We have designed an in vivo model in which murine hybridoma cell clones producing human Ig light chains (LC) are administred to mice. Depending on which monoclonal LC is expressed, this model mimicks either cast myeloma nephropathy or the pathological condition defined as myeloma-associated Fanconi’s syndrome (FS) with LC crystallization. Morphological alterations of the kidney cells are thus obtained in mice. All studied LC are closely related human monoclonal VκI proteins, which differ by a limited number of substitutions within the variable region. In the case of an FS monoclonal LC, we show that limited changes introduced through site-directed mutagenesis in the variable domain may suppress formation of intracellular crystals within tubular cells. We also show that multiple peculiarities of the variable region are simultaneously needed to allow LC crystallization; this property thus likely results from a unique LC tridimensional conformation imposed by concomitant somatic mutations of a specific germinally encoded framework.
CAST NEPHROPATHY IS the most frequent renal complication in myeloma patients with Bence-Jones proteinuria.1 It is characterized by casts made up of Tamm-Horsfall glycoprotein and monoclonal Ig light chains (LC) obstructing distal tubule lumens.2 By contrast, myeloma-associated Fanconi’s syndrome (FS) is a rare entity; it is characterized by alterations of proximal tubule functions and in most patients is accompanied by crystalline inclusions in proximal tubular cells, plasma cells, and macrophages. We recently studied and sequenced at the cDNA level the monoclonal κ LC CHEB involved in FS.3 Small protein-enriched gel filtration fractions from patient’s urine yielded crystals morphologically similar to those found in patient’s proximal tubular cells, with the same 60 Å periodicity on electron micrographs. N-terminal sequencing and mass spectrometry studies showed that the crystals contained a 107 amino acid fragment (with a C-terminal lysine) corresponding to the variable (V) domain together with a low proportion of the entire κ chain. In vitro trypsin, pepsin, or cathepsin B treatment of the native entire κ LC yielded a homogeneous V domain fragment that, in contrast to other monoclonal κ LC, was completely resistant to further proteolytic digestion.4 The patient’s κ chain also displayed an unusual self-reactivity by Western blotting. Genes encoding CHEB, as well as 2 other LCs from FS patients studied by our group, TRE and TRO,5 were found to be highly homologous to the same germline VκI gene O2/O12. All 3 patients (CHEB, TRO, and TRE) had numerous intracellular crystals. LC sequences in another patient (DEL) with myeloma associated FS, but no detectable crystals were homologous to another closely related VκI germline gene, O8/O18.5
We hypothesized that some specific and unusual V region sequences would result in the physicochemical properties of LC in FS and be responsible for their impaired trafficking and crystallization within proximal tubular cells. The presence of polar residues at positions 30 and 94 in the CHEB V region sequence was postulated to play a role in crystallization.3,5 With the aim of evaluating the in vivo potential nephrotoxicity of FS LCs, we developed an experimental model in which transfected hybridoma cells expressing such LCs were administred to mice. A similar strategy previously allowed us to reproduce renal deposits featuring LC deposition disease in mice.6 This model is based on the injection into mice of syngeneic transfected tumor cells expressing either the CHEB FS LC or closely related human κI LC (269) as a control. CHEBand 269 κI chains, mutated at positions 30 or 94, were also expressed in mice (Fig 1). This model allowed us to show that limited sequence peculiarities of the variable region can lead to the development of tubular lesions either typical of the FS with LC crystals or of cast myeloma nephropathy.
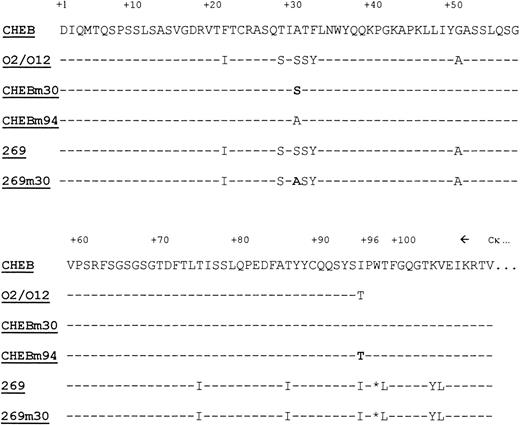
LCs sequences. Alignment of the primary sequences ofCHEB, 269, and mutated CHEBm30, CHEBm94, and 269m30 proteins. Dashes indicate identities with CHEB’s sequence. Bold residues indicate amino acids, which had been modified by site-directed mutagenesis. Stars indicate absent residues. Amino acids are numbered according to Kabat’s numbering.33
LCs sequences. Alignment of the primary sequences ofCHEB, 269, and mutated CHEBm30, CHEBm94, and 269m30 proteins. Dashes indicate identities with CHEB’s sequence. Bold residues indicate amino acids, which had been modified by site-directed mutagenesis. Stars indicate absent residues. Amino acids are numbered according to Kabat’s numbering.33
MATERIALS AND METHODS
Production of human LCs in vitro.
The sequence of the FS chain CHEB encoded by a rearranged VκI-Jκ1 gene was previously determined at the cDNA level.3 The control κI sequence 269 was cloned from human polyclonal B cells and carried an in-frame VκI-Jκ1 coding region. Sp2/0, a B-cell hybridoma lacking endogenous Ig, was transfected with a modified pAKκIV expression vector7 or with the pALI-Eμ vecto,8 carrying the Vκ sequence of either CHEB, 2 variants of CHEB (CHEBm30 [Ala 30 → Ser],CHEBm94 [Ile 94 → Thr]), 269, or a variant of 269 (269m30 [Ser 30 → Ala]). Cotransfection with a neogene was necessary, given the absence of resistance gene in pAKκIV and pALI Eμ. Geneticin-resistant cells were assayed for human LC production by enzyme-linked immunosorbent assay (ELISA) and clones with the highest secretion level of each κ I chain were selected: clones S-CHEB (producing 35 μg/mL/24 h/106 cells of LC), S-269 (2 μg/mL/24 h/106 cells of 269 LC), S-CHEBm30 [Ala 30 → Ser] (2.2 μg/mL/24 h/106 cells), S-CHEBm94 [Ile94 → Thr] (35 μg/mL/24h h/106 cells), and S-269m30 [Ser30 → Ala] (1.4 μg/mL/24 h/106cells).
Production of human LCs in vivo.
Selected clones, S-CHEB, S-269, S-CHEBm30, S-CHEBm94, and S-269m30 (4 × 106 cells) were injected intraperitoneally into 8-week old BALB/c mice (Iffa Credo, L’Arbresle, France). These mice were called CHEB, 269, CHEBm30,CHEBm94, or 269m30 mice. After development of tumor, urine and serum were collected and κ chain secretion was evaluated by ELISA. Mice were killed about 5 weeks after injection (mean, 35.6 days; standard deviation [SD], 4.75 days). At that time, the average body weight of mice was 35.13 g (SD, 2.53 g).
Immunochemical studies.
For Western blots, culture supernatants, urine, and sera were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose sheets, and shown with antihuman κ chain alkaline-phosphatase–conjugated polyclonal antiserum (Amersham, Buckinghamshire, UK).
Immunomorphologic analysis.
Light microscopic examination was performed on slides stained either with hematoxilin/eosin (HES), Periodic Acid Schiff, light green trichrome, silver methenamine, or toluidine blue. Frozen blocks of liver, kidney, spleen, heart, and tumor obtained at death were cut in 4-μm-thick slices. Organs were studied for the presence of human κ chains by immunofluorescence with fluorescein-conjugated polyclonal rabbit antisera against human κ or λ chain (DAKO, Glostrup, Denmark) or with a combination of mouse monoclonal antibody against human κ chain and a rabbit antimouse Ig-rhodamine conjugate (Zymed, San Francisco, CA).
For immunoelectronmicroscopic studies, samples were fixed in 4% glutaraldehyde. Staining with either biotinylated antihuman κ, antihuman λ sera, or biotinylated peptostreptococcus magnus protein L (Zymed), shown by gold-tagged streptavidin (10-nm EM grade; Zymed), was additionnally performed. Binding of rabbit anticathepsin D antiserum (DAKO) was shown by 10-nm gold-conjugated goat antirabbit IgG (Sigma, Aldrich Chimie, L’Isle d’Abeau Chesnes, France). Specificity was controlled using normal rabbit serum instead of anticathepsin D antibody. Sections were then stained with uranyl acetate and examined in a Jeol 100 CX electron microscope (Akishima, Japan).
Nucleic acids studies and sequence analysis.
Total RNA (10 μg) from untransfected Sp2/0 cells, S-CHEB, and S-269 transfectomas from 269-4 and CHEB-9 mice tumors and from patientCHEB’s bone marrow were analyzed on 1% agarose, 0.7 mol/L formaldehyde gels, transferred onto nylon sheets (Amersham), and hybridized with a human Cκ exon probe.
For sequencing, 2 μg of total RNA from 269-4 and CHEB-9 mice tumors were used as templates for synthesis of complementary DNA (cDNA) by reverse transcriptase (Boerhinger, Mannheim, Germany). cDNA were amplified by polymerase chain reaction (PCR),9 cloned inSma I cut M13 mp19 vector, and sequenced by the dideoxynucleotide method10 on an ABI 310 DNA sequencer (Perkin-Elmer, Branchburg, NJ).
Site-directed mutagenesis.
Site-directed mutagenesis was performed by PCR of the VJ segment cDNA: the first 2 amplifications were performed separately on both flanks of the mutation to be introduced, (1) on 1 side (PCR1) with a 5′ primer corresponding to the VκI leader and a 3′ primer introducing the mutated base and (2) on the other side (PCR2), with a 5′ primer introducing the desired mutation and a 3′ primer corresponding to Jκ. Complementary primers introducing the mutations were chosen so that both mutated fragments overlapped by 10 bp. The third round (PCR3) allowed mutated segments to anneal and restored a complete mutated VJ region by using a 5′ primer corresponding to the VκI leader and a 3′ primer corresponding to Jκ. PCR1 and 2 consisted of 35 cycles of 94°C for 30 seconds, 53°C for 30 seconds, and 72°C for 30 seconds. PCR3 consisted of 3 cycles of 94°C for 30 seconds, 45°C for 30 seconds, and 72°C for 45 seconds for the hybridization of the 2 mutated segments, followed by 30 cycles of 94°C for 30 seconds, 53°C for 30 seconds, and 72°C for 30 seconds for the amplification of the entire V domain.
The forward primer corresponding to the VκI leader and used for all κI sequences was 5′- AAGTCGACATGGACATGAGGGTGCC-3′. The backward primers corresponding to the 3′ end of the Jκ ofCHEB and L269 were as follows: JCHEB: 5′- TTCTCGAGACTTACGTTTGATTTCCACCTTGGT-3′; and J269: 5′- TTCTCGAGACTTACGTTTGATCTCCAGCTTGGT-3′.
Sequences of the other primers used in the directed mutagenesis were as follows: (1) mutant CHEB m30 (Ala 30 → Ser): PCR1 forward primer: VκI; backward primer: 5′- TTAAAAAGGTGCT AATGGTCTGA-3′; PCR2 forward primer: 5′- TCAGACCATTAG CACCTTTTTAA-3′; backward primer: JCHEB; PCR3 forward primer: VκI; backward primer: JCHEB; (2) mutant CHEB m94 (Ile 94 → Thr): PCR1 forward primer: VκI; backward primer: 5′-ACGTCCACGGGG TACTGTAACTC-3′; PCR2 forward primer: 5′-GAGTTACAGTAC CCCGTGGACGT-3′; backward primer: JCHEB; PCR3 forward primer VκI; backward primer: JCHEB; (3) mutant 269 m30 (Ser30 → Ala): PCR1 forward primer: VκI; backward primer: 5′- TTAAATAGCTGGC AATGCTCTGA-3′; PCR2 forward primer: 5′- TCAGAGCATTGC CAGCTATTTAA-3′; backward primer: J269; PCR3 forward primer: VκI; backward primer: J269.
ELISA.
96-well plates were coated with 100 μL of antihuman κ LC antibody (Kallestad, Sanofi Diagnostic, Pasteur, France) diluted 1/1,000 in phosphate-buffered saline (PBS) and then saturated with 200 μL of PBS/0.1% bovine serum albumin. After incubation with samples, the second antibody was an alkaline phosphatase-conjugated antihuman κ antibody (Sigma, L’Isle d’Abeau Chesnes, France). After addition of the substrate, plates were kept at room temperature in the dark for 15 minutes; reaction was stopped with 50 μL NaOH 3N and plates were read at 405 nm.
Tubule suspension and uptake studies.
Proximal tubules were isolated immediately after death: kidneys were decapsulated and kept at 4°C in Hank’s Balanced Salt Solution (HBSS) supplemented with 10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, and 5 mmol/L D-glucose (HBSS-HEPES). The cortex was separated from the medulla and dilacerated. Proximal tubules were isolated by filtering on a Nylon mesh (106 μm) and washed twice with HBSS-HEPES. Uptake of inorganic phosphate (Pi) was performed at 37°C in a buffered solution with the following composition: 137 mmol/L NaCl, 5.4 mmol/L KCl, 1 mmol/L CaCl2, 1.2 mmol/L MgSO4, and 15 mmol/L HEPES (pH 7.4). The sodium-free solution was made iso-osmotic by replacing sodium chloride with N-methyl-D-glutamine. Tubule suspensions were washed twice with the uptake solution and incubated in the presence of K2H32PO4 (0.5 μCi/mL) and 100 μmol/L KH2PO4 at 37°C. Uptake was stopped by washing the suspension twice with ice-cold solution 137 mmol/L NaCl, 15 mmol/L HEPES, pH 7.4. Cells were then solubilized in 0.5% Triton X-100 and aliquots were counted by liquid scintillation. Protein concentrations were determined by Bradford’s method.11
RESULTS
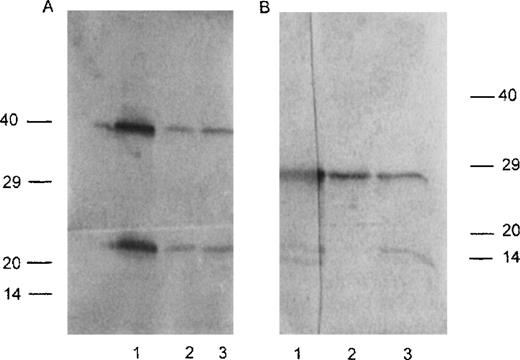
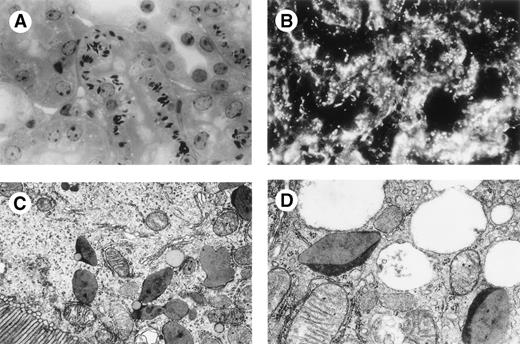
A high secretion rate of human κ chain was observed in vitro after transfection of Sp2/0 hybridoma cells with cDNA expression vectors encoding either the FS κI chain CHEB, the control κI chain 269, the 2 mutated CHEB chains (CHEBm30 andCHEBm94), or the mutated 269m30 chain. The mutated CHEBchains did not differ from the original CHEB κ chain in their apparent molecular mass in PAGE-SDS or in their ability to polymerize as dimers and tetramers (Fig 2A and B). In vivo, human κ chains were produced in mice injected with these transfected clones (Table1), but not in control mice injected with untransfected Sp2/0 cells (C mice; not shown). In C mice, kidneys were normal. In 7 of 10 CHEB mice, light microscopy examination of kidney samples showed marked proximal tubular cell lesions with clear cytoplasmic atrophy and numerous intracellular crystals; toluidine blue typically stained multiple rhomboid crystals within a large number of proximal tubular cells (Fig 3A). Rarely (2 of 10 mice) were crystals found in the tubular lumens, but no myeloma casts were found. By immunofluorescence, these intracellular crystals strongly stained with an antihuman κ chain (Fig 3B). Electron microscopic analysis of proximal tubular cells of CHEB mice showed that inclusions in proximal tubular cells were surrounded by smooth membranes and were located predominantly in the apical pole of cells (Fig 3C) and also confirmed the crystalline nature of the hexagonal inclusions with a regular striation of 60 Å periodicity (Fig 3D). By immunoelectronmicroscopy, these crystals were stained with antihuman κ chain, anticathepsin D sera, and protein L (which specifically binds Vκ domains). The results observed with CHEB mice were very similar to those observed in patient CHEB, either by immunofluorescence labeling pattern with the antihuman κ chain (Fig 4A) or by electronmicroscopic examination of intracellular crystals (Fig 4B and C). Transfected hybridoma cells themselves producing the CHEB κ chain did not contain any intracytoplasmic crystalline inclusions (data not shown). Similar analysis on kidney biopsies from CHEBm30 andCHEBm94 mice did not show crystals in tubular cells (Fig 5A and B), but only cytoplasmic droplets indicating internalization of the chain by the normal process of proximal tubular reabsorption (9 of 9 and 7 of 7, respectively).
Western blot pattern for CHEB LC and its mutants. Western blot analysis of LCs produced in vitro by transfected Sp2/0 cells. (A) Nonreduced conditions. Lane 1, CHEB chain; lane 2,CHEBm30; lane 3, CHEBm94. (B) Reduced conditions. Lane 1, CHEB chain; lane 2, CHEBm30; lane 3,CHEBm94. Molecular weight markers are indicated in kilodaltons. The difference in migration of molecular weight markers between (A) and (B) reflects nonreducing versus reducing conditions.
Western blot pattern for CHEB LC and its mutants. Western blot analysis of LCs produced in vitro by transfected Sp2/0 cells. (A) Nonreduced conditions. Lane 1, CHEB chain; lane 2,CHEBm30; lane 3, CHEBm94. (B) Reduced conditions. Lane 1, CHEB chain; lane 2, CHEBm30; lane 3,CHEBm94. Molecular weight markers are indicated in kilodaltons. The difference in migration of molecular weight markers between (A) and (B) reflects nonreducing versus reducing conditions.
Light Chain Production by the Mice and Microscopic Analysis of the Kidneys
| . | Mice . | Serum κ Chain (μg/mL) . | Urine κ Chain (μg/mL) . | κ Chain Crystals . | Myeloma Casts . |
|---|---|---|---|---|---|
| CHEB | 1 | 22 | 64 | − | − |
| 2 | 16 | 41 | − | − | |
| 3 | 6 | 138 | − | − | |
| 4 | 230 | 6500 | + | − | |
| 5 | 1040 | 1931 | + | − | |
| 6 | 82 | 130 | + | − | |
| 7 | 120 | 4630 | + | − | |
| 8 | 156 | 6328 | + | − | |
| 9 | 140 | 2530 | + | − | |
| 10 | ND | ND | + | − | |
| 269 | 1 | 82 | 517 | − | − |
| 2 | 120 | ND | − | + | |
| 3 | 65 | 44 | − | + | |
| 4 | 207 | ND | − | + | |
| 5 | 90 | 455 | − | + | |
| 6 | 121 | 1054 | − | + | |
| 7 | 25 | 120 | − | − | |
| 8 | 32 | 159 | − | − | |
| 9 | 13 | 159 | − | − | |
| 10 | ND | ND | − | − | |
| CHEBm30 | 1 | 91 | 328 | − | + |
| 2 | 99 | 338 | − | − | |
| 3 | 12.65 | ND | − | − | |
| 4 | 45.1 | ND | − | − | |
| 5 | 7.9 | 370 | − | − | |
| 6 | 740 | 89.2 | − | − | |
| 7 | 41.7 | 735 | − | − | |
| 8 | 12.4 | 258 | − | − | |
| 9 | 22.3 | 501 | − | ± (rare) | |
| CHEBm94 | 1 | 81 | 1160 | − | − |
| 2 | 112 | 372 | − | − | |
| 3 | 147 | 3080 | − | − | |
| 4 | 257 | 2980 | − | − | |
| 5 | 172 | 3000 | − | − | |
| 6 | 297 | 1400 | − | − | |
| 7 | 253 | 3050 | − | − | |
| 269m30 | 1 | 82 | 793 | − | − |
| 2 | 30.3 | 670 | − | + | |
| 3 | 28 | 840 | − | + | |
| 4 | 8 | 205 | − | − | |
| 5 | 14 | 206 | − | + | |
| 6 | 28.6 | ND | − | − | |
| 7 | 30.6 | ND | − | + | |
| 8 | 35.6 | ND | − | − |
| . | Mice . | Serum κ Chain (μg/mL) . | Urine κ Chain (μg/mL) . | κ Chain Crystals . | Myeloma Casts . |
|---|---|---|---|---|---|
| CHEB | 1 | 22 | 64 | − | − |
| 2 | 16 | 41 | − | − | |
| 3 | 6 | 138 | − | − | |
| 4 | 230 | 6500 | + | − | |
| 5 | 1040 | 1931 | + | − | |
| 6 | 82 | 130 | + | − | |
| 7 | 120 | 4630 | + | − | |
| 8 | 156 | 6328 | + | − | |
| 9 | 140 | 2530 | + | − | |
| 10 | ND | ND | + | − | |
| 269 | 1 | 82 | 517 | − | − |
| 2 | 120 | ND | − | + | |
| 3 | 65 | 44 | − | + | |
| 4 | 207 | ND | − | + | |
| 5 | 90 | 455 | − | + | |
| 6 | 121 | 1054 | − | + | |
| 7 | 25 | 120 | − | − | |
| 8 | 32 | 159 | − | − | |
| 9 | 13 | 159 | − | − | |
| 10 | ND | ND | − | − | |
| CHEBm30 | 1 | 91 | 328 | − | + |
| 2 | 99 | 338 | − | − | |
| 3 | 12.65 | ND | − | − | |
| 4 | 45.1 | ND | − | − | |
| 5 | 7.9 | 370 | − | − | |
| 6 | 740 | 89.2 | − | − | |
| 7 | 41.7 | 735 | − | − | |
| 8 | 12.4 | 258 | − | − | |
| 9 | 22.3 | 501 | − | ± (rare) | |
| CHEBm94 | 1 | 81 | 1160 | − | − |
| 2 | 112 | 372 | − | − | |
| 3 | 147 | 3080 | − | − | |
| 4 | 257 | 2980 | − | − | |
| 5 | 172 | 3000 | − | − | |
| 6 | 297 | 1400 | − | − | |
| 7 | 253 | 3050 | − | − | |
| 269m30 | 1 | 82 | 793 | − | − |
| 2 | 30.3 | 670 | − | + | |
| 3 | 28 | 840 | − | + | |
| 4 | 8 | 205 | − | − | |
| 5 | 14 | 206 | − | + | |
| 6 | 28.6 | ND | − | − | |
| 7 | 30.6 | ND | − | + | |
| 8 | 35.6 | ND | − | − |
Phosphate Uptake Experiments
| . | BALB/c . | CHEB . | CHEBm94 . | 269 . | Sp2/0 . |
|---|---|---|---|---|---|
| 16/06/97 | 6.49 | 4.17 | — | 4.56 | — |
| 11/10/97 | — | 4.26 | 3.15 | — | 5.6 |
| 21/10/97 | 5.19 | — | — | 4.87 | — |
| 22/10/97 | 6.42 | — | — | — | |
| 18/05/98 | 8.01 | — | — | 4.2 | — |
| 20/05/98 | 5.16 | 3.15 | 3.27 | 3.47 | — |
| M | 6.25 | 3.86 | 3.21 | 4.28 | 5.60 |
| N | 5 | 3 | 2 | 4 | 1 |
| SD | 1.17 | 0.62 | 0.08 | 0.60 | — |
| SEM | 0.52 | 0.36 | 0.06 | 0.30 | — |
| . | BALB/c . | CHEB . | CHEBm94 . | 269 . | Sp2/0 . |
|---|---|---|---|---|---|
| 16/06/97 | 6.49 | 4.17 | — | 4.56 | — |
| 11/10/97 | — | 4.26 | 3.15 | — | 5.6 |
| 21/10/97 | 5.19 | — | — | 4.87 | — |
| 22/10/97 | 6.42 | — | — | — | |
| 18/05/98 | 8.01 | — | — | 4.2 | — |
| 20/05/98 | 5.16 | 3.15 | 3.27 | 3.47 | — |
| M | 6.25 | 3.86 | 3.21 | 4.28 | 5.60 |
| N | 5 | 3 | 2 | 4 | 1 |
| SD | 1.17 | 0.62 | 0.08 | 0.60 | — |
| SEM | 0.52 | 0.36 | 0.06 | 0.30 | — |
Summary of the results obtained in phosphate uptake experiments. Results are expressed in nmol/5min/mg protein. N corresponds to the number of experiments; for each, 2 or 3 mice were killed for kidney cell preparations. Uptakes were measured 3 times each.
Abbreviations: M, mean; SD, standard deviation; SEM, standard error of the mean.
Mouse CHEB kidney. (A) Light microscopy, toluidine blue staining (original magnification × 500): strong staining of crystalline inclusions in the cytoplasm of proximal tubular cells. (B) Immunofluorescence microscopy, anti-κ human conjugate (original magnification × 500): these inclusions are strongly stained with a pattern similar to that found in patients’ CHEB tubular proximal cells. Electron microscopy: (C) original magnification × 1,200; (D) original magnification × 25,000: these crystalline inclusions are osmiophilic and similar to that found in patient CHEB with the same 60 Å periodic striation.
Mouse CHEB kidney. (A) Light microscopy, toluidine blue staining (original magnification × 500): strong staining of crystalline inclusions in the cytoplasm of proximal tubular cells. (B) Immunofluorescence microscopy, anti-κ human conjugate (original magnification × 500): these inclusions are strongly stained with a pattern similar to that found in patients’ CHEB tubular proximal cells. Electron microscopy: (C) original magnification × 1,200; (D) original magnification × 25,000: these crystalline inclusions are osmiophilic and similar to that found in patient CHEB with the same 60 Å periodic striation.
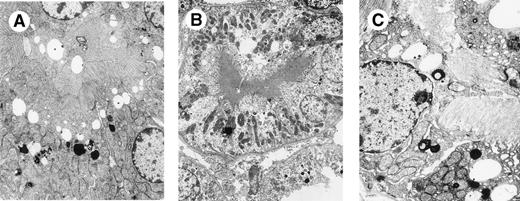
Patient CHEB, kidney biopsy. (A) Immunofluorescence microscopy, anti-κ conjugate (original magnification × 500), heavy staining of cytoplasmic inclusions in proximal tubular cells. (B) Electron microscopy (original magnification × 2,600): numerous crystalline and osmiophilic inclusions in the cytoplasm of proximal tubular cells. (C) Electron microscopy (original magnification × 16,000): details of intracytoplasmic crystalline inclusions.
Patient CHEB, kidney biopsy. (A) Immunofluorescence microscopy, anti-κ conjugate (original magnification × 500), heavy staining of cytoplasmic inclusions in proximal tubular cells. (B) Electron microscopy (original magnification × 2,600): numerous crystalline and osmiophilic inclusions in the cytoplasm of proximal tubular cells. (C) Electron microscopy (original magnification × 16,000): details of intracytoplasmic crystalline inclusions.
Mutant CHEB mice and 269 mice kidneys. (A) MouseCHEBm30 no. 1, kidney, electron microscopy (original magnification × 6,000). (B) Mouse CHEBm94 no. 1, kidney, electron microscopy (original magnification × 2,500). (C) Mouse 269 no. 10, kidney, electron microscopy (original magnification × 4,000): moderate or heavy increase of lysosomal component in proximal tubular cells with variable apical vacuolation secondary to reabsorption of mutated CHEB or 269 LCs. Crystalline inclusions are absent in the lysosomal compartment.
Mutant CHEB mice and 269 mice kidneys. (A) MouseCHEBm30 no. 1, kidney, electron microscopy (original magnification × 6,000). (B) Mouse CHEBm94 no. 1, kidney, electron microscopy (original magnification × 2,500). (C) Mouse 269 no. 10, kidney, electron microscopy (original magnification × 4,000): moderate or heavy increase of lysosomal component in proximal tubular cells with variable apical vacuolation secondary to reabsorption of mutated CHEB or 269 LCs. Crystalline inclusions are absent in the lysosomal compartment.
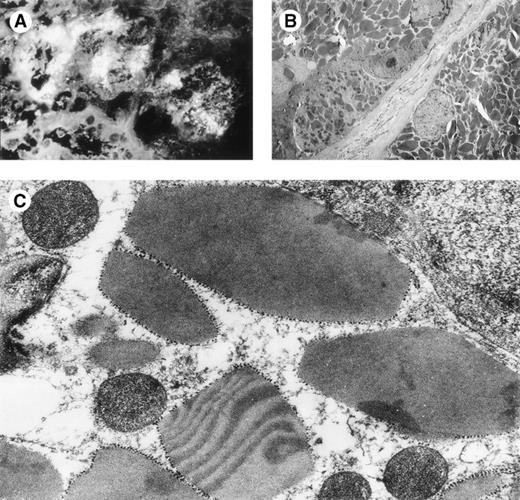
Finally, in κI 269 control mice, the proximal tubular cells contained droplets (8 of 10) and were sometimes atrophic, but no crystal inclusions were found (Fig 5C). Surprisingly, large tubular myeloma casts were found in 5 of 10 269 mice (Fig6A), which strongly stained with antihuman κ conjugate (Fig 6B and C). The same pattern was observed in mice expressing the mutant 269m30 of the control 269 chain, with myeloma casts in 4 of 8 mice and no crystals (Table 1). Interestingly, CHEBm30 κ chains, sharing both Ser30 and Ile94 residues with 269 LC, also led to the formation of myeloma casts in 2 injected mice (no. 1 and 9 in Table 1).
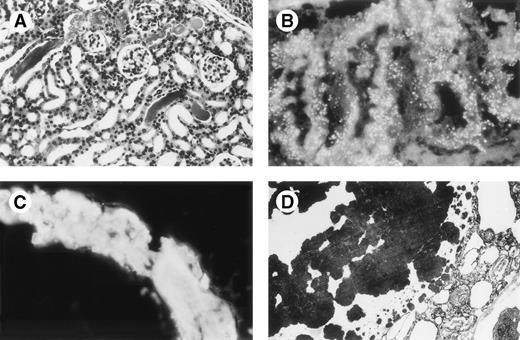
Mice 269 kidneys. (A) Mouse 269 no. 3, kidney, light microscopy, HES staining (original magnification × 200): glomeruli without abnormalities, tubular casts are large with fracture lines, tubular lumen are enlarged. (B) Mouse 269 no. 8, kidney, immunofluorescence microscopy, antihuman κ conjugate (original magnification × 500): numerous reabsorptive granules in the cytoplasm of proximal tubular cells with a pattern different from that noted in Fig 3 upper right quadrant. (C) Immunofluorescence microscopy, antihuman κ LC conjugate: strong staining of a cast in 1 tubular lumen. Note fracture lines in cast. Tubular basement membranes are unstained. Staining for human λ LC and mouse Igs were negative. (D) Electron microscopy (original magnification × 10,000): osmiophilic myeloma cast without crystalline substructure.
Mice 269 kidneys. (A) Mouse 269 no. 3, kidney, light microscopy, HES staining (original magnification × 200): glomeruli without abnormalities, tubular casts are large with fracture lines, tubular lumen are enlarged. (B) Mouse 269 no. 8, kidney, immunofluorescence microscopy, antihuman κ conjugate (original magnification × 500): numerous reabsorptive granules in the cytoplasm of proximal tubular cells with a pattern different from that noted in Fig 3 upper right quadrant. (C) Immunofluorescence microscopy, antihuman κ LC conjugate: strong staining of a cast in 1 tubular lumen. Note fracture lines in cast. Tubular basement membranes are unstained. Staining for human λ LC and mouse Igs were negative. (D) Electron microscopy (original magnification × 10,000): osmiophilic myeloma cast without crystalline substructure.
Identity of LC produced in mice with the expected translation products of the expression vectors was checked at the mRNA and protein levels. Northern blotting of total RNAs from the transfected clones grown in vitro or isolated from tumors showed κ mRNAs of the size expected from the splice sites used in the vectors (data not shown). No human κ mRNA was detected in C mice tumors. No staining was found in proximal tubular cells of C mice with antihuman κ conjugate. Staining with the antihuman λ conjugate was negative in all mice. Western blots of urines of CHEB and 269 mice showed normal sized κ chains (data not shown). The absence of any mutation in transfected genes during tumoral growth in mice was checked by sequencing κ cDNAs from representative CHEB and κI mice.
Preliminary uptake studies on renal tubule suspensions showed a significant decrease in the uptake of Pi in CHEB mice when compared with normal BALB/c control mice and BALB/c mice injected with untransfected Sp2/0 cells (P < .05). A similar reduction in the Pi uptake was also observed in CHEBm94 mice and in 269 mice, when compared with control mice injected with Sp2/0 and uninjected BALB/c mice (P < .05). Because there was no significant difference in the reduction of Pi uptake betweenCHEB mice and CHEBm94 mice or 269 mice (P = .10), the alteration of proximal tubule Pi uptake could not be ascribed to the presence or the absence of intracytoplasmic crystals within proximal tubular cells (Table 2).
DISCUSSION
We have established a murine model of human FS with κI LC crystallization in proximal tubular cells (CHEB mice expressing a human FS κ I chain) and by chance, a murine model of myeloma cast nephropathy without crystals in mice secreting a randomly-selected κI chain. Long ago, it had been postulated that peculiarities of certain LC V domains may promote their agregation into fibrils in AL-amyloidosis,12-22 their deposition along basement membranes in nonamyloid LC deposition disease,6,23-29 or their crystallization in the cytoplasm of proximal tubular cells in FS.3 5 We speculated that an in vivo model of FS, allowing site-directed mutagenesis on the sequence of a nephrotoxic human κI chain, would be ideally suited for addressing the issue of which particular residues are responsible for the abnormal behavior of the protein.
Indeed, when mice were injected with tumours secreting the CHEBFS κI chain, pathologic alterations of the kidneys typical of FS with intracellular crystals were readily induced and physiological alterations of phosphate tubular reabsorption also appeared. By contrast, mice injected with tumors secreting a control κI chain (269) yielded no crystals, but did develop intratubular formations with the typical aspect of myeloma casts. Although this control κI chain was chosen randomly among κI chain sequences from polyclonal B cells, its ability to induce cast nephropathy when expressed in high amounts was not unexpected, given the high frequency of this nephropathy in LC myeloma patients.
We then decided to express variants of these 2 κI chains in the same model. LCs are of the κ type in more than 90% of myeloma-associated FS with intracellular crystals, with a strong overrepresentation of the VκI subgroup.5 Although up to 56% of myeloma cases produce κ chains of the κI subgroup, FS stands as a rare complication of myeloma30,31; it thus appears likely that specific somatic mutations of κI genes are involved in nephrotoxicity and that subgroup-specific germline sequences are not sufficient to confer toxicity. This is in clear contrast with the situation of λ6 LCs in AL-amyloidosis, in which all known myeloma patients with free λ6 monoclonal LCs had amyloidosis.13 In FS, it is likely that a germinally encoded framework is needed, but that somatically created mutations finally confer a nephrotoxic potential to LC; indeed, among published FS LC sequences, 3 were derived from the same germline gene O2/O12 and were associated with LC crystallization, the fourth one derived from the closely related O8/O18 gene did not yield any crystal. Comparison of these sequences focused our attention on specific substitutions at position 30, where the unusual presence of nonpolar residues is shared by the 3 FS LC CHEB, TRE, andTRO5 featuring crystallization. In LC CHEB, Ala 30 is encoded by the codon GCC and may either result from allelic variation or from a block mutation of the original AGC codon of the published O2/O12 germline gene. In FS patient DEL5without crystallization, VκI position 30 was occupied by serine, the most frequent amino acid at this position.
The importance of Ala30 in protein CHEB is most clearly seen in mice expressing CHEBm30 LC with an Ala→Ser substitution introduced through site-directed mutagenesis, because these mice had no intracellular crystals. Indeed, 2 of these mice presented tubular cast nephropathy, thus showing that a single amino acid substitution can change the crystal-forming property into the ability to yield partially organized agregates. Another unusual nonpolar residue of proteinCHEB, Ile 94, was targeted in the same way: the CHEBm94 variant κI chain did not yield crystals either. Taken together, our data directly demonstrate that sequence peculiarities of V domains are implicated in the process of LC crystallization that occurs in most cases of myeloma-associated FS and show that slightly different V domain sequences may confer distinct pathogenetic properties on LC, leading either to FS or cast myeloma. Although FS κI chains likely originate from a limited set of germline genes, we show that, in the case of protein CHEB, concomitant alterations of such a germline sequence are needed at least at 2 different positions (30 and 94) for the process of crystallization to occur. Interestingly, simultaneous occurrence of these very same substitutions in the context of a closely related VκI framework is not sufficient to induce crystallization in κI variant 269m30. Because protein crystal formation probably requires nucleation-dependent initiation,32 unusual amino acids at position 30 may increase the stability of monomer or dimer interactions, as suggested for amyloidogenic LC21 and could thus initiate the formation of crystal-forming units.32 However, the rare ability of κ chains to form crystals also clearly involves the whole tridimensional structure of the V domain and a precise combination of germinally and somatically encoded residues.
In vivo studies and clearance measurements could not be performed. Consequently, proximal tubule function was evaluated ex vivo by uptake studies on freshly isolated proximal tubule suspensions. Phosphate proximal tubule uptake was significantly decreased in proximal tubule cells from CHEB mice, thus suggesting that transfection of κ LC induced 1 cardinal feature of the proximal tubule dysfunction characteristic of FS. In our model, the mechanisms of the alteration of phosphate proximal tubule uptake remain to be elucidated. Notably, tubular necrosis was not observed and inhibition of phosphate uptake could theoretically result either from inhibition of the Na/Pi brush border membrane cotransport or from decreased availability of the transporter. Interestingly, CHEBm94 mice and κI mice did not have any intracellular crystal formation in proximal tubular cells, but exhibited a significant reduction of the phosphate uptake. These results suggest that the ability of CHEB κ LC to inhibit tubular phosphate uptake may be independent from its property to crystallize within proximal tubule cells. Indeed, clinical reports have well established the absence of crystal formation in some patients with multiple myeloma-associated FS.5
C.D. and A.R. contributed equally to this work.
Supported by grants from Fondation contre la Leucémie (Grant No. 91001123), Ligue contre le Cancer (Comité Régional de la Vienne), Conseil Régional du Limousin, Association pour la Recherche sur la Cancer (Grant No. 9121), INSERM 4R001B, and PHRC AOM 96058. C.D. is the recipient of a fellowship from Association pour la Recherche sur le Cancer.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M. Cogné, MD, Laboratoire d’Immunologie, CNRS EP118, Faculté de Médecine, 2 rue du Dr Marcland, F87025 Limoges, France; e-mail:cogne@unilim.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal