The outcome of patients with non-Hodgkin’s lymphoma has been improved by current approaches to treatment. Nevertheless, many patients either do not have a complete remission or ultimately relapse. To identify such patients, it is important to be able to predict the outcome. We previously found that the differentiation inhibitory factor/nm23 was correlated with the prognosis of acute myeloid leukemia. To examine the prognostic effect of nm23 on non-Hodgkin’s lymphoma, we established an enzyme-linked immunosorbent assay procedure to determine nm23-H1 protein levels in plasma and assessed the association of this protein level with the response to chemotherapy, overall survival, and progression-free survival in patients with aggressive non-Hodgkin’s lymphoma. The plasma concentration of nm23-H1 was significantly higher in patients with malignant lymphoma than in normal controls, especially in aggressive non-Hodgkin’s lymphoma. The complete remission rate in patients with higher nm23-H1 levels was significantly worse than that in patients with lower nm23-H1 levels. Overall survival and progression-free survival were also lower in patients with higher nm23-H1 levels than in those with lower levels. The 3-year survival rates in patients with low and high nm23-H1levels were 79.5% and 6.7% (P = .0001). A multivariate analysis of prognostic factors showed that the plasma nm23-H1level was independently associated with the survival and progression-free survival. An elevated plasma nm23-H1concentration predicts a poor outcome of advanced non-Hodgkin’s lymphoma. Therefore, nm23-H1 in plasma may be useful for identifying a distinct group of patients at very high risk.
WE FOUND THAT A differentiation inhibitory factor purified from conditioned medium of a differentiation-resistant mouse myeloid leukemia cell line was identical to the nm23 protein.1-7nm23proteins are involved in tumor metastasis regulation and have nucleoside diphosphate kinase enzyme activity that catalyzes the ATP-dependent synthesis of nucleoside diphosphates.8,9 In humans, 5 nm23 isotypes (nm23-H1, nm23-H2, DR-nm23, nm23-H4, and nm23-H5) have been identified to date.8-13 Among them, nm23-H1 and nm23-H2show 88% amino acid sequence homology, and their genes are located on the same region of chromosome 17q21 in tandem.10,14-17 An inverse relationship between metastatic potential and the level ofnm23-H1 expression has been well established in various cancers.9nm23 homologs have been reported to be involved in various cellular processes, such as stimulating transcription, cell differentiation and proliferation, and apoptosis.5,7,11,18 In addition, a serine/threonine-specific protein phosphotransferase activity and a histidine protein kinase activity have also been detected.19,20 The nm23 protein inhibits the differentiation of murine and human myeloid leukemia cells, although the mechanism is unknown.1,2,21 The inhibition of differentiation may be associated with the aggressive behavior of leukemia. To clarify the role of nm23 in human myeloid leukemia, we investigated the relative levels of nm23 mRNA in bone marrow and blood samples from patients with acute myeloid leukemia (AML) using the reverse transcriptase-polymerase chain reaction. We reported that the mRNA expression levels of nm23 in bone marrow and blood samples from AML patients were significantly higher than those in normal blood cells, and a higher level of nm23-H1expression was correlated with a poor prognosis in AML patients.3-5 An analysis of the correlation betweennm23 expression and clinical parameters demonstrated that increased nm23-H1 mRNA levels were associated with resistance to initial chemotherapy and reduced overall survival. A multivariate analysis of putative prognostic factors showed that elevatednm23-H1 mRNA levels significantly influenced the prognosis of patients with AML.6 Furthermore, expression ofnm23-H1 mRNA was significantly higher in other hematological neoplasms, including acute lymphoblastic leukemia, myelodysplastic syndrome, and chronic myeloid leukemia in blastic crisis, than in normal blood cells.6 It has been shown using immunohistochemical analyses that high-grade non-Hodgkin’s lymphoma exhibits significantly higher levels of nm23-H1 expression than does low-grade non-Hodgkin’s lymphoma.22 Therefore,nm23-H1 may have prognostic value in hematological malignancies.
Advances in combination chemotherapy and supportive therapy have made a long-term survival rate of 60% to 70% possible for patients with non-Hodgkin’s lymphoma.23-25 The choice of treatment for this lymphoma has become increasingly broad and now ranges from first-generation to third-generation chemotherapy and further to extremely potent therapy involving stem cell transplantation. Selection of the appropriate therapy not only involves a consideration of the complete remission (CR) rate, but also treating each patient neither too much nor too little, so that the quality of life can be maintained at a high level while the disease is cured. If intractable non-Hodgkin’s lymphoma could be identified at diagnosis, this information would be useful for planning the best therapeutic strategy. Therefore, effective prognostic factors are needed to select the appropriate therapy. Many prognostic variables for predicting the outcome have been identified, and prognostic models based on various factors have been evaluated. Among them, the international prognostic index (IPI) reported by Shipp in 199326 has been accepted in many countries as a prognostic model that predicts fairly well the clinical outcome for intermediate- and high-grade non-Hodgkin’s lymphoma. The IPI model is based on 5 independent prognostic factors (age, performance status, number of extranodal sites, Ann Arbor stage, and serum lactate dehydrogenase concentration) and can identify patients with non-Hodgkin’s lymphoma in 4 different risk groups (ie, low [L], low-intermediate [L-I], high-intermediate [H-I], and high [H] risk). In an attempt to use the IPI model for treatment selection, peripheral blood stem cell transplantation has been administered early in the treatment of the H-I and H risk groups to improve the outcome of non-Hodgkin’s lymphoma. However, more accurate prognostic factors are required, because some patients in the L and L-I risk groups have a poor outcome, whereas some in the H-I and H risk groups have excellent results. Because prognostic factors need to be assessed before treatment to design a therapeutic strategy, it is essential that the tests should be easy to perform and provide results promptly.
With regard to the prognostic value of nm23-H1in malignant lymphoma, we tried to use a quantitative assay system to detectnm23 protein in the plasma of patients, because it was difficult to obtain lymphoma cells without contamination by normal cells. nm23 proteins are basically intracellular proteins, but these are sometimes found in the conditioned medium of certain tumor cells, although the secretion mechanism is unclear. We established a method to determine the nm23-H1 protein in plasma of patients with malignant lymphomas by enzyme-linked immunosorbent assay (ELISA). The plasma level of nm23-H1 was elevated in patients with aggressive non-Hodgkin’s lymphoma, and the plasma level was significantly correlated with the clinical outcome, suggesting thatnm23-H1 may be a valuable prognostic factor for predicting the outcome in aggressive non-Hodgkin’s lymphoma.
MATERIALS AND METHODS
Patients.
nm23-H1 was measured in 201 consecutive untreated patients who were diagnosed and treated at the First Department of Internal Medicine, Toho University School of Medicine (Tokyo, Japan) from 1987 to 1996. Of these 201 patients, 184 had non-Hodgkin’s lymphoma. Thirty-five patients had low-grade, 147 had intermediate-grade, and 2 had high-grade lymphoma according to the Working Formulation scheme.27 Nine of the remaining cases had Hodgkin’s disease, and 8 had adult T-cell leukemia/lymphoma. Clinical staging was performed according to the Ann Arbor classification system.28 Evaluation included a complete history and physical examination: chest roentgenography; bone marrow aspiration and biopsy; computed tomography of the chest, abdomen, and pelvis; hemogram and differential counts; and routine biochemical tests. One hundred eighty patients were treated with combination chemotherapy. Patients with intermediate- or high-grade lymphoma and disseminated disease were treated with cyclophosphamide, vincristine, prednisone, bleomycin, doxorubicin, and procarbazine (COP-BLAM)29 or with biweekly COP-BLAM with granulocyte colony-stimulating factor.30Low-grade lymphoma was treated with cyclophosphamide, vincristine, and prednisone (COP) or COP-BLAM. In addition to chemotherapy, 74 patients received megavoltage radiotherapy. All patients were followed-up at intervals of a few months at the department. Re-evaluation included physical examination, hemogram and differential counts, biochemical tests, and computed tomography of the chest, abdomen, and pelvis. The median follow-up time was 64 months (range, 24 to 120 months). Of the 149 patients with aggressive (intermediate- or high-grade) non-Hodgkin’s lymphoma, 109 were alive after follow-up for 88 to 120 months (median, 68 months). The other 40 patients died from 1 to 34 months (median, 12 months) after diagnosis.
Plasma samples from 21 healthy volunteers with a mean age of 34 years (range, 24 to 52 years) were analyzed for comparison. Samples were collected if the controls had not had fever within 1 week, were not receiving any medications, were not known to be pregnant, and did not have a history of any chronic or acute illnesses.
Venous blood samples.
Peripheral venous blood samples were collected into sterile test tubes with heparin and placed on ice for at least 10 minutes to avoid platelet activation. The samples were centrifuged at 2,000g for 15 minutes at 4°C, filtered through a 0.22-μm microfilter (Millipore, Molsheim, France), and stored at −80°C.
ELISA for human nm23-H1.
Ninety-six–well plates (Corning 25805-96; Corning, Corning, NY) were coated with 50 μL of 2.5 μg/mL of monoclonal anti–nm23-H1 antibody (Seikagaku Co, Tokyo, Japan) in 50 mmol/L bicarbonate buffer (pH 9.6) overnight, washed 4 times with phosphate-buffered saline (PBS), and incubated with 200 μL of 25% Block Ace (Dainihon Seiyaku, Oosaka, Japan) for 1 hour. Plasma samples were diluted 2-fold with PBS, and then 50 μL aliquots were added to the wells. After incubation at room temperature for 1 hour, the wells were washed 4 times with PBS containing 0.05% Tween 20 (T-PBS). The samples were then incubated with polyclonal anti-nm23-H1antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), washed 4 times with T-PBS, and incubated again with a 1:500 dilution of alkaline phosphatase-conjugated antirabbit IgG (Bio-Rad Lab, Richmond, CA) for 1 hour. After washing 4 times with T-PBS, alkaline phosphatase activity was detected using diethanolamine as a substrate and an alkaline phosphatase detection kit (Bio-Rad Lab). The absorbance was measured at 405 to 415 nm with a correction wavelength of 620 to 630 nm using a microplate-ELISA reader. Recombinant nm23-H1-GST protein (kindly provided by Prof H. Shiku, Nagasaki University, Nagasaki, Japan) was used as the standard.31 32
Figure 1A shows the standard curve fornm23-H1 protein in this assay, which specifically detectsnm23-H1 protein but not nm23-H2 protein, which has 88% amino acid sequence identity to nm23-H1. We first examined thenm23-H1 levels in the 2-fold–diluted plasma; next, for samples exhibiting higher levels outside of the linear range, a number of dilutions (2-, 4-, 8-, 16-, and 32-fold) were performed. We obtained similar results when we used serum instead of plasma (Fig 1B). Thenm23-H1 protein levels of all the normal plasma samples used (n = 21) were lower than 10 ng/mL, whereas those of the aggressive lymphoma varied (Fig 1C). Nucleoside diphosphate kinase activity, one of the activities of nm23 protein, could be detected in human plasma, probably due to a limited in vivo lysis of red blood cells.33 Therefore, to exclude the possible effect of hemolysis, we measured the contents of free plasma hemoglobin as a marker of hemolysis. Free plasma hemoglobin was determined according to the method described.34 The levels of free plasma hemoglobin in all of the plasma samples used were less than 120 μg/mL (Fig 1C). We examined the relation between levels of nm23-H1protein and free hemoglobin in human red blood cell extracts. Although a significant level of nm23-H1 protein was detected at a higher concentration of free hemoglobin/red blood cell extract, no significant levels of nm23-H1 protein were detected at concentrations lower than 120 μg/mL (Fig 1C). Soluble interleukin-2 (IL-2) receptor was measured with a sandwich ELISA as described in the literature.35 Soluble CD44 was measured with a sandwich ELISA using the sCD44std ELISA kit (Bender MedSystems, Vienna, Austria).
ELISA for human nm23-H1 protein in plasma. (A) Demonstration of the quantitative nature of this system. The linearity of the quantitation of nm23-H1 protein, but not nm23-H2protein, was determined using recombinant nm23 fusion protein. (B) Detection of nm23-H1 protein in human plasma/serum diluted with PBS. (C) Plasma nm23-H1 levels and free hemoglobin levels in 149 aggressive lymphomas (▩), 21 healthy controls (•), and blood red cell extracts with various amounts of free hemoglobin (▵).
ELISA for human nm23-H1 protein in plasma. (A) Demonstration of the quantitative nature of this system. The linearity of the quantitation of nm23-H1 protein, but not nm23-H2protein, was determined using recombinant nm23 fusion protein. (B) Detection of nm23-H1 protein in human plasma/serum diluted with PBS. (C) Plasma nm23-H1 levels and free hemoglobin levels in 149 aggressive lymphomas (▩), 21 healthy controls (•), and blood red cell extracts with various amounts of free hemoglobin (▵).
Statistical analysis.
The response to treatment was documented on completion of chemotherapy. CR was defined as the absence of detectable disease based on clinical, radiologic, and histologic criteria. Partial remission (PR) required a greater than 50% reduction of tumor volume. The duration of CR was calculated from the completion of chemotherapy to relapse or the last follow-up. Overall survival was the interval from the initiation of therapy to the time of death or the last follow-up. Progression was defined as a 25% increase in the product of the 2 largest diameters of the tumor. Progression-free survival (PFS) was calculated from the date of entry into the study to the date of the first physical or radiographic evidence of disease progrssion, death, or the last follow-up visit. Survival analysis was performed according to the Kaplan-Meier method.36 The statistical significance of differences among curves was determined by the log-rank and generalized Wilcoxon’s tests.37 Differences between groups were evaluated by the Mann-Whitney U-test (nonparametric analysis),38 and P < .05 indicated significance. A multivariate analysis of the prognosis was performed using Cox’s proportional-hazards regression model.39 All calculations were performed with SAS software, version 6.10 (SAS Institute, Cary, NC).
RESULTS
Measurement of nm23-H1 protein in plasma of patients and healthy controls.
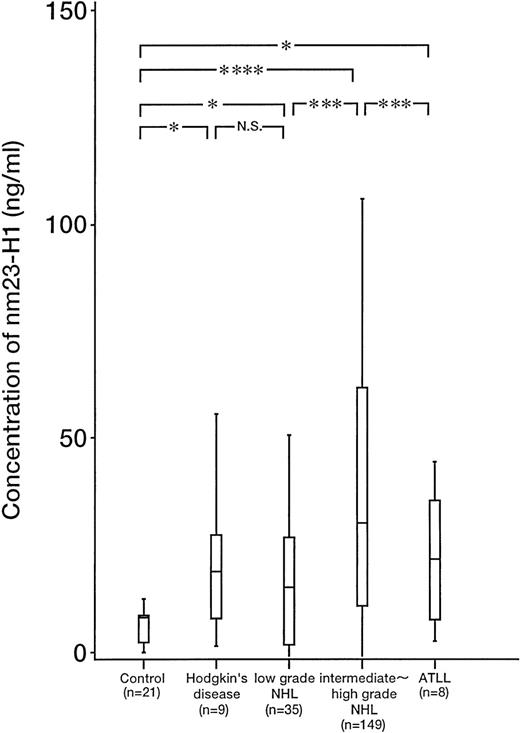
The plasma level of nm23-H1 was significantly elevated in patients with malignant lymphoma (n = 201, mean ± SD; 42.91 ± 59.69 ng/mL) compared with that in the healthy controls (n = 21, 6.13 ± 4.13 ng/mL; P = .0001). The plasma levels ofnm23-H1 in Hodgkin’s lymphoma (n = 9, 20.33 ± 17.15 ng/mL;P = .0195), low-grade non-Hodgkin’s lymphoma (n = 35, 18.93 ± 19.33 ng/mL; P = .0175 ), intermediate- and high-grade non-Hodgkin’s lymphoma (n = 149, 50.73 ± 66 ng/mL; P = .00001), and adult T-cell leukemia/lymphoma (n = 8, 27.65 ± 28.68 ng/mL; P = .0017) were significantly higher than those in the healthy controls. The plasma level of nm23-H1 in intermediate- and high-grade non-Hodgkin’s lymphoma was especially high and was significantly higher than that in low-grade non-Hodgkin’s lymphoma (P = .007; Fig 2). However, there was no significant difference in the nm23-H1 level between high- and intermediate-grade (P = .94) or among the subtypes of intermediate- and high-grade lymphoma such as diffuse large, diffuse mixed, diffuse small cleaved cell, lymphoblastic, etc (data not shown), indicating that the plasma level of nm23-H1is independent of the Working Formulation grade in aggressive non-Hodgkin’s lymphoma.
Plasma levels of nm23-H1 protein in malignant lymphoma (n = 201) and healthy controls (n = 21; P = .0001, Wilcoxon’s test). Upper and lower lines indicate the 10th and 90th percentiles, and boxes indicate the 25th and 75th percentiles. The line through each box indicates the median. NHL, non-Hodgkin’s lymphoma; ATLL, adult T-cell leukemia/lymphoma; NS, not significant. *P < .05; ***P < .001; ****P < .0001.
Plasma levels of nm23-H1 protein in malignant lymphoma (n = 201) and healthy controls (n = 21; P = .0001, Wilcoxon’s test). Upper and lower lines indicate the 10th and 90th percentiles, and boxes indicate the 25th and 75th percentiles. The line through each box indicates the median. NHL, non-Hodgkin’s lymphoma; ATLL, adult T-cell leukemia/lymphoma; NS, not significant. *P < .05; ***P < .001; ****P < .0001.
nm23-H1 and clinical characteristics in aggressive lymphoma.
Table 1 shows the clinical characteristics of the 149 patients with intermediate- and high-grade non-Hodgkin’s lymphoma. An elevated plasma level of nm23-H1 before treatment was correlated with poor prognostic features, such as a poor performance status, Ann Arbor stage III or IV, T-cell type, and an elevated serum level of soluble IL-2 receptor (P < .02 for all comparisons, Mann-Whitney U-test; Table 1). There was no significant difference in the proportion of patients with highnm23-H1 levels between the L + L-I risk group and the H-I + H risk group according to the IPI score (P = .24; Table 1). The plasma nm23-H1 level was 34.9 ± 40.6, 52.1 ± 66.7, 54.2 ± 81.9, and 78.8 ± 63.2 ng/mL in the L (n = 35), L-I (n = 65), H-I (n = 37), and H (n = 12) risk groups according to the IPI scores, respectively. The mean level tended to increase as the risk increased, but there was no statistically significant difference (P= .065).
Correlation of Plasma Levels of nm23-H1 With Clinical and Prognostic Characteristics in Intermediate- and High-Grade Non-Hodgkin’s Lymphoma
| Characteristic . | No. of Patients . | Mediannm23-H1 Level (ng/mL) . | P Value* . |
|---|---|---|---|
| Age at diagnosis (yr) | |||
| <60 | 59 | 29.1 | .5435 |
| ≥60 | 90 | 30.5 | |
| Gender | |||
| Male | 84 | 29.0 | 1.0000 |
| Female | 65 | 30.4 | |
| WHO performance status | |||
| 0, 1 | 131 | 28.4 | .0069 |
| 2 ∼ 4 | 18 | 58.8 | |
| Serum LDH level | |||
| Normal | 39 | 23.2 | .0926 |
| >Normal | 110 | 33.4 | |
| Ann Arbor stage | |||
| I, II | 61 | 21.3 | .0176 |
| III, IV | 88 | 35.2 | |
| Extranodal sites | |||
| ≤1 | 141 | 29.6 | .4310 |
| >1 | 8 | 52.3 | |
| IPI | |||
| Low/low-intermediate | 100 | 28.7 | .2429 |
| High-intermediate/high | 49 | 32.5 | |
| Immunophenotype | |||
| T | 37 | 39.1 | .0145 |
| B | 112 | 28.1 | |
| Soluble IL-2 receptor level (U/mL) | |||
| <1,000 | 83 | 24.4 | .0018 |
| ≥1,000 | 66 | 41.1 | |
| Soluble CD44 level (ng/mL) | |||
| <1,000 | 95 | 28.2 | .4932 |
| ≥1,000 | 17 | 35.0 | |
| Therapeutic response | |||
| CR | 126 | 37.4 | .0001 |
| PR + nonresponse | 23 | 93.6 |
| Characteristic . | No. of Patients . | Mediannm23-H1 Level (ng/mL) . | P Value* . |
|---|---|---|---|
| Age at diagnosis (yr) | |||
| <60 | 59 | 29.1 | .5435 |
| ≥60 | 90 | 30.5 | |
| Gender | |||
| Male | 84 | 29.0 | 1.0000 |
| Female | 65 | 30.4 | |
| WHO performance status | |||
| 0, 1 | 131 | 28.4 | .0069 |
| 2 ∼ 4 | 18 | 58.8 | |
| Serum LDH level | |||
| Normal | 39 | 23.2 | .0926 |
| >Normal | 110 | 33.4 | |
| Ann Arbor stage | |||
| I, II | 61 | 21.3 | .0176 |
| III, IV | 88 | 35.2 | |
| Extranodal sites | |||
| ≤1 | 141 | 29.6 | .4310 |
| >1 | 8 | 52.3 | |
| IPI | |||
| Low/low-intermediate | 100 | 28.7 | .2429 |
| High-intermediate/high | 49 | 32.5 | |
| Immunophenotype | |||
| T | 37 | 39.1 | .0145 |
| B | 112 | 28.1 | |
| Soluble IL-2 receptor level (U/mL) | |||
| <1,000 | 83 | 24.4 | .0018 |
| ≥1,000 | 66 | 41.1 | |
| Soluble CD44 level (ng/mL) | |||
| <1,000 | 95 | 28.2 | .4932 |
| ≥1,000 | 17 | 35.0 | |
| Therapeutic response | |||
| CR | 126 | 37.4 | .0001 |
| PR + nonresponse | 23 | 93.6 |
Abbreviation: LDH, lactate dehydrogenase.
Mann-Whitney test.
CR was achieved in 126 (84.6%) of the 149 patients, and the mean level of these 126 patients was 37.43 ± 48.55 ng/mL of nm23-H1 in plasma at diagnosis, which was significantly lower than the mean of those who showed a PR or failed to respond (93.60 ± 98.62 ng/mL,P = .0001), suggesting a close relationship between the plasma level of nm23-H1 and therapeutic responsiveness.
nm23-H1 and survival.
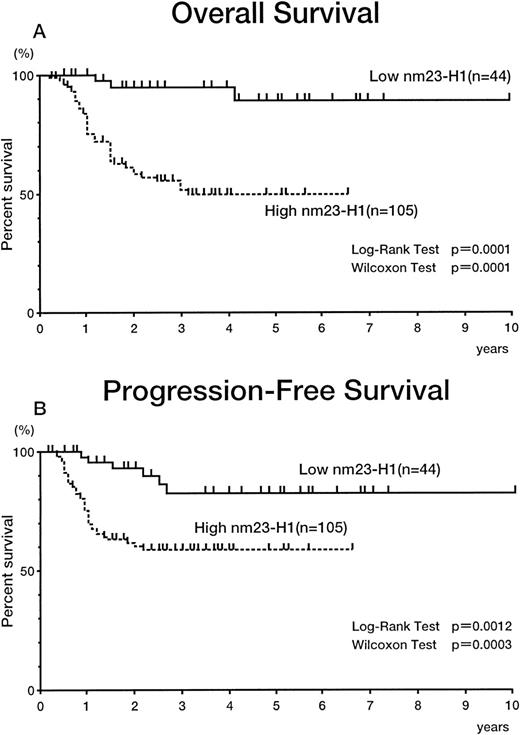
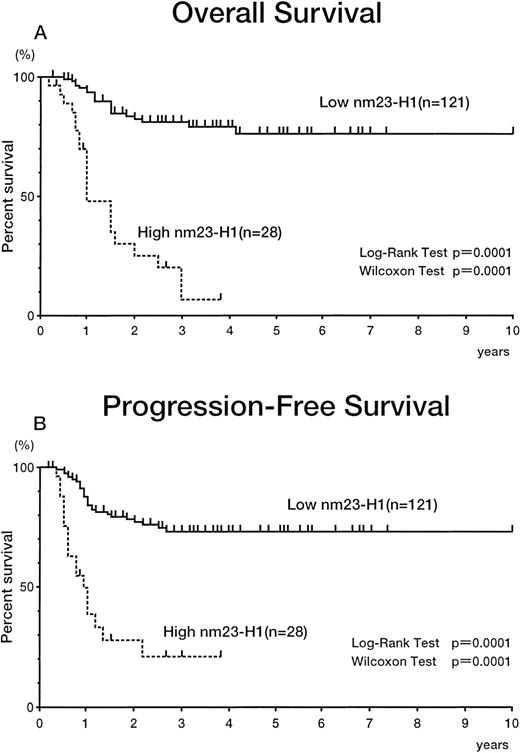
The 149 patients with aggressive (intermediate- and high-grade) non-Hodgkin’s lymphoma were divided into 2 groups with different plasma nm23-H1 levels. We tried to set the various cut-off points over 14.39 ng/mL, which was the upper limit in controls plasma (6.13 +2 SD). The cut-off points used here were 14.39 ng/mL (<14.39 [n = 44] v ≥14.39 [n = 105]), 50 ng/mL (<50 [n = 103]v ≥50 [n = 46]), 80 ng/mL (<80 [n = 121] v ≥80 [n = 28]), and 100 ng/mL (<100 [n = 132] v ≥100 [n = 17]). All of the cut-off values showed significant prognostic effects (data not shown). Figure 3 shows the results using 14.39 ng/mL as a cut-off value. The 5-year survival rates for the high (≥14.39 [n = 105]) and low nm23-H1 groups (<14.39 [n = 44]) were 49.2% and 90.6%, respectively (P = .0001 for both the log-rank test and generalized Wilcoxon’s test), with PFS values of 56.2% and 81.6% (P = .0012 for the log-rank test and P = .0003 for generalized Wilcoxon’s test; Fig 3). Figure 4 shows the results using 80 ng/mL as a cut-off value. The 3-year survival rates for the high (≥80 [n = 28]) and low nm23-H1 groups (<80 [n = 121]) were 6.7% and 79.5%, respectively (P = .0001 for both the log-rank test and generalized Wilcoxon’s test), with PFS values of 20.6% and 73.0% (P = .0001 for both the log-rank test and generalized Wilcoxon’s test; Fig 4). Even though the uppper limit of normal plasma nm23-H1 level was 14.39 ng/mL, as described above, the levels elevated enough to affect severely the survival and to select accurately the intractable non-Hodgkin’s lymphoma were greater than 50 ng/mL (data not shown). Because the statistical significance of 80 ng/mL and 100 ng/mL for survival (P = .0001 for the log-rank test) was greater than that of 50 ng/mL (P = .0013), we chose 80 ng/mL as a cut-off value for further analysis.
Overall survival (A) and PFS (B) curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. Highnm23-H1 (≥14.39 ng/mL) patients (n = 105) had a worse prognosis than low nm23-H1 (<14.39 ng/mL) patients (n = 44).
Overall survival (A) and PFS (B) curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. Highnm23-H1 (≥14.39 ng/mL) patients (n = 105) had a worse prognosis than low nm23-H1 (<14.39 ng/mL) patients (n = 44).
Overall survival (A) and PFS (B) curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. Highnm23-H1 (≥80 ng/mL) patients (n = 28) had a worse prognosis than low nm23-H1 (<80 ng/mL) patients (n = 121).
Overall survival (A) and PFS (B) curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. Highnm23-H1 (≥80 ng/mL) patients (n = 28) had a worse prognosis than low nm23-H1 (<80 ng/mL) patients (n = 121).
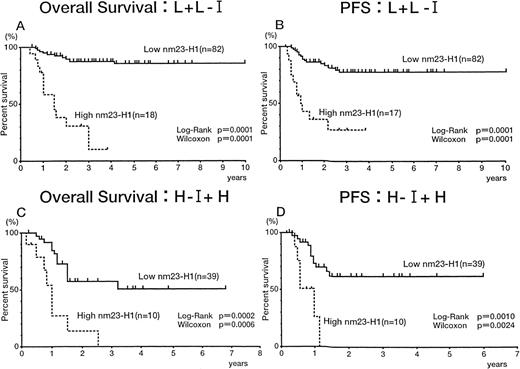
We evaluated the significance of the plasma nm23-H1 level in patients classified according to the IPI. In the L + L-I risk groups, both overall survival (Fig 5A) and PFS (Fig5B) were worse for patients with an nm23-H1 level of 80 ng/mL or greater than for patients with a level less than 80 ng/mL (P= .0001 for both the log-rank test and generalized Wilcoxon’s test), indicating that the therapeutic outcome was worse when thenm23-H1 level was high. Similar results were obtained in the H-I + H risk groups (Fig 5C and D). Thus, we could predict the therapeutic outcome in both risk groups of IPI using the cut-off value 80 ng/mL at diagnosis.
Overall survival and PFS curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. (A and C) Overall survival of patients in the low and low-intermediate (L + L-I) risk and high-intermediate and high (H-I + H) risk groups based on the IPI, respectively. (B and D) PFS of patients in the L + L-I and H-I + H risk groups based on the IPI, respectively.
Overall survival and PFS curves of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. (A and C) Overall survival of patients in the low and low-intermediate (L + L-I) risk and high-intermediate and high (H-I + H) risk groups based on the IPI, respectively. (B and D) PFS of patients in the L + L-I and H-I + H risk groups based on the IPI, respectively.
Prognostic factors in a univariate analysis in aggressive lymphoma.
The univariate analysis for intermediate- and high-grade non-Hodgkin’s lymphoma (n = 149) showed that the plasma nm23-H1 level, age, performance status, lactate dehydrogenase level, IPI score, immunophenotype, soluble IL-2 receptor level, and soluble CD44 level were significantly correlated with overall survival (P < .05; Table 2); among these, the nm23-H1level, age, performance status, lactate dehydrogenase level, IPI score, immunophenotype, and soluble IL-2 receptor level were also significantly correlated with PFS (P < .05; Table 2).
Prognostic Factors in a Univariate Analysis
| Characteristic . | No. of Patients . | 3-yr Survival (%) . | P Value . | 3-yr PFS (%) . | P Value . | ||
|---|---|---|---|---|---|---|---|
| Wilcoxon Test . | Log-Rank Test . | Wilcoxon Test . | Log-Rank Test . | ||||
| Age at diagnosis (yr) | |||||||
| <60 | 59 | 80.2 | .0096 | .0055 | 74.9 | .0343 | .0314 |
| ≥60 | 90 | 54.5 | 57.7 | ||||
| Gender | |||||||
| Male | 84 | 64.2 | .9083 | .8921 | 59.6 | .7351 | .4104 |
| Female | 65 | 67.9 | 71.8 | ||||
| WHO performance status | |||||||
| 0, 1 | 131 | 71.8 | .0001 | .0001 | 69.8 | .0001 | .0001 |
| 2 ∼ 4 | 18 | 0.0 | 22.0 | ||||
| Serum LDH level | |||||||
| Normal | 39 | 85.7 | .0160 | .0101 | 80.7 | .0447 | .0301 |
| >Normal | 110 | 58.2 | 58.4 | ||||
| Ann Arbor stage | |||||||
| I, II | 61 | 69.4 | .1167 | .2022 | 65.6 | .1923 | .3998 |
| III, IV | 88 | 62.6 | 64.2 | ||||
| Extranodal sites | |||||||
| ≤1 | 141 | 66.5 | .1283 | .1995 | 65.7 | .2874 | .2175 |
| >1 | 8 | 51.4 | 41.7 | ||||
| IPI | |||||||
| Low/low-intermediate | 100 | 73.8 | .0021 | .0016 | 69.7 | .0245 | .0273 |
| High-intermediate/high | 49 | 45.6 | 53.0 | ||||
| Immunophenotype | |||||||
| T | 37 | 41.1 | .0065 | .0023 | 49.7 | .0232 | .0214 |
| B | 112 | 72.7 | 69.3 | ||||
| Soluble IL-2 receptor level (U/mL) | |||||||
| <1,000 | 83 | 76.0 | .0002 | .0006 | 73.9 | .0080 | .0063 |
| ≥1,000 | 66 | 50.6 | 50.0 | ||||
| Soluble CD44 level (ng/mL) | |||||||
| <1,000 | 95 | 65.8 | .0321 | .0554 | 64.2 | .1016 | .1449 |
| ≥1,000 | 17 | 51.5 | 52.9 | ||||
| nm23-H1 level (ng/mL) | |||||||
| <80 | 121 | 79.5 | .0001 | .0001 | 73.0 | .0001 | .0001 |
| ≥80 | 28 | 6.7 | 20.6 | ||||
| Characteristic . | No. of Patients . | 3-yr Survival (%) . | P Value . | 3-yr PFS (%) . | P Value . | ||
|---|---|---|---|---|---|---|---|
| Wilcoxon Test . | Log-Rank Test . | Wilcoxon Test . | Log-Rank Test . | ||||
| Age at diagnosis (yr) | |||||||
| <60 | 59 | 80.2 | .0096 | .0055 | 74.9 | .0343 | .0314 |
| ≥60 | 90 | 54.5 | 57.7 | ||||
| Gender | |||||||
| Male | 84 | 64.2 | .9083 | .8921 | 59.6 | .7351 | .4104 |
| Female | 65 | 67.9 | 71.8 | ||||
| WHO performance status | |||||||
| 0, 1 | 131 | 71.8 | .0001 | .0001 | 69.8 | .0001 | .0001 |
| 2 ∼ 4 | 18 | 0.0 | 22.0 | ||||
| Serum LDH level | |||||||
| Normal | 39 | 85.7 | .0160 | .0101 | 80.7 | .0447 | .0301 |
| >Normal | 110 | 58.2 | 58.4 | ||||
| Ann Arbor stage | |||||||
| I, II | 61 | 69.4 | .1167 | .2022 | 65.6 | .1923 | .3998 |
| III, IV | 88 | 62.6 | 64.2 | ||||
| Extranodal sites | |||||||
| ≤1 | 141 | 66.5 | .1283 | .1995 | 65.7 | .2874 | .2175 |
| >1 | 8 | 51.4 | 41.7 | ||||
| IPI | |||||||
| Low/low-intermediate | 100 | 73.8 | .0021 | .0016 | 69.7 | .0245 | .0273 |
| High-intermediate/high | 49 | 45.6 | 53.0 | ||||
| Immunophenotype | |||||||
| T | 37 | 41.1 | .0065 | .0023 | 49.7 | .0232 | .0214 |
| B | 112 | 72.7 | 69.3 | ||||
| Soluble IL-2 receptor level (U/mL) | |||||||
| <1,000 | 83 | 76.0 | .0002 | .0006 | 73.9 | .0080 | .0063 |
| ≥1,000 | 66 | 50.6 | 50.0 | ||||
| Soluble CD44 level (ng/mL) | |||||||
| <1,000 | 95 | 65.8 | .0321 | .0554 | 64.2 | .1016 | .1449 |
| ≥1,000 | 17 | 51.5 | 52.9 | ||||
| nm23-H1 level (ng/mL) | |||||||
| <80 | 121 | 79.5 | .0001 | .0001 | 73.0 | .0001 | .0001 |
| ≥80 | 28 | 6.7 | 20.6 | ||||
Multivariate analysis of prognostic factors in aggressive lymphoma.
The 8 prognostic factors (nm23-H1 level, age, performance status, lactate dehydrogenase level, IPI score, immunophenotype, soluble IL-2 receptors level, and soluble CD44 level) that proved to be significant in the univariate analysis were further evaluated for their association with survival by a multivariate analysis using Cox’s proportional hazard model. This analysis showed that, in patients with intermediate- and high-grade non-Hodgkin’s lymphoma, the plasmanm23-H1 level was the most important independent prognostic factor (Table 3). Regarding the association with PFS, 7 factors (nm23-H1 level, age, performance status, lactate dehydrogenase level, IPI score, immunophenotype, and soluble IL-2 receptors level) were studied. An elevated plasma nm23-H1level was identified as the most important prognostic determinant similar to the performance status for poor PFS (Table 3). An additional multivariate analysis was used to assess the association of the 5 prognostic factors used to calculate the IPI score and the plasmanm23-H1 level with survival and PFS (data not shown). These results indicated that the nm23-H1 level was an independent prognostic factor that could predict both the overall survival and PFS.
Multivariate Analysis by Cox’s Proportional Hazards Model on Intermediate- and High-Grade Non-Hodgkin’s Lymphoma
| Category . | Estimate Value ± SE . | P Value . | Hazards Ratio . |
|---|---|---|---|
| Overall survival | |||
| nm23-H1(<80/≥80 ng/mL) | 1.96 ± 0.39 | .0001 | 7.087 |
| Age (<60/≥60 yr) | 1.35 ± 0.49 | .0053 | 3.877 |
| Performance status (0, 1/2 ∼ 4) | 1.05 ± 0.54 | .0511 | 2.847 |
| Immunophenotype (T/B) | 0.42 ± 0.43 | .3293 | 1.519 |
| Soluble CD44 (<1,000/≥1,000 ng/mL) | 0.39 ± 0.49 | .4278 | 1.473 |
| IPI (L, L-I/H-I, H) | 0.18 ± 0.53 | .7394 | 1.192 |
| Soluble IL-2 receptor (<1,000/≥1,000 U/mL) | 0.18 ± 0.53 | .7394 | 1.192 |
| LDH (normal/>normal) | 0.12 ± 0.62 | .8428 | 1.132 |
| PFS | |||
| nm23-H1 (<80/≥80 ng/mL) | 1.48 ± 0.33 | .0001 | 4.400 |
| Age (<60/≥60 yr) | 0.67 ± 0.38 | .0753 | 1.955 |
| Performance status (0, 1/2 ∼ 4) | 1.52 ± 0.43 | .0004 | 4.573 |
| Immunophenotype (T/B) | 0.37 ± 0.33 | .2710 | 0.693 |
| IPI (L, L-I/H-I, H) | 0.35 ± 0.45 | .4432 | 0.707 |
| Soluble IL-2 receptor (<1,000/≥1,000 U/mL) | 0.17 ± 0.36 | .6408 | 1.185 |
| LDH (normal/>normal) | 0.58 ± 0.48 | .2244 | 1.793 |
| Category . | Estimate Value ± SE . | P Value . | Hazards Ratio . |
|---|---|---|---|
| Overall survival | |||
| nm23-H1(<80/≥80 ng/mL) | 1.96 ± 0.39 | .0001 | 7.087 |
| Age (<60/≥60 yr) | 1.35 ± 0.49 | .0053 | 3.877 |
| Performance status (0, 1/2 ∼ 4) | 1.05 ± 0.54 | .0511 | 2.847 |
| Immunophenotype (T/B) | 0.42 ± 0.43 | .3293 | 1.519 |
| Soluble CD44 (<1,000/≥1,000 ng/mL) | 0.39 ± 0.49 | .4278 | 1.473 |
| IPI (L, L-I/H-I, H) | 0.18 ± 0.53 | .7394 | 1.192 |
| Soluble IL-2 receptor (<1,000/≥1,000 U/mL) | 0.18 ± 0.53 | .7394 | 1.192 |
| LDH (normal/>normal) | 0.12 ± 0.62 | .8428 | 1.132 |
| PFS | |||
| nm23-H1 (<80/≥80 ng/mL) | 1.48 ± 0.33 | .0001 | 4.400 |
| Age (<60/≥60 yr) | 0.67 ± 0.38 | .0753 | 1.955 |
| Performance status (0, 1/2 ∼ 4) | 1.52 ± 0.43 | .0004 | 4.573 |
| Immunophenotype (T/B) | 0.37 ± 0.33 | .2710 | 0.693 |
| IPI (L, L-I/H-I, H) | 0.35 ± 0.45 | .4432 | 0.707 |
| Soluble IL-2 receptor (<1,000/≥1,000 U/mL) | 0.17 ± 0.36 | .6408 | 1.185 |
| LDH (normal/>normal) | 0.58 ± 0.48 | .2244 | 1.793 |
DISCUSSION
Based on the biological activity of nm23 proteins for a differentiation inhibitory factor, we previously investigated the relative levels of nm23-H1 and nm23-H2 transcripts in AML cells. These transcripts were overexpressed in AML cells, and an elevated nm23-H1 expression level predicted the prognosis of AML.3,5,6 Most recently, it has been reported thatnm23-H1 overexpression (protein and mRNA) correlates with a high relapse rate and a short survival in acute lymphoblastic leukemia.40 In the present investigation, we found that an elevated plasma nm23-H1 protein level had prognostic value in aggressive non-Hodgkin’s lymphoma. On the other hand, reducednm23-H1 expression has been associated with reduced survival or with other histopathological indicators of a high metastatic potential in cohorts of breast, ovarian, cervical, gastric, and hepatocellular carcinoma and melanoma.9 However, an opposite trend has been identified in neuroblastoma and pancreatic carcinoma.9Although the reason for these differences is unknown, it might be worth examining the prognostic value of the plasma nm23-H1 level in several solid tumors in which nm23-H1 is overexpressed, as in acute leukemias.
It has been suggested that nm23 proteins can affect gene transcription.18nm23-H2 was cloned as a DNA-binding protein with a transactivating function that was identical to that of the previously described c-myc transcription factor PuF.18 Moreover, nm23-H2/PuF has been confirmed to play a role in c-myc activation in Burkitt’s lymphoma, in which the oncogene is activated as a result of translocation involving the c-myc gene and an Ig gene.41 We have not yet examined the expression levels of plasma nm23-H2 or evaluated its prognostic effect in lymphoma. In AML, we have reported that thenm23-H2 mRNA level may be a weak prognostic factor, whereas the combination of the nm23-H1 and nm23-H2 mRNA levels is a better prognostic factor than that of nm23-H1alone.4 We are interested in the nm23-H2 plasma level in addition to the nm23-H1 plasma level in aggressive lymphoma, although we have not yet succeeded in making the assay system specific for nm23-H2. The prognostic value of thenm23-H2 plasma level and the relative ratios of the two proteins are currently under investigation.
In the present study, we found that nm23-H1 protein was elevated in the plasma of patients with intermediate- and high-grade non-Hodgkin’s lymphoma. The nm23-H1 level was closely associated with the response to treatment; patients with a low level ofnm23-H1 frequently achieved CR, whereas a high level was closely associated with poor survival among unselected patients with intermediate- and high-grade non-Hodgkin’s lymphoma in treatment with the COP-BLAM regimen. A high nm23-H1 level was strongly associated with a poor performance status and a high Ann Arbor stage, but not with the Working Formulation grade, suggesting that the plasmanm23-H1 level may be correlated with lymphoma dissemination or possibly with the tumor burden rather than with the biological aggressiveness of lymphoma.
Recently, mutations of the p53 gene have been shown to be associated with a poor prognosis in patients with aggressive B-cell lymphoma.42 However, p53 mutations have not been shown to have any prognostic value in patients in the H-I and H risk groups of the IPI. Therefore, we assessed the association of the nm23-H1level with overall survival and PFS after stratification by the IPI score. As shown in Fig 5, the the plasma nm23-H1 level had significant prognostic value in both the L + L-I risk and H-I + H risk groups of the IPI. Both overall survival and PFS decreased in association with an increase in the nm23-H1 level (≥80 ng/mL) in 18 of the 100 patients in the L and L-I risk group and in 10 of the 49 patients in the H-I and H risk group. The CHOP regimen was associated with a CR rate of 87% in the L risk group, but this rate decreased to 67% in the L-I risk group.26 Although this regimen has been recommended as standard chemotherapy for L risk patients, it is not necessarily sufficient for L-I risk patients. Therefore, we should not be satisfied with using this regimen, and our efforts should be directed toward the development of a new therapeutic strategy for L-I risk non-Hodgkin’s lymphoma to improve the clinical outcome. Standard chemotherapy was unsatisfactory for H-I and H risk patients. However, high-dose chemotherapy with peripheral blood stem cell transplantation has achieved excellent results in clinical trials and has proved reasonably safe.43 44 Such high-dose chemotherapy should also be considered for patients with L and L-I risk non-Hodgkin’s lymphoma if the nm23-H1 level is greater than 80 ng/mL and the clinical outcome by standard chemotherapy is predicted to be poor.
The prognosis of intermediate- and high-grade non-Hodgkin’s lymphoma has improved as a result of recent progress in therapeutic strategies. However, there are still intractable cases that either fail to respond to initial treatment with standard chemotherapy (CHOP and COP-BLAM regimen) or recur after CR. Recently, prognostic factor models have been used to individualize treatment. The elevation of plasmanm23-H1 may be an important marker for a poor clinical outcome, because it is associated with intractability of the disease. Thenm23-H1 protein can be easily determined by ELISA, and the assay can be performed within several hours if a plasma sample is available. Therefore, consideration of the plasma nm23-H1 level may help guide the practitioner to select an appropriate therapy.
Supported in part by a grant from the Ministry of Health and Welfare, and Grants-in-Aid for Scientific Research (C) and Cancer Research, from The Ministry of Education, Science, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Junko Okabe-Kado, PhD, Saitama Cancer Center Research Institute, 818 Komuro, Ina, Kita-adachi, Saitama 362-0806, Japan; e-mail: jkado@cancer-c.pref.saitama.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal