Abstract
We have examined in whole blood the actions of 2 lipoxin A4 (LXA4) stable analogs, 15-R/S-methyl-LXA4 and 16-phenoxy-LXA4, for their impact on the expression of adhesion molecules on human leukocytes and coronary artery endothelial cells (HCAEC) and on neutrophil adhesion to HCAEC in vitro. Both LXA4 analogs in nanomolar to micromolar concentrations prevented shedding of L-selectin and downregulated CD11/CD18 expression on resting neutrophils, monocytes, and lymphocytes. Changes in CD11/CD18 expression were blocked by the mitogen-activated protein kinase kinase inhibitor PD98059. The LXA4 analogs also attenuated changes in L-selectin and CD11/CD18 expression evoked by platelet-activating factor (PAF), interleukin-8, or C-reactive protein-derived peptide 201-206 with IC50 values of 0.2 to 1.9 μmol/L, whereas they did not affect lipopolysaccharide (LPS)– or tumor necrosis factor-–stimulated expression of E-selectin and intercellular adhesion molecule-1 on HCAEC. These LXA4analogs markedly diminished adhesion of neutrophils to LPS-activated HCAEC. Inhibition of adhesion was additive with function blocking anti–E-selectin and anti–L-selectin antibodies, but was not additive with anti-CD18 antibody. Combining LXA4 analogs with dexamethasone (100 nmol/L) almost completely inhibited PAF-induced changes in adhesion molecule expression on leukocytes and gave additive inhibition of neutrophil adhesion to HCAEC. Culture of HCAEC with dexamethasone, but not with LXA4 analogs, also decreased neutrophil attachment. Together, these results indicate that LXA4 stable analogs modulate expression of both L-selectin and CD11/CD18 on resting and immunostimulated leukocytes and inhibit neutrophil adhesion to HCAEC by attenuating CD11/CD18 expression. These actions are additive with those of glucocorticoids and may represent a novel and potent regulatory mechanism by which LXA4 and aspirin-triggered 15-epi-LXA4 modulate leukocyte trafficking.
LIPOXINS (trihydroxytetraene-containing eicosanoids) formed by leukocytes during cell-cell interactions represent a unique class of lipid mediators with potent anti-inflammatory actions.1 In particular, lipoxin A4 (LXA4) was found to inhibit neutrophil and eosinophil chemotaxis in vitro,2,3 to block transmigration of neutrophil granulocytes (PMNL) across epithelial cells4and endothelial monolayers,5 and to reduce PMNL entry into inflamed renal tissues.6 Recent results showed that stable analogs of LXA4 resist rapid inactivation and retain biological activities of native LXA4, including inhibition of PMNL adhesion and transmigration across epithelial and endothelial monolayers,7 inhibition of interleukin-8 (IL-8) production with consequent impairment of the ability of bacteria-infected epithelia to direct PMNL movement,8 and attenuation of P-selectin–dependent PMNL adhesion and rolling on the mesenteric microvasculature.9 These studies described novel actions of LXA4 and aspirin-triggered LXA4 (15-epimer of LXA4) on isolated cells, intestinal epithelial cells, or isolated vessels after their topical application, yet the question of whether these lipid mediators are active within the microenvironment of human whole blood that possess a wide array of components that can bind lipophilic compounds as well as the issue of the cellular mechanisms that account for their novel inhibitory actions in leukocyte trafficking have not been addressed.
Leukocyte extravasation into inflamed areas involves a multistep interaction of leukocytes and endothelial cells via regulated expression of surface adhesion molecules.10,11 The initial capture and tethering of circulating neutrophils to endothelium is mediated by L-selectin. L-selectin is constitutively expressed by most leukocytes and is rapidly shed after cell activation with a concomitant upregulation of Mac-1 (CD11b/CD18).12 The CD18 integrins, Mac-1 and LFA-1 (CD11a/CD18), are largely responsible for subsequent tightening of the adhesion and transendothelial migration of neutrophils via interactions with their endothelial counterreceptors, intercellular adhesion molecule-1 (ICAM-1) and ICAM-2.10 11
In the present experiments, we studied the impact of stable LXA4 analogs in human whole blood and addressed their cellular mechanisms of action with isolated cells. We examined the expression of adhesion molecules on human leukocytes and human coronary artery endothelial cells (HCAEC) and on adhesion of PMNL to HCAEC. We used 16-phenoxy-LXA4, an analog of the native LXA4,7 and 15-R/S-methyl-LXA4, an analog of aspirin-triggered 15-epi-LXA4.13Platelet-activating factor (PAF) and IL-8 were chosen to activate leukocytes, because these mediators can serve as signals for neutrophils to bind tightly to the endothelium.14 15
MATERIALS AND METHODS
Antibodies and reagents.
In these studies, the monoclonal antibodies (MoAbs) used included fluorescein isothiocyanate (FITC)-conjugated mouse antihuman L-selectin MoAb DREG-56 (PharMingen, San Diego, CA), R-phycoerythrin–conjugated mouse antihuman CD18 MoAb MEM-48 (Monosan, Uden, The Netherlands), FITC-labeled mouse antihuman CD11a MoAb G-25.2 (Becton Dickinson Immunocytometry Systems, Mountain View, CA), FITC-labeled mouse antihuman E-selectin MoAb 1.2B6 (Serotec, Kidlington, UK), and R-phycoerythrin–conjugated mouse antihuman ICAM-1 MoAb HA58 (PharMingen). Appropriately labeled, class-matched irrelevant mouse IgG1 was used as a negative control for each staining. The following murine MoAbs were used in neutrophil-endothelial cell adhesion assays: anti–L-selectin MoAb DREG-56 (IgG1; PharMingen) at 20 μg/mL16; anti–E-selectin MoAb ENA-2 [IgG1, purified F(ab′)2 fragments; Monosan] at 10 μg/mL17; and anti-CD18 MoAb L130 (IgG1; Becton Dickinson) at 10 μg/mL.18 The irrelevant MoAb MOPC-21 (IgG1; PharMingen) at 20 μg/mL was used as a negative control.
The LXA4 analogs, 15-R/S-methyl-LXA4, 16-phenoxy-LXA4 and 15-deoxy-LXA4, were prepared by total organic synthesis7 and stored in ethanol at −70°C. An aliquot was removed and diluted in assay medium immediately before use. The highest concentration of ethanol (0.1%) had no detectable effects in any of the assays used.
Lipopolysaccharide (LPS; Escherichia coli O111:B4), C-reactive protein (CRP)-derived peptide 201-206 (Lys-Pro-Gln-Leu-Trp-Pro), dexamethasone 21-phosphate, wortmannin, and genistein were obtained from Sigma Chemical Co (St Louis, MO); PD98059, herbimycin A, and PAF were from Calbiochem (San Diego, CA); and human recombinant IL-8 and tumor necrosis factor-α (TNF-α) were purchased from R&D Systems (Minneapolis, MN).
Whole blood incubation.
Venous blood (anticoagulated with 50 U/mL sodium heparin) was obtained from nonsmoking healthy volunteers (male and female, 24 to 55 years of age) who had not taken any drugs for at least 10 days before the experiments. Informed consent was obtained from each volunteer, and the protocol was approved by the Clinical Research Committee. White blood cell counts were between 4,500 and 9,500 cells/μL. Whole blood aliquots were incubated with one of the LXA4 analogs for 30 minutes at 37°C, 95% air/5% CO2. Preliminary experiments showed that the maximum effects of LXA4 analogs could be achieved after 30 minutes of preincubation. Some blood samples were preincubated for 30 minutes with LXA4 analogs or treated for 120 minutes with dexamethasone (100 nmol/L) with or without LXA4 analogs and then challenged with PAF (1 μmol/L), IL-8 (10 nmol/L), or CRP peptide 201-206 (100 μg/mL), which downregulates L-selectin expression without inducing cell activation.18 In some experiments, blood samples were challenged with Mg2+ (1 mmol/L) and EGTA (2 mmol/L) to induce a higher affinity form of β2integrins.19
Analysis of surface antigen expression.
Direct immunofluorescence labeling of resting and treated leukocytes in whole blood was performed as described.18 20 Leukocytes were stained with saturating concentration of fluorescence dye-conjugated antihuman L-selectin or antihuman CD18 MoAb. Nonspecific binding was evaluated by using appropriately labeled mouse IgG1. Double- or single-color immunofluorescence staining was analyzed by a cytofluorometer (FACScan; Becton Dickinson) with Lysis II software. Antibody binding was determined as mean fluoresence intensity after gating for neutrophils, monocytes, and lymphocytes by their characteristic forward and side scatter properties. The results are presented as relative fluorescence units (RFU): RFU = (Fuexperimental − Fuisotype) × 100/(FUcontrol − Fuisotype), where FUexperimental and FUcontrol are the L-selectin and CD18 fluorescence intensity of treated cells and cells cultured in medium only, respectively, and FUisotypeis the fluorescence intensity of class-matched irrelevant antibody.
Isolation and treatment of neutrophils.
PMNL were isolated from peripheral blood by centrifugation through Ficoll-Hypaque (Pharmacia Diagnostics, Uppsala, Sweden), sedimentation through dextran (3%, wt/vol), and hypotonic lysis of erythrocytes. Neutrophils (5 × 106 cells/mL; purity, >97%) were suspended in a modified Hanks' balanced salt solution; incubated with the MAPK kinase (MEK) inhibitor PD98059, the phosphatidylinositol 3-kinase inhibitor wortmannin, or the protein tyrosine kinase inhibitors genistein or herbimycin A for 30 minutes at 37°C; and then challenged with 15-R/S-methyl-LXA4 for 30 minutes. Surface expression of L-selectin and CD18 was analyzed as just described.
Culture of endothelial cells.
Normal HCAEC obtained from Clonetics Corp (San Diego, CA) were cultured as described.18 HCAEC (passages 3 to 6) seeded into 24-well or 96-well microplates and grown to confluence were used in the experiments.
Neutrophil-endothelial cell adhesion assay.
The adhesion assay was performed as in Zouki et al.18 In brief, monolayers of HCAEC in 96-well microplates were stimulated with LPS (1 μg/mL) with or without LXA4 analogs or dexamethasone (100 nmol/L) for 6 hours at 37°C in a 5% CO2 atmosphere. The cells were then washed 3 times, and 2 × 10551Cr-labeled neutrophils in 100 μL were added. In some experiments, neutrophils were preincubated with one of the LXA4 analogs for 30 minutes or with dexamethasone (100 nmol/L) for 120 minutes with or without LXA4 analogs for 30 minutes before the addition to HCAEC. In another set of experiments, LPS-activated HCAEC were incubated for 15 minutes with ENA-2 or MOPC-21 MoAb before the addition of neutrophils. Radiolabeled neutrophils were incubated with DREG-56, L130, or MOPC-21 MoAb for 15 minutes before the addition to HCAEC. After incubation of HCAEC with neutrophils for 30 minutes at 37°C on an orbital shaker at 90 rpm, loosely adherent or unattached neutrophils were washed 3 times and the endothelial monolayer plus the adherent neutrophils were lysed in 200 μL of 0.1% Triton X-100. The number of adhered neutrophils in each experiment was estimated from the radioactivity of a control sample. Treatment of HCAEC with any of the LXA4 analogs did not affect the integrity of viable endothelial monolayers.
Expression of E-selectin and ICAM-1.
After incubation for 4 hours at 37°C in a 5% CO2atmosphere with LPS (1 μg/mL) or TNF-α (2.5 ng/mL) in the absence or presence of various concentrations of 15-R/S-methyl-LXA4, HCAEC were removed from the 24-well microplates by exposure to EDTA (0.01%) in phosphate-buffered saline (PBS) for 10 minutes at 37°C, followed by gentle trituration. Cells were resuspended in ice-cold saline containing sodium azide (0.02%), incubated with a saturating concentration of fluorescein dye-conjugated anti–E-selectin or anti–ICAM-1 MoAb for 30 minutes at 4°C, washed, and fixed in formaldehyde (3.9% in PBS). Nonspecific binding was evaluated by using appropriately labeled mouse IgG1. Immunofluorescence of HCAEC was then analyzed with a FACScan.
Data analysis.
Results are expressed as the means ± SEM. Statistical comparisons were made by ANOVA using ranks (Kruskal-Wallis test), followed by Dunn's multiple contrast hypothesis test to identify differences between various treatments, or by the Wilcoxon signed rank test for paired observations. P values less than .05 were considered significant for all tests.
RESULTS
LXA4 analogs in whole blood modulate expression of L-selectin and CD11/CD18 on resting and immunostimulated leukocytes.
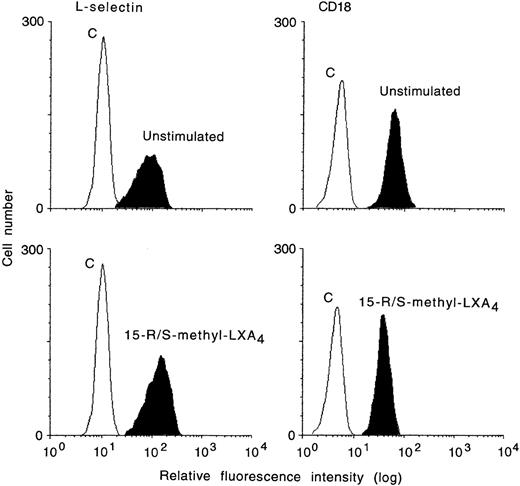
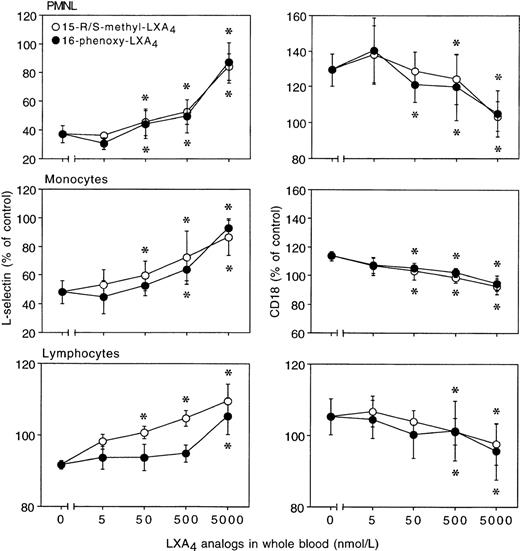
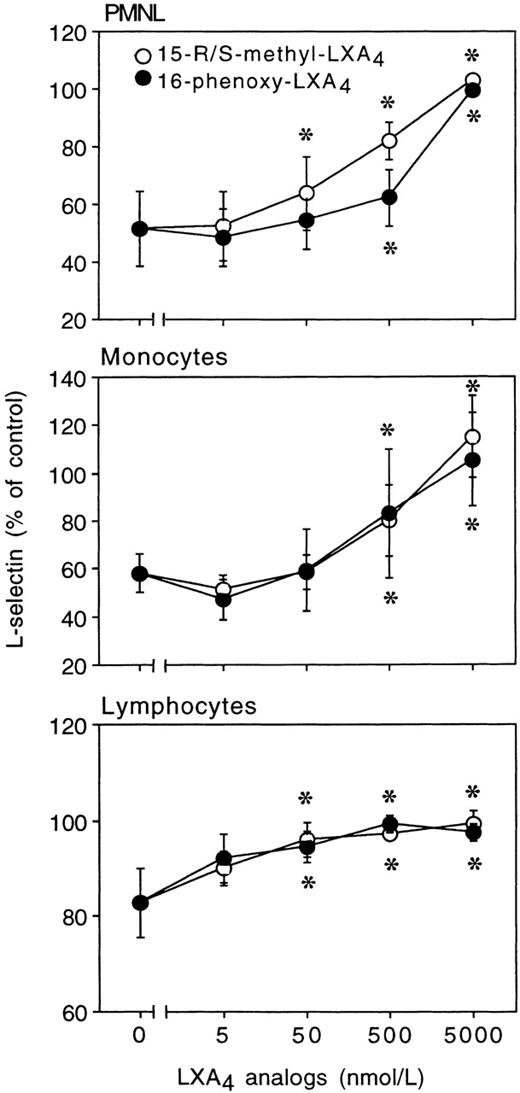
Incubation of heparinized whole blood with either 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 resulted in higher L-selectin expression on treated than control (untreated) cells and gave concentration-dependent downregulation of CD18 expression on PMNL. Figure 1 reports representative results illustrating the impact of 15-R/S-methyl-LXA4 added to whole blood. Incubation of blood samples for 30 minutes at 37°C with 5 μmol/L of 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 resulted in 31% ± 8% and 32% ± 8% higher levels of expression for L-selectin than on cells from untreated whole blood and gave 26% ± 5% and 27% ± 5% decreases in CD18 expression, respectively (Fig 2). Similar changes were observed with monocytes and lymphocytes (Fig 2). Neither 15-deoxy-LXA4(Fig 2) nor structurally degraded (by thermal degradation) 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 affected expression of adhesion molecules (data not shown). When blood samples were incubated at 4°C, none of the LXA4 analogs studied affected L-selectin expression. For instance, mean fluorescence intensity for L-selectin on PMNL was 129 ± 8 and 120 ± 11 (n = 3, P > .5) in control samples and in the presence of 15-R/S-methyl-LXA4, respectively.
Whole blood actions of aspirin-triggered LXA4analog (15-R/S-methyl-LXA4) on cell surface expression of L-selectin and CD18 by human neutrophils. Whole blood was incubated with 15-R/S-methyl-LXA4 (5 μmol/L) for 10 minutes at 37°C. In each histogram is also displayed the negative control of immunostaining with class-matched irrelevant antibodies (C). Shown is a representative experiment of 5 experiments.
Whole blood actions of aspirin-triggered LXA4analog (15-R/S-methyl-LXA4) on cell surface expression of L-selectin and CD18 by human neutrophils. Whole blood was incubated with 15-R/S-methyl-LXA4 (5 μmol/L) for 10 minutes at 37°C. In each histogram is also displayed the negative control of immunostaining with class-matched irrelevant antibodies (C). Shown is a representative experiment of 5 experiments.
In whole blood, LXA4 analogs modulate surface expression of L-selectin and CD11/CD18 on resting leukocytes. Blood aliquots were incubated with LXA4 analogs for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Control mean fluorescence intensity for L-selectin: PMNL, 108 ± 7; monocytes, 30 ± 2; lymphocytes, 55 ± 3; for CD18: PMNL, 52 ± 4; monocytes, 113 ± 11; lymphocytes, 20 ± 1; n = 8. The results are the mean ± SEM of 4 to 8 experiments with different donor cell preparations. *P < .05v control.
In whole blood, LXA4 analogs modulate surface expression of L-selectin and CD11/CD18 on resting leukocytes. Blood aliquots were incubated with LXA4 analogs for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Control mean fluorescence intensity for L-selectin: PMNL, 108 ± 7; monocytes, 30 ± 2; lymphocytes, 55 ± 3; for CD18: PMNL, 52 ± 4; monocytes, 113 ± 11; lymphocytes, 20 ± 1; n = 8. The results are the mean ± SEM of 4 to 8 experiments with different donor cell preparations. *P < .05v control.
The addition of PAF (1 μmol/L) to whole blood gave a significant decrease in L-selectin and a marked upregulation of CD11/CD18 on leukocytes (Figs 3 and4). Figure 3 shows a representative experiment on the effect of 15-R/S-methyl-LXA4 on PAF-induced changes in the expression of these adhesion molecules. Preincubation of blood with either 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 attenuated PAF-induced upregulation of CD18 expression on PMNL, monocytes, and lymphocytes in a concentration-dependent fashion, with IC50 values of approximately 1.5 μmol/L (Fig 4). Essentially complete inhibition was achieved with 5 μmol/L of the LXA4 analogs. PAF-induced decreases in L-selectin expression on PMNL and monocytes were also markedly, although not completely, inhibited by these LXA4analogs (Fig 4). Incubation of blood with IL-8 (10 nmol/L) downregulated L-selectin and upregulated CD18 expression on PMNL (Fig 5). As with PAF, both 15-R/S-methyl-LXA4 and 16-phenoxy-LXA4 at 5 μmol/L completely inhibited IL-8–induced upregulation of CD18, whereas they partially inhibited changes in L-selectin expression (Fig5). Immunostaining of leukocytes with an anti-CD11b MoAb showed similar changes as those observed with the anti-CD18 MoAb (data not shown).
Aspirin-triggered LXA4 analog (15-R/S-methyl-LXA4) inhibits PAF-induced changes in cell surface expression of L-selectin and CD18 by human neutrophils. Whole blood was incubated in medium only (unstimulated) or with 15-R/S-methyl-LXA4 (5 μmol/L) for 10 minutes and then with PAF (1 μmol/L) for 30 minutes at 37°C. In each histogram is also displayed the negative control of immunostaining with class-matched irrelevant antibodies (C). Shown is a representative experiment of 5 experiments.
Aspirin-triggered LXA4 analog (15-R/S-methyl-LXA4) inhibits PAF-induced changes in cell surface expression of L-selectin and CD18 by human neutrophils. Whole blood was incubated in medium only (unstimulated) or with 15-R/S-methyl-LXA4 (5 μmol/L) for 10 minutes and then with PAF (1 μmol/L) for 30 minutes at 37°C. In each histogram is also displayed the negative control of immunostaining with class-matched irrelevant antibodies (C). Shown is a representative experiment of 5 experiments.
Inhibition of PAF-induced changes in L-selectin and CD18 expression on leukocytes. Whole blood aliquots were incubated with LXA4 analogs for 30 minutes and then challenged with 1 μmol/L PAF for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: PMNL, control, 103 ± 9; PAF, 43 ± 5; monocytes, control, 30 ± 3; PAF, 20 ± 2; lymphocytes, control, 53 ± 3; PAF, 50 ± 2, n = 5, all P < .05. Mean fluorescence intensity for CD18: PMNL, control, 48 ± 3; PAF, 61 ± 6; P < .05; monocytes, control, 107 ± 12; PAF, 123 ± 13;P < .05; lymphocytes, control, 19 ± 1; PAF, 21 ± 1. The results are the mean ± SEM of 4 to 5 experiments with different donor cell preparations. *P < .05 vPAF-stimulated cells.
Inhibition of PAF-induced changes in L-selectin and CD18 expression on leukocytes. Whole blood aliquots were incubated with LXA4 analogs for 30 minutes and then challenged with 1 μmol/L PAF for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: PMNL, control, 103 ± 9; PAF, 43 ± 5; monocytes, control, 30 ± 3; PAF, 20 ± 2; lymphocytes, control, 53 ± 3; PAF, 50 ± 2, n = 5, all P < .05. Mean fluorescence intensity for CD18: PMNL, control, 48 ± 3; PAF, 61 ± 6; P < .05; monocytes, control, 107 ± 12; PAF, 123 ± 13;P < .05; lymphocytes, control, 19 ± 1; PAF, 21 ± 1. The results are the mean ± SEM of 4 to 5 experiments with different donor cell preparations. *P < .05 vPAF-stimulated cells.
Inhibition of IL-8–induced changes in L-selectin and CD18 expression on human PMNL. Whole blood was preincubated with LXA4 analogs for 30 minutes and then challenged with 10 nmol/L IL-8 for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: control, 115 ± 8; IL-8, 61 ± 5; CD18: control, 45 ± 4; IL-8, 59 ± 9; n = 3; bothP < .05. Values are the means ± SEM of 3 experiments with different donor cell preparations. *P < .05 vIL-8–stimulated cells.
Inhibition of IL-8–induced changes in L-selectin and CD18 expression on human PMNL. Whole blood was preincubated with LXA4 analogs for 30 minutes and then challenged with 10 nmol/L IL-8 for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: control, 115 ± 8; IL-8, 61 ± 5; CD18: control, 45 ± 4; IL-8, 59 ± 9; n = 3; bothP < .05. Values are the means ± SEM of 3 experiments with different donor cell preparations. *P < .05 vIL-8–stimulated cells.
We also investigated whether LXA4 analogs are able to modify the affinity of β2 integrins. Formation of a higher affinity form of LFA-1 and Mac-1 is thought to involve conformational changes and association of the I domain with the β-propeller domain.21,22 The MoAb G-25.2 recognizes this latter domain in LFA-1.22 Incubation of whole blood with PAF or IL-8 in the absence or presence of 15-R/S-methyl-LXA4 was not associated with a detectable increase in expression of MoAb G-25.2 (Fig6A and B). On the other hand, activation of cells with Mg2+and EGTA, which is known to induce the formation of a higher affinity form of LFA-1 without inducing clustering,19 resulted in increases in MoAb G-25.2 expression (Fig 6C) that was not affected by 15-R/S-methyl-LXA4 (Fig 6C and D) or 16-phenoxy-LXA4 (data not shown).
Effect of 15-R/S-methyl-LXA4 on the expression of LFA-1 epitope expressed by the β-propeller domain of LFA-1 on neutrophils. Mean fluorescence of MoAb G-25.2 on PMNL in whole blood challenged with PAF (1 μmol/L; A) or IL-8 (10 nmol/L; B) for 30 minutes at 37°C in the presence of 15-R/S-methyl-LXA4(5 μmol/L). The dotted line represents MoAb G-25.2 binding at 4°C. Shown is a representative of 3 experiments. (C) The expression of MoAb G-25.2 on PMNL in whole blood challenged with Mg2+ (1 mmol/L) and EGTA (2 mmol/L) for 30 minutes at 4°C (dotted line) and at 37°C in the absence or presence of 15-R/S-methyl-LXA4 (5 μmol/L). Shown is a representative of 3 experiments. (D) Mean fluorescence of MoAb G-25.2 on neutrophils challenged with Mg2+ and EGTA in the presence of 15-R/S-methyl-LXA4. Values are the means ± SEM of 3 experiments.
Effect of 15-R/S-methyl-LXA4 on the expression of LFA-1 epitope expressed by the β-propeller domain of LFA-1 on neutrophils. Mean fluorescence of MoAb G-25.2 on PMNL in whole blood challenged with PAF (1 μmol/L; A) or IL-8 (10 nmol/L; B) for 30 minutes at 37°C in the presence of 15-R/S-methyl-LXA4(5 μmol/L). The dotted line represents MoAb G-25.2 binding at 4°C. Shown is a representative of 3 experiments. (C) The expression of MoAb G-25.2 on PMNL in whole blood challenged with Mg2+ (1 mmol/L) and EGTA (2 mmol/L) for 30 minutes at 4°C (dotted line) and at 37°C in the absence or presence of 15-R/S-methyl-LXA4 (5 μmol/L). Shown is a representative of 3 experiments. (D) Mean fluorescence of MoAb G-25.2 on neutrophils challenged with Mg2+ and EGTA in the presence of 15-R/S-methyl-LXA4. Values are the means ± SEM of 3 experiments.
Next, we examined whether LXA4 analogs when added to whole blood could also affect the cell activation-independent downregulation of L-selectin expression. Incubation of blood with CRP peptide 201-206 (100 μg/mL) resulted in, on average, 49%, 42%, and 17% decreases in L-selectin expression on PMNL, monocytes, and lymphocytes, respectively (Fig 7). These changes were inhibited by 15-R/S-methyl-LXA4 in a concentration-dependent manner, with apparent IC50 values of approximately 250 nmol/L (Fig 6). Similar inhibition was observed with 16-phenoxy-LXA4 (Fig 7).
LXA4 analogs prevent cell activation-independent downregulation of L-selectin expression on human PMNL, monocytes, and lymphocytes. Whole blood aliquots were incubated with LXA4 analogs for 30 minutes at 37°C and then challenged with 100 μg/mL CRP peptide 201-206. Results are presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: PMNL, control, 97 ± 6; peptide 201-206, 50 ± 14; monocytes, control, 35 ± 4; peptide 201-206, 22 ± 7; lymphocytes, control, 61 ± 4; peptide 201-206, 51 ± 4; n = 3; allP < .05. Values represent the mean ± SEM of 3 independent experiments. *P < .05 v peptide 201-206–stimulated cells.
LXA4 analogs prevent cell activation-independent downregulation of L-selectin expression on human PMNL, monocytes, and lymphocytes. Whole blood aliquots were incubated with LXA4 analogs for 30 minutes at 37°C and then challenged with 100 μg/mL CRP peptide 201-206. Results are presented as the percentage of control (unchallenged cells). Mean fluorescence intensity for L-selectin: PMNL, control, 97 ± 6; peptide 201-206, 50 ± 14; monocytes, control, 35 ± 4; peptide 201-206, 22 ± 7; lymphocytes, control, 61 ± 4; peptide 201-206, 51 ± 4; n = 3; allP < .05. Values represent the mean ± SEM of 3 independent experiments. *P < .05 v peptide 201-206–stimulated cells.
Inhibition of MAPK kinase reverses LXA4-induced changes in CD11/CD18 expression on neutrophils.
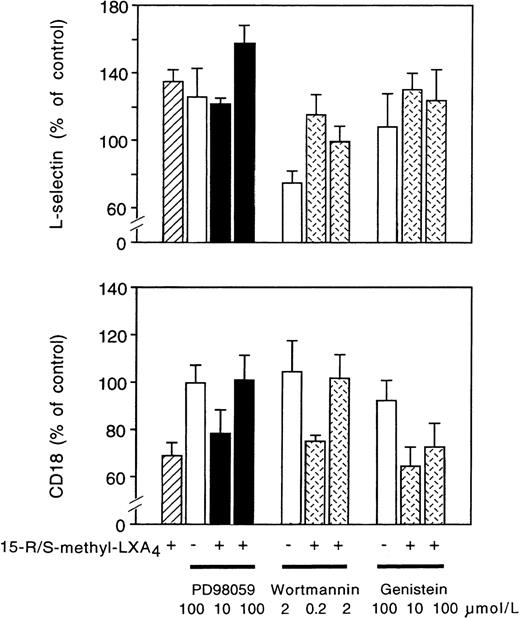
Treatment of neutrophils with the MAPK kinase inhibitor PD98059 increased by approximately 27% L-selectin expression without altering CD11/CD18 expression (Fig 8). PD98059 prevented 15-R/S-methyl-LXA4–induced downregulation of CD11/CD18 expression in a concentration-dependent fashion, whereas changes in L-selectin expression were not affected (Fig 8). Wortmannin at 0.2 μmol/L had no effect, whereas at 2 μmol/L, it prevented 15-R/S-methyl-LXA4–induced downregulation of CD18 (Fig 8). Furthermore, wortmannin (2 μmol/L) alone downregulated L-selectin expression and inhibited the action of 15-R/S-methyl-LXA4(Fig 8). 15-R/S-methyl-LXA4 induction was not affected by the tyrosine kinase inhibitor genistein (Fig 8) or herbimycin-A (data not shown).
Effects of MAPK kinase (MEK) and tyrosine kinase inhibitors on neutrophil adhesion molecules. Isolated neutrophils (107 cells/mL) were preincubated with PD98059, wortmannin, or genistein for 30 minutes at 37°C and then challenged with 5 μmol/L 15-R/S-methyl-LXA4 for 30 minutes. Adhesion molecule expression is presented as the percentage of control. Mean fluorescence intensity for control samples, L-selectin: 35 ± 1; CD18: 82 ± 14; n = 4. Values are the mean ± SEM of 4 independent experiments.
Effects of MAPK kinase (MEK) and tyrosine kinase inhibitors on neutrophil adhesion molecules. Isolated neutrophils (107 cells/mL) were preincubated with PD98059, wortmannin, or genistein for 30 minutes at 37°C and then challenged with 5 μmol/L 15-R/S-methyl-LXA4 for 30 minutes. Adhesion molecule expression is presented as the percentage of control. Mean fluorescence intensity for control samples, L-selectin: 35 ± 1; CD18: 82 ± 14; n = 4. Values are the mean ± SEM of 4 independent experiments.
LXA4 analogs do not affect E-selectin or ICAM-1 expression on LPS- or TNF-α–stimulated endothelial cells.
After stimulation by LPS, HCAEC increased 27- and 2.6-fold the expression of E-selectin and ICAM-1, respectively (n = 4, bothP < .05; Table 1). 15-R/S-methyl-LXA4 did not affect basal expression of these adhesion molecules (data not shown) and produced only a slight inhibition of LPS-induced changes (Table 1). The maximum inhibition did not exceed 10%. Similarly, 15-R/S-methyl-LXA4 failed to significantly inhibit TNF-α–induced E-selectin and ICAM-1 expression (Table 1).
15-R/S-methyl-LXA4 Does Not Alter E-Selectin and ICAM-1 Expression on Human Coronary Artery Endothelial Cells
| . | 15-R/S- methyl-LXA4 . | E-Selectin (RFI) . | ICAM-1 (RFI) . |
|---|---|---|---|
| Control | 0 | 2 ± 1 | 140 ± 31 |
| LPS | 0 | 50 ± 5 | 327 ± 41 |
| LPS | 5 nmol/L | 51 ± 5 | 324 ± 31 |
| LPS | 50 nmol/L | 49 ± 4 | 305 ± 21 |
| LPS | 500 nmol/L | 45 ± 4 | 296 ± 27 |
| LPS | 5,000 nmol/L | 45 ± 2 | 302 ± 21 |
| TNF-α | 0 | 24 ± 3 | 386 ± 27 |
| TNF-α | 500 nmol/L | 19 ± 2 | 335 ± 32 |
| TNF-α | 5,000 nmol/L | 20 ± 1 | 357 ± 12 |
| . | 15-R/S- methyl-LXA4 . | E-Selectin (RFI) . | ICAM-1 (RFI) . |
|---|---|---|---|
| Control | 0 | 2 ± 1 | 140 ± 31 |
| LPS | 0 | 50 ± 5 | 327 ± 41 |
| LPS | 5 nmol/L | 51 ± 5 | 324 ± 31 |
| LPS | 50 nmol/L | 49 ± 4 | 305 ± 21 |
| LPS | 500 nmol/L | 45 ± 4 | 296 ± 27 |
| LPS | 5,000 nmol/L | 45 ± 2 | 302 ± 21 |
| TNF-α | 0 | 24 ± 3 | 386 ± 27 |
| TNF-α | 500 nmol/L | 19 ± 2 | 335 ± 32 |
| TNF-α | 5,000 nmol/L | 20 ± 1 | 357 ± 12 |
Confluent HCAEC monolayers were left unstimulated (control) or challenged with 1 μg/mL LPS or 2.5 ng/mL TNF-α for 4 hours at 37°C in the presence of 15-R/S-methyl-LXA4. Values are the mean ± SEM of 3 independent experiments.
Abbreviation: RFI, relative fluorescence intensity.
LXA4 analogs inhibit neutrophil adhesion to endothelial cells.
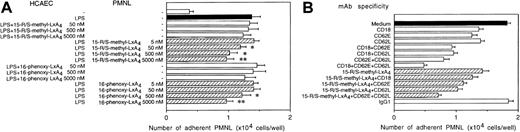
Activation of HCAEC with LPS resulted in a 4.1-fold increase in the number of adherent neutrophils (Fig 9). When HCAEC were challenged with LPS in the presence of one of the stable LXA4 analogs, only slight decreases in adhesion could be detected (Fig 9A). By contrast, addition of neutrophils preincubated with either 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 to LPS-activated HCAEC attenuated neutrophil attachment in a concentration-dependent fashion (Fig 9A). Significant inhibition of adhesion was detected with 15-R/S-methyl-LXA4 at a concentration as low as 50 nmol/L. At 5 μmol/L, 15-R/S-methyl-LXA4 and 16-phenoxy-LXA4 inhibited neutrophil adhesion by 38% ± 4% and 36% ± 3%, respectively (n = 5, both P < .05; Fig 9A). Neither 15-deoxy-LXA4 nor decomposed 15-R/S-methyl-LXA4 or 16-phenoxy-LXA4 affected neutrophil adhesion (data not shown).
Inhibition of neutrophil binding to endothelial cells by LXA4 analogs (A) or by anti–E-selectin, anti–L-selectin, and anti-CD18 MoAbs and 15-R/S-methyl-LXA4 (B). Confluent HCAEC monolayers were cultured with 1 μg/mL LPS with or without LXA4 analogs for 6 hours at 37°C. (A) Neutrophils were preincubated for 30 minutes with LXA4 analogs or medium as indicated before addition to activated HCAEC. Values are the means ± SEM of 3 to 6 experiments using neutrophils from different donors. *P < .05 v LPS-treated HCAEC. (B) Neutrophils were treated with 15-R/S-methyl-LXA4 (5 μmol/L) or the indicated MoAbs before and during the assay. Neutrophil adhesion to unstimulated HCAEC was 0.41 ± 0.03 × 104 cells per well. The irrelevant MoAb MOPC-21 (IgG1) was used as a negative control. The results represent the mean ± SEM of 5 experiments using neutrophils from different donors.
Inhibition of neutrophil binding to endothelial cells by LXA4 analogs (A) or by anti–E-selectin, anti–L-selectin, and anti-CD18 MoAbs and 15-R/S-methyl-LXA4 (B). Confluent HCAEC monolayers were cultured with 1 μg/mL LPS with or without LXA4 analogs for 6 hours at 37°C. (A) Neutrophils were preincubated for 30 minutes with LXA4 analogs or medium as indicated before addition to activated HCAEC. Values are the means ± SEM of 3 to 6 experiments using neutrophils from different donors. *P < .05 v LPS-treated HCAEC. (B) Neutrophils were treated with 15-R/S-methyl-LXA4 (5 μmol/L) or the indicated MoAbs before and during the assay. Neutrophil adhesion to unstimulated HCAEC was 0.41 ± 0.03 × 104 cells per well. The irrelevant MoAb MOPC-21 (IgG1) was used as a negative control. The results represent the mean ± SEM of 5 experiments using neutrophils from different donors.
Because multiple receptors are involved in neutrophil adhesion to LPS-stimulated HCAEC18 and LXA4 analogs affected expression of both L-selectin and CD11/CD18 on PMNL, we assayed the contribution of L-selectin, E-selectin, and CD18 to the binding interaction. A significant proportion of neutrophil-HCAEC attachment was blocked by MoAbs binding to L-selectin (44% ± 4%, n = 5), CD18 (31% ± 4%), or E-selectin (38% ± 5%; Fig 9B). The combination of these MoAbs inhibited neutrophil adhesion by 87% to 98%. Treatment of neutrophils with 15-R/S-methyl-LXA4 and anti-CD18 MoAb resulted in only a slightly greater inhibition of adhesion than those observed with neutrophils treated with either 15-R/S-methyl-LXA4 or anti-CD18 MoAb (Fig 9B). The combination of 15-R/S-methyl-LXA4 with either anti–L-selectin MoAb or anti–E-selectin MoAb resulted in additive inhibition, and the degree of inhibition was similar to those observed when anti–L-selectin MoAb or anti–E-selectin MoAb was combined with anti-CD18 MoAb, respectively (Fig 9B). Combining 15-R/S-methyl-LXA4, anti–L-selectin MoAb, and anti–E-selectin MoAb blocked approximately 80% of adhesion. Similar results were obtained with 16-phenoxy-LXA4 (data not shown).
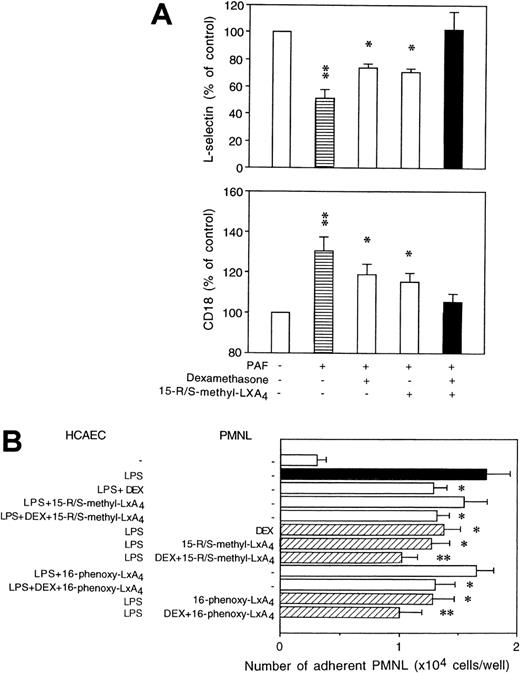
Additive inhibition by LXA4 analogs and dexamethasone of PAF-induced changes in neutrophil adhesion molecule expression and binding to endothelial cells.
Dexamethasone was used at a concentration of 100 nmol/L, because our previous results showed that the maximum inhibitory effect of dexamethasone on neutrophil adhesion molecule expression can be achieved with this concentration.20 As expected, dexamethasone added alone attenuated by 35% to 45% PAF-induced changes in L-selectin and CD11/CD18 expression on PMNL (Fig 10A). Combining dexamethasone with 15-R/S-methyl-LXA4 resulted in an almost complete inhibition (Fig 10A). Similar inhibition was observed with both monocytes and lymphocytes (data not shown). Culture of HCAEC with dexamethasone diminished the number of adherent neutrophils (Fig 10B). However, no further decrements in neutrophil adhesion were detected in the presence of LXA4 analogs. On the other hand, the inhibitory actions of dexamethasone and LXA4 analogs were additive when neutrophils were preincubated with dexamethasone and LXA4 analogs before exposure to LPS-activated HCAEC (Fig10B).
Cooperative actions of dexamethasone and LXA4 analogs. (A) Human whole blood aliquots were incubated with 100 nmol/L dexamethasone for 120 minutes in the presence of 15-R/S-methyl-LXA4 (5 μmol/L) for the last 30 minutes of incubation, as indicated, and then challenged with 1 μmol/L PAF for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control. Mean fluorescence intensity for L-selectin: control, 37 ± 3; PAF, 26 ± 4; CD18: control, 61 ± 4; PAF, 73 ± 7; n = 5; both P < .05. *P < .05; **P < .01 v control (untreated). L-selectin and CD18 expression on dexamethasone plus 15-R/S-methyl-LXA4–treated cells did not differ significantly from those of control. (B) Confluent HCAEC monolayers were cultured for 6 hours with LPS (1 μg/mL) in the presence of dexamethasone (DEX; 100 nmol/L), 15-R/S-methyl-LXA4, or 16-phenoxy-LXA4 (5 μmol/L). Neutrophils pretreated with dexa- methasone and/or LXA4 analogs as described for whole blood were added to activated HCAEC and then challenged with PAF (1 μmol/L). The results are expressed as the mean ± SEM of 5 experiments using neutrophils from different donors. *P < .05 vLPS-treated.
Cooperative actions of dexamethasone and LXA4 analogs. (A) Human whole blood aliquots were incubated with 100 nmol/L dexamethasone for 120 minutes in the presence of 15-R/S-methyl-LXA4 (5 μmol/L) for the last 30 minutes of incubation, as indicated, and then challenged with 1 μmol/L PAF for 30 minutes at 37°C. Adhesion molecule expression is presented as the percentage of control. Mean fluorescence intensity for L-selectin: control, 37 ± 3; PAF, 26 ± 4; CD18: control, 61 ± 4; PAF, 73 ± 7; n = 5; both P < .05. *P < .05; **P < .01 v control (untreated). L-selectin and CD18 expression on dexamethasone plus 15-R/S-methyl-LXA4–treated cells did not differ significantly from those of control. (B) Confluent HCAEC monolayers were cultured for 6 hours with LPS (1 μg/mL) in the presence of dexamethasone (DEX; 100 nmol/L), 15-R/S-methyl-LXA4, or 16-phenoxy-LXA4 (5 μmol/L). Neutrophils pretreated with dexa- methasone and/or LXA4 analogs as described for whole blood were added to activated HCAEC and then challenged with PAF (1 μmol/L). The results are expressed as the mean ± SEM of 5 experiments using neutrophils from different donors. *P < .05 vLPS-treated.
DISCUSSION
In this report, we describe a novel mechanism(s) by which stable LXA4 analogs can affect the inflammatory response, namely via the modulation of surface expression of adhesion molecules on resting and immunostimulated leukocytes, and inhibition of neutrophil adhesion to the activated endothelium. This inhibition is predominantly mediated via actions of LXA4 analogs on leukocytes and is distinct from those of glucocorticoids. Moreover and of particular interest, these actions of stable LXA4 analogs were monitored for the first time within the whole blood environment, where they clearly remain bioactive.
Because dehydrogenation of native lipoxins by human leukocytes results in their rapid inactivation,23 we used LXA4stable analogs that carry substituents at the carbon 15 through ω 20 end of the native LXA4 structure, resist conversion,7,24 and retain the biological activities of native LXA4 in leukocyte adhesion and migration assays.5,24 15-R/S-methyl-LXA4 is an analog of aspirin-triggered 15-epi-LXA4 and 16-phenoxy-LXA4 is an analog of native LXA4.7 13 The effects of 15-R/S-methyl-LXA4 and 16-phenoxy-LXA4 observed in this study are attributed to the molecules themselves, because neither degraded LXA4 analogs nor 15-deoxy-LXA4caused detectable changes in the assays used.
Our study documents the complex nature of actions of stable LXA4 analogs on expression of leukocyte adhesion molecules. These analogs upregulated L-selectin and downregulated CD11/CD18 expression on human resting neutrophils, monocytes, and, to a lesser extent, lymphocytes in whole blood and markedly attenuated changes evoked by immunostimulation. These results are consistent with earlier reports, which showed that native LXA4 inhibits formyl-Met-Leu-Phe–stimulated upregulation of CD11b/CD18 on human isolated neutrophils.25 The present results indicated that this action is also demonstrable within whole blood with nanomolar to micromolar concentrations of LXA4 analogs. As expected, higher concentrations were required in whole blood to inhibit the upregulation of CD18 expression than in isolated cells. This might reflect interactions of LXA4 analogs with serum components such as albumin. Nevertheless, it is impressive that these lipophilic compounds are active in the microenvironment of whole blood and overcome interactions with blood components to specifically regulate leukocytes. Downregulation of CD11/CD18 expression on resting neutrophils by LXA4 analogs does not involve phosphatidylinositol 3-kinase or tyrosine kinases; rather, it appears to be mediated via activation of MAPK kinase, as suggested by the experiments with PD98059 and wortmannin, which, at a concentration of 2 μmol/L, also inhibits MAPK kinase.26 The LXA4analogs did not initiate detectable changes in tyrosin phosphorylation of either Erk-2 or p38 MAPK in resting human neutrophils as evaluated by immunoblotting using specific antibodies to the phosphorylated forms of these enzymes (Hachicha et al, unpublished observations). Recent results suggest that intracellular arachidonic acid induces integrin-dependent homotypic adhesion of neutrophils via the Raf-1/MAPK kinase/Erk pathway.27 With respect to LXA4 receptor activation, it is possible that other Erk or p38 MAPK-independent pathways may be involved in signaling neutrophil adhesion molecule expression.
Physiological stimuli regulate adhesion by either altering the affinity of the individual integrin molecule or by inducing clustering of β2 integrins, thereby increasing the overall strength of binding.19,28,29 Increasing intracellular Ca2+concentration does not induce detectable changes in the affinity of LFA-1.22 Because leukocyte activation evoked by PAF or IL-8 is mediated through increases in intracellular Ca2+concentration, these agonists would not increase integrin affinity. Indeed, neither PAF nor IL-8 affected MoAb G-25.2 expression. Activation of leukocytes with Mg2+ and EGTA, which results in the formation of a higher affinity form of LFA-1,19increased the expression of MoAb G-25.2, which did not appear to be affected by the LXA4 analogs studied. These results suggest that LXA4 analogs do not alter the affinity of either LFA-1 and probably of Mac-1, but rather they interfere with activation-induced clustering of β2 integrins. However, our results do not preclude the possibility that, in the presence of integrin ligands, LXA4 analogs might affect a ligand-induced affinity increase secondary to integrin clustering.
Comparison of L-selectin expression at 4°C and at 37°C indicated that, within whole blood, these LXA4 analogs prevented temperature-induced L-selectin shedding on unstimulated leukocytes. For minutes of activation by either PAF or IL-8 or challenge with CRP peptide 201-206, which does not activate cells, leukocytes release L-selectin from their surface by a proteolytic enzyme. Because this enzyme appears to be constitutively active, formation of an appropriate 3-dimensional structure of L-selectin near the membrane is thought to regulate this proteolytic process.30,31 Whereas it remains to be established whether the conformational changes induced by cell activation dependent or independent stimuli are identical, our results demonstrate that these events are effectively blocked by LXA4 analogs. A recent study has reported that the intracellular tail of L-selectin is phosphorylated on serine upon activation with chemoattractants.32 It is of interest that calmodulin inhibitors directly induce proteolytic shedding of L-selectin.33 Whether this action of LXA4involves calmodulin or interference with phosphorylation step(s) remains a key area of inquiry.
Despite inhibition of L-selectin shedding from neutrophils within whole blood, which is thought to promote neutrophil-HCAEC attachment, LXA4 analogs actually inhibit isolated neutrophil adhesion to HCAEC. This inhibition can primarily be attributed to their actions on neutrophils rather than on HCAEC in this interaction, because only slight decreases in the number of adherent neutrophils were observed after culture of HCAEC with LPS in the presence of LXA4analogs. Consistently, LXA4 analogs have little effect on LPS- or TNF-α–stimulated expression of E-selectin and ICAM-1 on HCAEC. Our results indicate that inhibition of neutrophil-HCAEC adherence by LXA4 analogs is predominantly attributable to inhibition of CD11/CD18 expression on neutrophils. The LXA4analogs or a function-blocking anti-CD18 MoAb resulted in similar decreases in neutrophil adhesion to HCAEC. The action of LXA4 analogs and anti-CD18 MoAb was not additive, whereas the inhibition with LXA4 analogs was additive with anti–E-selectin and anti–L-selectin MoAbs. These results suggest that LXA4 analogs had little, if any, effect on E-selectin or L-selectin function and did not interfere with E-selectin ligand. Taken together, our data indicate that, in addition to inhibition of P-selectin expression on rat intestinal venular endothelial cells,8 lipoxins are potent regulators of both leukocyte L-selectin and CD11/CD18 expression. Whereas inhibition of P-selectin–dependent capture of neutrophils may be a key mechanism by which LXA4 analogs inhibit neutrophil-endothelial interactions in the mesenteric circulation,8 functions of adhesion molecules may overlap, and even neutrophil recruitment into other vascular beds may not rely on a P-selectin–dependent adhesion.34
The present study and previous studies20,35 indicate that the mechanisms of action of LXA4 analogs differ from those of glucocorticoids. Adhesion molecule expression on resting neutrophils can be modulated by LXA4 analogs, but not by dexamethasone.20 Both dexamethasone and LXA4analogs alone inhibited by approximately 20% to 45% PAF-induced changes in L-selectin and CD11/CD18 expression on neutrophils, representing the near maximum inhibition that can be achieved with these compounds (Filep et al20 and the present study). Interestingly, the inhibitory actions of dexamethasone and LXA4 analogs were additive, resulting in almost complete inhibition of PAF-stimulated changes. The inhibitory actions of dexamethasone on PMNL requires de novo protein synthesis.20Preincubation of neutrophils with either dexamethasone or LXA4 analogs before addition to LPS-activated HCAEC resulted in partial inhibition of adhesion, and the inhibitory actions of dexamethasone and LXA4 analogs were additive. Culture of HCAEC with LPS and dexamethasone, but not with LXA4analogs, resulted in marked decreases in the number of adherent neutrophils. These findings are consistent with the glucocorticoid inhibition of LPS-induced expression of ICAM-1 and E-selectin on endothelial cells.35 By contrast, LXA4 analogs had little, if any, effect on expression of these adhesion molecules. Interestingly, the mechanism by which LXA4 analogs inhibit neutrophil-endothelial adhesion also differs from that of several nonsteroidal anti-inflammatory drugs, which induce shedding of L-selectin from neutrophils and inhibit L-selectin–mediated attachment.36 The actions of LXA4 analogs on β2 integrins appear to be similar to those reported for piroxicam,37 tepoxalin, a dual cyclooxygenase/lipoxygenase inhibitor,38 and the anti-inflammatory agent leumedin NPC 15669.39 Piroxicam prevents expression of a CD11b activation neo-epitope,37 whereas tepoxalin and leumedin NPC 15669 inhibit upregulation of Mac-1.38 39 However, unlike the LXA4 analogs, none of these agents affected β2 integrin expression on resting leukocytes.
In conclusion, this study demonstrates that stable LXA4analogs modulate adhesion molecule expression on leukocytes monitored within the microenvironment of human whole blood and inhibit neutrophil adhesion to HCAEC via downregulation of CD11/CD18 expression. These effects are distinct from those of either glucocorticoids or nonsteroidal anti-inflammatory drugs. Therefore, stable analogs of native LXA4 and aspirin-triggered 15-epi-LXA4may represent a novel therapeutic approach for selective regulation of leukocyte trafficking in host defense, inflammation, and reperfusion injury.
ACKNOWLEDGMENT
The authors thank Valery V. Fokin for expert assistance in the synthesis of the lipoxin analogs.
Supported by grants from the Medical Research Council of Canada (MT-12573 to J.G.F.) and from the National Institutes of Health (GM-38765 and DK50305 to C.N.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to János G. Filep, MD, Research Center, Maisonneuve-Rosemont Hospital, 5415 boulevard de l'Assomption, Montréal, Québec, Canada H1T 2M4; e-mail:filepj@ere.umontreal.ca.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal