Abstract
The detection of residual molecular and cytogenetic disease was prospectively compared in patients with Philadelphia-chromosome (Ph1) positive first chronic phase chronic myelogenous leukemia (CML) who underwent allogeneic transplantation of unmanipulated peripheral blood stem cells (PBSCT) (n = 29) or bone marrow (BM) (n = 62) using genotypically HLA-identical sibling donors or partially HLA-matched extended family donors. A molecular relapse (MR), as defined by two consecutive positive polymerase chain reaction (PCR) assays for the detection of M-bcr-abl transcripts in a 4-week interval, was found in two of 29 (7%) patients after PBSCT compared with 20 of 62 (32%) patients after bone marrow transplantation (BMT). This corresponds to a 4-year molecular relapse estimate (± standard error) of 7% ± 5% after PBSCT and of 44% ± 8% after BMT (P < .009). With identical follow-up periods of survivors in both patient subsets between 6 and 55 months (median, 28 months), 14 of the 20 patients with MR after BMT progressed to an isolated cytogenetic (n = 10) or a hematologic (n = 4) disease recurrence, resulting in a 4-year cytogenetic relapse estimate of 47% ± 11%, while none of the patients after PBSCT has so far relapsed (P < .006). Multivariate analysis including all potential influencial factors of posttransplant disease recurrence identified the source of stem cells (P < .02) as the only independent predictor of molecular relapse. In conclusion, this prospective comparison of molecular and cytogenetic residual disease demonstrates that peripheral blood stem cell transplants have a more pronounced activity against residual CML cells than bone marrow transplants. Prospective randomized trials comparing PBSCT and BMT in patients with first chronic phase Ph1-positive CML are strictly required to further substantiate differences in the antileukemic activity of the two stem cell sources.
BECAUSE IT IS APPARENT that recombinant human granulocyte colony-stimulating factor (rhG-CSF) can safely and efficiently be administered to healthy individuals for the mobilization of sufficient numbers of hematopoietic progenitor cells, allogeneic peripheral blood stem cell transplantation (PBSCT) becomes a rapidly developing alternative method to allogeneic bone marrow transplantation (BMT) as a broadly applied treatment modality of hematological diseases. For transplant recipients, accelerated hematologic and immune reconstitution constitute potential advantages of peripheral blood stem cell transplants over marrow grafts.1-4 These potential benefits, however, still need to be balanced against the potentially increased hazards of acute and chronic graft-versus-host disease (GVHD), which may result from the one log10 higher donor T-cell numbers in transplants of peripheral blood stem cells (PBSCs) compared with marrow grafts.3
In 15% to 25% of patients with Philadelphia-chromosome (Ph1) positive first chronic phase chronic myelogenous leukemia (CML) who underwent unmanipulated allogeneic marrow transplantation from genotypically HLA-identical sibling donors, leukemic relapse remains the primary cause of treatment failure.5 6 Relapse is considered to evolve from residual malignant cells that survive the pretransplant conditioning regimen and escape immune surveillance by allogeneic effector cells.
Residual CML cells can be detected through amplification of the unique M-bcr-abl fusion messenger (m) RNA transcript as the molecular correlate of transcriptional active Ph1-positive cells by the polymerase chain reaction (PCR). The detection of bcr-abl-mRNA in transplant patients is associated with an increased risk of CML relapse, if repeated PCR assays show positive results later than 100 days posttransplant.7 Additionally, it has been reported that the detection of bcr-abl transcripts between 6 and 12 months posttransplant correlates with an increased relapse rate.8Sustained PCR-negativity in the posttransplant course, in turn, is generally accepted to be associated with an excellent prognosis with regard to cytogenetic or hematologic disease recurrence.7-9
In the present prospective single-center study, we assessed minimal residual disease as detected by regularly performed analyses of reverse-transcription (RT)-PCR amplification of M-bcr-abl mRNA, as well as cytogenetic evaluations in patients with first chronic phase Ph1-positive CML after PBSCT (n = 29) and compared these results with those obtained in patients who underwent allogeneic BMT (n = 62) during the same observation period.
MATERIALS AND METHODS
Patients.
All patients (n = 91) undergoing BMT (n = 62) or PBSCT (n = 29) in the first chronic phase of CML with genotypically HLA-identical sibling donors or partially HLA-identical extended family donors at the University Hospital of Essen between October 1994 and June 1998 were consecutively included in the present study. Approval for all aspects of this study had been obtained by the Institutional Review Board on Medical Ethics at the Essen University Hospital. Patients were preferentially transplanted with PBSCs if at least one of the following inclusion criteria was fulfilled: (1) HLA-A,B,DRβ1,DQβ1,DPβ1disparities between recipient and family donor with a maximum of one class I and II antigen disparities in graft-versus-host (GVH) direction; (2) prolonged pretransplant interferon-α therapy; (3) a history of documented serious infectious complications within 4 months pretransplant; and (4) organ functional impairment of the donor precluding anesthesia or marrow harvest.4
All patients who were discharged after transplantation were enrolled in our long-term follow-up program. Outpatient visits were performed in at least monthly intervals during the first 6 months and at 3-month intervals during the the first 2 years posttransplant. After 2 years, patients were usually seen at yearly intervals.
Conditioning regimen.
The conditioning regimen consisted of intravenous cyclophosphamide (60 mg/kg of body weight per day x 2) in combination with fractioned total body irradiation (TBI) delivered by a60cobalt source in four daily fractions of 2.5 Gy (n = 85) or oral busulfan (BU) (1 mg/kg of body weight every 6 hours over 4 days) in combination with intravenous cyclophosphamide (60 mg/kg of body weight per day x 2) (n = 6).
All transplants were performed without ex vivo removal of donor lymphocytes from the graft. Irradiated (30 Gy) and leukocyte-depleted blood products were exclusively used for blood component substitution throughout the posttransplant course. Prophylaxis of acute GVHD consisted of intravenous methotrexate (15 mg/m2, day 1; 10 mg/m2, days 3, 6, and 11) in combination with continuous intravenous cyclosporine in all patients.10 Patient demographic characteristics are summarized in Table 1.
Demographic and Treatment Characteristics of Patients Undergoing Transplantation of Allogeneic PBSCT or BM
| . | BMT . | PBSCT . | P Value* . |
|---|---|---|---|
| No. of patients | 62 | 29 | |
| Median age (range) [yrs] | |||
| Patients | 42 (24-58) | 42 (20-58) | NS |
| Donors | 38 (9-63) | 52 (18-66) | <.0005 |
| Donor → recipient gender† | |||
| F → F | 16 (26) | 6 (21) | |
| M → M | 18 (29) | 10 (34) | NS |
| M → F | 10 (16) | 5 (17) | |
| F → M | 18 (29) | 8 (28) | |
| Donor type† | |||
| HLA-identical sibling donor | 51 (82) | 16 (55) | <.01 |
| Partially HLA-matched family donor | 11 (18) | 13 (45) | |
| Median time interval (range) between diagnosis and transplant (mos) | 18 (1-42) | 19 (5-53) | NS |
| Interferon-α pretransplant† | 29 (47) | 16 (55) | NS |
| Median graft size (range) | |||
| Nucleated cell dose (×108/kg) | 3.1 (1.0-19.9) | 9.1 (4.1-27.8) | <.0001 |
| CD34+ cell dose (×108/kg) | 3.4 (1.2-9.7) | 9.5 (3.4-25.5) | <.0001 |
| GVHD prophylaxis† | |||
| Short methotrexate + cyclosporine | 62 (100) | 29 (100) | NS |
| Myeloablative regimen† | |||
| TBI + cyclophosphamide | 58 (94) | 27 (93) | NS |
| Busulfan + cyclophosphamide | 4 (6) | 2 (7) | |
| Median follow-up (range) of survivors (mos) | 28 (6-55) | 28 (6-55) | NS |
| . | BMT . | PBSCT . | P Value* . |
|---|---|---|---|
| No. of patients | 62 | 29 | |
| Median age (range) [yrs] | |||
| Patients | 42 (24-58) | 42 (20-58) | NS |
| Donors | 38 (9-63) | 52 (18-66) | <.0005 |
| Donor → recipient gender† | |||
| F → F | 16 (26) | 6 (21) | |
| M → M | 18 (29) | 10 (34) | NS |
| M → F | 10 (16) | 5 (17) | |
| F → M | 18 (29) | 8 (28) | |
| Donor type† | |||
| HLA-identical sibling donor | 51 (82) | 16 (55) | <.01 |
| Partially HLA-matched family donor | 11 (18) | 13 (45) | |
| Median time interval (range) between diagnosis and transplant (mos) | 18 (1-42) | 19 (5-53) | NS |
| Interferon-α pretransplant† | 29 (47) | 16 (55) | NS |
| Median graft size (range) | |||
| Nucleated cell dose (×108/kg) | 3.1 (1.0-19.9) | 9.1 (4.1-27.8) | <.0001 |
| CD34+ cell dose (×108/kg) | 3.4 (1.2-9.7) | 9.5 (3.4-25.5) | <.0001 |
| GVHD prophylaxis† | |||
| Short methotrexate + cyclosporine | 62 (100) | 29 (100) | NS |
| Myeloablative regimen† | |||
| TBI + cyclophosphamide | 58 (94) | 27 (93) | NS |
| Busulfan + cyclophosphamide | 4 (6) | 2 (7) | |
| Median follow-up (range) of survivors (mos) | 28 (6-55) | 28 (6-55) | NS |
Abbreviations: F, female; M, male; HLA, human leukocyte antigen; NS, not significant; TBI, (fractionated) total body irradiation.
Comparison between continuous variables by Wilcoxon rank-sum test; comparisons between frequencies by Fisher exact-test.
No. of patients (rounded percent of patients).
Donors.
Sixteen blood stem cell donors (55%) and 51 bone marrow donors (82%) were genotypically HLA-identical with their respective recipients, while 13 blood stem cell donors (45%) and 11 bone marrow donors (18%) had a single class I- or II-antigen mismatch with regard to acute GVHD. Peripheral blood stem cells were mobilized by daily subcutaneous injections of 10 to 16 μg rhG-CSF/kg of donor body weight over 5 to 6 consecutive days. Details of stem cell aphereses performed in this study have been published previously.4
Isolation of mRNA and M-bcr-abl detection.
RNA was prepared from peripheral blood cells, bone marrow buffy coat cells, and K562 cells (positive control). RNA was extracted by the acid guanidium/phenol/chloroform method.11 The RNA was reverse transcribed into complementary DNA (cDNA) with Moloney murine leukemia virus (Mo-MuLV) reverse transcriptase (GIBCO-BRL, Gaithersburg, MD) using random hexamers (Boehringer, Mannheim, Germany). A nested PCR technique was used as previously described.12 Briefly, the cDNA was divided into two tubes, each containing a final volume of 25 μL with 1 × PCR buffer consisting of 50 mmol/L KCl, 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCL2, 0,001% gelatin (Perkin Elmer-Cetus, Weiterstadt, Germany), 0.2 mmol/L each of deoxynucleoside triphosphates (dNTP), 2.5 U Taq polymerase (Perkin Elmer-Cetus), and 12 pmol/L of each of the primers. To one half of the cDNA, a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA or β-actin mRNA specific primer set was added. This results in a positive 200 bp or 420 bp band from all human RNA, thereby controlling for the quality of mRNA and successful PCR amplification, especially in the absence of any detectable bcr-abl transcript amplification.13 To the other half, bcr-1 and abl-1 primers were added and amplification with a total of 35 cycles was performed with 45 seconds at 94°C, 45 seconds at 60°C, and 90 seconds at 72°C.12 The second round of PCR was performed using internal primers bcr-2 and abl-2 with 1 μL of the first round PCR mix as a template for an additional 35 cycles.12 PCR products were visualized by electrophoresis in ethidium bromide stained agarose gels under ultraviolet (UV) light and photographed.
PCR controls.
Elaborate measures were taken to minimize contamination by following the recommendations of Kwok and Higuchi.14 All PCR products were kept in separate laboratory rooms from patients samples, RNA, and PCR reagents. Negative control cells and blank controls were included in all RNA extraction procedures as controls to assess quality and cross-contamination between each samples. Cells from the K562 cell line were included as positive controls. Oligonucleotide primers were synthesized on an Applied Biosystems 380 B DNA synthesizer (Foster City, CA). All PCR amplifications were performed with a Perkin-Elmer model 9600 thermocycler.
Sensitivity of the PCR.
The PCR assay for the detection of BCR/ABL transcript is reported to have a sensitivity of 0.0001%.12
Definition of positive and negative PCR assays.
The PCR assays were performed without knowledge of the patient’s cytogenetic results or hematologic remission status or type of transplantation. A positive PCR test required a correct size of the bcr-abl PCR product, as well as a negative “blank” and a positive K562 amplification. A negative assay required the absence of a bcr-abl PCR product, as well as no amplification of the “blank”, but a positive K562- and β-actin- or GAPDH-PCR amplification. If a positive or negative control did not amplify as expected, the entire panel of patient samples performed in one amplification “batch” was discarded and patient samples were reevaluated. No attempts were made to quantify the PCR products in this study. For the diagnosis of molecular relapse or persistence, only those positive bcr-abl-PCR results, which were confirmed in a consecutive PCR assay from a patient sample taken within a 4-week interval were considered positive. A total of 811 evaluable peripheral blood (n = 519) or marrow samples (n = 292) were prospectively included in the present study. Blood samples were evaluated monthly within the first 6 months posttransplant and in 3-months intervals during the first 2 posttransplant years thereafter. After 2 years, those patients without evidence of molecular or cytogenetic disease were evaluated once per year. Marrow samples were evaluated by bcr-abl-PCR according to the schedule of cytogenetic analyses or in case of repeatedly positive blood samples.
Cytogenetic analysis.
Cytogenetic evaluations for the detection of the Ph1-chromosome were performed by standard metaphase karyotyping technique as previously described.15 Karyotypes of bone marrow samples were performed routinely every 3 months during the first posttransplant year and in 6-month intervals thereafter. In case of a positive bcr-abl-PCR assay in peripheral blood or bone marrow samples, an additional cytogenetic bone marrow evaluation was performed within a 4-week interval. A minimum of 20 metaphases was analyzed in all bone marrow samples. All 91 patients included in the present study had a completely Ph1-positive karyotype in pretransplant cytogenetic bone marrow evaluations.
Definition of relapse.
Hematologic relapse was diagnosed on the basis of standard hematologic criteria. An isolated cytogenetic relapse was assumed if Ph1-positive metaphases were detected in repeated cytogenetic analyses without evidence of hematologic disease.
Clinical evaluation and statistical analysis.
The diagnosis of acute and chronic GVHD was based on the characteristic clinical appearance of the symptoms of organ involvement. In case of doubt, the clinical diagnosis had to be confirmed by histologic examinations of the suspected organ whenever possible. Grading of acute or chronic GVHD followed the commonly accepted criteria.16,17 For the estimates of molecular or cytogenetic posttransplant disease, the event times of those patients who never achieved a bcr-abl- or Ph1-negative status or converted to a bcr-abl- or Ph1-positive status were calculated as the time intervals between transplant and the first date at which a positive bcr-abl- or Ph1-assay was detected. Conversely, those patients who attained a sustained bcr-abl- or Ph1-negative posttransplant status were right-censored at the last time point at which they were at risk for molecular or cytogenetic disease recurrence. Thus, patients surviving without molecular or cytogenetic disease were censored at the time point of the last molecular or cytogenetic evaluation or at the time of death. Cumulative estimates (± standard errors) were calculated by the product-limit method.18 Differences between time-to-event distribution functions were compared by the log-rank test. Comparisons between continuous covariates were performed by the two-tailed Wilcoxon rank-score test across strata. Differences between frequencies were compared by the two-tailed Fisher’s exact test (2 × 2 contingency tables) or the Mantel-Haentzel test (2 × 4 contingency table). Stepwise proportional hazards general linear model (PHGLM) analysis was used to evaluate interactions of different covariates on the analytical endpoints of molecular relapse.19 Covariates in PHGLM analyses were the stratified pretransplant disease duration (≤ 12 months v > 12 months), the stratified pretransplant treatment duration with hydroxycarbamide, busulfan, or interferon-α (≤ 12 months v > 12 months), patient and donor age (≤ median value v > median value), type of donor (genotypically HLA-identical sibling donor vpartially HLA-matched extended family donor), patient and donor gender combinations, transplanted nucleated and CD34+ cell dose/kg of recipient body weight (≤ median value v > median value), acute GVHD (grades 0 to I v grades II to IV) and chronic GVHD (absent v present), and the type of stem cell source (BMTv PBSCT). Acute and chronic GVHD were analyzed as time-dependent covariates in PHGLM analyses. Conditional risk ratios (RR) and their 95% confidence intervals (95% CI) were derived from PHGLM analyses after adjustment for significant covariates in the models.20
RESULTS
Bcr-abl-PCR results.
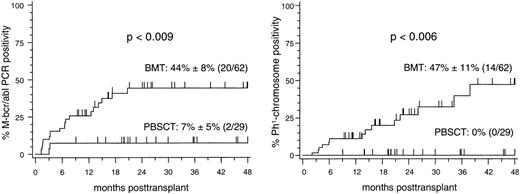
Twenty-eight of 62 patients (45%) after BMT and six of 29 patients (21%) after PBSCT were positive in at least one bcr-abl-PCR assay (P < .04). According to the applied definition of MR, a repeatedly positive bcr-abl-PCR assay within a 4-week interval was detectable in 20 patients (32%) after BMT compared with 2 patients (7%) after PBSCT (P < .009). The median time interval between transplant and the detection of MR was 181 days (range, 38 to 627 days) after BMT. In the two patients who developed MR after PBSCT, this was detected at 91 and 98 days posttransplant, respectively. The 4-year estimate (± standard error) of MR was 44% ± 8% after BMT and 7% ± 5% after PBSCT (P < .009) (Fig 1).
Four-year cumulative estimates of molecular relapse (left panel) and cytogenetic relapse (right panel) after transplantation of allogeneic bone marrow (BMT) or peripheral blood stem cells (PBSCT) in patients with first chronic phase CML. Molecular relapse was defined as two consecutive M-bcr-abl-positive PCR assays within a 4-week interval. Cytogenetic relapse was defined as reappearance of Philadelphia (Ph1) chromosome-positive marrow metaphases after transplant. Tick marks indicate patients who survive in continuous molecular (left panel) or cytogenetic (right panel) remission of CML.
Four-year cumulative estimates of molecular relapse (left panel) and cytogenetic relapse (right panel) after transplantation of allogeneic bone marrow (BMT) or peripheral blood stem cells (PBSCT) in patients with first chronic phase CML. Molecular relapse was defined as two consecutive M-bcr-abl-positive PCR assays within a 4-week interval. Cytogenetic relapse was defined as reappearance of Philadelphia (Ph1) chromosome-positive marrow metaphases after transplant. Tick marks indicate patients who survive in continuous molecular (left panel) or cytogenetic (right panel) remission of CML.
In recipients of sibling donor transplants, the estimate of MR was not significantly different from those of extended family donor transplants (37% ± 7% v 19% ± 9%) in this study. In patients with sibling donors, this estimate appeared higher after BMT (45% ± 9%) compared with PBSCT (14% ± 9%), but this difference was not significant. Patients with extended family donors, however, had a significantly higher estimate of MR after BMT (44% ± 17%) than after PBSCT (0%) (P < .02).
Because acute or chronic GVHD may interfere with disease recurrence, we further examined the association between the development of acute or chronic GVHD and the detection of MR. No significant difference between the estimate of MR in patients with grades 0 to I acute GVHD (38% ± 7%) and in those with grades II to IV acute GVHD (15% ± 9%) was detectable. In addition, the estimate of MR between patients with (28% ± 6%) or without chronic GVHD (47% ± 15%) was not significant. The influence of acute or chronic GVHD on MR in patients after BMT or PBSCT is summarized in Table2.
Influence of Acute and Chronic GVHD on Molecular Relapse of CML Leukemia After Allogeneic BMT or PBSCT
| . | BMT . | PBSCT . | P Value* . | ||
|---|---|---|---|---|---|
| Patient No. With MR/ Patient No. With GvHD (%) . | Cumulative 4-yr Estimate of MR . | Patient No. With MR/ Patient No. With GvHD (%) . | Cumulative 4-yr Estimate of MR . | ||
| Acute GVHD | |||||
| Grades 0-I | 17/46 (37) | 47% ± 9% | 2/18 (11) | 13% ± 8% | <.07 |
| Grades II-IV | 3/16 (19) | 28% ± 15% | 0/11 (0) | 0% | NS |
| Chronic GVHD | |||||
| Absent | 6/16 (37) | 63% ± 18% | 1/8 (12) | 17% ± 15% | NS |
| Present | 14/46 (30) | 39% ± 9% | 1/21 (5) | 5% ± 5% | <.03 |
| Acute or chronic GVHD | |||||
| Absent | 6/13 (46) | 65% ± 18% | 1/8 (12) | 17% ± 15% | NS |
| Present | 14/49 (29) | 39% ± 9% | 1/21 (5) | 5% ± 5% | <.03 |
| . | BMT . | PBSCT . | P Value* . | ||
|---|---|---|---|---|---|
| Patient No. With MR/ Patient No. With GvHD (%) . | Cumulative 4-yr Estimate of MR . | Patient No. With MR/ Patient No. With GvHD (%) . | Cumulative 4-yr Estimate of MR . | ||
| Acute GVHD | |||||
| Grades 0-I | 17/46 (37) | 47% ± 9% | 2/18 (11) | 13% ± 8% | <.07 |
| Grades II-IV | 3/16 (19) | 28% ± 15% | 0/11 (0) | 0% | NS |
| Chronic GVHD | |||||
| Absent | 6/16 (37) | 63% ± 18% | 1/8 (12) | 17% ± 15% | NS |
| Present | 14/46 (30) | 39% ± 9% | 1/21 (5) | 5% ± 5% | <.03 |
| Acute or chronic GVHD | |||||
| Absent | 6/13 (46) | 65% ± 18% | 1/8 (12) | 17% ± 15% | NS |
| Present | 14/49 (29) | 39% ± 9% | 1/21 (5) | 5% ± 5% | <.03 |
Abbreviations: MR, molecular relapse; GVHD, graft-versus-host disease.
Significances derived by log-rank test of event distribution functions across strata (BMT v PBSCT).
To evaluate interactions between different potential influencial factors of MR, multivariate analysis including acute and chronic GVHD as time-dependent covariates was applied (Table 3). This analysis confirmed that the relative risk (RR) of MR was significantly higher after BMT compared with PBSCT (RR, 5.7; [95% CI, 1.3 to 24.3]) (P < .02). After adjustment for the influence of the stem cell source, no other factor had a significant influence on the occurence of MR.
Proportional Hazards General Linear Model of Potential Influencial Factors of Molecular Relapse of CML After Allogeneic BMT or PBSCT
| Factors3-150 . | Univariate3-151 . | Multivariate3-152 . | RR (95% CI) . |
|---|---|---|---|
| Patient age | 0.396 | 0.286 | — |
| Donor age | 0.013 | 0.090 | — |
| Interval diagnosis to transplant | 0.015 | 0.071 | — |
| Interferon-α pretransplant | 0.964 | 0.770 | — |
| Nucleated cell dose | 0.034 | 0.495 | — |
| CD34+ cell dose | 0.059 | 0.522 | — |
| Type of donor | 0.348 | 0.973 | — |
| Donor-recipient gender combination | 0.418 | 0.431 | — |
| Acute GVHD | 0.119 | 0.244 | — |
| Chronic GVHD | 0.083 | 0.052 | — |
| Stem cell source | 0.008 | 0.019 | 5.7 (1.3-24.3) |
| Factors3-150 . | Univariate3-151 . | Multivariate3-152 . | RR (95% CI) . |
|---|---|---|---|
| Patient age | 0.396 | 0.286 | — |
| Donor age | 0.013 | 0.090 | — |
| Interval diagnosis to transplant | 0.015 | 0.071 | — |
| Interferon-α pretransplant | 0.964 | 0.770 | — |
| Nucleated cell dose | 0.034 | 0.495 | — |
| CD34+ cell dose | 0.059 | 0.522 | — |
| Type of donor | 0.348 | 0.973 | — |
| Donor-recipient gender combination | 0.418 | 0.431 | — |
| Acute GVHD | 0.119 | 0.244 | — |
| Chronic GVHD | 0.083 | 0.052 | — |
| Stem cell source | 0.008 | 0.019 | 5.7 (1.3-24.3) |
Factors were stratified according to the definitions given in the Materials and Methods section.
Significances derived by log-rank test of event distribution functions across stratified factors.
Significances, relative risk (RR) estimates, and their 95% confidence intervals (CI) derived from proportional hazards general linear model analysis after adjustment for significant factors included in model building.
Cytogenetic results.
Cytogenetic relapse developed in 14 of 62 patients after BMT (23%), but in none of the patients after PBSCT (Fig 1). The median number of Ph1-positive metaphases was 10 (range, 1 to 20) of 20 marrow metaphases at time of the diagnosis of cytogenetic relapse. With a median interval of 86 days (range, 15 to 997), diagnosis of cytogenetic relapse followed the detection of MR in 13 of the 14 cytogenetically relapsing patients after BMT. A repeated positive bcr-abl-PCR analysis had a specificity of 84%, a sensitivity of 90%, and a negative predictive value of 99% with respect to the development of cytogenetic relapse in this study. No significant association of cytogenetic relapse with either acute or chronic GVHD or the type of donor was detectable by univariate analysis (data not shown).
Clinical results.
With a median follow-up of 28 months (range, 6 to 55), 41 of 61 patients after BMT (66%) and 19 of 29 patients after PBSCT (66%) are currently alive (not significant). This translates into 4-year survival estimates of 64% ± 6% after BMT and 61% ± 11% after PBSCT, respectively (n.s.). The estimate of survival in continuous cytogenetic and hematologic remission at 4 years posttransplant is 36% ± 9% after BMT and 61% ± 11% after PBSCT (n.s.). Of the 14 patients who developed a cytogenetic or a hematologic relapse after BMT, 10 patients are currently alive. Eight of these patients achieved a molecular remission after withdrawal of cyclosporine, treatment with interferon-α or donor buffy-coat transfusions, while two patients survive in hematologic relapse. The remaining four patients died of chronic GVHD (n = 2), blastic phase CML, or influenza A virus interstitial pneumonia after donor buffy-coat transfusion as the leading cause of death.
DISCUSSION
This is the first prospective study, which compared the detection of residual disease by means of sequentially performed bcr-abl-PCR assays and cytogenetic analyses in patients with first chronic phase CML after allogeneic transplantation of unmanipulated bone marrow or PBSCs.
As the most important finding, the present study demonstrates that chronic phase CML patients have a significantly lower risk of residual molecular disease after PBSCT than patients after BMT. This finding was based on at least two consecutive positive bcr-abl-PCR assays within a 4-week time interval, because this approach has been shown to be more predictive with regard to posttransplant CML relapse than isolated findings of bcr-abl transcripts.7 The clinical significance of this approach is best illustrated by the high specificity and sensitivity between the detection of MR by bcr-abl-PCR and the development of cytogenetic relapse in this study: as expected from the molecular analyses of residual disease, patients after BMT had a significantly increased risk of cytogenetic disease recurrence compared with patients after PBSCT. Because multivariate analysis including all potential influencial factors of disease recurrence confirmed that the source of the transplant was the only independent predictor of MR, it appears justified to conclude that allogeneic transplantation of PBSCs in first chronic phase CML patients is associated with a lower risk of posttransplant disease recurrence than allogeneic BMT.
The rate of cytogenetic and hematologic relapses in patients who underwent BMT in this study (23%) is in the expected range of 15% to 25%, which has been described in most larger series of chronic phase CML patients after unmanipulated sibling donor BMT. The median time intervals from allogeneic BMT to cytogenetic or hematologic relapse have been reported to be 9 and 12 months, respectively.21The median follow-up of surviving patients in both of our patient subsets is now 28 months, and it is therefore apparent that the time frame in which the majority of cytogenetic or hematologic relapses typically manifest after BMT has been covered by the present analysis. It remains to be seen, however, whether the lower risk of MR after PBSCT is a consequence of a more effective elimination of residual CML cells or will be counterbalanced by delayed relapse events.
The biological basis for the observed differences in the rates of molecular and cytogenetic relapse after allogeneic BMT and PBSCT in first chronic phase CML patients is currently not clear. Two major differences between grafts of marrow cells and PBSCs may contribute to a higher antileukemic potential of allogeneic PBSCT: first, as in the present study, PBSC grafts contain higher numbers of hematopoietic progenitor cells compared with marrow grafts, and this may result in a more rapid and more stable donor cell engraftment.22 This hypothesis is supported by our previous preliminary observation that complete donor chimerism is attained earlier and minimal residual disease persists less frequently after PBSCT compared with BMT.23 In recipients of marrow cells from unrelated donors for treatment of high-risk acute leukemia, marrow cell doses above the median were independently associated with a better leukemia-free survival, if the transplant was performed in remission.24Both observations are consistent with a stem cell competition effect, by which a rapidly expanding normal progenitor cell compartment can inhibit or displace residual clonogenic leukemic cells after transplant. Second, unmanipulated PBSC grafts contain one log10 higher numbers of donor T and accessory cells.3,22,25,26 Because donor T cells are the most important effector cells of graft-versus-leukemia reactions in humans, transfusion of substantially higher donor T-cell numbers along with PBSCs may be associated with a more pronounced antileukemic effect against residual leukemic cells. In considering the improved numerical and functional T-cell reconstitution after allogeneic PBSCT compared with BMT and the particularly high antileukemic activity of donor buffy-coat transfusions demonstrated in patients with posttransplant CML relapse, it is tempting to speculate that the substantially lower risk of residual disease observed in the present study is at least partially mediated by donor T cells with antileukemic activity against residual CML cells.3,27 An improved graft-versus-leukemia effect associated with allogeneic PBSCT has been convincingly demonstrated in a murine leukemia model.28
Neither acute nor chronic GVHD had a significant influence on residual molecular or cytogenetic disease in the present study. This may be taken as indirect evidence that the higher antileukemic activity associated with allogeneic PBSCT does not simply rely on a graft-versus-host reaction, which acts against residual recipient hematopoietic tissue. The hypothetical mechanism of stem cell competition thus represents a new concept for a graft-versus-leukemia reaction, which is separate from GVHD and may be especially operative after allogeneic PBSCT.
In conclusion, the present study provides laboratory and clinical evidence that peripheral blood stem cell transplants have a more pronounced activity against residual CML cells than bone marrow transplants. Prospective randomized trials comparing PBSCT and BMT in patients with first chronic phase Ph1-positive CML are strictly required to further substantiate differences in the antileukemic activity of the two stem cell sources.
ACKNOWLEDGMENT
The authors thank Jitka Stockova, Melanie Kroll, and Susanne Hiebel for their excellent technical performance of the PCR analyses and are indebted to Annette Parr and Anneliese Patzelt at the cytogenetic laboratory of the Department of Internal Medicine (Tumor Research), University Hospital of Essen, for the continuous support in cytogenetic evaluations.
Supported by a grant from ‘Aktion Kampf dem Krebs’ of the German Cancer Society and a grant from ‘Deutsche Krebshilfe’ (Project-No. 70-1669-EL I).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Dietrich W. Beelen, MD, Department of Bone Marrow Transplantation, University Hospital of Essen, Hufelandstr. 55, 45122 Essen, Germany; e-mail: dietrich.beelen@uni-essen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal