We showed in a phase I trial that the maximum tolerated dose of the ProMACE-CytaBOM regimen in patients with aggressive lymphoma was 200% (Gordon et al, J Clin Oncol 14:1275, 1996). Based on these observations, we initiated a phase II trial designed to determine response, toxicity, and dose intensity using this regimen. We analyzed 74 patients with advanced-stage (III or IV) or bulky stage II aggressive lymphoma. The overall complete response rate was 69% (72% in evaluable patients). With a median follow-up of 4.5 years, the median survival has not yet been reached. The 4-year survival rate is 73% (95% confidence interval [CI] 62, 83%) and no difference was observed among International Prognostic Index (IPI) groups. The 4-year disease-free survival was 71% (95% CI 58, 84%) with no statistical difference between patients with IPI 0 to 1 versus 2 to 4. The toxicity was acceptable, though the grade 4 hematologic toxicity rate for this regimen was 100%. Grade 4 nonhematologic toxicity was 36%. Three cases of either myelodysplastic syndrome or acute leukemia occurred at 7 months, 3.4 years, and 4.2 years after registration. Cytogenic analysis was available in two cases, showing inv(16) without French American British classification (FAB) M4 EO histology in one patient and a 5q-syndrome in the other. These data suggest that 200% ProMACE-CytaBOM with either granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF results in a high complete remission rate and a disease-free survival comparable to any prior risk-based analysis in aggressive lymphoma. Before using this regimen in general practice, phase III clinical trials should be conducted.
INTENSIVE COMBINATION chemotherapy for patients with aggressive non-Hodgkin’s lymphoma results in complete response rates of between 60% to 80% with long-term disease-free survival in 30% to 40%.1 It has become clear that inherent prognostic features are better predictors of outcome than the choice of chemotherapy regimen.2-16 Because there are data to support the concept that dose intensity may be important in the treatment of cancer in general and in malignant lymphoma in particular,17-22 we intensified the dose of the ProMACE-CytaBOM regimen in a phase I dose-finding study,23and found that patients could tolerate up to 200% dose of the myelosuppressive agents with growth factor support without the need for stem cell infusion. We now report mature phase II response, toxicity, and survival data in a cohort of patients with aggressive lymphoma treated with 200% ProMACE-CytaBOM.
MATERIALS AND METHODS
Patient characteristics.
A total of 77 patients (70 in the phase II and 7 from the phase I trial treated at 200% ) were entered. There were 2 pathology exclusions and 1 patient was canceled and never received treatment, leaving 74 patients, 67 treated in the phase II portion of the study and 7 treated in the phase I portion (Table 1). Included in this group were 3 patients who were declared ineligible because they were not registered to an ancillary laboratory study. They were nevertheless treated according to protocol and were considered evaluable for analysis of toxicity, response, and survival.
Cases
| Cases entered | 77 |
| Canceled | 1 |
| Ineligible | 0* |
| Pathology Exclusion | 2 |
| Cases Eligible | 74 |
| Cases entered | 77 |
| Canceled | 1 |
| Ineligible | 0* |
| Pathology Exclusion | 2 |
| Cases Eligible | 74 |
Three cases were eligible for analysis of response, toxicity and survival, but were ineligible because of failure to register to an ancillary laboratory study.
Eligibility.
Patients were eligible if they had a tissue diagnosis of non-Hodgkin’s lymphoma of intermediate or high-grade histology other than Burkitts, non-Burkitts undifferentiated, or lymphoblastic lymphoma (Working Formulation categories D to H), had at least bulky stage II (mediastinal mass greater than 33% of chest diameter on chest radiograph or any mass greater than 10 cm), stage III or IV disease by Ann Arbor criteria, and at least one objective measurable parameter, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, and age less than 65. Patients could not have other malignancies, central nervous system lymphoma or human immunodeficiency virus (HIV)-related lymphoma, or significant organ compromise. Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Response criteria.
Complete response (CR) was defined as regression of all disease (by physical examination and radiograph). Repeat bilateral bone marrow examinations were required if the marrow was initially involved. A partial response (PR) is defined as a 50% reduction in the sum of the products of the dimensions of the measurable lesions, whereas stable disease (SD) included patients who did not satisfy the requirements for CR or PR. Progressive disease (PD) is an increase of 25% of the sum of the products of the pretreatment lesions or the appearance of new lesions. Relapse is defined as reappearance of any lymphoma in patients who have had CR, and relapse for patients with PR is defined as progression. Duration of response is measured from the documentation of response to the time of relapse or progression.
Patients who had residual computed tomography (CT) scan abnormalities were carefully evaluated. If the scans were stable and/or biopsies negative, patients were judged to have a “clinical” CR (CCR).
Treatment regimen.
The regimen and dose adjustment guidelines are described in Table 2. The growth factor used in the phase I study was granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering-Plough, Nutley, NJ), an Esherichia coli–derived nonglycosylated protein. In this trial, the GM-CSF was used in the 7 patients on the phase I portion treated at the maximum tolerated disease (MTD) (200%) and the first 39 patients treated on the phase II study (total, n = 46), whereas the remaining 28 patients received G-CSF (Amgen, Thousand Oaks, CA) when it became clear that GM-CSF was associated with more toxicity. No differences in myelotoxicity were observed, however, after the change from GM-CSF to G-CSF. Intrathecal chemotherapy was allowed but not required if the marrow was involved with large cells or if there was testicular involvement; however, no patient received intrathecal prophylaxis. Radiotherapy was not part of the protocol, but one patient had radiation to a bulky mediastinal mass.
200% ProMACE-CytaBOM
| Cyclophosphamide | 1300 mg/m2, day 1 |
| Doxorubicin* | 50 mg/m2, day 1 |
| Etoposide | 240 mg/m2, day 1 |
| Prednisone | 60 mg/m2 PO, days 1-14 |
| Cytarabine | 600 mg/m2, day 8 |
| Bleomycin | 5 mg/m2, day 8 |
| Vincristine† | 1.4 mg/m2, day 8 |
| Methotrexate | 120 mg/m2, day 8 |
| Leucovorin | 25 mg/m2 PO every 6 hours for four doses to begin 24 hours after Methotrexate |
| Plus | |
| rH-GM-CSF‡ or | 5 μg/kg/day SC days 9 to 19 |
| rH-G-CSF2-153 | 5 μg/kg/day SC days 9 to 19 |
| Cyclophosphamide | 1300 mg/m2, day 1 |
| Doxorubicin* | 50 mg/m2, day 1 |
| Etoposide | 240 mg/m2, day 1 |
| Prednisone | 60 mg/m2 PO, days 1-14 |
| Cytarabine | 600 mg/m2, day 8 |
| Bleomycin | 5 mg/m2, day 8 |
| Vincristine† | 1.4 mg/m2, day 8 |
| Methotrexate | 120 mg/m2, day 8 |
| Leucovorin | 25 mg/m2 PO every 6 hours for four doses to begin 24 hours after Methotrexate |
| Plus | |
| rH-GM-CSF‡ or | 5 μg/kg/day SC days 9 to 19 |
| rH-G-CSF2-153 | 5 μg/kg/day SC days 9 to 19 |
| Dose Modifications2-155 . | |||||
|---|---|---|---|---|---|
| ANC . | Methotrexate . | Ara-C . | Cyclo- phosphamide . | Doxo- rubicin . | VP-16 . |
| ≥1,500 | 100% | 100% | 100% | 100% | 100% |
| 1,500 to 1,999 | 100% | 100% | 100% | 100% | 100% |
| 1,000 to 1,499 | 100% | 75% | 50% | 50% | 50% |
| 500 to 999 | 100% | 25% | 25% | 25% | 25% |
| <500 | 50% | Hold | Hold | Hold | Hold |
| Platelets | Methotrexate | Ara-C | Cyclo- phosphamide | Doxo- rubicin | VP-16 |
| ≥100,000 | 100% | 100% | 100% | 100% | 100% |
| 50,000 to 99,999 | 100% | 50% | 75% | 75% | 50% |
| <50,000 | 50% | Hold | Hold | Hold | Hold |
| Dose Modifications2-155 . | |||||
|---|---|---|---|---|---|
| ANC . | Methotrexate . | Ara-C . | Cyclo- phosphamide . | Doxo- rubicin . | VP-16 . |
| ≥1,500 | 100% | 100% | 100% | 100% | 100% |
| 1,500 to 1,999 | 100% | 100% | 100% | 100% | 100% |
| 1,000 to 1,499 | 100% | 75% | 50% | 50% | 50% |
| 500 to 999 | 100% | 25% | 25% | 25% | 25% |
| <500 | 50% | Hold | Hold | Hold | Hold |
| Platelets | Methotrexate | Ara-C | Cyclo- phosphamide | Doxo- rubicin | VP-16 |
| ≥100,000 | 100% | 100% | 100% | 100% | 100% |
| 50,000 to 99,999 | 100% | 50% | 75% | 75% | 50% |
| <50,000 | 50% | Hold | Hold | Hold | Hold |
Total dose not to exceed 550 mg/m2.
Cap = 2 mg.
n = 46.
n = 28.
Modification based on ANC and platelet count on day of treatment.
Statistical methods.
Disease-free survival (DFS) was defined as the time from documentation of a CR to the date of relapse or death from any cause. If a patient did not relapse or die, DFS was censored at the last known date in remission, or if unknown, the last survival date. Overall survival was defined from the time of entry into the study to the date of death from any cause. Time to treatment failure (TTF) was defined as the interval from the date of entry on study to the date of relapse after a CR, the date of progression from PR/SD, or the date of death from any cause without documented relapse. Therefore, this analysis includes all patients, not only those in CR. If a patient in CR did not relapse, they were censored at the date last known in remission or the survival date if the date last known in remission was missing. The survival distribution for overall survival, TTF, and DFS was estimated by the method of Kaplan and Meier.24 CR rates were compared by patient characteristics using Fisher’s Exact test.25
RESULTS
Patient characteristics.
Table 3 summarizes the distribution of selected baseline patient characteristics at on-study for the 74 eligible patients. The median age was 43 years with a range of 19 to 65 years. There were 43 (58%) male patients and 67 (91%) of the patients were white. Forty-five (61%) had a performance status (PS) of 0 and 29 (39%) had a PS of 1; 43 (58%) had no B symptoms. Sixty-one (82%) had stage III/IV disease by Ann Arbor criteria.
Baseline Characteristics of Eligible Patients
| Patient Characteristic . | N . | % . |
|---|---|---|
| Age (in years) | ||
| Median | 43 | |
| Range | 19 to 65 | |
| Gender | ||
| Male | 43 | 58% |
| Female | 31 | 42% |
| Race | ||
| White | 67 | 91% |
| Black | 1 | 1% |
| Other | 6 | 8% |
| Performance Status | ||
| 0 | 45 | 61% |
| 1 | 29 | 39% |
| B-Symptoms | ||
| Yes | 29 | 40% |
| No | 43 | 60% |
| Missing | 2 | |
| Stage | ||
| II | 13 | 18% |
| III | 21 | 28% |
| IV | 40 | 54% |
| Number of Cycles | ||
| 1 to 5 | 11 | 15% |
| 6 | 39 | 53% |
| 7 | 6 | 8% |
| 8 | 18 | 24% |
| International Index | ||
| 0 to 1 | 29 | 43% |
| 2 | 29 | 43% |
| 3 | 9 | 13% |
| 4 | 1 | 2% |
| Missing | 6 | |
| Age Adjusted International Index | ||
| 0 to 1 | 37 | 54% |
| 2 | 31 | 46% |
| Missing | 6 | |
| Pathology | ||
| Follicular large cell | 10 | 14% |
| Diffuse small cleaved | 1 | 1% |
| Diffuse mixed | 6 | 8% |
| Diffuse large cell | 37 | 50% |
| Large cell immunoblastic | 17 | 23% |
| Composite | 1 | 1% |
| Other (Ki-1 anaplastic) | 2 | 3% |
| Patient Characteristic . | N . | % . |
|---|---|---|
| Age (in years) | ||
| Median | 43 | |
| Range | 19 to 65 | |
| Gender | ||
| Male | 43 | 58% |
| Female | 31 | 42% |
| Race | ||
| White | 67 | 91% |
| Black | 1 | 1% |
| Other | 6 | 8% |
| Performance Status | ||
| 0 | 45 | 61% |
| 1 | 29 | 39% |
| B-Symptoms | ||
| Yes | 29 | 40% |
| No | 43 | 60% |
| Missing | 2 | |
| Stage | ||
| II | 13 | 18% |
| III | 21 | 28% |
| IV | 40 | 54% |
| Number of Cycles | ||
| 1 to 5 | 11 | 15% |
| 6 | 39 | 53% |
| 7 | 6 | 8% |
| 8 | 18 | 24% |
| International Index | ||
| 0 to 1 | 29 | 43% |
| 2 | 29 | 43% |
| 3 | 9 | 13% |
| 4 | 1 | 2% |
| Missing | 6 | |
| Age Adjusted International Index | ||
| 0 to 1 | 37 | 54% |
| 2 | 31 | 46% |
| Missing | 6 | |
| Pathology | ||
| Follicular large cell | 10 | 14% |
| Diffuse small cleaved | 1 | 1% |
| Diffuse mixed | 6 | 8% |
| Diffuse large cell | 37 | 50% |
| Large cell immunoblastic | 17 | 23% |
| Composite | 1 | 1% |
| Other (Ki-1 anaplastic) | 2 | 3% |
The International Prognostic Index (IPI) defines five pretreatment characteristics including age (≤60 v >60), Ann Arbor stage (I/II v III/IV), number of extranodal sites (0 to 1 v>1), PS (0 to 1 v >2) and serum lactic dehydrogenase (LDH) ( normal vs > normal). The age-adjusted index defines three pretreatment characteristics, excluding age and extranodal sites, so that LDH, Ann Arbor stage, and performance status are the predictors of outcome.26 Patients were analyzed both by index and by age-adjusted index. Of the 68 eligible patients with data (6 were missing LDH values), 29 (43%) had an IPI of 0 to 1, and 39 (57%) had an IPI of 2 to 4, whereas 37 (54%) had an age-adjusted IPI of 0-1, and 31 (46%) had an age-adjusted IPI of 2 to 3. Only 1 patient had an IPI score of 4 and no patients had an age-adjusted IPI score of 3. Median follow-up is 4.5 years.
Number of cycles.
A total of 461 cycles of therapy were administered to 74 eligible patients. The median number of cycles was 6 (range, 1 to 8 cycles). Eleven patients (15%) received less than the protocol-specified minimum of 6 cycles: 1 patient received only 1 cycle, 2 received 3 cycles, 6 received 4 cycles, and 2 received 5 cycles. Reasons for early treatment termination include excessive complications/toxicity (8 cases) and patient withdrawal/refusal (3 cases, all received 4 cycles).
Dose intensity.
Dose intensity was calculated as the dose received/m2 body surface area (BSA) for each cycle. The normalized dose intensity is defined as the actual dose/intended dose, and serves as a measure of the ability to deliver the regimen as designed. These data are summarized in Table 4. For the most part, the escalated doses of cyclophosphamide, etoposide, and doxorubicin could be administered with minimal cycle delays and at close to 95% normalized dose intensity for up to cycle 8. However, the dose of the day-8 drug, cytosine arabinoside, could be maintained at about 95% dose intensity for 6 cycles, but had to be reduced to 82% by cycle 7 and 50% by cycle 8.
Normalized Dose Intensity4-150 (Number of Patients)
| . | Cycle 1 . | Cycle 2 . | Cycle 3 . | Cycle 4 . | Cycle 5 . | Cycle 6 . | Cycle 7 . | Cycle 8 . |
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | 100.0 (74) | 100.0 (71) | 100.0 (72) | 100.0 (68) | 100.0 (60) | 100.0 (56) | 97.2 (23) | 96.3 (19) |
| Doxorubicin | 100.0 (74) | 100.0 (71) | 100.0 (72) | 100.0 (69) | 100.0 (62) | 100.0 (57) | 98.5 (23) | 96.3 (19) |
| VP-16 | 100.0 (73) | 100.0 (71) | 99.9 (71) | 99.9 (68) | 99.9 (52) | 99.9 (59) | 98.5 (23) | 96.3 (19) |
| Ara-C | 100.0 (71) | 98.5 (61) | 97.8 (68) | 99.7 (58) | 99.4 (56) | 96.6 (54) | 82.1 (20) | 50.3 (14) |
| Bleomycin | 100.0 (74) | 100.0 (70) | 100.0 (70) | 100.0 (63) | 100.0 (61) | 100.0 (56) | 100.0 (23) | 100.0 (18) |
| Vincristine4-151 | 100.0 (74) | 100.0 (70) | 100.0 (65) | 100.0 (59) | 100.0 (59) | 100.0 (53) | 100.0 (23) | 100.0 (18) |
| Methotrexate | 99.6 (72) | 98.5 (69) | 99.2 (69) | 99.2 (64) | 99.1 (62) | 99.2 (58) | 99.5 (24) | 96.8 (19) |
| Cycle duration (days)‡ | 22 | 22 | 23 | 22 | 27 | 22 | 22 | 21 |
| . | Cycle 1 . | Cycle 2 . | Cycle 3 . | Cycle 4 . | Cycle 5 . | Cycle 6 . | Cycle 7 . | Cycle 8 . |
|---|---|---|---|---|---|---|---|---|
| Cyclophosphamide | 100.0 (74) | 100.0 (71) | 100.0 (72) | 100.0 (68) | 100.0 (60) | 100.0 (56) | 97.2 (23) | 96.3 (19) |
| Doxorubicin | 100.0 (74) | 100.0 (71) | 100.0 (72) | 100.0 (69) | 100.0 (62) | 100.0 (57) | 98.5 (23) | 96.3 (19) |
| VP-16 | 100.0 (73) | 100.0 (71) | 99.9 (71) | 99.9 (68) | 99.9 (52) | 99.9 (59) | 98.5 (23) | 96.3 (19) |
| Ara-C | 100.0 (71) | 98.5 (61) | 97.8 (68) | 99.7 (58) | 99.4 (56) | 96.6 (54) | 82.1 (20) | 50.3 (14) |
| Bleomycin | 100.0 (74) | 100.0 (70) | 100.0 (70) | 100.0 (63) | 100.0 (61) | 100.0 (56) | 100.0 (23) | 100.0 (18) |
| Vincristine4-151 | 100.0 (74) | 100.0 (70) | 100.0 (65) | 100.0 (59) | 100.0 (59) | 100.0 (53) | 100.0 (23) | 100.0 (18) |
| Methotrexate | 99.6 (72) | 98.5 (69) | 99.2 (69) | 99.2 (64) | 99.1 (62) | 99.2 (58) | 99.5 (24) | 96.8 (19) |
| Cycle duration (days)‡ | 22 | 22 | 23 | 22 | 27 | 22 | 22 | 21 |
Dose Intensity is expressed as a percentage of the designed dose. The figures above represent median % dose.
VCR had a dose cap of 2 mg. Therefore, the normalized dose intensity for those patients receiving 2 mg (with a BSA >1.43) was set to 100%.
Median cycle duration.
Toxicity.
Table 5 summarizes the acute toxicity associated with the phase II portion of the study. Toxicity information is available on 76 patients (including the 2 pathology exclusions). Seventy-four (97%) of the patients had grade 4 leukopenia, 37 (49%) experienced grade 4 thrombocytopenia, and 63 (83%) had grade 4 granulocytopenia. The nonhematologic grade 4 toxicity included 16 (21%) cases of anemia, 7 (9%) cases of infection, 3 (4%) cases of liver toxicity, and 1 (1%) case each of pulmonary embolus, allergy, and neuromotor toxicity. The grade 4 hematologic toxicity rate for the ProMACE-CytaBOM regimen was 100%. The grade 4 nonhematologic toxicity rate was 36% (27 of the 76 patients had at least 1 grade 4 nonhematologic toxicity) with an exact 95% confidence interval (CI) of (25%, 47%). No toxic deaths occurred.
Incidence of Acute Toxicity5-150 (N = 76)
| Type . | Grade . | ||
|---|---|---|---|
| 1, 2 . | 3 . | 4 . | |
| Leukopenia | — | 2 | 74 |
| Thrombocytopenia | 18 | 18 | 37 |
| Anemia | 17 | 36 | 16 |
| Hemorrhage | 11 | 1 | — |
| Infection | 35 | 9 | 7 |
| Fever (no infection) | 47 | 3 | — |
| GU | 21 | 1 | — |
| Nausea | 45 | 5 | — |
| Vomiting | 40 | 2 | — |
| Diarrhea | 35 | 1 | — |
| Stomatitis | 37 | 9 | — |
| Liver | 46 | 4 | 3 |
| Pulmonary | 25 | 4 | 1 |
| Cardiac | 15 | 2 | — |
| Hypertension | 4 | — | — |
| Hypotension | 12 | 2 | — |
| Skin | 25 | 1 | — |
| Allergy | 6 | 2 | 1 |
| Phlebitis | 6 | 3 | — |
| Local (no phlebitis) | 5 | — | — |
| Alopecia | 57 | — | — |
| Weight gain | 10 | 2 | — |
| Weight loss | 30 | 1 | — |
| Neurosensory | 33 | 1 | — |
| Neuromotor | 33 | 3 | 1 |
| Neuropsych | 14 | 2 | — |
| Neuroclinical | 27 | 5 | 1 |
| Metabolic | 38 | 4 | — |
| Coagulation | 2 | — | — |
| Worst degree (nonhematologic) | 9 | 40 | 27 |
| Type . | Grade . | ||
|---|---|---|---|
| 1, 2 . | 3 . | 4 . | |
| Leukopenia | — | 2 | 74 |
| Thrombocytopenia | 18 | 18 | 37 |
| Anemia | 17 | 36 | 16 |
| Hemorrhage | 11 | 1 | — |
| Infection | 35 | 9 | 7 |
| Fever (no infection) | 47 | 3 | — |
| GU | 21 | 1 | — |
| Nausea | 45 | 5 | — |
| Vomiting | 40 | 2 | — |
| Diarrhea | 35 | 1 | — |
| Stomatitis | 37 | 9 | — |
| Liver | 46 | 4 | 3 |
| Pulmonary | 25 | 4 | 1 |
| Cardiac | 15 | 2 | — |
| Hypertension | 4 | — | — |
| Hypotension | 12 | 2 | — |
| Skin | 25 | 1 | — |
| Allergy | 6 | 2 | 1 |
| Phlebitis | 6 | 3 | — |
| Local (no phlebitis) | 5 | — | — |
| Alopecia | 57 | — | — |
| Weight gain | 10 | 2 | — |
| Weight loss | 30 | 1 | — |
| Neurosensory | 33 | 1 | — |
| Neuromotor | 33 | 3 | 1 |
| Neuropsych | 14 | 2 | — |
| Neuroclinical | 27 | 5 | 1 |
| Metabolic | 38 | 4 | — |
| Coagulation | 2 | — | — |
| Worst degree (nonhematologic) | 9 | 40 | 27 |
There was 1 grade-4 pulmonary embolus, 1 grade-4 pancreatitis, and 63 grade-4 granulocytopenia.
Late effects.
Three patients later developed myelodysplastic syndrome (MDS) or acute leukemia at 7 months, 3.4 years, and 4.2 years after registration on study. The patient who developed acute nonlymphocytic leukemia (ANLL) at 7 months had achieved PR of lymphoma, and died of leukemia (no cytogenetics were available). The other two cases were in CR and are still alive. The patient who developed ANLL at 3.4 years had inv(16) without FAB M4 EO histology and the patient with MDS at 4.2 years had the 5q- abnormality. The incidence of either MDS or acute leukemia is therefore 4.1 %, with 90% CI (1.1, 10.2). Three cases (incidence of 4.1%, 90% CI 1.1, 10.2) of aseptic necrosis of the femoral head also occurred.
Response.
The objective response is summarized in Table 6. There were 51 CR/CCR (69%), 17 PR (23%), 2 SD (3%), 1 PD (1%), and 3 (4%) with missing or unevaluable response. The CR/CCR rate among evaluable cases was therefore 72% with a 95% CI (60, 82%).
Objective Response
Including six cases who were considered clinical CR, and had either stable residual mass on CT or rebiopsy was negative for tumor.
CR rate in evaluable patients is 72%.
Table 7 summarizes the CR rate by selected baseline patient characteristics. There were slightly higher CR rates observed for patients who were younger, had lower anatomic stage and lower IPI scores, but none of these differences were statistically significant.
CR Rate by Patient Characteristics
| Patient Characteristics . | N . | % . |
|---|---|---|
| Age | ||
| <55 (n = 60) | 42 | 70% |
| ≥55 (n = 14) | 9 | 64% |
| Sex | ||
| Male (n = 43) | 28 | 65% |
| Female (n = 31) | 23 | 74% |
| Race | ||
| White (n = 67) | 46 | 69% |
| Other (n = 7) | 5 | 71% |
| Performance status | ||
| 0 (n = 45) | 32 | 71% |
| 1 (n = 29) | 19 | 66% |
| B-symptoms | ||
| Yes (n = 29) | 18 | 62% |
| No (n = 43) | 32 | 74% |
| Missing (n = 2) | 1 | |
| Stage | ||
| II (n = 13) | 8 | 62% |
| III/IV (n = 61) | 43 | 70% |
| International index | ||
| 0-1 (n = 29) | 20 | 69% |
| 2-4 (n = 39) | 26 | 67% |
| Missing (n = 6) | 5 | |
| Age adjusted international index | ||
| 0-1 (n = 37) | 25 | 68% |
| 2 (n = 31) | 21 | 68% |
| Missing (n = 6) | 5 | |
| Pathology | ||
| Follicular large cell (n = 10) | 7 | 70% |
| Diffuse small cleaved (n = 1) | 0 | 0% |
| Diffuse mixed (n = 6) | 4 | 67% |
| Diffuse large cell (n = 37) | 25 | 68% |
| Large cell immunoblastic (n = 17) | 13 | 76% |
| Composite (n = 1) | 1 | 100% |
| Other (Ki-1 anaplastic) (n = 2) | 1 | 50% |
| Patient Characteristics . | N . | % . |
|---|---|---|
| Age | ||
| <55 (n = 60) | 42 | 70% |
| ≥55 (n = 14) | 9 | 64% |
| Sex | ||
| Male (n = 43) | 28 | 65% |
| Female (n = 31) | 23 | 74% |
| Race | ||
| White (n = 67) | 46 | 69% |
| Other (n = 7) | 5 | 71% |
| Performance status | ||
| 0 (n = 45) | 32 | 71% |
| 1 (n = 29) | 19 | 66% |
| B-symptoms | ||
| Yes (n = 29) | 18 | 62% |
| No (n = 43) | 32 | 74% |
| Missing (n = 2) | 1 | |
| Stage | ||
| II (n = 13) | 8 | 62% |
| III/IV (n = 61) | 43 | 70% |
| International index | ||
| 0-1 (n = 29) | 20 | 69% |
| 2-4 (n = 39) | 26 | 67% |
| Missing (n = 6) | 5 | |
| Age adjusted international index | ||
| 0-1 (n = 37) | 25 | 68% |
| 2 (n = 31) | 21 | 68% |
| Missing (n = 6) | 5 | |
| Pathology | ||
| Follicular large cell (n = 10) | 7 | 70% |
| Diffuse small cleaved (n = 1) | 0 | 0% |
| Diffuse mixed (n = 6) | 4 | 67% |
| Diffuse large cell (n = 37) | 25 | 68% |
| Large cell immunoblastic (n = 17) | 13 | 76% |
| Composite (n = 1) | 1 | 100% |
| Other (Ki-1 anaplastic) (n = 2) | 1 | 50% |
Survival.
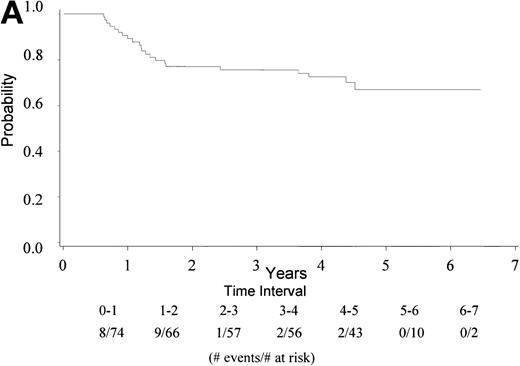
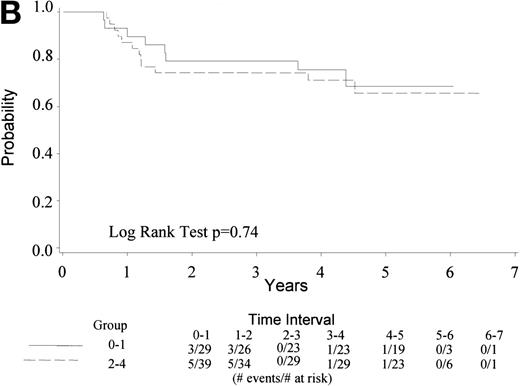
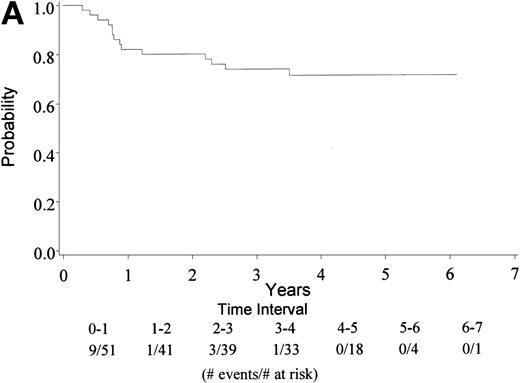
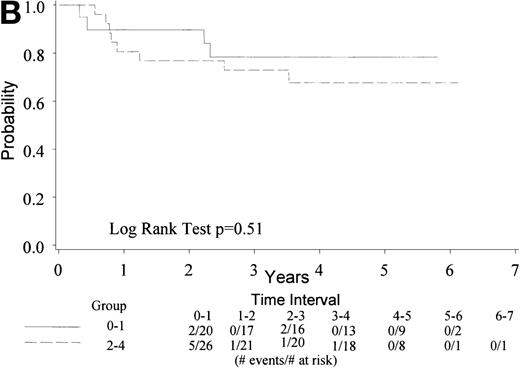
With a median follow-up of 4.5 years, 22 of 74 eligible patients have died (30%). The median survival has not yet been reached (range, 0.6 to 6.5 + years). The 4-year survival rate is 73% with a 95% CI (62, 83%). Figure 1A gives the Kaplan-Meier estimate of the survival distribution for the overall group. Figure 1B depicts the overall survival by IPI groupings. There was no significant difference in survival among patients with an IPI of 0 to 1 versus 2 to 4 (only 1 patient had an IPI score of 4), though the sample size for each group was small. Analysis of prognostic factors by the age-adjusted IPI groups (0 to 1 v 2) also showed no differences in survival (data not shown).
(A) Kaplan Meier estimate of the overall survival for all 74 patients is shown here. (B) The same analysis depicting overall survival by IPI groupings 0 to 1 and 2 to 4. IPI low (0 to 1), low intermediate (2), high intermediate (3), and high (4). Only 1 patient had an IPI score of 4.
(A) Kaplan Meier estimate of the overall survival for all 74 patients is shown here. (B) The same analysis depicting overall survival by IPI groupings 0 to 1 and 2 to 4. IPI low (0 to 1), low intermediate (2), high intermediate (3), and high (4). Only 1 patient had an IPI score of 4.
TTF.
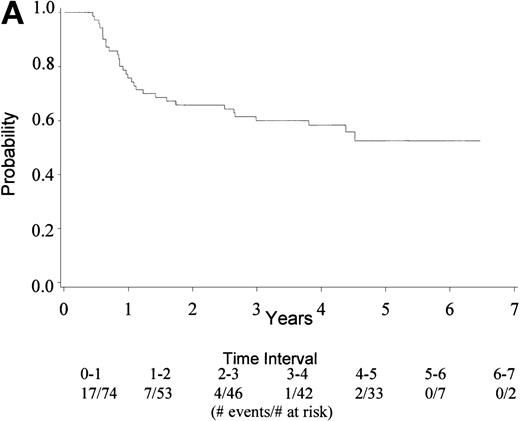
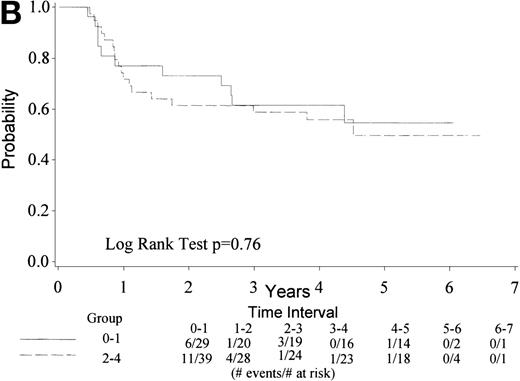
Median TTF for the 74 eligible patients has not yet been reached; 26 have died/relapsed/progressed (35%) at a median follow-up of 4.5 years. TTF ranges from 0.3+ to 6.5+ years. Figure 2A gives the Kaplan-Meier estimate of the survival distribution for TTF. The 4-year TTF is 58% with a 95% CI (47, 70%). Figure 2B shows similar data by IPI groupings, showing no difference by IPI score. There is no statistical difference between patients with age-adjusted IPI groups 0 to 1 versus 2 (data not shown).
(A) Kaplan Meier estimate of the survival distribution for TTF, showing a 4-year TTF of 58% with 95% confidence interval (47, 70%). (B) The same analysis depicting TTF by IPI groupings 0 to 1 and 2 to 4. IPI low (0 to 1), low intermediate (2), high intermediate (3), and high (4).
(A) Kaplan Meier estimate of the survival distribution for TTF, showing a 4-year TTF of 58% with 95% confidence interval (47, 70%). (B) The same analysis depicting TTF by IPI groupings 0 to 1 and 2 to 4. IPI low (0 to 1), low intermediate (2), high intermediate (3), and high (4).
DFS.
Median DFS for the 51 patients who achieved CR has not yet been reached. Of these 51 cases, 14 have died or relapsed (27%). The DFS ranges from 0.3 to 6.1 + years. The 4-year DFS rate is 71% with 95% CI (58, 84%). Figure 3A depicts the Kaplan-Meier estimates of the survival distributions for DFS and Fig 3B shows similar data by IPI score. There were no differences in DFS by IPI groups or age-adjusted IPI groups (data not shown).
(A) Kaplan Meier estimate of DFS for the 51 patients who achieved CR showing a 4-year DFS of 71% with 95% CI (58, 84%). (B) The same analysis depicting DFS by IPI groupings 0 to1 and 2 to 4. IPI low (0 to1), low intermediate (2), high intermediate (3), and high (4).
(A) Kaplan Meier estimate of DFS for the 51 patients who achieved CR showing a 4-year DFS of 71% with 95% CI (58, 84%). (B) The same analysis depicting DFS by IPI groupings 0 to1 and 2 to 4. IPI low (0 to1), low intermediate (2), high intermediate (3), and high (4).
DISCUSSION
The treatment of aggressive lymphomas has not changed dramatically over the past 20 years despite the use of aggressive combination chemotherapy, better support with antibiotics, and more precise staging. Although single institution studies have suggested improvement in CR rates and DFS, randomized trials balancing prognostic features have failed to validate these smaller trials of third generation chemotherapy regimens.6 10
Studies conducted by ECOG in conjunction with Cancer and Acute Leukemia Group B (CALGB) showed that there were no significant differences in response or survival when cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) was compared with methotrexate, bleomycin, cyclophosphamide, doxorubicin, vincristine, and dexamethasone (m-BACOD), but there was more toxicity associated with the m-BACOD regimen.10 Further, it was found that doses of some active drugs (cyclophosphamide and doxorubicin) had to be reduced to allow for the addition of other drugs at midcycle, perhaps obscuring any potential benefit that might have accrued from the newer regimen. Similarly, Fisher et al7 found that CHOP resulted in equivalent response and survival when compared with three other regimens in patients with aggressive lymphomas. Additional data show there is no evidence that there is a “tail” in survival curves in the more intensively treated groups, nor do patients who are “sicker” benefit from more intensive therapy.6 27
In an attempt to improve response and disease-free survival, a series of studies using non-cross–resistant drugs was developed.28 One approach was to use non-cross–resistant chemotherapy administered midcycle to prevent the emergence of drug resistance. An alternate idea was to exploit the dose-response relationship in malignant lymphoma. Some studies suggested improved outcome with higher doses,17-20,29-32 but this impression remains controversial.22 33-35
This trial was initiated for a high-risk group of patients for whom standard CHOP therapy was thought to be inadequate. Thus, patients were required to have advanced (Ann Arbor stage III or IV or bulky stage II) disease. Indeed, 84% of patients had either stage III or IV disease. Because of the concern about toxicity in the higher-dose regimen, patients greater than 65 years or patients with poor performance status were excluded. Thus, when we analyzed our data by the IPI criteria (not available when this trial was designed), we found that 43% were in index groups 0 to 1, and 57% were in the higher-risk groups 2 to 4. By age-adjusted index, 54% were in groups 0 to 1, and 46% were in group 2. Only one patient had an IPI score of 4 and no patients had an age-adjusted IPI score of 3. These data, then, do not apply to patients with lymphoma in the highest risk groups by the IPI.
At 4 years, the overall survival was 73% and TTF was 58%, with no differences between the index groups. Importantly, the overall 4-year DFS rate was 71% and was greater than 60% for the index groups 2 to 4, with no significant differences between the lower- and the higher-risk groups. These data compare favorably to any prior risk-based analysis in aggressive lymphoma.13,26,31,36 The 5-year relapse-free survival for index group 2 to 4 in a large retrospective analysis reported by Shipp26 was 40% to 50%, and 5-year survival was between 26% to 51%. The 5-year disease-free survival for age-adjusted index group 1 was 66% and for group 2 was 53%, and 5-year survival was 69% and 46% respectively.26 These data cannot be compared with those reported in the current study nor across any study, but such comparisons provide a framework to help determine which randomized phase III trials need to be pursued.
The toxicity of this regimen was acceptable. We found that the day-1 drugs were administered at nearly 100% dose intensity, whereas the day-8 drug cytosine arabinoside had to be modified according to the dose-adjustment criteria specified in the protocol. Nevertheless, there were no grade 5 (fatal) toxicities in any of the patients treated both in the phase I and the phase II protocols.
There was 1 case of myelodysplastic syndrome and there were 2 cases of acute leukemia in the 74 patients available for analysis, with an incidence of 4.1% for the 74 patients. One of the cases of leukemia occurred as early as 7 months after the treatment for lymphoma, and although no cytogenetics were done, this clinical syndrome is consistent with treatment-related leukemia with 11q23 rearrangement.37 The recent demonstration of secondary leukemias arising either after alkylating agent exposure38or developing with specific cytogenetic abnormalities shortly after exposure to topoisomerase inhibitors and growth factors37raises concerns about the routine use of high-dose therapy and growth factors. If the incidence of secondary leukemias proves to be high, then clearly any superiority in CR rates and DFS rates in this and in any other dose-intense regimen, including stem-cell transplantation,39 would have to balance morbidity and mortality that might increase with these important late effects.
These data suggest that this regimen of 200% ProMACE-CytaBOM with growth factor is tolerated without grade 5 toxicity, and that the results of the phase II study compare favorably with other phase II trials in aggressive lymphomas. We found that even in the index risk groups 2 to 4, the survival and disease-free survival compare favorably to other regimens. Phase III randomized trials are needed to determine whether this approach to the treatment of aggressive lymphomas will prove superior to CHOP.
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service grants CA 17145, CA 23318, CA 13650, CA 15488, and CA 21115 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Leo I. Gordon, MD, Northwestern University Medical School, Department of Medicine, Chief, Division of Hematology/Oncology and Associate Director for Clinical Sciences, Robert H. Lurie Comprehensive Cancer Center, 303 E. Chicago Ave, Chicago, IL 60611-3008.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal