Abstract
Hemopexin (Hx) is a plasma glycoprotein mainly expressed in liver and, less abundantly, in the central and peripheral nervous systems. Hx has a high binding affinity with heme and is considered to be a major transport vehicle of heme into the liver, thus preventing both heme-catalyzed oxidative damage and heme-bound iron loss. To determine the physiologic relevance of heme-Hx complex formation, Hx-deficient mice were generated by homologous recombination in embryonic stem (ES) cells. The Hx-deficient mice were viable and fertile. Their plasma iron level and blood parameters were comparable to those of control mice and they showed no evidence of tissue lesions caused by oxidative damage or abnormal iron deposits. Moreover, they were sensitive to acute hemolysis, as are wild-type mice. Nevertheless, Hx-null mice recovered more slowly after hemolysis and were seen to have more severe renal damage than controls. After hemolytic stimulus, Hx-deficient mice presented prolonged hemoglobinuria with a higher kidney iron load and higher lipid peroxidation than control mice. Moreover, Hx-null mice showed altered posthemolysis haptoglobin (Hp) turnover in as much as Hp persisted in the circulation after hemolytic stimulus. These data indicate that, although Hx is not crucial either for iron metabolism or as a protection against oxidative stress under physiologic conditions, it does play an important protective role after hemolytic processes.
HEMOPEXIN (Hx) is a 60-kD plasma glycoprotein with a high binding affinity to heme in an equimolar ratio.1,2 It is mainly expressed in liver and belongs to the family of the acute-phase proteins whose synthesis may be induced by several cytokines as a result of inflammatory processes.3,4 Other than in the liver, Hx is expressed in the CNS,5 in the retina,6,7 and in peripheral nerves.8 Subsequent to heme binding, Hx undergoes a conformational change that allows for interaction with a specific receptor mainly expressed on the hepatocyte membrane and its internalization. In the cytosol, heme is catabolized into bilirubin, biliverdin, and iron, and the Hx-Hx receptor complex is then recycled.9 Receptors for heme-Hx are expressed not only by liver parenchymal cells,10 but also by retinal pigment epithelia cells,6 lymphocytes, and several cell lines.11
Hx is the plasma protein that has the highest affinity for heme (affinity constant [kd]< 1 pmol/L). Heme is composed of protoporphyrin IX and iron forming a rigid hydrophobic planar structure, which rapidly intercalates into lipid membranes and other hydrophobic compartments when not associated with proteins.12 As heme is able to intercalate into lipid membranes and participate in the Fenton reaction in the production of hydroxyl radicals, it is a potent catalyst for injury from hydrogen peroxide, oxidized low-density lipoprotein, and activated neutrophils.13,14 Under normal conditions, the presence of heme in plasma is due to oxidation of hemoglobin, which is released during the enucleation of erythroblasts or in states of intramedullary or intravascular hemolysis. When this process produces a low hemoglobin concentration, most of it dissociates in αβ dimers, which rapidly bind to haptoglobin (Hp) and metabolize in the liver. The plasma hemoglobin that remains unbound to Hp is quickly oxidized into ferrihemoglobin that, in turn, dissociates into globin and ferriheme. Ferriheme can then be bound by albumin (kd ∼10 nmol/L), transferred to Hx, and transported to the liver.9 Heme concentration increases in plasma after hemolysis and this state, in humans, is associated with several pathologic conditions such as reperfusion injury and/or ischemia.9
The strength of the binding between heme and Hx, together with the presence of specific receptors for the heme-Hx complex on liver parenchyma cells, have led to the belief that Hx is mainly responsible for the transportation of heme into the liver. Consequently, it may be hypothesized that the function of Hx is that of preventing both heme-bound iron loss and heme-catalyzed oxidative damage.15,16 Such a hypothesis is supported by data on cultured primary hepatocytes and hepatoma cell lines demonstrating that, once the heme-Hx complex is bound and internalized by the Hx receptor, several events involved in cellular response to stress take place. These include (1) induction of heme oxygenase 1 (HO-1), ferritin, and metallothionein 1 (MT-1) genes that function at an intracellular level as antioxidants17,18; and (2) rapid c-jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activation with phosphorylation of c-jun and nuclear translocation of NFkB together with the induction and maintenance of p21 and p53.19 These events may help cell survival, in as much as the cell neutralizes the oxidative potential of heme and iron. Therefore, it would logically follow that Hx would tend to target heme towards a discrete set of tissues that express Hx receptor and are able to catabolize heme and would also protect receptor-null cells, including endothelial cells, by preventing the diffusion of free heme.
On the other hand, the pivotal role of Hx in scavenging free heme from circulation was recently questioned by Taketani et al,20who showed that primary rat hepatocytes are able to uptake heme from culture medium directly from albumin, and that concentrations of Hx in excess of heme inhibit both heme uptake and heme-mediated HO-1 induction. Moreover, cultured adult rat hepatocytes exhibit only about 2,000 Hx receptors per cell, which is not consistent with the magnitude of the heme flux into hepatocytes.21 When these data are considered together, it suggests that heme may be transferred from albumin with the help of a plasma membrane heme transporter and that, having crossed the hepatocyte membrane, it is then bound by cytosolic heme-binding proteins.
In an effort to determine the physiologic relevance of the heme-Hx complex formation in vivo, Hx-deficient mice were generated by homologous recombination in embryonic stem (ES) cells. It was observed that, under physiologic conditions, Hx depletion is not crucial for viability, fertility, or heme-iron metabolism. Nevertheless, following acute hemolysis, Hx-deficient mice recover less efficiently than control mice and suffer major renal damage. Even several days after hemolytic stimulus, they still present elevated hemoglobinuria and accumulate renal iron causing oxidative damage. Moreover, Hp turnover after hemolysis is altered in Hx-null mice, since Hp persisted in the circulation for several days after hemolytic stimulus, suggesting the existence of compensatory mechanisms between Hx and Hp in the acute-phase reaction.
MATERIALS AND METHODS
Hx-targeting vector.
Genomic DNA from the Hx locus was isolated by a 129/SV genomic library using a human Hx cDNA probe. A 12-kb ClaI-BamHI clone containing exons 1 to 4 of the Hx gene and approximately 10-kb upstream sequences was used to construct a targeting vector. A restriction site for SalI was inserted instead of the ATG start codon in the first exon of the Hx gene using polymerase chain reaction (PCR). This restriction site was used to insert a 5-kb lacZ-PGKneo cassette.22
Generation of Hx −/− mice.
Gene targeting in ES cells and the generation of Hx-mutant mice were performed as previously described.23 Briefly, the targeting vector was linearized with NotI and introduced by electroporation into approximately 1.5 × 107 R1 ES cells. After 24 hours, the cells were placed under selection with 400 μg/mL G418 (GIBCO, Gaithersburg, MD) for 7 to 9 days. The genomic DNAs of resistant clones were digested with EcoRV and analyzed by Southern blotting with a 3′ 1-kb external probe encompassing exons 5 and 6 of the Hx gene. Three targeted ES cell clones were injected into the blastocysts of C57B6 mice and transferred into pseudopregnant females as described previously.23 Chimeric male offspring were bred to C57B6 or 129/SV females and F1offspring were tested for transmission of the disrupted allele by Southern blot analysis. Homozygous F2 mutant mice were obtained by the heterozygous mating of the F1 mice.
Northern blot.
Total RNA was extracted from the liver with the RNeasy Mini Kit (Qiagen, Chatsworth, CA). A 10-μg quantity of RNA was electrophoresed on a 1% agarose gel under denaturing conditions, transferred to a nylon membrane (Hybond N+; Amersham, Buckinghamshire, UK) and hybridized with a 32P-labeled probe corresponding to a fragment of murine Hx cDNA. The entire human Hp cDNA and the mouse β-actin cDNA were also used as probes. Quantitation of the band intensity was performed by densitometry using a scanner connected to a personal computer.
Western blot.
For Western blot analysis, mice were bled from the tail vein and 1 μL of plasma was separated on 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose filter (Amersham), and probed with a goat antiserum against human Hx (AES-217; Harlan Sera-Lab, Crawley Down, Sussex, UK) or a goat antiserum against human Hp (Sigma H5015; Sigma, St Louis, MO) that also cross-reacts with mouse Hx or Hp, respectively. Filters were then incubated with horseradish peroxidase–conjugated rabbit antigoat IgG (Southern Biotechnology Associates, Birmingham, AL) and developed with an ECL detection system (Amersham).
Phenylhydrazine treatment.
Phenylhydrazine hydrochloride (Sigma P6926) was dissolved in phosphate-buffered saline (PBS) at 20 mg/mL and pH was adjusted to 7.4 with NaOH. Age- and sex-matched 5- to 7-week-old mice were injected intraperitoneally with freshly prepared phenylhydrazine ranging from 0.2 to 0.25 mg/g body weight.
Histology and histochemistry.
Tissues were dissected, fixed in 10% formalin or Bouin’s fluid for 24 hours, and embedded in paraffin. Microtome sections, 7 to 10 μm thick, were mounted onto TESPA (3-aminopropyl-triethoxysilane; Sigma)-treated slides and stained with hematoxylin and eosin or Prussian blue to detect ferric iron according standard procedures.24 Measurement of differences in ferric iron deposition was performed by counting on a microscope at high magnification (1,000×, immersion oil) the number of discrete blue spots per cell. Blood smears were obtained from tail vein and stained with May-Grünwald Giemsa as described previously.24For β-galactosidase histochemistry, dissected tissues were snap-frozen in 10% vol/vol embedding medium (Bio-Optica, Milan, Italy)/PBS on the surface of liquid nitrogen and sectioned at 15 μm on a cryostat at −20°C to −25°C. Sections were fixed in 2.5% glutaraldehyde in PBS (vol/vol) in a microwave oven at maximum power for 10 seconds and processed for β-galactosidase activity detection according standard procedures.5
Hematological parameters.
Blood was obtained by retroorbital sampling from anesthetized mice and blood cell counts were determined using an automatic cell counter. Iron, albumin, total protein, and bilirubin in serum were determined by colorimetric detection systems (Sigma) according to standard procedures on an automatic analyzer.
Analysis of oxidative damage.
Lipid peroxidation from tissue extracts was measured using the colorimetric assay kit Bioxytech LPO-586 from Oxis International (Portland, OR) according to the manufacturer’s instructions. Briefly, tissue samples were homogenated (20% to 30% wt/vol) in ice-cold 20 mmol/L Tris-HCl pH 7.4 containing 5 mmol/L butylated hydroxytoluene and protein content was determined using the Biorad protein assay system (Biorad, München, Germany). A 200-μL quantity of sample was assayed for malonaldehyde (MDA) content in hydrochloride using the chromogenic reagent N-methyl-2-phenylindole. Absorbance was measured at 586 nm and the results were expressed as nanomoles of MDA per milligram of protein using a molar extinction coefficient of 110.
Hemoglobinuria.
Syringes (1 mL) were used to collect urine samples from spontaneous urination or directly from the bladder of anesthetized mice by needle aspiration. The values were then determined with Bayer Multistix 10 SG (Milano, Italy).
RESULTS
Generation of Hx-null mice.
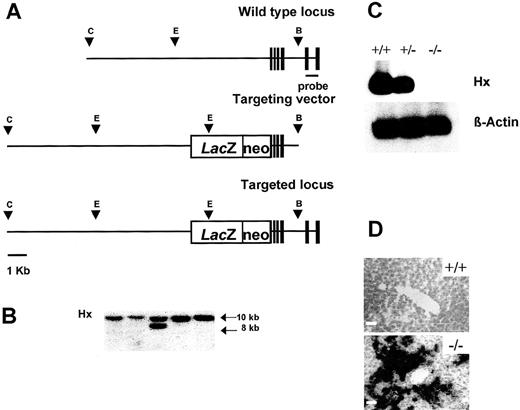
A targeting vector containing a lacZ-PGKneo cassette on the ATG start codon located in the first exon of the Hx gene was designed to disrupt the Hx gene. This targeting vector had a total of 12 kb of homologous genomic sequence flanking the cassette, with 10 kb upstream and 2 kb downstream (Fig 1A). The construct was linearized and introduced into R1 ES cells by electroporation. After selection with G418, resistant clones were screened for homologous recombination by Southern blotting with a 3′ external probe. Eleven of 200 screened clones contained the 8-kb EcoRV band diagnostic of homologous recombination in one allele in addition to the 10-kb fragment from the wild-type allele (Fig 1B). Three targeted ES clones were injected into the blastocysts of C57B6 mice. All of the chimeric males showed germ-line transmission of the disrupted allele. F1 offspring from chimeras were independently intercrossed to generate F2 homozygous mice of 129/SV and hybrid strain backgrounds. All of the mice that were heterozygous and homozygous for the Hx gene disruption appeared healthy, grew, reproduced normally, and produced homozygous offspring in the expected number. Northern blot analysis on total RNA extracted from the liver of wild-type, heterozygous, and homozygous littermates was performed to confirm that Hx −/ mice did not express the Hx gene product. Hx mRNA was absent in the liver of Hx −/− mice and was about half normal in +/− mice, indicating that there was no compensation for the reduced gene dosage of Hx in heterozygous mice (Fig 1C). β-Galactosidase staining on histologic sections was used to demonstrate that the reporter gene was correctly expressed in the hepatocytes (Fig 1D). Apart from the liver, the only other site in which β-galactosidase was detectable was the CNS, where staining was present in the ependymal cells and choroid plexi (not shown).
Targeted disruption of the Hx gene. (A) Structure of the Hx gene (top), the targeting vector (middle) containing a LacZ-PGKneo cassette in the first exon of the Hx gene, and the predicted structure of the disrupted allele after homologous recombination (bottom). Only the relevant restriction sites are shown: C, ClaI site; E,EcoRV site; B, BamHI site. Solid boxes represent exons 1-6. The position of the 3′ external probe is indicated. (B) Southern blot analysis of EcoRV-digested genomic DNA from ES clones. Filter was hybridized with the 3’ external probe shown in (A). The wild-type and mutant alleles are indicated by 10- and 8-kbEcoRV fragments, respectively. (C) Northern blot analysis of total RNA extracted from the liver of a wild-type, an Hx +/−, and an Hx −/− mouse. Filter was hybridized sequentially with an Hx probe and a β-actin probe. Hx transcript was reduced in Hx +/− liver and absent in Hx −/− liver. (D) β-Galactosidase staining of liver sections from a wild-type and an Hx −/− mouse. The majority of hepatocytes were labeled in Hx knockouts. Bar, 10 μm.
Targeted disruption of the Hx gene. (A) Structure of the Hx gene (top), the targeting vector (middle) containing a LacZ-PGKneo cassette in the first exon of the Hx gene, and the predicted structure of the disrupted allele after homologous recombination (bottom). Only the relevant restriction sites are shown: C, ClaI site; E,EcoRV site; B, BamHI site. Solid boxes represent exons 1-6. The position of the 3′ external probe is indicated. (B) Southern blot analysis of EcoRV-digested genomic DNA from ES clones. Filter was hybridized with the 3’ external probe shown in (A). The wild-type and mutant alleles are indicated by 10- and 8-kbEcoRV fragments, respectively. (C) Northern blot analysis of total RNA extracted from the liver of a wild-type, an Hx +/−, and an Hx −/− mouse. Filter was hybridized sequentially with an Hx probe and a β-actin probe. Hx transcript was reduced in Hx +/− liver and absent in Hx −/− liver. (D) β-Galactosidase staining of liver sections from a wild-type and an Hx −/− mouse. The majority of hepatocytes were labeled in Hx knockouts. Bar, 10 μm.
Analysis of Hx-deficient mice under physiologic conditions.
To test the possibility that Hx acts in vivo by scavenging free heme from the plasma, preventing oxidative damage, and contributing to the conservation of body iron, iron stores in the plasma and in the tissues were analyzed. As an altered iron status may affect hematopoiesis, peripheral blood was also analyzed and a histologic assessment of hematopoietic organs was made.
Plasma levels of bilirubin, iron, total proteins, and albumin were unaffected by the Hx mutation, as were all blood cell lineages (Table1).
Serum Bilirubin, Iron, Total Protein and Albumin Levels and Hematological Parameters of Hx-Null Mice
| Parameter . | Hx −/− . | Wild-Type . |
|---|---|---|
| Bilirubin (mg/dL) | 2.97 ± 0.9 | 3.18 ± 1.5 |
| Iron (μg/dL) | 153 ± 24 | 158 ± 37 |
| Total protein (mg/dL) | 4.6 ± 0.3 | 4.6 ± 0.5 |
| Albumin (mg/dL) | 2.8 ± 0.2 | 2.8 ± 0.2 |
| WBC (× 106/mL) | 3.82 ± 2.17 | 3.95 ± 1.61 |
| RBC (× 109/mL) | 8.77 ± 0.48 | 8.71 ± 0.64 |
| HGB (g/dL) | 14.1 ± 0.8 | 13.9 ± 0.9 |
| HCT (%) | 45.5 ± 2.0 | 45.3 ± 2.8 |
| MCV (fL) | 52.0 ± 2.4 | 52.1 ± 2.4 |
| MCH (pg) | 16.1 ± 0.9 | 16.0 ± 0.9 |
| RDW-SD (fL) | 26.2 ± 1.7 | 27.4 ± 4.0 |
| PLT (× 106/mL) | 335 ± 121 | 368 ± 124 |
| PDW (fL) | 6.3 ± 0.4 | 6.3 ± 0.3 |
| MPV (fL) | 6.5 ± 0.3 | 6.5 ± 0.2 |
| Parameter . | Hx −/− . | Wild-Type . |
|---|---|---|
| Bilirubin (mg/dL) | 2.97 ± 0.9 | 3.18 ± 1.5 |
| Iron (μg/dL) | 153 ± 24 | 158 ± 37 |
| Total protein (mg/dL) | 4.6 ± 0.3 | 4.6 ± 0.5 |
| Albumin (mg/dL) | 2.8 ± 0.2 | 2.8 ± 0.2 |
| WBC (× 106/mL) | 3.82 ± 2.17 | 3.95 ± 1.61 |
| RBC (× 109/mL) | 8.77 ± 0.48 | 8.71 ± 0.64 |
| HGB (g/dL) | 14.1 ± 0.8 | 13.9 ± 0.9 |
| HCT (%) | 45.5 ± 2.0 | 45.3 ± 2.8 |
| MCV (fL) | 52.0 ± 2.4 | 52.1 ± 2.4 |
| MCH (pg) | 16.1 ± 0.9 | 16.0 ± 0.9 |
| RDW-SD (fL) | 26.2 ± 1.7 | 27.4 ± 4.0 |
| PLT (× 106/mL) | 335 ± 121 | 368 ± 124 |
| PDW (fL) | 6.3 ± 0.4 | 6.3 ± 0.3 |
| MPV (fL) | 6.5 ± 0.3 | 6.5 ± 0.2 |
Values represent the mean ± SD for at least 20 adult animals per genotype.
Abbreviations: HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; WBC, white blood cells; RBC, red blood cells; PDW, platelet density width. PLT, platelet count; MCH, mean corpuscular hemoglobin; RDW-SD, red cell distribution width-standard deviation; MPV, mean platelet volume.
Histologic analysis of liver, kidney, heart, brain, spleen, and bone marrow showed no evident abnormalities or tissue lesions due to oxidative damage in Hx −/− mice, nor did Prussian blue staining of tissue sections show any abnormal iron deposition (not shown). These data indicate that, under physiologic conditions, Hx does not protect from oxidative stress and is not crucial for iron metabolism.
Susceptibility of Hx-deficient mice to acute hemolysis.
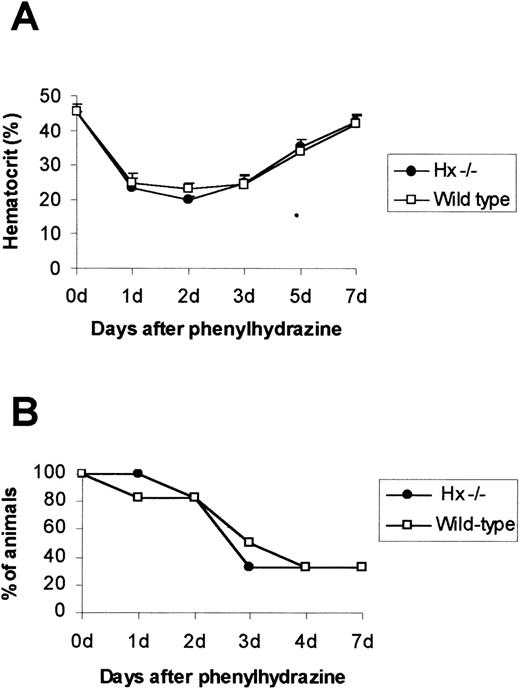
To investigate the capability of Hx in the prevention of oxidative stress, Hx-deficient and wild-type mice were subjected to acute hemolysis by administration of a single dose of phenylhydrazine (φhyd) ranging from 0.2 mg/g to 0.25 mg/g body weight. Hemolysis was evident on day 1 by a marked depression in hematocrit level (Fig2A) and dark brown coloration of plasma and urine, indicating hemoglobinemia and hemoglobinuria, respectively. Susceptibility to hemolysis did not differ in wild-type and Hx-null mice: after an injection of 0.23 mg/g of φhyd, 80% of mice from both genotypes began to die from day 1 to day 4 (Fig 2B).
Effect of phenylhydrazine injection. (A) Mean hematocrit ± SD after a single dose of phenylhydrazine of 0.2 mg /g. A total of 4 mice for each genotype was used. (B) Survival after phenylhydrazine treatment. A single dose of phenylhydrazine of 0.23 mg/g was intraperitoneally injected and mice were monitored for 7 days; 20 animals per genotype were used.
Effect of phenylhydrazine injection. (A) Mean hematocrit ± SD after a single dose of phenylhydrazine of 0.2 mg /g. A total of 4 mice for each genotype was used. (B) Survival after phenylhydrazine treatment. A single dose of phenylhydrazine of 0.23 mg/g was intraperitoneally injected and mice were monitored for 7 days; 20 animals per genotype were used.
Spleens of both wild-type and Hx-deficient mice, after hemolysis, were 3 to 4 times normal size due to the hyperplasia of the reticuloendothelial system. Histologic analysis of several organs (liver, kidney, spleen, heart, and brain) from dying mice showed hydropic degeneration as evidenced by numerous cytoplasmic vacuoles in the liver and kidney of both +/+ and −/− mice. Prussian blue staining showed considerable nonheme iron deposits, in the form of hemosiderin and ferritin, in renal cortical tubules and in splenic macrophages and, to a less extent, in the Kupffer cells of the liver in all the mice. Other organs, such as heart and brain, showed no signs of degeneration or evident iron deposits.
On the basis of hemoglobinuria and histologic data, renal failure was assumed to be the cause of death of φhyd-treated mice.
Recovery of Hx-deficient mice following acute hemolysis.
To further assess the protective role of Hx after hemolysis, the recovery after hemolytic stimulus in wild-type and Hx-deficient mice was analyzed by administering a sublethal dose of φhyd of 0.2 mg/g and killing them after 7 days. This dose of φhyd was effective in producing hemolysis as indicated by the reduced hematocrit, hemoglobinemia, and hemoglobinuria. Seven days after injection, blood parameters were within normal limits in both wild-type and Hx-null mice (Fig 2A). Moreover, blood parameters and blood smears obtained from Hx −/− and controls 7 days after the hemolytic stress were morphologically identical (data not shown).
Hp turnover.
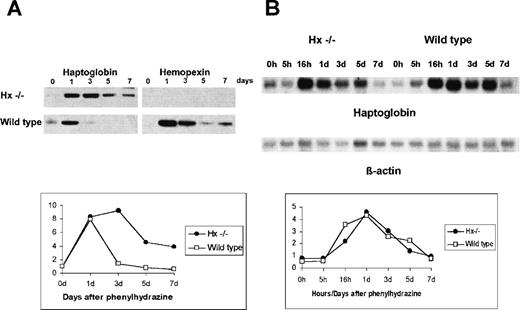
As a depletion of Hp in the circulation is a clinical marker of a hemolytic status, follow-up evaluation of recovery was made by monitoring Hp plasma levels after hemolysis. Surprisingly, while in control mice Hp levels decreased 3 days after the φhyd injection, they remained elevated for 7 days after treatment in Hx-null mice (Fig3A). In wild-type mice, Hx increased 1 day after the φhyd injection and decreased slowly to the seventh day (Fig3A). Therefore, to assess whether the difference in plasma Hp content was due to a different transcriptional activation of the Hp gene in Hx −/− mice, Northern blot was used to analyze Hp mRNA expression in the liver. As shown in Fig 3B, there was no difference in Hp mRNA induction and turnover after the φhyd injection in either wild-type or Hx-null mice: Hp mRNA level increased 1 day after the φhyd injection and remained elevated for the following 4 days. These data indicated that, after hemolysis, the Hx mutation altered Hp turnover at the protein level.
Hp turnover after a single dose of phenylhydrazine of 0.2 mg/g. (A) Western blot: mice were monitored for 7 days after injection and plasma from tail vein analyzed with an antibody against Hp (left) and Hx (right). (B) Northern blot: mice were monitored for 7 days after injection and total liver RNA analyzed with a probe for Hp (top) and β-actin (bottom). Diagrams refer to Hp turnover at the plasma (A) and mRNA level (B).
Hp turnover after a single dose of phenylhydrazine of 0.2 mg/g. (A) Western blot: mice were monitored for 7 days after injection and plasma from tail vein analyzed with an antibody against Hp (left) and Hx (right). (B) Northern blot: mice were monitored for 7 days after injection and total liver RNA analyzed with a probe for Hp (top) and β-actin (bottom). Diagrams refer to Hp turnover at the plasma (A) and mRNA level (B).
Hemoglobinuria.
Seven days after the φhyd injection, Hx −/− mice had a higher hemoglobinuria than did controls: 6 of 8 Hx −/− presented high hemoglobin urine concentration while 6 of 8 wild-type mice had no hemoglobin in the urine and only 2 of 8 animals had low hemoglobin content in the urine (Table 2).
Hemoglobinuria in Hx −/− and Wild-Type Mice 7 Days After Phenylhydrazine Treatment
| Hemoglobinuria . | Hx −/− . | Wild-Type . |
|---|---|---|
| Negative | 2/8 | 6/8 |
| 150-300 μg/L | 0/8 | 2/8 |
| 300-450 μg/L | 2/8 | 0/8 |
| 450-620 μg/L | 4/8 | 0/8 |
| Hemoglobinuria . | Hx −/− . | Wild-Type . |
|---|---|---|
| Negative | 2/8 | 6/8 |
| 150-300 μg/L | 0/8 | 2/8 |
| 300-450 μg/L | 2/8 | 0/8 |
| 450-620 μg/L | 4/8 | 0/8 |
Histology.
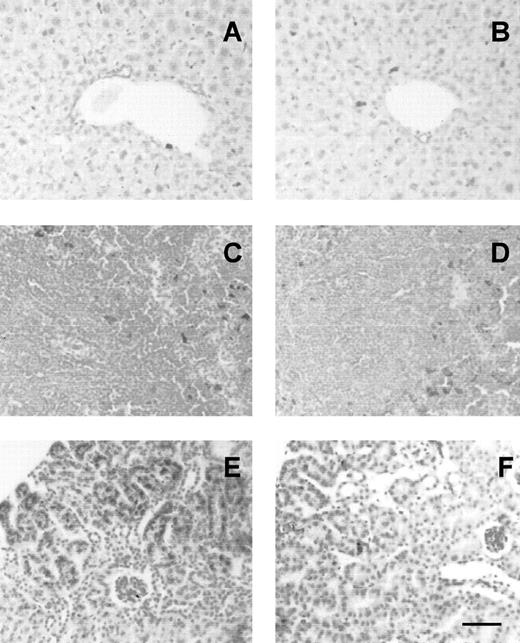
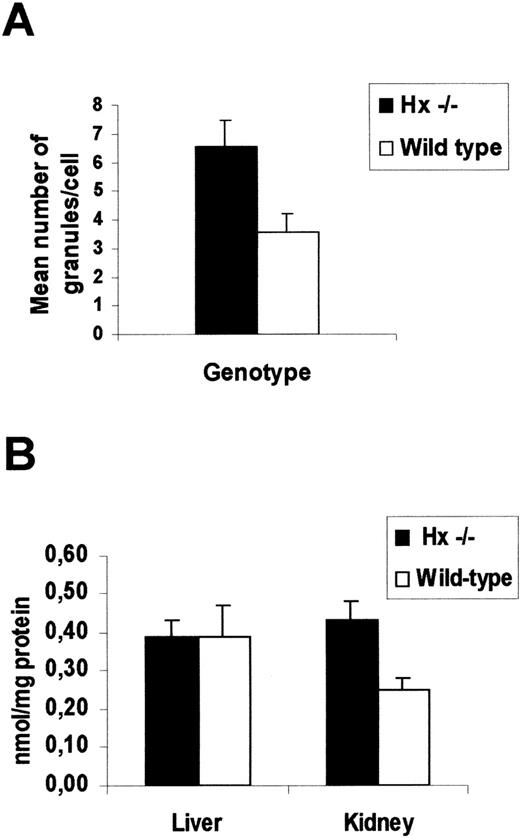
When the organs of φhyd-treated mice were examined for iron content, it was observed that liver, spleen, bone marrow, and kidney of all mice contained nonheme iron deposits, in the form of hemosiderin and ferritin. Liver and spleen, where iron deposits were present in Kupffer cells and macrophages, respectively, showed no differences between Hx −/− and wild-type mice. On the contrary, a significantly higher iron load was found in the kidney of Hx-deficient mice than in the control group: blue-stained hemosiderin was evident in proximal tubules (Fig 4). To quantify the extent of the renal damage, the number of blue-stained, Perls’ positive granules, detected by focusing on a microscope at high magnification, was counted in 200 tubular cells for each animal. In Hx −/− mice, we observed 6.58 ± 0.89 granules/cell, as compared with 3.59 ± 0.6 granules/cell in wild types (Fig5A).
Iron loading in Hx −/− tissues. Liver (A,B), spleen (C,D) and kidney (E,F) sections from Hx −/− (A,C,E) and wild-type (B,D,F) mice stained with Prussian blue for detection of ferric iron. Liver and spleen show iron deposits in Kupffer cells and macrophages, respectively, without differences between knockouts and controls. Kidney of Hx −/− has much more iron loading compared with controls: intense blue staining is evident in proximal cortical tubules. Bar, 20 μm.
Iron loading in Hx −/− tissues. Liver (A,B), spleen (C,D) and kidney (E,F) sections from Hx −/− (A,C,E) and wild-type (B,D,F) mice stained with Prussian blue for detection of ferric iron. Liver and spleen show iron deposits in Kupffer cells and macrophages, respectively, without differences between knockouts and controls. Kidney of Hx −/− has much more iron loading compared with controls: intense blue staining is evident in proximal cortical tubules. Bar, 20 μm.
Analysis of renal damage after injection of 0.2 mg/g of phenylhydrazione. (A) Measurement of iron loading in the kidney of Hx −/− and wild-type mice 7 days after phenylhydrazine treatment. Mean number ± SD of Perls’ positive granules per tubular cell. At least 200 cells for each animal were counted. A total of 6 mice for each genotype was used (P < .001). (B) Lipid peroxidation estimated as MDA levels of tissue homogenates of Hx −/− and wild-type mice 7 days after phenylhydrazine treatment. Data represent mean ± SEM from 5 mice for each genotype (P < .01). Kidney from Hx −/− mice, which consistently shows iron loading, has significantly greater oxidative damage than that from control mice.
Analysis of renal damage after injection of 0.2 mg/g of phenylhydrazione. (A) Measurement of iron loading in the kidney of Hx −/− and wild-type mice 7 days after phenylhydrazine treatment. Mean number ± SD of Perls’ positive granules per tubular cell. At least 200 cells for each animal were counted. A total of 6 mice for each genotype was used (P < .001). (B) Lipid peroxidation estimated as MDA levels of tissue homogenates of Hx −/− and wild-type mice 7 days after phenylhydrazine treatment. Data represent mean ± SEM from 5 mice for each genotype (P < .01). Kidney from Hx −/− mice, which consistently shows iron loading, has significantly greater oxidative damage than that from control mice.
Analysis of oxidative stress.
To determine any oxidative stress that may have arisen as a result of iron deposition, oxidized lipid levels were analyzed by measuring the MDA content in the liver and kidney of φhyd-treated mice. To avoid new lipid peroxidation that can occur during homogenization and detection of MDA, the antioxidant butylated-hydroxytoluene at a final concentration of 5 mmol/L was used during all steps of the procedure. The degree of lipid peroxidation in the kidney of Hx −/− was higher than that in controls, whereas the MDA level in the liver was similar (Fig 5B). These data indicated that Hx-deficient mice presented an altered Hp turnover after hemolysis and suffered more renal damage than did the control group.
DISCUSSION
Hx is the plasma protein that has the highest affinity for heme. Due to the strength of the binding between heme and Hx and the presence of specific receptors on hepatocyte membrane, Hx is believed to be the major factor responsible for the scavenging of free heme from plasma, thus preventing oxidative damage and contributing to the conservation of body iron.9,15 16
Analysis of Hx-deficient mice showed that the presence of Hx was not crucial for heme catabolism under physiologic conditions; indeed, the plasma levels of iron and bilirubin, the major products of heme catabolism, were unaffected by the Hx mutation. Hematopoietic organs and blood parameters were normal. There was no abnormal iron deposition or oxidative lesions at the tissue level. These data are consistent with the absence of a known human inherited disorder caused by Hx deficiency. On the contrary, HO-1 knockout mice25 with ageing develop an anemia associated with abnormally low serum iron content, and accumulate hepatic and renal iron that causes oxidative damage, tissue injury, and chronic inflammation. Our data demonstrated that Hx is not essential for the transport of heme into the hepatic cells where HO-1 catabolizes it. In plasma, heme can be bound by albumin, present at higher concentrations than Hx (30 to 55 mg/mLv 0.5 to 1 mg/mL).9 Recently, Noyer et al21 showed that heme can be transferred directly from the albumin to primary rat hepatocytes in culture and suggested the presence of a transmembrane heme transporter. A similar mechanism may also take place in vivo. After crossing the hepatocyte membrane, heme could then be bound by cytosolic heme-binding proteins. Several candidate heme-binding proteins have been suggested: fatty acid binding protein,26 glutathione S-transferases,27 MSP23 or the rat homologous HBP 23,28-30 or even the recently cloned p22 HBP.31
The role of Hx became evident after φhyd-induced hemolysis. The susceptibility of mice lacking Hx to φhyd treatment was the same as that of wild-type mice. On the other hand, mice lacking the Hp gene32 showed increased susceptibility to hemolysis, ie, 55% of them died within 5 days of φhyd treatment compared with 18% of wild-type mice. These data indicate that Hp is more efficient than is Hx in protecting from hemolytic stress. The slight difference in the lethal dose between Hp and Hx knockouts (0.2 mg/g v 0.23 mg/g) is probably due to a different φhyd batch or to differences in the genetic background of the mice. Other than susceptibility to hemolysis, also recovery of blood parameters after φhyd injection was unaffected by Hx mutation; indeed, hematocrit normalized within 1 week in knockouts, as in wild-type mice. Nevertheless, Hx-deficient mice responded to hemolysis in a different way, since Hp persisted in the circulation for several days after the φhyd injection. The prolonged presence of Hp in the plasma of Hx −/− after φhyd could be the consequence of a major Hp protein stability and/or of a major rate of translation. This conclusion is supported by the fact that Hp is not recycled after internalization of the Hp-hemoglobin complex and that Hp mRNA induction and turnover in Hx −/− after φhyd is unaltered. Alternatively, Hx null mice may have an alteration in the kinetic of internalization of the hemoglobin-Hp complex, thus suggesting interactions between the Hp and Hx receptor systems. It would, therefore, be interesting to analyze Hx turnover in Hp −/− mice after hemolysis. Considered together, the data on the susceptibility to hemolysis in Hx and Hp-deficient mice suggest that Hp is in the first line of defense against hemolytic stress, followed by Hx, and that the 2 proteins cooperate with each other in the response to hemolysis.
To the best of our knowledge, this is the first time that data have been presented on a possible cross-talk between Hx and Hp in the acute-phase response. This could be mediated by several cytokines such as interleukin-6, which has cis-acting responsive elements in the promoter of both Hx and Hp.33 34 However, an altered Hp turnover cannot explain the lack of phenotype in Hx −/− under physiologic conditions, since basal Hp plasma levels are unaffected by the Hx mutation.
Based on histologic data, hemoglobinemia, hemoglobinuria, and clinical reports we assumed that renal failure was the cause of mortality after φhyd, as reported for Hp-null mice.32 The kidney is also the organ that suffered the most damage after the sublethal dose of φhyd, as demonstrated by prolonged hemoglobinuria, high lipid peroxidation, and iron loading. It is unlikely that prolonged hemoglobinuria is due to a continued hemolysis in Hx −/− mice since blood parameters normalized at the same rate as they did in control mice. It may be due to a reduced capability of the Hp system to remove hemoglobin from the circulation. Hemoglobin accumulates at the tubular level and causes iron loading. The presence of ferric iron in the kidney could then account for the higher lipid peroxidation observed in Hx-deficient mice compared with controls. Consistent with our results, Hp-null mice that recovered after hemolysis presented impaired renal regeneration and repair.32 These data are in agreement with those observed in acute renal tubular necrosis in patients with a high degree of hemolysis.35
Iron loading was present exclusively in renal tubular cells and in macrophages or Kupffer cells of the reticuloendothelial system. The latter sites of deposition, where no difference was found between Hx −/− and wild-type mice, are to be expected due to the large expansion of the reticuloendothelial system after hemolytic processes. The absence of iron loading in hepatocytes was also predictable, unlike HO-1 −/−, β2-microglobulin −/−, and HFE −/− mice25,36 37 that suffered from specific defects in their iron metabolism.
In conclusion, our data demonstrate that the most significant role of Hx is that of protecting cells against heme toxicity, rather than participating in iron metabolism. This is in agreement with previously reported biochemical studies showing the protective role of Hx in heme-catalyzed oxidations.38,39 The role of Hx becomes evident after a hemolytic process, particularly in the kidney, which is also the most compromised organ involved in human hemolytic pathologies. These results are in agreement with those previously reported for Hp knockout mice, which showed that the major function of Hp is to protect renal tubules from hemoglobin-mediated oxidative damage rather than to clear free plasma hemoglobin under normal conditions.32 Therefore, when considering the data on Hp and Hx knockouts, we may conclude that the response to hemolysis occurs in 2 stages: first, Hp and Hx are rapidly induced to protect from increased plasma hemoglobin and heme concentrations, then, once Hp disappears from the circulation, the delayed presence of Hx in the plasma takes on a relevant role in the protection against heme derived from hemoglobin oxidation.
Although the data reported herein refer only to the “systemic” physiologic role of Hx, it may well act locally at the level of peripheral nerves after an injury. Indeed, in transected rat sciatic nerves, Hx is expressed by fibroblasts, Schwann cells, and invading blood macrophages, and is accumulated in the extracellular matrix, thus suggesting that it may protect injured tissues from oxidative damage.8,40,41 Moreover, Hx is synthesized in the retina, which is isolated from the circulation by the blood-retinal barrier, and the Hx receptor is expressed by the retinal pigment epithelia cells.6,7 The binding between the heme-Hx complex and the Hx receptor results in the increased expression of several proteins that all interact in the degradation of heme, the storage of iron from heme catabolism, and as intracellular antioxidants.42Hx-deficient mice could be useful in characterizing other Hx functions, particularly at injury sites in the nervous system.
ACKNOWLEDGMENT
The authors thank Drs V. Poli, G. Pescarmona, F. Di Cunto, and G. Saglio for stimulating discussion, and Drs G. Topley and B. Wade for critical reading of the manuscript. We would also like to thank Dr A. Gariboldi and Dr A. Grillo for performing hematologic analyses. The technical help of L. Cavarretta and I. Carfora is gratefully acknowledged.
Supported by grants from the National Research Council (Target Project on Biotechnology) to F.A. and from the Ministry of University and Scientific Research to F.A.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fiorella Altruda, PhD, Department of Genetics, Biology and Biochemistry, Via Santena 5bis, 10126, Turin, Italy; e-mail: altruda@molinette.unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal