Primary mediastinal large B-cell lymphoma (PMBL) appears to be a distinct clinicopathologic entity among diffuse large B-cell lymphomas (DLBLs). To find molecular alterations associated with this disease, we compared the mRNAs expressed in 3 PMBLs and 3 peripheral DLBLs by differential display-reverse transcription (DDRT) and identified a mRNA specifically expressed in PMBLs. Sequence analysis showed that this mRNA is encoded by the MAL gene, the expression of which was shown to be restricted to the T-cell lineage during hematopoiesis. MAL gene expression was demonstrated by Northern blot and reverse transcription-polymerase chain reaction (RT-PCR) in 8 of 12 PMBLs. However, there was little or no MAL gene expression in 8 peripheral DLBLs. Immunohistochemical analysis evidenced expression of MAL protein in tumoral B cells restricted to the PMBL subtype. Finally, Southern blot studies did not demonstrate rearrangement of the MAL gene. Altogether, our results indicate that MAL expression is recurrent in PMBLs, providing further evidence that PMBL represents a distinct entity among DLBLs. Because MAL protein is located in detergent-insoluble glycolipid-enriched membrane (GEM) domains involved in lymphocyte signal transduction, abnormal expression of MAL protein in the B-lymphoid lineage may have significant implications in PMBL lymphomagenesis.
DIFFUSE LARGE B-CELL lymphomas (DLBLs) account for 30% to 40% of adult non-Hodgkin’s lymphomas and constitute a heterogeneous group of lymphoid neoplasms with a wide spectrum of morphological and clinical features, treatment response, and prognosis.1 The genetic basis of this heterogeneity remains poorly understood, and identification of new molecular markers has been the focus of numerous studies in the past few years. In the Revised European-American classification of Lymphoid neoplasms (REAL classification), DLBLs encompass 3 major histological subtypes, namely centroblastic, immunoblastic, and anaplastic large B-cell lymphomas.1 This morphological subclassification may help in prognosis stratification, but its usefulness is limited by a poor interobserver reproducibility.2 At the molecular level, several genes have been implicated in the pathogenesis of these lymphomas. The most frequent genetic lesions (30% to 40%) are rearrangements of the LAZ3/Bcl6 gene, which encodes a zinc finger transcription factor that participates in B-cell differentiation.3,4 Besides these frequent genomic alterations, approximately 20% of DLBLs harbor a t(14;18) involving the Bcl2 gene, 20% of cases have mutations of the p53 gene, and a small percentage of DLBLs display rearrangements and/or mutations of the c-myc gene.5-7 Recently, a comparative genomic hybridization analysis associated with a candidate gene approach evidenced amplification of rel, myc, Bcl2, gl1, cdk4, andmdm2 genes, providing additional genetic information on DLBLs.8
Among DLBLs, primary mediastinal large B-cell lymphoma (PMBL) was individualized as a distinct subtype in the REAL classification.1 These lymphomas may be distinguished from peripheral DLBLs on clinical, morphological, and immunophenotypic features. Clinically, they are characterized by a female predominance and a median age at diagnosis in the fourth decade. Patients present with a prominent mediastinal tumoral mass, commonly associated with symptoms of airway compromise and superior vena cava syndrome. The tumor mass is usually bulky (>10 cm) and extension remains most frequently localized to the adjacent intrathoracic structures. Morphologically, the tumors consist of clear large cells and exhibit a diffuse growth pattern associated with a variable degree of sclerosis. Thymic remnants may be found within the tumor. These lymphomas display a particular immunophenotype CD19+CD20+CD79a+CD10−CD21−, with variable expression of CD23 and CD30, and absence of expression of cytoplasmic or surface Ig, despite Ig genes rearrangements.1,9-12 The Ig− and CD21− immunophenotype bears similarity to the thymic medullary B cells, and it was postulated that these lymphomas may arise from this particular subset of B cells.13 14
Specific genetic alterations involved in the pathogenesis of PMBLs are presently unknown. Alterations of the c-myc gene consisting of major rearrangements, and mutations or small rearrangements in the 5′ noncoding region were reported in a substantial proportion of cases in 2 studies (3 of 6 and 3 of 16 cases, respectively).15,16 Other reported molecular alterations include rearrangement of the Bcl6 gene (1 of 16 cases) and missense point mutations of the p53 gene (3 of 16 cases).16 A comparative genomic hybridization approach performed in a series of 26 PMBLs demonstrated frequent gains of chromosomal material involving chromosomes 2p, 9p, 12q, and Xq and amplification of the proto-oncogenerel in 2 cases.17 Hence, in addition to distinct clinicopathologic features, PMBLs generally lack the molecular alterations involved in peripheral DLBLs.
To identify molecular alterations associated with this disease, we compared the mRNAs expressed in PMBLs and peripheral DLBLs by differential display-reverse transcription (DDRT). We identified MAL mRNA as being differentially expressed between these 2 entities and further confirmed by immunohistochemistry the specific expression of MAL protein in PMBL neoplastic cells.
MATERIALS AND METHODS
Tissue specimens and cell lines.
Tumor samples from 12 patients with PMBL and 8 patients with peripheral DLBL were collected from the archives of 2 departments of Pathology (Hôpital Henri Mondor [Créteil, France] and Hôpital Laennec [Paris, France]). The clinical and pathologic features of all cases are summarized in Table 1. All patients with PMBL presented with an initial prominent bulky mediastinal mass. Representative tumor samples from PMBLs were obtained at mediastinoscopy for 9 cases and from supraclavicular and axillary lymph nodes for 3 cases. Biopsy specimens of peripheral DLBLs consisted of peripheral lymph nodes in all cases. Disseminated B-cell lymphomas with bulky mediastinal involvement were excluded. The morphologic features were assessed on hematoxylin-eosin–stained sections of Bouin’s or formalin-fixed, paraffin-embedded tissue. Expression of B- and T-cell–associated differentiation antigens were evaluated on deparaffinized tissue sections using the alkaline-phosphatase/anti-alkaline-phosphatase (APAAP) procedure with the CD3ε, L26/CD20, and Ber-H2/CD30 antibodies (Dako SA, Glostrup, Denmark).18 All cases had a CD20+CD3− immunophenotype and occasionally expressed CD30 after microwave antigen retrieval. Diagnosis of PMBLs and peripheral DLBLs were established in each case by standard clinical, histological, and immunohistochemical criteria, and lymphomas were classified according to the REAL and updated Kiel classifications.1 19
Clinical and Pathologic Characteristics of PMBLs (Cases No. 1 Through 12) and Peripheral DLBLs (Cases No. 13 Through 20)
| Case No. . | Age/ Sex . | Clinical Stage . | Bulky Mediastinal Mass . | Site of Biopsy . | Other Localizations at Diagnosis . | Tumor Cell Morphology (Kiel)* . |
|---|---|---|---|---|---|---|
| 1 | 30/M | I | + | Mediastinum | — | cb polymorphic |
| 2† | 37/F | IV | + | Mediastinum | Pleura, lung, cervical LN | cb polymorphic with anaplastic large-cell component |
| 3 | 48/M | II | + | Mediastinum | — | cb polymorphic |
| 4† | 34/F | IV | + | Mediastinum | Lung | cb polymorphic |
| 5† | 32/F | I | + | Mediastinum | — | cb polymorphic |
| 6† | 26/M | I | + | Mediastinum | — | cb polymorphic |
| 7 | 31/M | I | + | Mediastinum | — | cb type with clear-cell component |
| 8† | 23/F | I | + | Mediastinum | — | cb polymorphic |
| 9 | 38/M | I | + | Mediastinum | — | cb polymorphic |
| 10† | 41/F | II | + | Axillary LN | Axillary LN | cb type with clear-cell component |
| 11† | 27/F | IV | + | Supra-clavicular LN | Lung, pericardium, axillary, supra-clavicular, and retroperitoneal LN | cb type with clear-cell component |
| 12 | 38/M | II | + | Supra-clavicular LN | Supra-clavicular LN | cb type with clear-cell component |
| 13 | 68/M | II | − | Inguinal LN | Retroperitoneal LN | T-cell–rich B-cell lymphoma |
| 14 | 74/F | II | − | Supra-clavicular LN | Axillary LN | cb polymorphic |
| 15 | 62/M | IV | − | Supra-clavicular LN | Spleen, BM, peripheral, and retroperitoneal LN | cb polymorphic |
| 16 | 45/F | IV | − | Cervical LN | Stomach, lung, peripheral, and retroperitoneal LN | immunoblastic |
| 17 | 60/F | I | − | Inguinal LN | — | cb multilobated subtype |
| 18 | 57/F | IV | − | Inguinal LN | Spleen, BM, peripheral LN | cb polymorphic, high-grade transformation of a B-CLL |
| 19 | 59/F | I | − | Inguinal LN | — | cb polymorphic |
| 20 | 46/M | I | − | Cervical LN | — | cb polymorphic |
| Case No. . | Age/ Sex . | Clinical Stage . | Bulky Mediastinal Mass . | Site of Biopsy . | Other Localizations at Diagnosis . | Tumor Cell Morphology (Kiel)* . |
|---|---|---|---|---|---|---|
| 1 | 30/M | I | + | Mediastinum | — | cb polymorphic |
| 2† | 37/F | IV | + | Mediastinum | Pleura, lung, cervical LN | cb polymorphic with anaplastic large-cell component |
| 3 | 48/M | II | + | Mediastinum | — | cb polymorphic |
| 4† | 34/F | IV | + | Mediastinum | Lung | cb polymorphic |
| 5† | 32/F | I | + | Mediastinum | — | cb polymorphic |
| 6† | 26/M | I | + | Mediastinum | — | cb polymorphic |
| 7 | 31/M | I | + | Mediastinum | — | cb type with clear-cell component |
| 8† | 23/F | I | + | Mediastinum | — | cb polymorphic |
| 9 | 38/M | I | + | Mediastinum | — | cb polymorphic |
| 10† | 41/F | II | + | Axillary LN | Axillary LN | cb type with clear-cell component |
| 11† | 27/F | IV | + | Supra-clavicular LN | Lung, pericardium, axillary, supra-clavicular, and retroperitoneal LN | cb type with clear-cell component |
| 12 | 38/M | II | + | Supra-clavicular LN | Supra-clavicular LN | cb type with clear-cell component |
| 13 | 68/M | II | − | Inguinal LN | Retroperitoneal LN | T-cell–rich B-cell lymphoma |
| 14 | 74/F | II | − | Supra-clavicular LN | Axillary LN | cb polymorphic |
| 15 | 62/M | IV | − | Supra-clavicular LN | Spleen, BM, peripheral, and retroperitoneal LN | cb polymorphic |
| 16 | 45/F | IV | − | Cervical LN | Stomach, lung, peripheral, and retroperitoneal LN | immunoblastic |
| 17 | 60/F | I | − | Inguinal LN | — | cb multilobated subtype |
| 18 | 57/F | IV | − | Inguinal LN | Spleen, BM, peripheral LN | cb polymorphic, high-grade transformation of a B-CLL |
| 19 | 59/F | I | − | Inguinal LN | — | cb polymorphic |
| 20 | 46/M | I | − | Cervical LN | — | cb polymorphic |
Patients were staged according to the Ann Arbor system.
Abbreviations: cb, centroblastic; LN, lymph node; BM, bone marrow; CLL, chronic lymphocytic leukemia.
All cases were classified as DLBL in the REAL classification; all cases had a CD20+CD3− B-cell phenotype.
Cases showing variable expression of CD30 after antigen microwave retrieval.
Cell lines used in the present study included Jurkat T-cell line, B-cell lines at various stages of differentiation (Raji, Ramos, RL, 697, and RS 4:11), erythroleukemic cell lines (K562 and HEL), and an epithelial cell line (Hela). Hematopoietic cell lines were maintained in RPMI 1640 medium, and Hela cell line was maintained in Dulbecco’s modified Eagle medium, supplemented with 10% fetal calf serum. Cell lines were grown at 37°C in 5% CO2.
DDRT-polymerase chain reaction (DDRT-PCR).
DDRT was performed as described.20 Total RNAs were extracted from frozen tumor samples of 3 patients presenting with peripheral DLBL and 3 patients presenting with PMBL using the TRIZOL reagent (GIBCO-BRL Life Technologies, Cergy-Pontoise, France). After poly(A) enrichment on oligod(T) cellulose, 50 ng mRNAs were reverse-transcribed with 200 U Superscript II reverse transcriptase (GIBCO-BRL) in the presence of 50 pmol anchored T12MN primers (in which M represents A, C, or G and N is T, A, C, or G) in 20 μL RT buffer (50 mmol/L Tris, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol [DTT]) containing 20 μmol/L dNTP for 50 minutes at 37°C. After heat inactivation of the RT at 95°C for 5 minutes, subsequent PCR amplification was performed using 1 μL of the cDNA with 50 pmol of the appropriate T12NM primer and 10 pmol of arbitrary decamer. The PCR reaction was performed with 2 U Taq DNA polymerase (Perkin Elmer Applied Biosystems, Courtaboeuf, France) and 0.075 μL [α-33P]dATP (1,000 Ci/mmol) in 20 μL PCR buffer (10 mmol/L Tris, pH 8.3, 50 mmol/L KCl, 1.25 mmol/L MgCl2) containing 2 μmol/L dNTP. The cycling parameters were as follows: 94°C for 4 minutes, and then 40 cycles of denaturation (94°C for 15 seconds), annealing (42°C for 1 minute), and elongation (72°C for 30 seconds), followed by an elongation step at 72°C for 7 minutes. The amplified cDNAs were separated on a 6% polyacrylamide sequencing gel, which was then dried and exposed to a Kodak X-OMAT AR film (Eastman Kodak, Rochester, NY) overnight. Differential bands were excised from the dried sequencing gel, boiled in 100 μL H2O for 15 minutes, precipitated with ethanol, and suspended in 10 μL of water. One fourth of the recovered cDNA was used for reamplification in a 40 μL reaction volume using the same primer set and PCR conditions as used in the mRNA display, except a higher concentration of dNTP (20 μmol/L). Reamplified cDNA fragments were subcloned into pBS SK plasmid.
DNA sequencing.
Plasmid DNA and PCR products were Taq cycle sequenced using the Applied Biosystems PRISM ready reaction Dye-dideoxy Terminator and Dye-Primer sequencing kits, and samples were run on an ABI 373A DNA sequencer (Applied Biosystems, Foster City, CA).
Northern blot analysis.
Total RNAs were extracted using TRIZOL (GIBCO-BRL) according to the manufacturer’s instructions. RNAs extracted from lymphomas or cell lines (15 μg) were denatured for 10 minutes at 68°C and run in 1% agarose gel containing 2 mol/L formaldehyde in 10 mmol/L phosphate buffer, pH 7. RNAs were transferred on Hybond-N+ membranes (Amersham Pharmacia Biotech, Orsay, France) and cross-linked by UV irradiation. Prehybridization and hybridization were performed in Church buffer (140 mmol/L NaH2PO4, 360 mmol/L Na2HPO4, pH 7, 7% sodium dodecyl sulfate [SDS], and 1 mmol/L EDTA) with random-primed, α-32P–labeled probe. The MAL probe was obtained by PCR amplification of a fragment of MAL cDNA (nt 62 to 585, according to the nucleotide sequence published21). Control hybridizations were performed using a probe specific for glyceraldehyde-phosphate deshydrogenase (GAPDH) mRNA.
RT-PCR analysis.
One microgram of total RNA was reverse transcribed to cDNA using 200 U Superscript Plus (GIBCO-BRL) and 300 ng random primers in 20 μL RT buffer containing 0.5 mmol/L dNTP. MAL cDNA was amplified together with an internal standard, consisting of S14 ribosomal protein cDNA, in 1 tube. The PCR reactions were performed in 2 steps: the first step consisted of the amplification of MAL cDNA with 5 pmol of sense primer (5′CTTGCCCGACTTGCTCTTCA3′) and antisense primer (5′GGGGGGGTGGTTGTTTTCTT3′) and 0.5 U Taq Gold DNA polymerase (Perkin Elmer) in 20 μL PCR buffer containing 0.2 mmol/L dNTP. Thermocycling was performed as follows: 12 minutes at 95°C and 8 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute +2 seconds per cycle. The second step consisted of the addition of 10 μL PCR buffer containing 0.2 mmol/L dNTP, 2.5 pmol MAL sense and antisense primers, 5 pmol of S14 ribosomal protein sense primer (5′GGCAGACCGAGATGAATCCTCA3′) and antisense primer (5′CAGGTCCAGGGGTCTTGGTCC3′), and 0.5 U Taq Gold DNA polymerase (Perkin Elmer). The second thermocycling was performed as follows: 12 minutes at 95°C and 26 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute +2 seconds per cycle. Ten microliters of the PCR products was run on a 2% agarose gel in 1× TBE buffer (100 mmol/L Tris, 90 mmol/L Boric acid, and 1 mmol/L EDTA, pH 8.3). Samples containing distilled water and Jurkat cDNA were used as negative and positive controls, respectively.
DNA extraction and Southern blot analysis.
DNA from tumor samples of 6 PMBLs and 3 peripheral DLBLs was extracted by proteinase K digestion, phenol/chloroform extraction, and ethanol precipitation.22 After digestion with EcoRI, DNA fragments were electrophoresed on 0.8% agarose gels in 1× TAE buffer (40 mmol/L Tris-acetate, 1 mmol/L EDTA, pH 8.3) and transferred onto nylon N+ membrane (Amersham). Prehybridization and hybridization were performed in Quick Express Hyb Solution (Clontech, Palo Alto, CA) with α-32P probes, which were labeled by random priming according to the manufacturer instructions (GIBCO-BRL). The first probe consisted of an 1.5-kbEcoRI-HindIII fragment of the MAL gene derived from a plasmid containing the MAL first exon and 5.5 kb of upstream sequences.23 The second probe was obtained by PCR amplification of a fragment of MAL cDNA (nt 62 to 585, according to the nucleotide sequence published21).
Immunohistochemistry.
Immunohistochemistry was performed on paraffin sections using the APAAP method and an anti-MAL monoclonal antibody directed against amino acids 114-123 of the human MAL protein.18 24 Rabbit antimouse Igs and APAAP complexes were obtained from Dako. Paraffin sections from normal kidney were used as positive control.
RESULTS
Identification of MAL mRNA through differential screening of lymphomas mRNAs.
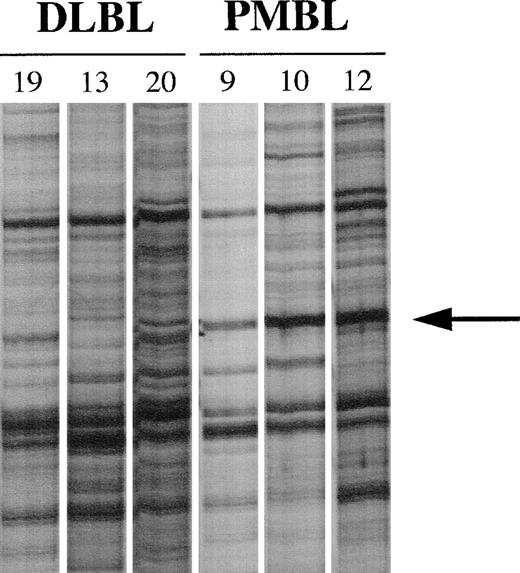
We compared the mRNAs expressed in tumor samples of 3 PMBL patients (patients no. 9, 10, and 12) with the mRNAs expressed in tumor samples of 3 other patients with peripheral DLBL (patients no. 19, 13, and 20). Total RNAs extracted from frozen tissue samples were reverse transcribed using T12GC anchor primer. Subsequent PCR amplification of the cDNAs were performed using T12GC anchor primer and OPA 18 arbitrary decamer (5′AGGTGACCGT3′). Comparative analysis of the PCR products identified a band that was present in 3 of 3 PMBLs but not in the 3 peripheral DLBLs (Fig 1). This band was eluted from the gel, reamplified by PCR with the set of primers used in the corresponding DDRT experiment, and sequenced. This sequence was compared with the nucleotide databases and proved to correspond to the 3′ end of MAL mRNA.
PCR differential screening. mRNA from tumor samples of 3 patients with peripheral DLBLs (patients no. 19, 13, and 20) and 3 patients with PMBLs (patients no. 9, 10, and 12) were reverse transcribed and amplified by PCR using the T12GC anchor primer and the OPA 18 arbitrary primer. Amplified cDNAs were run side by side on a 6% sequencing gel. The arrow indicates the band seen only in amplified cDNAs corresponding to patients with PMBL.
PCR differential screening. mRNA from tumor samples of 3 patients with peripheral DLBLs (patients no. 19, 13, and 20) and 3 patients with PMBLs (patients no. 9, 10, and 12) were reverse transcribed and amplified by PCR using the T12GC anchor primer and the OPA 18 arbitrary primer. Amplified cDNAs were run side by side on a 6% sequencing gel. The arrow indicates the band seen only in amplified cDNAs corresponding to patients with PMBL.
Northern blot analysis of MAL expression.
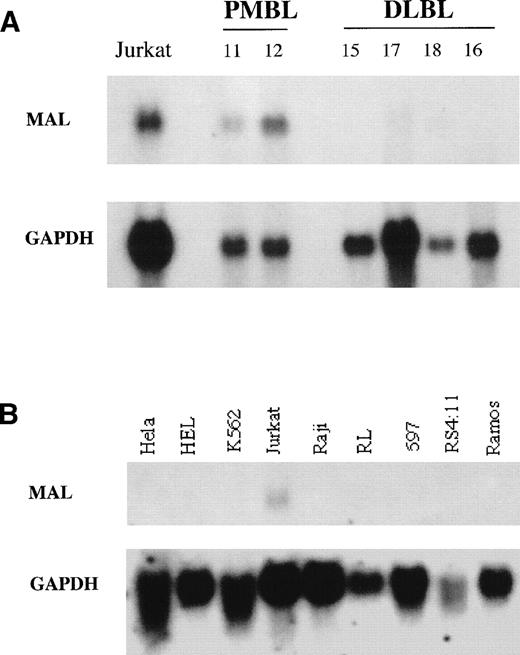
To confirm that the differential product observed corresponded to a differential mRNA, we studied MAL gene expression in tumor samples of patients with PMBL or peripheral DLBL by Northern blot analysis. As shown in Fig 2A, hybridization with MAL cDNA showed high expression of MAL 1.1-kb transcripts in 2 PMBLs (cases no. 11 and 12) and very low or undetectable expression in 4 peripheral DLBLs (cases no. 15, 17, 18, and 16). In addition, we tested MAL expression in a panel of human hematopoietic cell lines, including B-cell lines at various stages of differentiation. MAL mRNAs were detected in the Jurkat T-cell line, but were absent in the B-cell lines (Raji, RL, 697, RS 4:11, and Ramos), erythroleukemic cell lines (HEL and K562), and epithelial cell line (Hela) studied (Fig 2B). These results showed that MAL is not expressed in B-cell lines and thus confirmed that MAL expression is restricted to the T-cell lineage during hematopoiesis.
Northern blot analysis of MAL expression. Fifteen micrograms of total RNAs extracted from PMBLs, peripheral DLBLs, and human cell lines was loaded per lane. Hybond N+ membranes were hybridized with a PCR-derived MAL cDNA fragment (upper panel), stripped, and rehybridized with a GAPDH probe (lower panel) to check the RNA amounts loaded and transferred to the membrane. (A) RNAs from Jurkat T-cell line, 2 PMBLs (no. 11 and 12), and 4 peripheral DLBLs (no. 15, 17, 18, and 16). (B) RNAs from human cell lines. Hela is nonhematopoietic. HEL and K562 are erythroleukemic cell lines. Jurkat is a T-cell line. Raji and Ramos are derived from Burkitt’s lymphoma. RL bears the t(14;18) translocation associated with follicular lymphoma. 697 is a pre-B–cell line and RS 4:11 a pro-B–cell line.
Northern blot analysis of MAL expression. Fifteen micrograms of total RNAs extracted from PMBLs, peripheral DLBLs, and human cell lines was loaded per lane. Hybond N+ membranes were hybridized with a PCR-derived MAL cDNA fragment (upper panel), stripped, and rehybridized with a GAPDH probe (lower panel) to check the RNA amounts loaded and transferred to the membrane. (A) RNAs from Jurkat T-cell line, 2 PMBLs (no. 11 and 12), and 4 peripheral DLBLs (no. 15, 17, 18, and 16). (B) RNAs from human cell lines. Hela is nonhematopoietic. HEL and K562 are erythroleukemic cell lines. Jurkat is a T-cell line. Raji and Ramos are derived from Burkitt’s lymphoma. RL bears the t(14;18) translocation associated with follicular lymphoma. 697 is a pre-B–cell line and RS 4:11 a pro-B–cell line.
Analysis of MAL expression by RT-PCR.
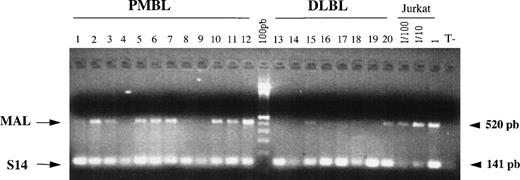
The small quantity of biopsy material obtained at mediastinocopy did not yield enough RNA to perform Northern blot analysis in a large series of lymphomas. Therefore, we studied MAL expression in 12 PMBLs and 8 peripheral DLBLs by RT-PCR analysis. As shown in Fig 3, a specific MAL PCR product of 520 bp was detected in 8 of 12 PMBLs (cases no. 2, 3, 5, 6, 7, 10, 11, and 12) and in only 2 of 8 peripheral DLBLs (cases no. 15 and 20). In these 2 cases, the level of MAL expression appeared lower than that observed in PMBLs. Efficient amplification of the S14 internal control was detected in all cases. This analysis confirmed the differential expression of MAL mRNA in PMBLs compared with peripheral DLBLs.
RT-PCR analysis of MAL expression in PMBLs and peripheral DLBLs. One microgram of RNA extracted from 12 PMBLs and 8 peripheral DLBLs was reverse transcribed and the cDNAs were coamplified with MAL and S14 primers. PCR products were run on a 2% agarose gel stained with ethidium bromide. Specific amplification of MAL and S14 cDNAs produced 520- and 141-bp bands, respectively. Positive control represented by serial dilutions of Jurkat cDNA and a negative control without template (T−) are included.
RT-PCR analysis of MAL expression in PMBLs and peripheral DLBLs. One microgram of RNA extracted from 12 PMBLs and 8 peripheral DLBLs was reverse transcribed and the cDNAs were coamplified with MAL and S14 primers. PCR products were run on a 2% agarose gel stained with ethidium bromide. Specific amplification of MAL and S14 cDNAs produced 520- and 141-bp bands, respectively. Positive control represented by serial dilutions of Jurkat cDNA and a negative control without template (T−) are included.
MAL protein expression in PMBLs and peripheral DLBLs.
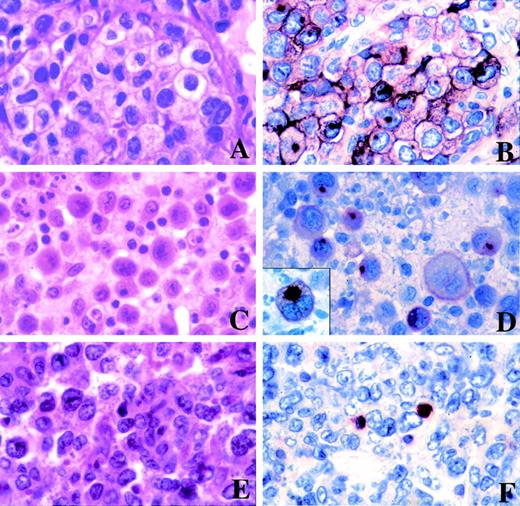
To examine whether MAL protein was expressed in tumoral B cells and to rule out the possibility that MAL mRNA expression observed in PMBLs was due to intratumoral reactive T cells, we analyzed the expression of MAL protein by immunohistochemistry on paraffin sections of tumor samples. The results are shown in Table 2. MAL protein was detected in neoplastic cells in 7 of 12 PMBLs (cases no. 2, 5, 6, 7, 10, 11, and 12). Two cases were negative, although internal positive controls, represented by small lymphoid cells, consistent with reactive T cells, were present. Three cases were not interpretable because of the absence of internal positive control. The staining pattern was associated with the surface membrane and there was granular cytoplasmic positivity, with accentuation in the Golgi area in most neoplastic cells (Fig 4B and D). The majority of positive PMBLs (5/7) displayed more than 50% positive neoplastic cells. Two PMBLs (cases no. 5 and 11) demonstrated only 10% to 20% positive neoplastic cells; however, the possibility of partial antigenic denaturation due to Bouin’s fixative cannot be ruled out in these 2 cases. In comparison, all peripheral DLBLs tested were negative, although internal positive control were present in all cases (Fig 4F).
MAL Protein Expression in Neoplastic Cells of PMBLs and Peripheral DLBLs
| PMBLs . | Peripheral DLBLs . | ||
|---|---|---|---|
| Patients . | MAL . | Patients . | MAL . |
| 1 | 0 | 13 | 0 |
| 2 | +++ | 14 | 0 |
| 3 | NI | 15 | 0 |
| 4 | NI | 16 | 0 |
| 5 | + | 17 | 0 |
| 6 | +++ | 18 | 0 |
| 7 | +++ | 19 | 0 |
| 8 | NI | 20 | 0 |
| 9 | 0 | ||
| 10 | +++ | ||
| 11 | + | ||
| 12 | +++ | ||
| PMBLs . | Peripheral DLBLs . | ||
|---|---|---|---|
| Patients . | MAL . | Patients . | MAL . |
| 1 | 0 | 13 | 0 |
| 2 | +++ | 14 | 0 |
| 3 | NI | 15 | 0 |
| 4 | NI | 16 | 0 |
| 5 | + | 17 | 0 |
| 6 | +++ | 18 | 0 |
| 7 | +++ | 19 | 0 |
| 8 | NI | 20 | 0 |
| 9 | 0 | ||
| 10 | +++ | ||
| 11 | + | ||
| 12 | +++ | ||
The number of large neoplastic cells positive with the anti-MAL antibody was scored as follows: 0, negative; +, <20%; ++, 20% to 50%; +++, >50%.
Abbreviation: NI, not interpretable (absence of internal positive control).
Morphological features and immunostaining for MAL of PMBLs and peripheral DLBLs. (A) PMBL of centroblastic subtype with a clear-cell component (case no. 10). (B) Case no. 10 stained for the MAL protein showing surface membrane and granular cytoplasmic immunoreactivity with accentuation in the Golgi area of neoplastic cells. (C) PMBL of centroblastic polymorphic subtype with a anaplastic large-cell component (case no. 2). (D) Staining for MAL of case no. 2 showing membrane and striking paranuclear dot-like positivity in neoplastic cells (inset). (E) Peripheral DLBL of centroblastic multilobated subtype (case no. 17). (F) Staining for MAL of case no. 17 showing that the tumor cells lack expression of the MAL protein. Positive internal control are present, represented by small lymphoid cells, consistent with reactive T cells. (A, C, and E, hematoxylin-eosin stain; B, D, and F, immunohistochemical staining of paraffin section with the anti-MAL antibody, APAAP method.)
Morphological features and immunostaining for MAL of PMBLs and peripheral DLBLs. (A) PMBL of centroblastic subtype with a clear-cell component (case no. 10). (B) Case no. 10 stained for the MAL protein showing surface membrane and granular cytoplasmic immunoreactivity with accentuation in the Golgi area of neoplastic cells. (C) PMBL of centroblastic polymorphic subtype with a anaplastic large-cell component (case no. 2). (D) Staining for MAL of case no. 2 showing membrane and striking paranuclear dot-like positivity in neoplastic cells (inset). (E) Peripheral DLBL of centroblastic multilobated subtype (case no. 17). (F) Staining for MAL of case no. 17 showing that the tumor cells lack expression of the MAL protein. Positive internal control are present, represented by small lymphoid cells, consistent with reactive T cells. (A, C, and E, hematoxylin-eosin stain; B, D, and F, immunohistochemical staining of paraffin section with the anti-MAL antibody, APAAP method.)
The different methods used to detect MAL expression in PMBLs and peripheral DLBLs yielded comparable results. Among the 8 RT-PCR–positive PMBLs, 7 were positive by immunohistochemistry and 1 case (case no. 3) was not interpretable. Two cases of peripheral DLBLs (cases no. 15 and 20) showed low levels of MAL mRNA expression by RT-PCR analysis, but the neoplastic cells remained negative by immunohistochemistry. In these 2 cases, the faint signal observed upon RT-PCR analysis is presumably related to the presence of intratumoral reactive T cells.
Southern blot analysis.
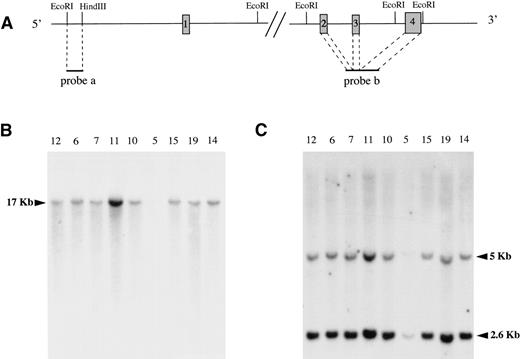
To study if MAL gene expression in tumoral B cells was due to genomic rearrangements, DNAs extracted from tumor samples of PMBLs and peripheral DLBLs were digested with EcoRI and subjected to Southern blot analysis. The blot was first hybridized with a 1.5-kbEcoRI-HindIII fragment located 3.5 kb upstream of MAL first exon, showing the same 17-kb DNA fragment in all lymphomas tested (Fig 5B). The blot was stripped and subsequently hybridized with a second probe obtained by PCR amplification of a fragment of MAL cDNA spanning exons 2, 3, and 4 and thus exploring the 3′ part of the MAL gene. In peripheral DLBLs as well as in PMBLs, this probe hybridized with the expected 5- and 2.6-kb DNA fragments (Fig 5C). These experiments did not demonstrate any rearrangement of the MAL gene in PMBLs. Furthermore, quantitative analysis of the signals correlated with the amount of DNA loaded in each lane (data not shown), thereby ruling out the possibility of an overexpression related to gain of chromosomal material.
Southern blot analysis. Genomic DNAs extracted from 6 PMBLs (no. 12, 6, 7, 11, 10, and 5) and 3 peripheral DLBLs (no. 15, 19, and 14) were subjected to EcoRI digestion, agarose gel electrophoresis, Nylon N+ membrane transfer, and hybridization with 2 MAL probes. (A) The physical map of the MAL gene and localization of the 2 probes are represented at the top of the figure. The boxes represent the exons, which are not drawn to scale for the clarity of the figure. (B) A 1.5-kb EcoRI-HindIII fragment (probe a) exploring the 5′ part of the MAL gene showed the same 17-kb DNA fragment in all samples. (C) A PCR-generated fragment of MAL cDNA (probe b) spanning exons 2, 3, and 4 showed 2 DNA fragments of 5 and 2.6 kb. The faint bands observed for case no. 5 in (B) and (C) are due to the lower amount of DNA loaded on the gel. However, longer autoradiographic expositions demonstrated the same DNA fragments in this sample.
Southern blot analysis. Genomic DNAs extracted from 6 PMBLs (no. 12, 6, 7, 11, 10, and 5) and 3 peripheral DLBLs (no. 15, 19, and 14) were subjected to EcoRI digestion, agarose gel electrophoresis, Nylon N+ membrane transfer, and hybridization with 2 MAL probes. (A) The physical map of the MAL gene and localization of the 2 probes are represented at the top of the figure. The boxes represent the exons, which are not drawn to scale for the clarity of the figure. (B) A 1.5-kb EcoRI-HindIII fragment (probe a) exploring the 5′ part of the MAL gene showed the same 17-kb DNA fragment in all samples. (C) A PCR-generated fragment of MAL cDNA (probe b) spanning exons 2, 3, and 4 showed 2 DNA fragments of 5 and 2.6 kb. The faint bands observed for case no. 5 in (B) and (C) are due to the lower amount of DNA loaded on the gel. However, longer autoradiographic expositions demonstrated the same DNA fragments in this sample.
DISCUSSION
Using a differential screening method, we identified a transcript expressed in PMBLs, which proved upon sequencing to correspond to the 3′ end of MAL mRNA. We demonstrated by Northern blot and RT-PCR the recurrent and differential expression of the MAL gene in PMBLs among DLBLs and identified the MAL protein at the surface membrane and in the Golgi area of PMBL neoplastic B cells.
The MAL gene was first described as differentially expressed during T-cell development and was subsequently shown to be expressed in thymus, thyroid, kidney, and brain.21,24-26 During hematopoiesis, MAL gene expression has been shown in leukemic T-cell lines, T-cell clones, and peripheral blood lymphocytes, and our study is the first report describing MAL expression in B cells.21,23 It has been postulated that PMBLs arise from B cells at a terminal stage of differentiation.14 Because MAL expression has been mostly studied in B-cell lines derived from bone marrow precursors or germinal center B cells, the possibility that MAL expression in PMBLs is related to terminal differentiation cannot be formally excluded. Our results could support the hypothesis that PMBLs arise from a specific subset of resident thymic medullary B cells expressing MAL. An immunohistochemical analysis of serial paraffin sections of a neonatal thymus with anti-MAL and anti-CD20 antibodies demonstrated that MAL protein expression was restricted to the thymic cortex in the T-cell areas with very few positive cells in the medulla, where the population of B cells is concentrated (data not shown). The absence of superimposed staining pattern suggests that MAL expression is not a common feature of normal thymic medullary B cells. Furthermore, MAL expression is found in PMBL biopsy samples issued both from the mediastinum and peripheral lymph nodes. These data indicate that MAL gene expression is not related to the anatomic site of origin of these lymphomas. Thus, MAL gene expression in PMBLs could be related to genomic alterations. Southern blot analysis did not show MAL gene rearrangements or gain of chromosomal material. This latter result is in agreement with comparative genomic hybridization studies of PMBLs that demonstrated gains of chromosomal material involving the short arm of chromosome 2 but not the long arm, where the MAL gene was mapped.16 27 However, ponctual mutations, microdeletions, or more distant rearrangements of the gene cannot be excluded, and further experiments are needed to elucidate the origin of MAL expression in PMBL neoplastic B cells.
The MAL gene encodes a highly hydrophobic integral transmembrane protein of 17 kD that belongs to the proteolipid group of proteins.23 MAL protein was found in detergent-insoluble glycolipid-enriched membrane (GEM) microdomains of T lymphocytes, myelin-forming cells, and both polarized kidney and thyroid epithelial cells.24,28,29 GEM domains are lipid rafts enriched in glycosylphosphatidylinositol (GPI)-anchored proteins and other proteins involved in signal transduction, including Src-family kinases and heterotrimeric guanosine triphosphate-binding proteins (G proteins).30 The essential role of rafts in signal transduction in the lymphoid lineage was recently highlighted by several reports. Upon stimulation, activated T-cell receptor and additional signal-transducing molecules are recruited to GEM domains, and disruption of raft structure inhibits the early stages of T-cell receptor signaling.31 Furthermore, Viola et al32 demonstrated that the costimulatory effect of CD28 in resting T cells is mediated to a large extent by its effect on raft redistribution. In addition, molecules participating in B- and T-cell activation, ie, CD44, CD45/lyn (B cells), and CD45/lck/CD4 (T cells), were shown to cofractionate in the GEM domains. 33,34
Several studies suggest that MAL is involved in both membrane trafficking and signaling. MAL was identified in trans-Golgi network-derived transport vesicles in epithelial cells and ectopic expression of MAL in a heterologous cell system induces extensive vesiculation of GEM.28,35 Besides its role as an element of the machinery for apical sorting in polarized epithelial cells, involvement of MAL in membrane signaling was suggested by coimmunoprecipitation experiments that demonstrated a specific association of MAL with the GPI-anchored CD59 protein and the Lck tyrosine kinase in GEM microdomains of T lymphocytes.36,37It was proposed that MAL could act as a transmembrane linker protein that mediates the interactions between GPI-anchored proteins and the Src-like kinases. It is tempting to speculate that MAL abnormal expression in B cells may interfere with GEM specialized functions and have significant implications in cell growth. In support of this hypothesis, caveolin transmembrane proteins, which are chief structural elements of GEM domains in many cell types, were linked to cell proliferation and oncogenic transformation in fibroblast and epithelial cell systems.38-43
In conclusion, our study shows recurrent expression of the MAL gene in PMBLs and suggests that PMBLs may not only have distinct clinicopathologic features, but also harbor particular molecular alterations. These results further support the idea that PMBL is a distinct lymphoma subtype among DLBLs, as proposed in the REAL classification. By analogy to the role of caveolins in tumor cell growth and in view of the putative functions of MAL in GEM vesiculation and signal transduction, it is tempting to speculate that abnormal expression of MAL protein in the B-lymphoid lineage may be related to PMBL lymphomagenesis. Further studies are needed to provide additional insights in the role of MAL in PMBLs.
ACKNOWLEDGMENT
The authors thank Marie-Laure Boulland, Nadine Martin, and Marie-Claude Labastie for their technical assistance.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Fondation de France, and the Association pour la Recherche contre le Cancer (ARC).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Karen Leroy, MD, PhD, INSERM U 474, Hôpital Henri Mondor, 51 av du Mal de Lattre de Tassigny, 94010 Créteil, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal