25 in risk group R3). In 10 of these patients, progression occurred during therapy; in 15 patients, progression occurred within 3 months after completion of therapy. Local manifestations were the most frequent site of tumor failure (19 of 27 cases), followed by BM and new sites (Table 3). Two patients developed a secondary malignancy. The histology of both second malignancies was again NHL. A girl who was 6.6 years of age at the first diagnosis Burkitt‘s lymphoma developed a second Burkitt‘s lymphoma 3.5 years later. The light-chain restriction of the first tumor was κ, whereas the light-chain was λ in the second tumor. The second case was an 8.5-year-old boy with a first diagnosis of B-ALL. Three years later, he developed a T-cell lymphoblastic lymphoma. Two boys (1 with B-NHL, not further classifiable, and 1 with Burkitt‘s lymphoma) suffered from unusual late recurrences 3.5 and 4 years after completion of therapy. One of them was very recently diagnosed as suffering from X-linked lymphoproliferative syndrome. In both cases, the clonal identity or difference of the first and the second tumor could not be investigated due to the lack of appropriate material.
Adverse Events by Risk Groups
| . | Total . | Risk Group . | ||
|---|---|---|---|---|
| R1 . | R2 . | R3 . | ||
| No. of patients | 413 | 71 | 167 | 175 |
| Early death | 3 | 0 | 0 | 3 |
| Progression | 27 | 0 | 2 | 25 |
| Death unrelated to tumor | 11 | 0 | 3 | 8 |
| Second malignancy | 2 | 0 | 1 | 1 |
| Lost to follow-up5-150 | 21 | 4 | 12 | 5 |
| . | Total . | Risk Group . | ||
|---|---|---|---|---|
| R1 . | R2 . | R3 . | ||
| No. of patients | 413 | 71 | 167 | 175 |
| Early death | 3 | 0 | 0 | 3 |
| Progression | 27 | 0 | 2 | 25 |
| Death unrelated to tumor | 11 | 0 | 3 | 8 |
| Second malignancy | 2 | 0 | 1 | 1 |
| Lost to follow-up5-150 | 21 | 4 | 12 | 5 |
After event-free follow-up duration of 0.2 to 5.46 years (median, 1.73 years).
EFS according to stage of disease and pretreatment LDH values.
pEFS according to stage is depicted in Fig4. Table 6 summarizes adverse events according to stage. Of the 56 B-ALL patients, 17 had 25% to 69% L3-blasts in the BM, 1 of them died early, 3 died of toxicity, and 2 failed to respond to therapy. Thirty-nine B-ALL patients had greater than 70% L3 blasts in the BM, 2 of them died early, 1 died of toxicity, and 4 failed to respond to therapy. Figure 2 shows the incidence of events according to stage and pretreatment LDH values. pEFS of patients with stage III (pEFS, 88% + 3%; n = 169) was significantly higher than pEFS of patients with stage IV+B-ALL (pEFS, 74% ± 5%; n = 80; P = .0059). However, in patients with stage III and IV B-ALL, the LDH values varied over a wide range. pEFS was significantly lower for patients with stage III/IV/B-ALL, with LDH values greater than 1,000 U/L (pEFS, 70% ± 5%; n = 84) as compared with those with LDH values less than 1,000 U/L (pEFS, 90% ± 3%; n = 158; P = .0001). In a Cox regression analysis with the covariables stage (stage III v stage IV+B-ALL) and LDH (<1,000 v ≥1,000 U/L), LDH greater than 1,000 U/L was the superimposed predictor for treatment outcome (risk ratio, 6.450; P = .0017), whereas the prognostic impact of stage IV+B-ALL was not significant (risk ratio, 2.726;P = .3802).
Adverse Events by Stages
| . | Stage . | ||||||
|---|---|---|---|---|---|---|---|
| I . | II . | III . | IV CNS− . | IV CNS+ . | B-ALL CNS− . | B-ALL CNS+ . | |
| No. of patients | 49 | 115 | 169 | 12 | 12 | 42 | 14 |
| Early death | 1 | 2 | |||||
| Progression | 1 | 15 | 2 | 3 | 6 | ||
| Death unrelated to tumor | 1 | 5 | 1 | 1 | 3 | ||
| Second malignancy | 1 | 1 | |||||
| . | Stage . | ||||||
|---|---|---|---|---|---|---|---|
| I . | II . | III . | IV CNS− . | IV CNS+ . | B-ALL CNS− . | B-ALL CNS+ . | |
| No. of patients | 49 | 115 | 169 | 12 | 12 | 42 | 14 |
| Early death | 1 | 2 | |||||
| Progression | 1 | 15 | 2 | 3 | 6 | ||
| Death unrelated to tumor | 1 | 5 | 1 | 1 | 3 | ||
| Second malignancy | 1 | 1 | |||||
CNS disease.
The pEFS at 6 years for the 26 patients with initial CNS disease (17 BM positive and 9 BM negative) was 65% ± 9%. Two patients died early of acute cell lysis syndrome, and 3 patients died of sepsis after the first course of therapy before an Ommaya-reservoir was implanted (Table6). Of the remaining 21 patients, 3 suffered from progression, but only 1 of them within the CNS.
Testicular involvement.
In 2 of the 4 boys with testicular involvement, no local therapy was performed and both remained event-free. In 2 cases, orchiectomy was performed after 3 courses of therapy due to suspected residual disease. In both cases, histology did not show evidence for residual lymphoma. One of these 2 patients died later in the course of sepsis, and the second one remained event-free.
Outcome according to the kinetics of response to therapy.
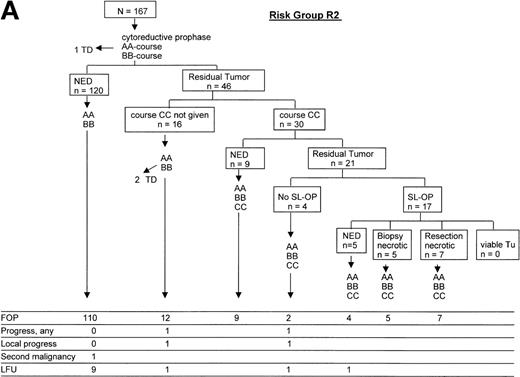
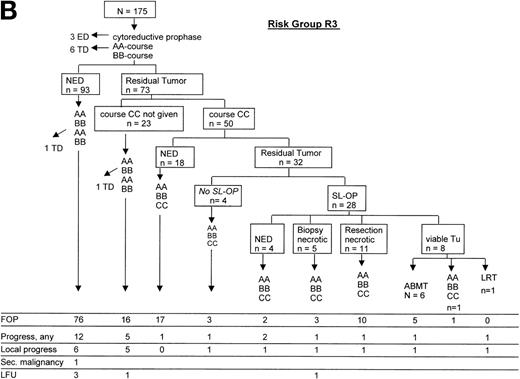
The courses of patients in risk groups R2 and R3 are depicted in Fig 5A and B, respectively. One patient in risk group R2 and 9 patients in risk group R3 died of initial complications or of infection before the response could be evaluated, after the second course of therapy. One hundred sixty-six patients each were evaluable for response after the first 2 therapy courses in risk groups R2 and R3. Using imaging methods, 46 patients (28%) in risk group R2 and 73 patients (44%) in risk group R3 had tumor remnants after 2 courses of therapy (P = .002). No patient with initial BM or/and CNS involvement had persistent blasts in the BM or the CSF after 2 courses. Of the 46 patients in risk group R2 with tumor remnants, 2 suffered from subsequent progression as compared with 13 of 73 patients in risk group R3 with tumor remnants (P = .031). In risk group R3, 8 patients had viable residual tumor after course CC: 6 received autologous BMT (ABMT); 1 suffered from progression, 1 patient received local radiotherapy and suffered from progression, and 1 patient had viable residual tumor in an ovary that was completely resected. This patient received 3 more courses AA-BB-CC and remained free of progression.
Direction of treatment intensity and second-look surgery according to response. (A) Course of patients in risk group R2; (B) course of patients in risk group R3. ED, early death by acute cell lysis syndrome; TD, toxic death; NED, no evidence of disease; SL-OP, second-look operation; ABMT, autologous BMT; LRT, local radiotherapy; FOP, free of progress; LFU, lost to follow-up.
Direction of treatment intensity and second-look surgery according to response. (A) Course of patients in risk group R2; (B) course of patients in risk group R3. ED, early death by acute cell lysis syndrome; TD, toxic death; NED, no evidence of disease; SL-OP, second-look operation; ABMT, autologous BMT; LRT, local radiotherapy; FOP, free of progress; LFU, lost to follow-up.
EFS according to histological subtypes.
pEFS at 6 years was 89% ± 2% for the 322 patients with Burkitt-type lymphomas or B-ALL, 95% ± 3% for the 56 patients with diffuse large B-cell lymphomas (including 8 patients with mediastinal [thymic] B-cell lymphoma), and 84% ± 7% for the 35 patients with B-NHL not further classified (P > .14 for all comparisons).
Toxicity.
Apart from myelosuppression, mucositis was the leading toxic side effect in this therapy and was related to the dose of MTX. Mucositis World Health Organization (WHO) grade ≥2 occurred after 12%, 19%, 47%, 48%, and 13% of courses A, B, AA, BB, and CC, respectively. Febrile episodes were observed after 7%, 12%, 7%, 37%, 31%, and 24% of courses A, B, AA, BB, and CC, respectively. Nine patients died of septicemia and enterocolitis (3 Pseudomonas aeruginosa, 1 Citrobacter, and 4 blood culture negative). Of these, 6, 2, and 1 patients died after the first, second, and third course of chemotherapy, respectively. On July 1, 1993, the number of cyclophosphamide doses of the cytoreductive prephase was reduced from 5 to 2. Five of the 6 toxic deaths during the neutropenic phase after the first course of therapy occurred before this amendment, with only 1 occurring thereafter. One patient died of acute cardiac insufficiency after the fifth course of therapy. One patient died of acquired respiratory distress syndrome after autologous BMT.
Comparison of results: NHL-BFM 90 and NHL-BFM 86.
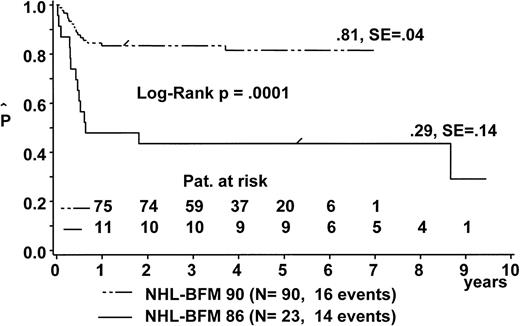
The pEFS at 6 years for B-NHL/-B-ALL patients was 89% ± 2% in study NHL-BFM 90 as compared with 82% ± 3% in study NHL-BFM 86 (P = .0087; Table 7). pEFS was not significantly different between both studies for patients with stage I, stage II, stage IV, and B-ALL, respectively. However, for patients with stage III disease, pEFS at 6 years was significantly higher in study NHL-BFM 90. The improvement in stage III patients was an increase of pEFS from 43% ± 10% in study NHL-BFM 86 to 81% ± 4% in study NHL-BFM 90 for those patients with abdominal disease and LDH ≥500 U/L (Fig 6).
Comparisons of pEFS of Patients Between Studies NHL-BFM 90 and NHL-BFM 86
| Group of Patients . | Study NHL-BFM 90 . | Study NHL-BFM 866 7-150 . | PLog Rank . | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients . | No. of Events . | % pEFS ± SE at 6 yrs . | No. of Patients7-150 . | No. of Events . | % pEFS ± SE at 6 yrs . | ||
| Total | 413 | 43 (10%) | 89 ± 2 | 197 | 37 (19%) | 82 ± 3 | .0087 |
| Stage I | 49 | 1 | 97 ± 3 | 24 | 0 | 100 | .43 |
| Stage II | 115 | 2 | 98 ± 1 | 41 | 1 | 98 ± 2 | .80 |
| Stage III | 169 | 20 (12%) | 88 ± 3 | 72 | 21 (29%) | 72 ± 5 | .0017 |
| Stage III-LDH <500 U/L7-151 | 77 | 4 (5%) | 95 ± 3 | 35 | 5 (17%) | 85 ± 6 | .09 |
| Stage III-LDH ≥500 U/L7-151 abdominal | 90 | 16 (18%) | 81 ± 4 | 23 | 14 (61%) | 43 ± 10 | .0001 |
| Stage IV | 24 | 6 (25%) | 73 ± 10 | 19 | 6 (32%) | 67 ± 10 | .58 |
| B-ALL | 56 | 14 (25%) | 74 ± 6 | 41 | 9 (22%) | 78 ± 6 | .64 |
| Group of Patients . | Study NHL-BFM 90 . | Study NHL-BFM 866 7-150 . | PLog Rank . | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients . | No. of Events . | % pEFS ± SE at 6 yrs . | No. of Patients7-150 . | No. of Events . | % pEFS ± SE at 6 yrs . | ||
| Total | 413 | 43 (10%) | 89 ± 2 | 197 | 37 (19%) | 82 ± 3 | .0087 |
| Stage I | 49 | 1 | 97 ± 3 | 24 | 0 | 100 | .43 |
| Stage II | 115 | 2 | 98 ± 1 | 41 | 1 | 98 ± 2 | .80 |
| Stage III | 169 | 20 (12%) | 88 ± 3 | 72 | 21 (29%) | 72 ± 5 | .0017 |
| Stage III-LDH <500 U/L7-151 | 77 | 4 (5%) | 95 ± 3 | 35 | 5 (17%) | 85 ± 6 | .09 |
| Stage III-LDH ≥500 U/L7-151 abdominal | 90 | 16 (18%) | 81 ± 4 | 23 | 14 (61%) | 43 ± 10 | .0001 |
| Stage IV | 24 | 6 (25%) | 73 ± 10 | 19 | 6 (32%) | 67 ± 10 | .58 |
| B-ALL | 56 | 14 (25%) | 74 ± 6 | 41 | 9 (22%) | 78 ± 6 | .64 |
Patients with ALCL and patients with precursor-B-cell lymphoblastic lymphoma treated in therapy group B-NHL of trial NHL-BFM 86 are not included here.
Only patients with LDH available are considered.
Kaplan-Meier estimate of EFS for patients with abdominal stage III B-NHL and LDH ≥500 U/L in trial NHL-BFM 90 and in the preceding trial NHL-BFM 86. SE, standard error.
Kaplan-Meier estimate of EFS for patients with abdominal stage III B-NHL and LDH ≥500 U/L in trial NHL-BFM 90 and in the preceding trial NHL-BFM 86. SE, standard error.
DISCUSSION
In trial NHL-BFM 90, a total of 431 children and adolescents with mature B-cell neoplasms were enrolled, of whom 413 were evaluable for response. The 6-year pEFS was 88% ± 2% for all 431 eligible patients and was 89% ± 2% for the 413 evaluable patients. The therapy strategy applied proved to be a highly efficacious treatment not only for patients with Burkitt-type lymphomas and B-ALL, but also for patients suffering from diffuse large B-cell lymphomas.
The results of trial NHL-BFM 90 demonstrate that the outcome of B-NHL patients who were at high risk of progression could be significantly improved by intensification of therapy. The improvement of treatment results in study NHL-BFM 90 as compared with study NHL-BFM 866 was exclusively due to a significant increase of pEFS for patients with abdominal stage III and LDH ≥500 U/L, who were the target group for treatment intensification in study NHL-BFM 90. Compared with our preceding studies, these patients received HD-MTX therapy at 5 g/m2 instead of ID-MTX at 0.5 g/m2. Furthermore, 44% of them received high-dose cytarabine/etoposide therapy because of an incomplete tumor resolution after 2 courses of therapy, and 6.6% of them received ABMT due to a persistent viable tumor after 3 courses of therapy. pEFS for these patient subgroup increased from 43% ± 10% in study NHL-BFM 86 to 81% ± 4% in study NHL-BFM 90.
Our data confirm that, in addition to stage, LDH as a parameter of tumor burden is appropriate for stratification of treatment intensity for patients with B-cell neoplasms. Approximately 40% of patients present with stage III in the St. Jude system,11 which includes patients with varying tumor burden and, therefore, who may need different treatment intensities.25 In our previous studies, a cut-off of 500 U/L of the pretherapeutic LDH serum concentration distinguished 2 subgroups of stage III patients with significantly different outcomes when they received uniform treatment, including ID-MTX at 0.5 g/m2. The pEFS of patients with LDH less than 500 U/L was comparable to that of patients with stage II disease, whereas the pEFS of patients with abdominal stage III and LDH ≥500 U/L was less than 50%. Therefore, in study NHL-BFM 90, the pretherapeutic LDH was used for stratification of treatment intensity together with resectability and stage (BM and CNS involvement). As a consequence, patients with stage III were subdivided into risk groups R2 and R3. This stratification system enables the pretherapeutic identification of 60% of patients (risk groups R1 + R2) with a probability of EFS of ≥95% with limited therapy intensity. Almost all patients at risk for failure are assembled in risk group R3.
As in many malignancies, the speed of the initial response to therapy has prognostic impact for B-NHL-patients. In our previous studies, as in other studies, patients with incomplete tumor regression after 2 or 3 therapy courses had an increased risk of failure, especially those with viable residual tumors.1,5,6,9,26 Therefore, in study NHL-BFM 90, we examined a strategy of stepwise intensification of therapy for patients with incomplete response after 2 courses of therapy. The first step of intensification was HD-cytarabine/etoposide, which was described as being effective in relapsed patients.27 Patients who still had residual tumor thereafter underwent second-look surgery. Those patients with viable tumor received megadose chemotherapy with hematological stem cell rescue as a second step of intensification.28 Although the contribution of each step to patient outcome is difficult to judge, some conclusions are possible. In patients with incomplete early tumor regression, the risk of subsequent progression depends on the patient’s initial tumor mass. In risk group R2, only 2 of 46 patients with residual tumor after 2 courses of therapy suffered from subsequent progression, compared with 13 of 73 such patients in risk group R3. Moreover, in risk group R3, the probability of progression-free survival of patients with a residual tumor after 2 courses was significantly higher for patients with pretreatment LDH values less than 1,000 U/L as compared with those with LDH values ≥1,000 U/L (data not shown).
Megadose chemotherapy with autologous stem cell rescue may be beneficial for patients with histologically active tumor after 3 therapy courses. Of 6 patients who received ABMT, 1 suffered from progression, whereas 4 of 5 such patients in study NHL-BFM 86 who did not receive ABMT died of progressive disease.6 However, this ambitious approach including invasive monitoring of tumor regression was not sufficiently predictive of the course of the disease. We observed local recurrences among patients with complete resolution of tumors as determined by imaging and second-look surgery. Contrarily, the majority of patients with tumor remnants who did not receive second-look surgery remained subsequently free of progression. Even the finding of viable residual tumor on second-look operation may be not of unequivocal value, considering the total group of patients. To identify 8 patients with viable residual tumor, 45 second-look operations had to be performed. Our conclusion from this prospective trial is that the strategy of guiding treatment according to the clinical response to therapy is of limited value and carries substantial risks. The interpretation of imaging and histological material may also be biased by the investigators’ knowledge that their judgement has consequences on patient treatment.
We agree with others that patients with localized stages I and II should have limited therapy.29 However, in view of the poor prognosis of relapsed patients, recurrences should be avoided completely.30 In trial NHL-BFM 90, it was shown that 2 5-day courses containing ID-MTX are enough therapy to avoid relapse in patients with completely resected localized B-NHL. The cumulative dose of doxorubicin (50 mg/m2) and that of oxazaphorins is below the threshold of late gonadotoxicity31 for these patients. However, it should be emphasized that resectability is determined by the extent of the disease. However, because the extent of the disease is the overriding predictor of outcome,9 32 disabling surgical procedures to achieve complete resection are not justified. For patients with no or incomplete resection but rather low tumor mass (stage I and II and stage III with LDH <500 U/L), who constitute risk group R2, treatment duration was reduced from 6 courses in the previous trials to 4 courses in study NHL-BFM 90. Hereby, the cumulative doses of cyclophosphamide, ifosfamide, and doxorubicin were reduced to 3 g/m2, 8 g/m2, and 100 mg/m2, respectively. As compensation, ID-MTX was replaced by HD-MTX. Only 2 of 167 patients in risk group R2 failed to respond to therapy. Thirty of the 167 patients (18%) in risk group R2 (2, 10, and 18 patients of stage I, II, and III, LDH <500 U/L, respectively) received HD-cytarabine/etoposide and a total of 6 therapy courses because of incomplete tumor regression after 2 courses. However, the fact that, in risk group R2, only 2 of 46 patients with residual tumor after 2 courses suffered from subsequent progression suggests that this strategy was most probably an overtreatment of these patients. Hence, further reduction of therapy for these patients has been integrated into our ongoing trial NHL-BFM 95.
Initial CNS disease remains a challenge in patients with B-cell neoplasms. Intriguing results were reported from the French study LMB 89 using HD-cytarabine, HD-MTX at 8 g/m2, and cranial irradiation.33 However, the benefit of CNS irradiation for CNS+ patients may be uncertain. Intraventricularly applied chemotherapy provides a better distribution within the CSF,34 and a higher concentration over time by fractionation of dose35 can be achieved. In trial NHL-BFM 90, we investigated whether fractionated, intraventricularly applied chemotherapy in combination with systemic HD-MTX therapy can provide efficacious CNS-protection for CNS+ patients without using radiotherapy. This strategy proved to be highly effective. Of the 26 CNS+ patients, 2 patients died of acute cell lysis syndrome and 3 patients died of sepsis after the first course of therapy before an Ommaya-reservoir was implanted. Of the remaining 21 patients, 3 suffered from progression, and only 1 of them within the CNS.
Whereas potential late risks of the therapy were reduced at least for part of the patients through reduction of the cumulative doses of critical drugs and omission of cranial radiotherapy, the acute toxicity of this treatment program is still a challenge. The contribution of hematopoetic growth factors to ameliorate morbidity and mortality is uncertain.8,36,37 Most deaths from infection occurred after the first course of therapy. The incidence of toxic death at this point was diminished after reduction of the intensity of the cytoreductive prephase. Metabolic complications of acute cell lysis and impaired renal function may also contribute to the risk of the early treatment phase. Urate oxidase is able to reduce these complications and may be most efficacious in reducing early deaths.38,39Unfortunately, urate oxidase is not available in all countries and could not be used in our trial. Severe orointestinal mucositis is the most likely pathway for life-threatening or fatal infections. In our therapy, severe mucositis is clearly related to HD-MTX. MTX is a keystone component of all successful treatment programs for B-cell neoplasms of childhood.7,8,33 40-42 However, doses and schedules of the MTX therapies differ from 1 g/m2 to 8 g/m2 as IV infusions over 3 to 24 hours. Both antitumor efficacy and toxicity may correlate with dose and time of exposure to the drug. We consider it an important goal to find out the most efficacious and least toxic schedule of MTX therapy for these children. Therefore, in our ongoing trial, we are investigating in a randomized fashion whether a reduced infusion time of the same dose of MTX will result in less toxicity without jeopardizing treatment outcome.
APPENDIX
Study Committee of Trial NHL-BFM 90.
W. Dörffel, Berlin; W. Ebell, Berlin; N. Graf, Homburg; H. Gadner, Vienna; G. Henze, Berlin; G. Janka-Schaub, Hamburg; T. Klingebiel, Tübingen; St. Müller-Weihrich, Munich; I. Mutz, Leoben; H.J. Plüss, Zürich; R. Parwaresch, Kiel; A. Reiter, Hannover; H. Riehm, Hannover; G. Schellong, Münster; M. Schrappe, Hannover; F. Zintl, Jena.
Principal Investigators.
R. Mertens (Aachen); R. Angst (Aarau); A. Gnekow (Augsburg); R. Dickerhoff (St. Augustin); P. Imbach (Basel); G.F. Wündisch (Bayreuth); W. Dörffel (Berlin-Buch); G. Henze (Berlin, Charité); E. Hilgenfeld (Berlin, Charité); U. Bode (Bonn); W. Eberl (Braunschweig); H.J. Spaar (Bremen); H. Jacobi (Celle); I. Krause (Chemnitz); J.-D. Thaben (Coburg); D. Möbius (Cottbus); W. Andler (Datteln); H. Breu (Dortmund); G. Weiβbach (Dresden, University); W. Kotte (Dresden); U. Göbel (Düsseldorf); J.D. Beck (Erlangen); W. Havers (Essen); G. Müller (Feldkirch); B. Kornhuber (Frankfurt); C. Niemeyer (Freiburg); F. Lampert (Gieβen); M. Lakomek (Göttingen); C. Urban (Graz); H. Reddemann (Greifswald); P. Exadaktylos (Halle); K. Winkler (Hamburg); H. Riehm (Hannover); K-M. Debatin (Heidelberg); N. Graf (Homburg); C. Tautz (Herdecke); F.M. Fink (Innsbruck); F. Zintl (Jena); I. Krüger (Kaiserslautern); G. Nessler (Karlsruhe); H. Wehinger (Kassel); R. Schneppenheim (Kiel); H. Meβner (Klagenfurt); M. Rister (Koblenz); F. Berthold (Köln); W. Sternschulte (Köln); C. Schulte-Wissermann (Krefeld); M. Domula (Leipzig); I. Mutz (Leoben); G. Schmitt (Linz); O. Stöllinger (Linz); L. Nobile (Locarno); P. Bucsky (Lübeck); H. Rütschle (Ludwigshafen); U. Caflisch (Luzern); U. Mittler (Magdeburg); P. Gutjahr (Mainz); O. Sauer (Mannheim); C. Eschenbach (Marburg); W. Tillmann (Minden); S. Müller-Weihrich (München, Technical University); C. Bender-Götze (München); R.J. Haas (München); P. Klose (München-Harlaching); H. Jürgens (Münster); A. Feldmann (Neunkirchen); A. Jobke (Nürnberg); U. Schwarzer (Nürnberg); G. Eggers (Rostock); R. Geib-König (Saarbrücken); H. Grienberger (Salzburg); H. Haas (Schwarzach); F.J. Göbel (Siegen); R. Poier (Steyr); J. Treuner (Stuttgart); R. Schumacher (Schwerin); A. Feldges (St. Gallen); H. Rau (Trier); D. Niethammer (Tübingen); G. Hartmann (Ulm); D. Franke (Vechta); H. Gadner (Wien, St. Anna Kinderspital); F. Haschke (Wien); J. Weber (Wiesbaden); H.P. Krohn (Wilhelmshaven); J. Otte (Wolfsburg); A. Dohrn (Wuppertal); J. Kühl (Würzburg); H.J. Plüss (Zürich).
Pathologists.
H. Mittermeyer (Aachen); B. Stamm (Aarau); R. Backmann (Augsburg); H. Ohnacker (Basel); M. Stolte (Bayreuth); H. Stein (Berlin); M. Dietel (Berlin); W. Schneider (Berlin); H.J. Födisch, (Bonn); R. Donhuisen (Braunschweig); F.K. Köβling (Bremen); J.O. Habeck (Chemnitz); P. Stosiek (Cottbus); E.W. Schwarze (Dortmund); M. Müller (Dresden); W. Hort (Düsseldorf); V. Becker (Erlangen); L.D. Leder (Essen); S. Falk (Frankfurt); H.E. Schäfer (Freiburg); W. Schultz (Gieβen); E. Kunze (Göttingen); C. Schmid (Graz); G. Lorenz (Greifswald); F.W. Rath (Halle); U. Helmchen (Hamburg); A. Georgii (Hannover); F. Otto (Heidelberg); K. Remberger (Homburg); G. Mikuz (Innsbruck); D. Katenkamp (Jena); R. Wagner (Kaiserslautern); W. Gusek (Karlsruhe); O. Klinge (Kassel); R. Parwaresch (Kiel); W. Wagner (Klagenfurt); F. deLeon (Koblenz); R. Fischer (Köln); O.M. Gokel (Krefeld); C. Wittekind (Leipzig); G. Leitner (Leoben); M. Weber (Linz); E. Pedrinis (Locarno); K. Wegener (Ludwigshafen); A. Feller (Lübeck); J.O. Grebbers (Luzern); A. Roesser (Magdeburg); W. Thoenes (Mainz); U. Bleyl (Mannheim); C. Thomas (Marburg); E. Jehn (Minden); K. Wurster (München, Technical University); U. Löhrs (München); P. Meister (München-Harlaching); M. Grundmann (Münster); P.H. Wünsch (Nürnberg); H. Nizze (Rostock); H. Mitschke (Saarbrücken); O. Dietze (Salzburg); A. Hittmair (Schwarzach); G. Wittstock (Schwerin); D. Kunde (Siegen); A. Diener (St. Gallen); J. Feichtinger (Steyr); B. Kraus-Hounder (Stuttgart); H. Mäusle (Trier); P. Kaiserling (Tübingen); O. Haferkamp (Ulm); M. Respondek (Vechta); Th. Radasckiewicz (Wien); W. Remmle (Wiesbaden); G. Fischer (Wilhelmshaven); H.K. Müller-Hermelink (Würzburg); T. Stallmach (Zürich).
Reference Laboratories.
Pathology: Lymphnode Registry of the Society of German Pathologists, Institut of Hematopathology, University of Kiel (R. Parwaresch/M. Tiemann); Institut of Pathology, University of Vienna (T. Radasckiewicz/A. Chott). Immunophenotyping: W.-D. Ludwig, Berlin; W. Knapp, Vienna.
ACKNOWLEDGMENT
The authors acknowledge the expert work of Edelgard Odenwald (cytomorphology), Ulrike Meyer, U. Regelsberger (data management), and Eckehard Schirg (central diagnostic radiology). We thank Jennifer Meyers for proofreading the English text.
Supported by the Deutsche Krebshilfe, Bonn, Grant-No.M109/91/Re1.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alfred Reiter, MD, Justice-Liebig-University, Department of Pediatric Hematology and Oncology, Feulgenstrasse 12, D-353, Giessen, Germany; e-mail:alfred.reiter@paediat.med.uni-giessen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal