Abstract
After hematopoietic stem cell transplantation, the persistence and expansion of grafted mature postthymic T cells allow both transfer of donor immunologic memory and generation of a diverse T repertoire. This thymic-independent process, which is particularly important in humans, because most transplant recipients present severe thymus atrophy, is impaired by graft-versus-host disease (GVHD). The goal of this study was to decipher how GVHD influences the fate of grafted postthymic T cells. Two major findings emerged. First, we found that, after a brisk proliferation phase, alloreactive antihost T cells underwent a massive activation-induced cell death (AICD). For both CD4+ and CD8+ T cells, the Fas pathway was found to play a major role in this AICD: alloreactive T cells upregulated Fas and FasL, and AICD of antihost T cells was much decreased in the case of lpr (Fas-deficient) donors. Second, whereas non–host-reactive donor T cells neither upregulated Fas nor suffered apoptosis when transplanted alone, they showed increased membrane Fas expression and apoptosis when coinjected with host-reactive T cells. We conclude that GVHD-associated AICD of antihost T cells coupled with bystander lysis of grafted non–host-reactive T cells abrogate immune reconstitution by donor-derived postthymic T lymphocytes. Furthermore, we speculate that massive lymphoid apoptosis observed in the acute phase of GVHD might be responsible for the occurrence of autoimmunity in the chronic phase of GVHD.
IN NORMAL INDIVIDUALS, the size of peripheral T-cell compartments is maintained by thymic and extrathymic pathways. First, thymic output is responsible for initial seeding of secondary lymphoid organs in young subjects.1 Continuous thymic output in later life contributes to the diversity of the T-cell repertoire,2,3 although the level of thymic function is known to decrease with age as the thymus undergoes progressive involution.4 Second, most T lymphocytes produced daily in adults derive from the proliferation of postthymic T cells that retain an impressive proliferative potential.5-7 Finally, extrathymic differentiation of hematopoietic progenitors has been detected in selected organs.8-12 The possible contribution of the latter pathway to T-cell homeostasis remains to be better defined but is generally believed to play a minor role compared with the first two other pathways.
It has been shown that, after transplantation of syngeneic hematopoietic stem cells, both thymic and extrathymic pathways can contribute to the reconstitution of peripheral T-cell compartments. In this model, the relative contribution of each pathway depends on the recipient’s thymic function and the number of postthymic T cells in the donor inoculum.13,14 In contrast, when the transplant is performed into an allogeneic recipient, graft-versus-host disease (GVHD) initiated by the graft postthymic T cells causes severe lymphoid hypoplasia.15-17 Disappearance of host lymphoid cells at the time of GVHD is easily accounted for by the generation of antihost alloreactive T cells that eradicate recipient mature lymphocytes and hematopoietic progenitors via both perforin and Fas-mediated cytotoxicity.16,18,19 However, there is no satisfactory explanation for the failure of donor-derived T cells to reconstitute GVHD+ hosts. It has been suggested that, during the course of GVHD, Fas/FasL interactions may contribute to failure of donor T-cell reconstitution, because donor T-cell hypoplasia is less severe when the donor is FasL defective or when the recipient is treated with anti-FasL monoclonal antibody (MoAb).16,20,21 However, interpretation of these interesting observations is limited by the fact that Fas/FasL interactions costimulate T-cell activation and proliferation in such a way that signaling through Fas in the early stages of an immune response augments the generation of effector cells.22,23 Indeed, after transplantation of FasL-defective cells, the generation of antihost effector cells has been shown to be severely impaired.20 Thus, it is not clear whether interference with Fas signaling decreases the severity of GVHD-related T-cell hypoplasia because (1) hypoplasia involves Fas-dependent apoptotic events or (2) because, similar to other molecules (eg, CD28, CD40, etc),24 25 the costimulatory effect of Fas is required for the expansion of alloreactive T cells.
The severity of GVHD-associated T-cell hypoplasia implies that GVHD abrogates both thymic and extrathymic T-cell reconstitution. Mechanistically, each differentiation pathway should be approached separately. It has been shown that the thymus stroma is a direct target of GVHD, and damage to the host thymus provides a rational explanation for the failure of donor-derived progenitors to differentiate via the classic central pathway.15,17,26 The problem in this case is probably one of soil rather than seed. In contrast, the failure of the graft mature postthymic T cells to expand and repopulate the host peripheral compartments, as observed in athymic syngeneic recipients, remains unexplained. This question is particularly relevant in the case of human recipients. Indeed, because of age- and disease-related factors such as chemotherapy, radiotherapy, or infections, most patients have thymus hypoplasia.27-31 In addition, T-cell reconstitution has been shown to depend mostly on proliferation of grafted postthymic T cells, at least during the first year posttransplant.32-34 Consequently, the failure of grafted postthymic T cells to expand in GVHD+ recipients can have a dramatic impact on immune reconstitution and is probably responsible to a large extent for the high frequency and severity of infections in these patients. Moreover, impairment of donor T-cell expansion could represent a major obstacle for the implementation of adoptive immunotherapy strategies. The latter point is increasingly important, because innovative approaches aimed at transferring activated/memory T cells specific for pathogens such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV) hold great promise for the treatment of infections in immunocompromised hosts.35-37Thence, the goal of the present work was to determine the fate of grafted postthymic T cells in GVHD+ recipients and to understand why these mature donor T cells fail to repopulate the host’s peripheral T-cell compartments.
MATERIALS AND METHODS
Mice.
The following strains of mice were purchased from the Jackson Laboratory (Bar Harbor, ME): A/J, C57BL/6J (B6), B6.SJL-PtprcaPep3b/BoyJ (Ly5a) (B6.SJL), B6.PL-Thy1a/Cy (Thy1.1) (B6.PL), B6.MRL-Faslpr (lpr), and B6Smn.C3H-Faslgld (gld). Mice with a B6 background have the H2b haplotype, whereas A/J mice are H2a. All inbred mice are Ly5.2+, except for B6.SJL mice, which are Ly5.1+. F1 hybrids from B6.SJL and A/J mice (B6.SAF1), and from B6 and A/J mice (B6AF1) were bred at the Guy-Bernier Research Center (Montreal, Quebec, Canada). Animals were maintained in specific pathogen-free conditions according to the standards of the Canadian Committee for Animal Protection. Lpr donors were between 5 and 8 weeks old, whereas other mice used as cell donors or recipients were between 5 and 16 weeks of age.
Induction of GVHD.
Spleen and lymph nodes (cervical, axillary, and inguinal) from donor mice with a B6 background were pooled into single-cell suspensions in which the number of Thy1+ T cells was assessed by flow cytometry. GVHD was induced by intravenous injection of a spleen/lymph node cell suspension containing 60 × 106 T cells into unirradiated F1 hybrid recipients.
Production of non–host-reactive cells.
Bone marrow cells were obtained from the tibiae and femurs of B6.PL donor mice and T-cell–depleted with specific anti–Thy-1 antiserum (Cedarlane, Hornby, Ontario, Canada) as previously described.13 38 Then, 107 bone marrow cells were intravenously injected into irradiated (12 Gy total body irradiation from a 60Co source at a dose rate of 128 cGy/min) B6AF1recipients on day 0. On day 60, spleen and lymph nodes of these B6AF1 recipients were harvested and used as a source of non–host-reactive cells.
MoAbs.
The following MoAbs were obtained from Pharmingen (San Diego, CA): fluorescein isothiocyanate (FITC)-labeled anti-Vβ panel, FITC- and phycoerythrin (PE)-labeled anti-Thy1.1 (MRC OX-7) and anti-Thy1.2 (53-2.1 and 30-H12, respectively), FITC-labeled and biotin-conjugated anti-Ly5.1 (A20) and anti-Ly5.2 (104), PE-labeled antibodies against CD19 (1D3), CD44 (1M7) and Fas (Jo2), Cy-chrome–labeled anti-CD4 (RM4-5) and anti-CD8 (53-7.7), purified anti-FasL (MFL3), biotin-conjugated goat-antihamster IgG, PE-conjugated streptavidin, and isotypic controls.
Flow cytometric analysis.
Cells were analyzed on a FACScalibur (Becton Dickinson, San Jose, CA) using the CellQuest program (Becton Dickinson) or on a FACScan (Becton Dickinson) using the Lysis II program (Becton Dickinson). Lymphocytes were gated by forward and side scatter, and fluorescence data were collected for 10,000 cells. Studies of selected T-cell populations were performed on 5,000 to 10,000 gated cells that were CD4+ or CD8+ and expressed the Ly5/Thy1 phenotype of interest. Direct immunofluorescence staining was performed as previously described.38 A sensitive three-step indirect staining method was used to assess the low-level expression of cell surface FasL. First, cells were stained with purifed anti-FasL Ab. After two washes, cells were incubated with biotinylated second-step reagent and then, after two additional washes, PE-conjugated streptavidin was added together with other selected fluorochrome-conjugated antibodies for three-color staining.
Measurement of FITC-labeled Annexin-V binding.
Apoptosis was analyzed by quantifying phosphatidylserine residues exposed on the cell membrane. Spleen cells were first stained with membrane-specific antibodies. After two washes with phosphate-buffered saline, 3 μL of recombinant FITC-labeled Annexin-V (Pharmingen) was added to cells resuspended in 100 μL of binding buffer (10 mmol/L HEPES/NaOH, pH 7.4, 140 mmol/L NaCl, 5 mmol/L CaCl2). After 15 minutes of incubation in the dark at room temperature, 500 μL of binding buffer was added and the samples were analyzed on a FACScan flow cytometer. In control samples, propidium iodide (2 μg/mL) staining was performed to help set the limit used to discriminate Annexin-V–positive and –negative cells. In some experiments, Annexin-V staining was performed after stimulation with Concanavalin A (Con A). Briefly, cells diluted at 2 × 106/mL in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 5 × 10−5mol 2-mercaptoethanol (2-ME), and 2 μg/mL of Concanavalin were incubated at 37°C in a humidified atmosphere of 5% CO2. After 40 hours of culture, cells were washed twice before antibodies and Annexin-V staining.
Statistical analysis.
Results for group means were compared using the Student’st-test.
RESULTS
Experimental model.
GVHD was induced by injecting a mixture of C57BL/6 or B6.SJL (H-2b) lymph node + spleen cells containing 6 × 107 T lymphocytes into unirradiated B6AF1 or B6.SAF1 recipients (H-2b/a). The origin of cells in chimeras was determined according to their Ly5 phenotype: C57BL/6J, A/J, and B6AF1 mice are Ly5.1−Ly5.2+, B6.SJL are Ly5.1+Ly5.2−, and B6.SAF1 mice are Ly5.1+Ly5.2+. We elected to use unirradiated recipients because irradiation per se can induce a number of effects that could have confounded our analyses. Indeed, irradiation can (1) increase Fas expression,39 (2) induce extrathymic T-cell development,40 (3) impair the function of thymus stromal cells,41 and (4) trigger the release of inflammatory cytokines.42 By using unirradiated recipients, we ensured that changes found in our chimeras were caused only by GVHD.
Expansion and disappearance of postthymic T cells during the course of GVHD.
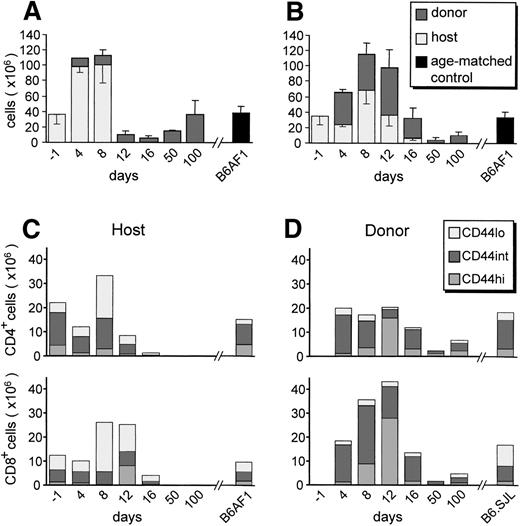
After injection of B6.SJL cells into B6AF1 hosts, a transient increase of host lymphocytes was followed by total and irreversible disappearance of host B cells by day 12 (Fig 1A) and of host T cells between days 16 and 50 (Fig 1B). Between days 4 and 12, donor-derived T cells showed a notable proliferation, involving mainly the CD8+ subset (Fig 1B and D). This was followed by a conspicuous lymphoid hypoplasia of longer duration for T cells than for B cells. On day 100, (donor-derived) B-cell numbers had regained normal levels, whereas the numbers of (donor-derived) T cells were decreased by 70% compared with age-matched controls. These observations are concordant with the description of T-cell reconstitution reported by Hakim et al43 in a similar model of GVHD.
Expansion and disappearance of postthymic T cells during the course of GVHD. A cell suspension containing 6 × 107B6.SJL T cells was injected into unirradiated B6AF1 recipients. Shown is a time course evaluation of the numbers of host- and donor-derived B cells (A) and T cells (B) found in the spleen of GVHD+recipients. CD44 phenotype of host (C) and donor (D) T cells found in the spleen of GVHD+ recipients. Three-color staining was performed using PE-labeled anti-CD19, anti-Thy1.2, or anti-CD44; Cy-chrome–labeled anti-CD4 or anti-CD8; and FITC-labeled anti-Ly5.1 or anti-Ly5.2. Results are presented as the mean ± SD in (A) and (B); for the sake of clarity, only the mean is shown in (C) and (D). There were three to four mice per group.
Expansion and disappearance of postthymic T cells during the course of GVHD. A cell suspension containing 6 × 107B6.SJL T cells was injected into unirradiated B6AF1 recipients. Shown is a time course evaluation of the numbers of host- and donor-derived B cells (A) and T cells (B) found in the spleen of GVHD+recipients. CD44 phenotype of host (C) and donor (D) T cells found in the spleen of GVHD+ recipients. Three-color staining was performed using PE-labeled anti-CD19, anti-Thy1.2, or anti-CD44; Cy-chrome–labeled anti-CD4 or anti-CD8; and FITC-labeled anti-Ly5.1 or anti-Ly5.2. Results are presented as the mean ± SD in (A) and (B); for the sake of clarity, only the mean is shown in (C) and (D). There were three to four mice per group.
T-cell proliferation can be observed after cognate interactions with antigen-presenting cells or after exposure of bystander cells to high concentrations of cytokines (such as interferon [IFN], interleukin-2 [IL-2], IL-6, and tumor necrosis factor [TNF]) produced by antigen-specific T cells.44-46 Acquisition of an activated/memory phenotype, characterized by upregulation of CD44, is observed in antigen-specific T cells after cognate interactions, but not in proliferating bystander T cells.44,47,48Nevertheless, at least in the context of some viral infections, T cells that are antigen-experienced, and thus have already upregulated CD44, may be more susceptible to bystander activation than naı̈ve (CD44lo) T cells.47 Analysis of the CD44 phenotype of expanded host- and donor-derived postthymic T-cell populations showed divergent phenotypes. At its time of maximal expansion (day 12), the population of donor-derived T cells was composed mainly of CD44hi cells, with very few CD44lo elements (Fig 1). In contrast, at its peak (day 8), expansion of recipient T cells involved mainly CD44locells, with a lesser increase in the number of CD44intcells and a notable decrease in the amount of CD44hielements. These results suggest that the transient expansion of host T cells was a bystander effect induced by the GVHD-associated cytokine storm, whereas donor T-cell proliferation was induced by cognate interactions with host antigens. Considering that we infused 6 × 107 donor T cells and that approximately 3% of T cells respond to a given allo-H2 haplotype,49 the grafted inoculum used in the preceding experiments contained ≤2 × 106 host-reactive T cells and approximately 5.8 × 107 non–host-reactive T cells. Taking into account that only a portion of infused T cells home to the spleen, host-reactive T cells (≤2 × 106) would have to expand more than 30-fold to account for the approximately 6 × 107donor T cells that were found in the spleen of day-12 recipients (Fig1). By comparison, the bystander expansion of host T cells was much more modest, because the number of host T cells increased to a maximum of twofold on day 8 (Fig 1).
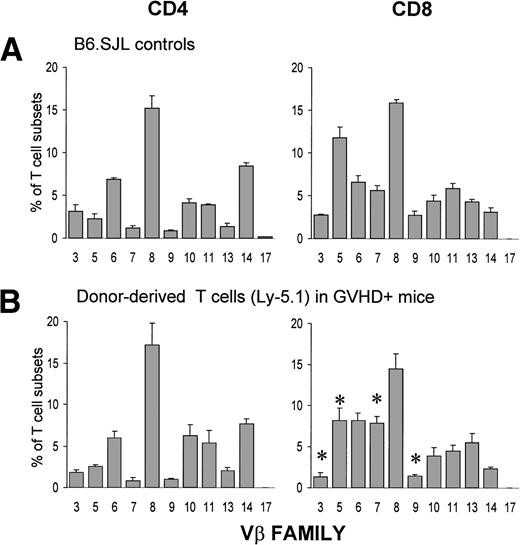
Assessment of Vβ usage by CD4+ and CD8+ cell populations on day 12 showed that expansion of donor T cells involved all Vβ families tested. Indeed, the Vβ profile of expanded donor CD4+ T cells was similar to that of normal B6.SJL controls, whereas the Vβ usage by the expanded CD8+ subset showed only minor differences when compared with controls (Fig 2). These observations show that the cell expansion was of polyclonal origin and was not elicited by a superantigen.50 51
Donor-derived postthymic T cells express a diverse Vβ repertoire. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. Three-color staining was performed using the following antibodies: FITC-labeled anti-Vβ, Cy-chrome–labeled anti-CD4 or anti-CD8, and biotin-coupled anti-Ly5.1 plus Streptavidin-PE. Chimeras were studied on day +12. Results are presented as the mean ± SD (3 mice per group). *P< .05 when compared with B6.SJL controls.
Donor-derived postthymic T cells express a diverse Vβ repertoire. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. Three-color staining was performed using the following antibodies: FITC-labeled anti-Vβ, Cy-chrome–labeled anti-CD4 or anti-CD8, and biotin-coupled anti-Ly5.1 plus Streptavidin-PE. Chimeras were studied on day +12. Results are presented as the mean ± SD (3 mice per group). *P< .05 when compared with B6.SJL controls.
Expansion of donor T cells essentially involves host-reactive cells and is thus antigen-specific.
To evaluate more directly the importance of antigen-specific versus bystander proliferation in donor T-cell expansion, we produced and studied the fate of a donor (B6.PL) T-lymphocyte population selectively depleted in host (H2a)-reactive cells. These (Ly5.2+Thy1.1+) non–host-reactive T cells were harvested from the spleen and lymph nodes of B6AF1 mice that had been irradiated (12 Gy) and injected 60 days before with 107T-cell–depleted B6.PL bone marrow cells. After differentiation of B6.PL progenitors in the B6AF1 thymus, the lymphoid organs of these chimeras are repopulated with B6.PL T cells from which anti-H2a reactive elements have been deleted. As expected,13 the proliferative activity of these T cells was normal when assessed after in vitro stimulation with mitogens (Con A and phytohemagglutinin [PHA]) or third-party major histocompatibility complex (MHC) antigens (H2d; data not shown).
A cell suspension containing 4 × 107non–host-reactive T cells (of B6.PL origin) and 4 × 107 B6.SJL T cells (containing host-reactive T cells) was injected into B6.AF1 hosts, and we compared the expansion of these two donor T-cell populations. The rationale was that, if donor T-cell proliferation was host antigen-specific, B6.PL-derived non–host-reactive T cells would not proliferate and the donor T-cell compartment would contain almost exclusively B6.SJL cells. On the contrary, if donor T-cell proliferation were mainly due to bystander activation, the ratio of B6.PL vs. B6.SJL cells would approach 1:1. When assessed on days 8 and 12 posttransplant, the ratio of B6.PL versus B6.SJL cells was 1:99 (Table 1). At the time of maximal T-cell expansion, on day 12, the mean absolute numbers of B6.PL-versus B6.SJL-derived T cells found in the spleen of GVHD+ recipients were 61 × 106 and 0.5 × 106, respectively (data not shown). This indicates that donor T-cell expansion involved host-reactive cells and that the importance of bystander proliferation was negligible. Thus, the intense donor T-cell proliferation was consecutive to cognate interactions with host antigens and not the result of paracrine stimulation of bystander (non–host-reactive) T cells.
Importance of Antigen-Specific Versus Bystander Proliferation in Donor T-Cell Expansion
| . | Donor Cell Origin . | |
|---|---|---|
| Ly5.1+Thy1.2+ (host-reactive +) . | Ly5.2+Thy1.1+ (host-reactive −) . | |
| Day 8 | ||
| CD4 | 28% | 0.8% |
| CD8 | 71% | 0.2% |
| Total | 99% | 1% |
| Day 12 | ||
| CD4 | 33% | 0.6% |
| CD8 | 66% | 0.4% |
| Total | 99% | 1% |
| . | Donor Cell Origin . | |
|---|---|---|
| Ly5.1+Thy1.2+ (host-reactive +) . | Ly5.2+Thy1.1+ (host-reactive −) . | |
| Day 8 | ||
| CD4 | 28% | 0.8% |
| CD8 | 71% | 0.2% |
| Total | 99% | 1% |
| Day 12 | ||
| CD4 | 33% | 0.6% |
| CD8 | 66% | 0.4% |
| Total | 99% | 1% |
B6AF1 recipients (Ly5.2+Thy1.2+) received a cell suspension containing 4 × 107 B6.SJL T cells (containing host-reactive T cells) (Ly5.1+Thy1.2+) and 4 × 107Ly5.2+Thy1.1+ non–host-reactive T cells. Results show the mean (N = 3) proportion of donor-derived T cells that were Ly5.2+Thy1.1+ (non–host-reactive) versus Ly5.1+Thy1.2+. Non–host-reactive T cells were harvested from day-60 lethally irradiated B6AF1 recipients transplanted with 107 T-cell–depleted bone marrow cells from B6.PL donors. Trace amounts of B6AF1-derived T cells that could have contaminated the inoculum of non–host-reactive cells were not enumerated by the Ly5.2+Thy1.1+ phenotype.
Host-reactive cells show massive apoptosis involving Fas/FasL interactions.
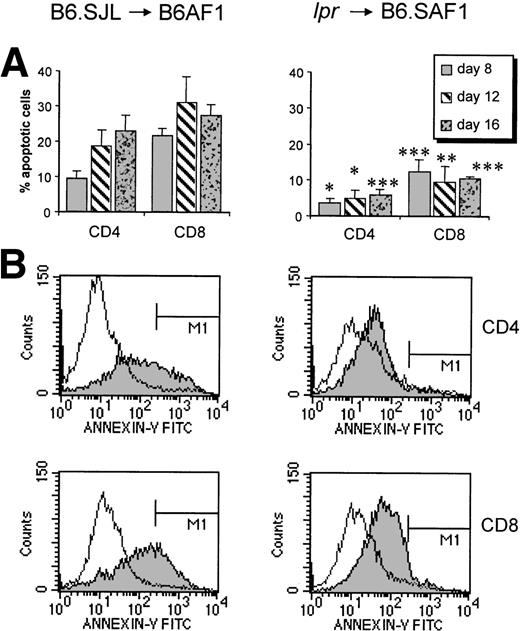
The observation that brisk expansion of host-reactive T cells is followed by severe and prolonged lymphoid hypoplasia suggests that activation of donor T cells leads to massive activation-induced cell death (AICD). We therefore used Annexin-V staining to measure the number of apoptotic B6.SJL donor T cells in the spleen of B6AF1 hosts. The percentage of apoptotic T cells in fresh spleen cell suspensions reached 22% for the CD4+ subset on day 16 and 32% for the CD8+ subset on day 12 (Fig 3). These numbers of apoptotic cells are significantly higher than those observed in control B6.SJL T cells (3% to 4.5%). Because the in vivo clearance of apoptotic cells is very rapid,52 53 our results suggest that extensive amounts of donor T cells are most likely eliminated by apoptosis during the course of acute GVHD.
Host-reactive cells show massive apoptosis involving Fas/FasL interactions. (A) Proportion of apoptotic (Annexin-V–positive) T cells in F1 recipients injected with B6.SJL vs. lpr cells. The mean numbers of apoptotic CD4+/CD8+ T cells was 4.5%/3% in B6.SJL controls and 5.8%/3.4% in lpr controls (n = 3; data not shown). Results are expressed as the mean ± SD, n = 3. *P < .05, **P < .01, ***P < .005 relative to B6.SJL donors. (B) Histograms showing results for a representative F1 recipient studied on day 16 after injection of B6.SJL or lpr cells (shaded diagram). The clear histogram represents a negative control (untreated B6.SJL or lpr mouse).
Host-reactive cells show massive apoptosis involving Fas/FasL interactions. (A) Proportion of apoptotic (Annexin-V–positive) T cells in F1 recipients injected with B6.SJL vs. lpr cells. The mean numbers of apoptotic CD4+/CD8+ T cells was 4.5%/3% in B6.SJL controls and 5.8%/3.4% in lpr controls (n = 3; data not shown). Results are expressed as the mean ± SD, n = 3. *P < .05, **P < .01, ***P < .005 relative to B6.SJL donors. (B) Histograms showing results for a representative F1 recipient studied on day 16 after injection of B6.SJL or lpr cells (shaded diagram). The clear histogram represents a negative control (untreated B6.SJL or lpr mouse).
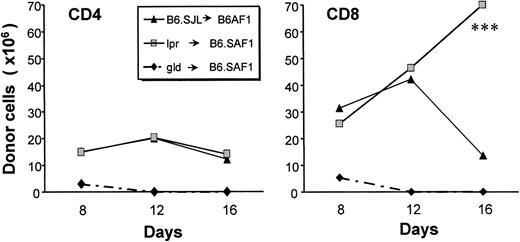
With this in mind, we asked whether Fas/FasL interactions played a significant role in the apoptosis of donor T cells. We inferred that, if apoptosis was Fas-dependent, it should not occur when the donor is Fas- or FasL-deficient. In a first set of experiments, we determined that only the study of Fas-deficient (lpr), but not of FasL-deficient (gld) donors would be appropriate to address this question. Indeed, after injection into B6.SAF1 hosts, T cells from gld donors showed no measurable expansion and were rapidly eliminated (Fig 4). This observation might be explained by the fact that Fas/FasL interactions have a costimulatory effect on T-cell activation and generation of effector cells.20,22 23 Moreover, the failure of T cells from gld donors to proliferate demonstrates that, at least in our model, the absence of GVHD with gld donors may not be accepted as an evidence that Fas-mediated apoptosis (as opposed to Fas-mediated costimulation) plays a role in GVHD. In contrast to gld donors, cells of lpr donors expanded vigorously in recipients (Fig 4). Furthermore, from day 12 to 16, the number of lpr-derived CD8+ T cells increased, in contrast to CD8+ cells obtained from normal donors, which decreased. More importantly, the number of apoptotic donor-derived CD4+ and CD8+ T cells was strikingly decreased in the case of lpr donors as compared with normal donors (Fig 3). These observations suggest that Fas signaling plays a prominent role in the AICD of host-reactive T cells. However, it should be noted that, in the case of lpr donors, CD4+ T cells did not expand to the same extent as CD8+ cells between days 12 and 16 (Fig 4). Although the reason for this observation is unclear, it suggests that there may be differences in the mechanisms controlling CD4+versus CD8+ cells in this setting.
Influence of Fas/FasL interactions on donor T-cell expansion. Expansion of donor T cells obtained from B6.SJL, gld (FasL-deficient), or lpr (Fas-deficient) mice after transplantation in B6AF1 or B6.SAF1 recipients. Three-color staining was performed with the following fluorochrome-conjugated antibodies: anti-Ly5.1 or anti-Ly5.2, anti-CD4 or anti-CD8, and anti-Thy1.1 or anti-Thy1.2. On day 16, the number of donor-derived CD8+ T cells was greater with lpr donors than with B6.SJL donors (***P < .005).
Influence of Fas/FasL interactions on donor T-cell expansion. Expansion of donor T cells obtained from B6.SJL, gld (FasL-deficient), or lpr (Fas-deficient) mice after transplantation in B6AF1 or B6.SAF1 recipients. Three-color staining was performed with the following fluorochrome-conjugated antibodies: anti-Ly5.1 or anti-Ly5.2, anti-CD4 or anti-CD8, and anti-Thy1.1 or anti-Thy1.2. On day 16, the number of donor-derived CD8+ T cells was greater with lpr donors than with B6.SJL donors (***P < .005).
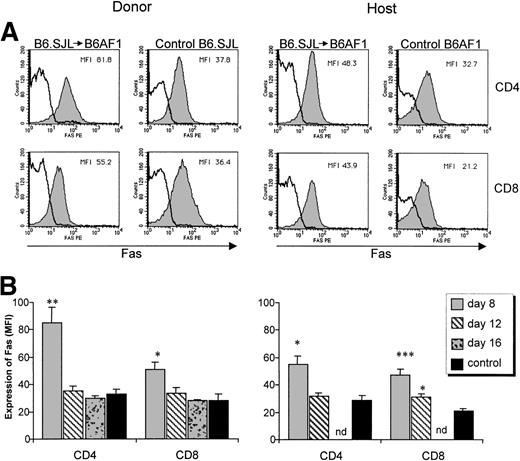
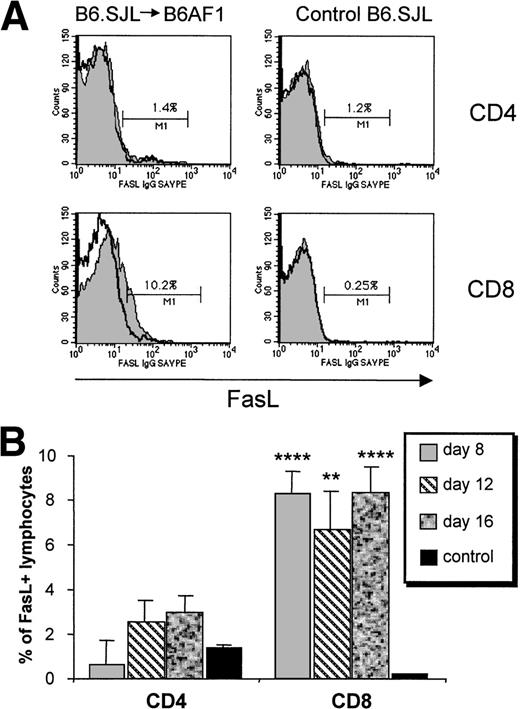
We then analyzed the kinetics of Fas and FasL expression on T-cell subsets between days 8 and 16 in B6AF1 recipients of a B6.SJL graft. A significant upregulation of Fas expression was found in CD4+ and CD8+ host and donor T cells (Fig 5). Fas upregulation was most noticeable on donor CD4+ T cells and was of limited duration, being observed on day 8 but not later. In addition, a significant increase in the proportion of FasL+ cells was found specifically among the donor CD8+ T-cell subset (Fig 6). Donor CD4+ T cells showed only a minimal increase in FasL expression that did not reach significance. Upregulation of FasL on donor CD8+ T cells was present at the three time points studied from days 8 to 16. These results suggest that Fas-dependent AICD of donor T cells is initiated specifically by FasL+ CD8+ host-reactive T cells.
Expression of Fas on donor and host T-cell subsets. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. (A) Increased Fas expression on day 8. Three-color staining was performed with the following antibodies: FITC-labeled anti-Ly5.1 or anti-Ly5.2, Cy-chrome–labeled anti-CD4 or anti-CD8, and PE-labeled anti-Fas (shaded histogram) or its isotypic control (clear histogram). Results from one representative experiment of three are shown. (B) Time course evaluation of Fas expression. Mean ± SD, N = 3; nd, not done. *P < .05, **P < .01, ***P < .005 relative to control.
Expression of Fas on donor and host T-cell subsets. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. (A) Increased Fas expression on day 8. Three-color staining was performed with the following antibodies: FITC-labeled anti-Ly5.1 or anti-Ly5.2, Cy-chrome–labeled anti-CD4 or anti-CD8, and PE-labeled anti-Fas (shaded histogram) or its isotypic control (clear histogram). Results from one representative experiment of three are shown. (B) Time course evaluation of Fas expression. Mean ± SD, N = 3; nd, not done. *P < .05, **P < .01, ***P < .005 relative to control.
Upregulation of FasL expression on CD8+ T cells. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. (A) Increased FasL expression on donor (Ly5.1+) CD8+ T cells on day 8. Cells were first labeled with hamster anti-FasL antibody, then with biotin-coupled goat antihamster antibody, and lastly with PE-conjugated streptavidin together with FITC-labeled anti-Ly5.1 and Cy-chrome–labeled anti-CD4 or anti-CD8 antibodies; only the last two steps were performed in staining control. FasL staining is represented by a shaded histogram and staining control as a clear histogram. The value shown in each box represents the specific staining of FasL+ cells. Results from one representative experiment of three are shown. (B) Time course evaluation of FasL expression. Mean ± SD, N = 3. **P < .01, ****P< .001 relative to control.
Upregulation of FasL expression on CD8+ T cells. A cell suspension containing 6 × 107 B6.SJL T cells was injected into unirradiated B6AF1 recipients. (A) Increased FasL expression on donor (Ly5.1+) CD8+ T cells on day 8. Cells were first labeled with hamster anti-FasL antibody, then with biotin-coupled goat antihamster antibody, and lastly with PE-conjugated streptavidin together with FITC-labeled anti-Ly5.1 and Cy-chrome–labeled anti-CD4 or anti-CD8 antibodies; only the last two steps were performed in staining control. FasL staining is represented by a shaded histogram and staining control as a clear histogram. The value shown in each box represents the specific staining of FasL+ cells. Results from one representative experiment of three are shown. (B) Time course evaluation of FasL expression. Mean ± SD, N = 3. **P < .01, ****P< .001 relative to control.
The influence of host-reactive cells on the fate of non–host-reactive cells.
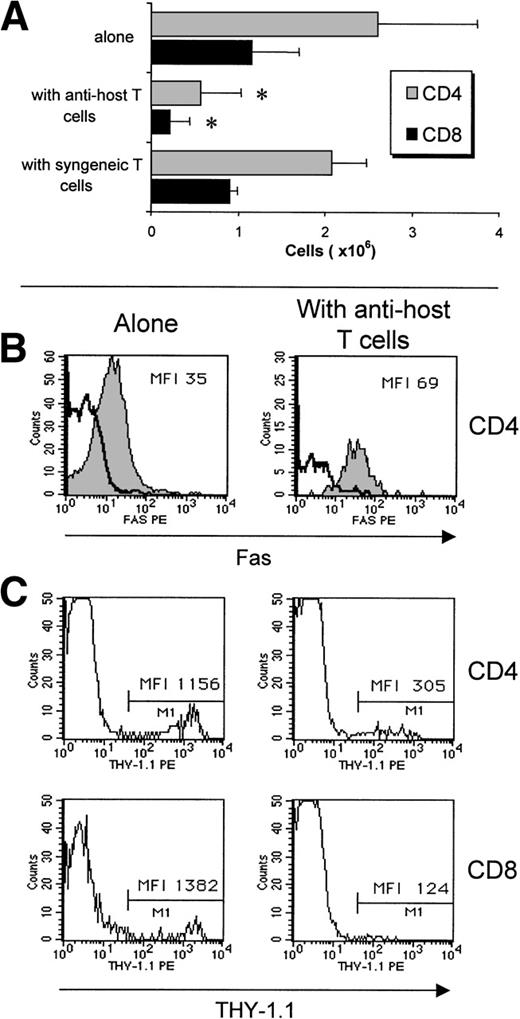
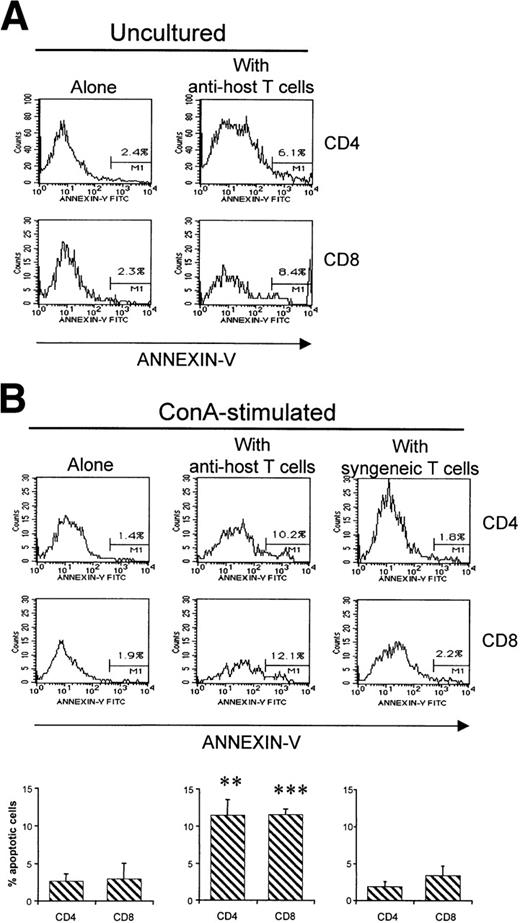
Although AICD entails the disappearance of most, if not all, host-reactive T cells, what is the fate of non–host-reactive donor T cells? To evaluate this question, we compared the fate of non–host-reactive T cells when injected either alone or with host-reactive T cells. After injection of 2 × 107(B6.PL→B6AF1) non–host-reactive T cells into B6AF1 hosts, 3.8 × 106 Ly5.2+Thy1.1+ T cells were found in day-12 recipients (Fig 7A). Strikingly, the recovery of non–host-reactive T cells was decreased by approximately fivefold when 4 × 107 B6.SJL T cells were coinjected (Fig 7A). As a negative control, we verified that coinjection of syngeneic (B6.SAF1) T cells did not decrease the recovery of non–host-reactive T cells. Thus, clearance of non–host-reactive T cells was accelerated in the presence of host-reactive T cells. We hypothesized that two mechanisms could be responsible for the rapid demise of non–host-reactive T cells in the presence of host-reactive T cells: (1) competition for space between host-reactive and nonreactive T cells and (2) apoptosis of bystander cells induced by host-reactive T cells. Three series of observations showed characteristic features of apoptosis in non–host-reactive T cells when coinjected with host-reactive T cells, but not when injected alone: (1) upregulation of Fas expression (Fig 7B), (2) downregulation of Thy1 (Fig 7C; decreased expression of several cell surface membrane antigens such as Thy1 is one of the characteristic changes found on apoptotic cells54), and (3) increased numbers of Annexin-V–positive T cells (Fig 8). Increased numbers of Annexin-V–positive elements among non–host-reactive T cells coinjected with host reactive T cells were observed when analyses were performed on fresh recipients’ splenocyte suspensions, but became more impressive when analyses were performed on mitogen-stimulated T cells (Fig 8). In a control group, we observed that coinjection of syngeneic (B6.SAF1) T cells did not increase the proportion of apoptotic elements among non–host-reactive cells (Fig8B). These results lead us to contend that the failure of grafted non–host-reactive postthymic T cells to reconstitute the immune system of GVHD+ hosts is a consequence of bystander killing mediated by host-reactive effector T cells.
Accelerated clearance of non–host-reactive donor (Ly5.2+Thy1.1+) T cells when coinjected with host-reactive (Ly5.1+Thy1.2+) T cells. (A) Numbers of CD4+ and CD8+non–host-reactive T cells found in the spleen of B6AF1 recipients on day 12. Host-reactive and non–host-reactive cells were prepared as described for Fig 3A. Non–host-reactive T cells (20 × 106) were injected alone or with either 40 × 106 host-reactive T cells or 40 × 106syngeneic (B6.SAF1) T cells. N = 3; *P < .05 when compared with results in mice transplanted with non–host-reactive T cells alone or non–host-reactive T cells + syngeneic T cells. No differences were found between mice transplanted with non–host-reactive T cells alone versus non–host-reactive T cells + syngeneic T cells. (B) Non–host-reactive grafted CD4+ T cells overexpress Fas when coinjected with host-reactive T cells (right panel), but not when injected alone (left panel). Fas expression was assessed on day 8 as described in Fig 5. Results from one representative experiment of three are shown. (C) Downregulation of Thy1 expression was observed on CD4+ and CD8+ non–host-reactive grafted T cells when coinjected with host-reactive T cells (right panel), but not when injected alone (left panel). Two- and three-color staining was performed with the following fluorochrome-conjugated antibodies: anti-Ly5.2 or anti-Thy1.1, anti-Fas, and anti-CD4 or anti-CD8. Results from one representative experiment of three are shown.
Accelerated clearance of non–host-reactive donor (Ly5.2+Thy1.1+) T cells when coinjected with host-reactive (Ly5.1+Thy1.2+) T cells. (A) Numbers of CD4+ and CD8+non–host-reactive T cells found in the spleen of B6AF1 recipients on day 12. Host-reactive and non–host-reactive cells were prepared as described for Fig 3A. Non–host-reactive T cells (20 × 106) were injected alone or with either 40 × 106 host-reactive T cells or 40 × 106syngeneic (B6.SAF1) T cells. N = 3; *P < .05 when compared with results in mice transplanted with non–host-reactive T cells alone or non–host-reactive T cells + syngeneic T cells. No differences were found between mice transplanted with non–host-reactive T cells alone versus non–host-reactive T cells + syngeneic T cells. (B) Non–host-reactive grafted CD4+ T cells overexpress Fas when coinjected with host-reactive T cells (right panel), but not when injected alone (left panel). Fas expression was assessed on day 8 as described in Fig 5. Results from one representative experiment of three are shown. (C) Downregulation of Thy1 expression was observed on CD4+ and CD8+ non–host-reactive grafted T cells when coinjected with host-reactive T cells (right panel), but not when injected alone (left panel). Two- and three-color staining was performed with the following fluorochrome-conjugated antibodies: anti-Ly5.2 or anti-Thy1.1, anti-Fas, and anti-CD4 or anti-CD8. Results from one representative experiment of three are shown.
Apoptosis of non–host-reactive T cells when coinjected with host-reactive T cells. Non–host-reactive cells were injected alone or with either host-reactive T cells or syngeneic T cells as described in Fig 7. (A) The proportion of non–host-reactive cells stained by Annexin-V was evaluated in fresh uncultured spleen cell suspensions from day-8 B6AF1 hosts. Three-color staining was performed with the following antibodies: PE-labeled anti-Ly5.2, Cy-chrome–labeled anti-CD4 or anti-CD8, and FITC-labeled Annexin-V. Results from one representative experiment of three are shown. (B) Annexin-V staining was performed after culturing recipient spleen cells during 40 hours in the presence of Con A. Results are expressed as the mean ± SD (N = 3) in the lower panel, and results from one representative experiment are depicted in the upper panel. **P< .01, ***P < .005 when compared with the results obtained in mice transplanted with non–host-reactive T cells alone or non–host-reactive T cells + syngeneic T cells. No differences were found between mice transplanted with non–host-reactive T cells alone versus non–host-reactive T cells + syngeneic T cells.
Apoptosis of non–host-reactive T cells when coinjected with host-reactive T cells. Non–host-reactive cells were injected alone or with either host-reactive T cells or syngeneic T cells as described in Fig 7. (A) The proportion of non–host-reactive cells stained by Annexin-V was evaluated in fresh uncultured spleen cell suspensions from day-8 B6AF1 hosts. Three-color staining was performed with the following antibodies: PE-labeled anti-Ly5.2, Cy-chrome–labeled anti-CD4 or anti-CD8, and FITC-labeled Annexin-V. Results from one representative experiment of three are shown. (B) Annexin-V staining was performed after culturing recipient spleen cells during 40 hours in the presence of Con A. Results are expressed as the mean ± SD (N = 3) in the lower panel, and results from one representative experiment are depicted in the upper panel. **P< .01, ***P < .005 when compared with the results obtained in mice transplanted with non–host-reactive T cells alone or non–host-reactive T cells + syngeneic T cells. No differences were found between mice transplanted with non–host-reactive T cells alone versus non–host-reactive T cells + syngeneic T cells.
DISCUSSION
The fate of alloreactive antihost T cells.
After transplantation of T-cell–replete hematopoietic cell grafts, host-reactive T cells are initially sequestered in the spleen, where they proliferate before reentering the circulation and disseminating to other organs.55,56 By examining host spleen cell suspensions, we observed, in the early days posttransplant, a brisk expansion of alloreactive T cells followed by massive in situ AICD. Expanded populations of donor T cells comprised more CD8+than CD4+ T cells and were quite diverse in terms of Vβ repertoire. Expansion selectively involved antihost T cells. Non–host-reactive T cells did not expand when transplanted alone or with host-reactive cells. The latter observation indicates that T-cell proliferation triggered by host H2 antigens basically involves antigen-specific and not bystander T cells. Our finding is consistent with other recent studies on T-cell responses to viral antigens.46 57-61
The very high numbers (up to 32%) of Annexin-V–labeled donor T cells found in fresh spleen cell suspensions from GVHD+ mice point to a massive GVHD-associated AICD. AICD is a physiologically important process that contributes to the termination of immune responses and can be mediated by numerous molecular pathways, including Fas, TNF, TRAIL, galectin, and CTLA-4.62-67 Analyses of T-cell responses to various antigens have shown that Fas usually plays a dominant role in AICD of CD4+ T cells.68-70The situation is more complex regarding AICD of CD8+ T cells. In the latter case, the role of Fas in various systems ranges from negligible to predominant.68,71-76 In models in which the Fas pathway seems to be of little significance, AICD is mediated predominantly by the TNF pathway.71 74
Our results demonstrate the upregulation of FasL on CD8+host-reactive T cells, an increased expression of Fas on donor CD4+ and CD8+ T cells, and a major decrease in apoptotic donor CD4+ and CD8+ T cells in the case of lpr donors. They provide strong evidence that the Fas pathway plays a critical role in the AICD associated with GVHD. Furthermore, although it appears that both CD4+ and CD8+ T cells are targets of this AICD, effector function (FasL expression) seems to be performed only by host-reactive CD8+ T cells. Our data are concordant with results from others showing that the level of FasL upregulation detected on antigen reactive T cells after massive in vivo expansion is inferior to that observed on T cells after short-term in vitro culture with mitogens.18 This may well be explained by the recent observation that upregulation of FasL is an early and transient event that is maximal after one cell division but decreases after two to three cell divisions.77 Thus, when studies are performed on T-cell populations that have undergone massive in vivo expansion (here ≥30-fold for host-reactive T cells, ie, ≥5 cell divisions), FasL expression may not be at its maximum level. The molecular interactions responsible for the low levels of apoptosis observed in T cells from lpr donors remain to be defined. This could represent Fas-dependent apoptosis mediated by the low levels of Fas proteins expressed by lpr cells.78 Alternatively, residual levels of apoptosis detected with lpr donors could result from AICD mediated by TNF or other pathways and/or from passive cell death caused by deprivation of survival stimuli leading to decreased expression of antiapoptotic proteins, mainly of the Bcl family.64
The fate of non–host-reactive T cells.
When injected alone, non–host-reactive T cells neither proliferated nor upregulated expression Fas expression. However, coinjection of host-reactive T cells entailed an increased expression of Fas on, in addition to apoptosis and accelerated disappearance of, non–host-reactive T cells. Therefore, apoptosis of non–host-reactive T cells and their subsequent failure to repopulate host secondary lymphoid organs likely represent collateral damage induced by FasL-expressing CD8+ host-reactive T cells. Bystander lysis of Fas+ T cells by FasL+ antigen-specific T cells has been reported in the course of viral infections.79-83 However, this collateral damage is usually of minimal significance because of its limited magnitude and because normal thymic output can replenish the peripheral T-cell pool. In contrast, we propose that, in the context of GVHD, bystander lysis of postthymic T cells can likely have far-reaching consequences. Indeed, severe thymic hypoplasia is commonly found in recipients of allogeneic hematopoietic stem cell transplants, particularly those with GVHD. Thymic-independent T-cell reconstitution via expansion of grafted postthymic T cells should be able to compensate for thymic failure.13 14 However, by abrogating this salvage pathway, bystander lysis of non–host-reactive donor T cells can dramatically compromise immune reconstitution.
Two different signaling pathways can increase Fas expression on T cells: TCR ligation and cytokines such as IFN-γ, IL-2, IL-7, and TNF-α.84-87 By definition, non–host-reactive T cells do not express antihost specific T-cell receptors. Thus, upregulation of Fas on these cells is presumably caused by secretion of IFN-γ, IL-2, IL-7, and TNF-α during the GVHD-associated cytokine storm.18,42,88,89 Interestingly, it has recently been shown in humans that acute GVHD, but not infection, is associated with increased soluble Fas serum levels.90 In the latter case, it remains to be determined whether increased serum Fas levels are caused by upregulation of Fas on donor T cells.
Patients with GVHD are profoundly immunodeficient. Therefore, they represent prime candidates for adoptive immunotherapy based on injection of donor-derived T cells specific for pathogens such as CMV or EBV.35-37 The results presented here suggest that, at least during the acute phase of GVHD, the brisk expansion of activated antihost CD8+ T cells can severely curtail the survival of transferred postthymic T cells. It will therefore be important to determine whether lysis of bystander postthymic T cells can be avoided by delaying adoptive transfer after the cytokine storm has subsided or after host-reactive T cells have gone through AICD.
Massive apoptosis: A link between GVHD and autoimmunity?
In normal individuals, apoptotic cells are readily cleared by the monocyte-macrophage system and do not elicit immune responses.52,53 However, under circumstances in which the clearance of apoptotic cells is impaired, it has been proposed that accumulation of high numbers of apoptotic cells could lead to immunogenic presentation of intracellular self-antigens and thereby initiate autoimmune responses.91,92 Direct evidence supporting this concept has recently been presented. Indeed, it has been shown that normal mice injected with large amounts of apoptotic syngeneic thymocytes (107 cells intravenously weekly for a total of 4 injections) develop a picture similar to that of systemic lupus erythematosus, characterized by autoantibodies and IgG deposition in the glomeruli.93 The investigators speculate that, when confronted with massive amounts of apoptotic (lymphoid) cells, the clearance capacity of macrophages could be overwhelmed and that abnormal (because of its level and/or duration) autoantigen presentation could pave the way to autoimmunity.93Additionally, studies in mice and humans have shown that chronic GVHD is more prevalent in individuals who have presented acute GVHD and shares many clinical, histologic, and immunologic features with autoimmune diseases such as systemic lupus erythematosus and Sjogren’s syndrome.94-96 How an alloimmune reaction (acute GVHD) can initiate autoimmunity (chronic GVHD) remains elusive. Our observations raise the possibility that massive apoptosis of donor T cells (via AICD and bystander lysis) could represent the missing link between these two processes.
Supported by the National Cancer Institute of Canada (C.P.). D.-C.R. is a senior scholar of the Fonds de la Recherche en Santé du Québec.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Claude Perreault, MD, Guy-Bernier Research Center, Maisonneuve-Rosemont Hospital, 5415 de l’Assomption Blvd, Montreal, Quebec, Canada H1T 2M4; e-mail: c.perreault@videotron.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal